Abstract
Two series of 2-(3,5-diaryl-4,5-dihydropyrazol-1-yl)-1-methyl-6-oxo-4-phenyl-1,6-dihydropyrimidine-5-carbonitriles 5a–h and 4-(4-chlorophenyl)-2-(3,5-diaryl-4,5-dihydropyrazol-1-yl)-1-methyl-6-oxo-1,6-dihydropyrimidine-5-carbonitriles 6a–h were synthesized via a cyclocondensation reaction of the corresponding 2-hydrazinopyrimidines 3a,b with the appropriate 2-propen-1-ones 4a–h. The target compounds were screened for their antiproliferative activity against A 549 (lung), HT 29 (colon), MCF 7 and MDA-MB 231 (breast) cell lines. The two most susceptible cell lines were the colon (HT 29) and breast (MDA-MB 231). Generally, the 4-unsubstitutedphenylpyrimidine derivatives 5a–h were more active than their 4-chlorophenylpyrimidine analogs 6a–h. Compounds 5e and 5g, showed high activity against three of the cell lines. The most active compound 5c possessed IC50 = 1.76 μM against A 549 cell line.
Keywords: Pyrimidine, Pyrazoline, Antiproliferative activity, Human colon (HT 29) cell line, Human breast (MDA-MB 231) cell line
1. Introduction
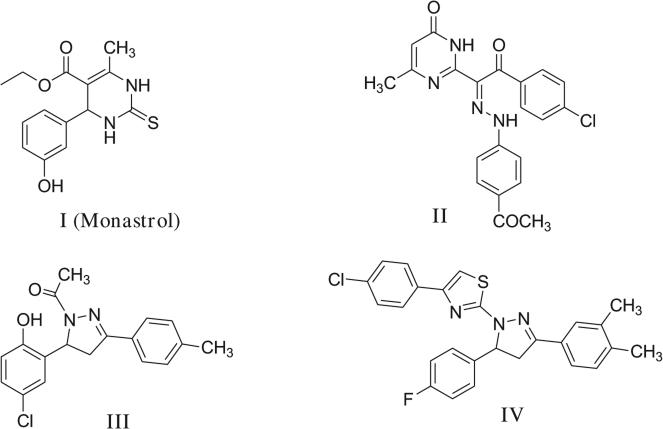
Cancer is a major worldwide health problem. Although there has been a progress in the development of treatment and prevention of cancer, this disease remains the second major cause of death in the world. Still, the successful treatment of cancer remains a challenge in the 21st century, and there is a need to search for newer and safer anticancer agents that have a broader spectrum of cytotoxicity to tumor cells [1]. With the discovery of multicomponent reaction, the dihydropyrimidinones (DHPMs) were reported for the first time by Biginelli over a century ago [2]. The multi-functionalized dihydropyrimidinones scaffold represents a class of heterocyclic compounds with significant pharmacological efficiency and are receiving considerable amount of interest. They exhibit a diverse pharmacological profile like calcium channel blockade, α1a-adrenoreceptor antagonism, antibacterial, antifungal and other related properties [3–6]. From natural marine sources, several alkaloids containing the dihydropyrimidine core unit were isolated such as batzelladine alkaloids, which were found to be potent HIV gp-120-CD4 inhibitors [7]. With the advent of combinatorial synthesis, which is particularly useful for multi-component reactions like Biginelli condensation, diverse DHMPs libraries have been synthesized and subjected to high throughput screening for biological activity [8]. By the end of the last century a structurally simple compound, monastrol I has been identified on screening a large library of diverse small molecules, as a novel cell permeable molecule that causes mitotic arrest by blocking bipolar mitotic spindle in mammalian cells [9]. Moreover, many diversely substituted dihydropyrimidinones have been synthesized showing promising antitumor activity among which the ethanehydrazonoylpyrimidinone II is an example [10] (Fig. 1).
Fig. 1.
Structures of some DHPMs and pyrazolines with antitumor activity.
On the other hand, many pyrazoline derivatives are acknowledged to possess a wide range of bioactivities. The pyrazoline motif makes up the core structure of numerous biologically active compounds. Thus, some representatives of this heterocycle exhibit antiviral/antitumor [11–13], antibacterial [14,15], anti-inflammatory [16], analgesic [17], fungistatic [18], and anti-hyperglycemic activities [19]. A series of novel 3,5-diarylpyrazolines III [20] and thiazolylpyrazoline derivatives IV were recently reported as potent anticancer agents [21] (Fig. 1).
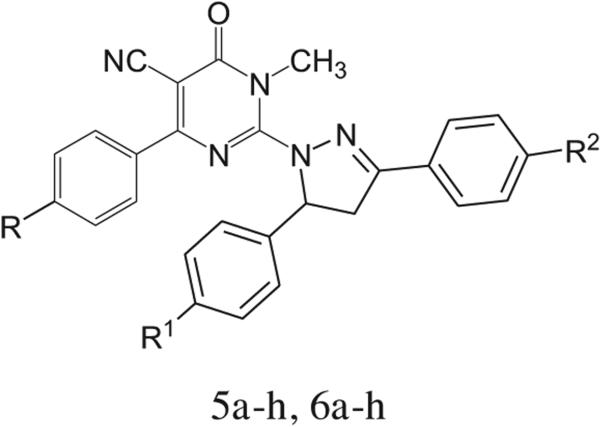
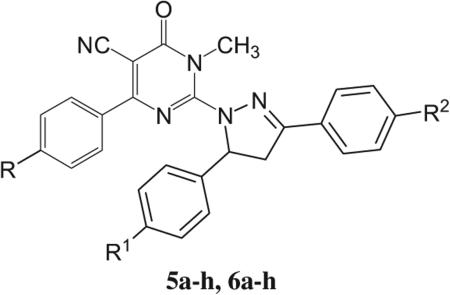
In view of these observations it was thought of interest to study the influence of pyrazoline moiety and dihydropyrimidinone scaffold combination, so that the two combined substructures, might exhibit synergistic antitumor effect. Therefore, two series of new pyrazolinyl-dihydropyrimidine derivatives, 5a–h and 6a–h, were synthesized and screened for their antitumor activity. On the molecular design level, the substitutions on the two phenyl rings on the pyrazoline moiety (R1 and R2) were subject to modifications regarding their electronic and lipophilic nature (Fig. 2). The impact of these molecular manipulations was studied from the results obtained from the cytotoxic biological assessment of all the synthesized compounds against the human lung cell line A 549, colon cancer cell line HT 29 and the breast cancer cell lines MCF 7 and MDA-MB 231.
Fig. 2.
General structure of target compounds.
2. Results and discussion
2.1. Chemistry
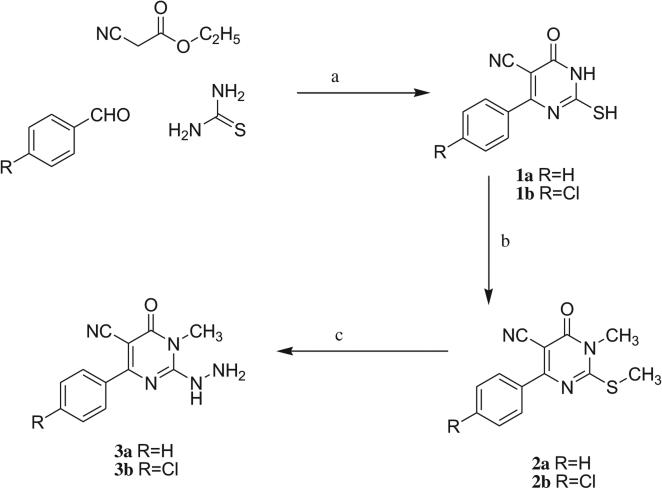
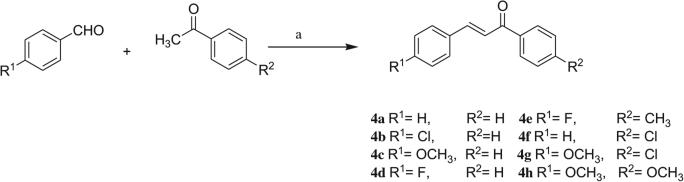
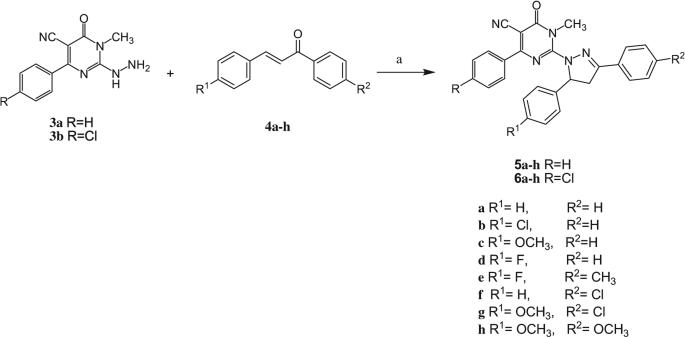
The starting 1-methyl-2-(methylthio)-6-oxo-4-pheny/(4-chlorophenyl)-1,6-dihydropyrimidine-5-carbonitriles 2a,b were synthesized in a one pot reaction from the corresponding aldehyde, ethyl cyanoacetate and thiourea followed by methylation. The corresponding 2-hydrazino-1-methyl-6-oxo-4-phenyl/(4-chlorophenyl)-1,6-dihydropyrimidine-5-carbonitriles 3a,b were obtained through hydrazinolysis of the precursor methylthio derivatives (Scheme 1). The propen-1-one derivatives 4a–h were synthesized via a base-catalyzed Claisen–Schmidt condensation of the appropriate benzaldehyde and acetophenone derivatives (Scheme 2).
Scheme 1.
Preparation of compounds 3a and 3b. Reagents and conditions: a: potassium carbonate/absolute ethanol, reflux, 12 h; b: methyl iodide, potassium carbonate, dry DMF, RT, 3 h; c: hydrazine hydrate/absolute ethanol, reflux, 12 h.
Scheme 2.
Preparation of compounds 4a–h. Reagents and conditions: a: sodium hydroxide/absolute ethanol, RT, 24 h.
The target compounds 5a–h and 6a–h were obtained through a cyclocondensation reaction of the corresponding hydrazinopyrimidine derivatives 3a,b and the appropriate propenones 4a–h in absolute ethanol in the presence of sodium hydroxide (Scheme 3). Postulated structures of the newly synthesized compounds were in good agreement with their IR, 1H NMR, 13C NMR, mass spectral and elemental analyses data. The 1H NMR spectra of the target compounds showed a prominent AMX system for the protons at C-4 and C-5 of the pyrazoline ring. Proton at C-4 (HA) appeared as doublet of doublet at δ 2.65–3.23 ppm, proton at C-4 (HM) appeared as doublet of doublet at δ 3.11–3.92 ppm, and proton at C-5 (HX) appeared as doublet of doublet at δ 4.10–5.30 ppm. There are three coupling constants: JAX = 6.3–6.9 Hz, JAM = 17.1–18.2 Hz, and JMX = 11.6–12.3 Hz. Other protons (N–CH3, OCH3, aromatic Hs) were shown in their usual range. 13C NMR showed the characteristic C-4 and C-5 carbon signals of the pyrazoline ring at δ 40.3–44.3 and 52.5–60.6 ppm, respectively, in addition to the other signals attributed to the carbon skeleton of the target compounds (c.f. Experimental section).
Scheme 3.
Synthesis of compounds 5a–h and 6a–h. Reagents and conditions: a: sodium hydroxide/absolute ethanol, reflux, 72 h.
2.2. Antiproliferative activity
The antiproliferative activity of all synthesized compounds were investigated against four human cell lines, namely, lung (A 549), colon (HT 29) and breast (MCF 7 and MDA-MB 231) using doxorubicin (Dox) as positive control. Compounds were first evaluated in triplicate for their percent proliferation inhibition. All the tested compounds revealed percentage inhibition above 60% and subsequently, their IC50 values were calculated in μM from a graph displaying the dose–survival percentage curve obtained after testing 8 concentrations for each tested compound with four replicates per concentration (Table 1). Generally, results showed that the two most susceptible cell lines were the colon (HT 29) and the breast (MDA-MB 231) cell lines that were inhibited by the tested compounds at IC50 values ranging from 2.49 to 19.51 μM and 3.99–29.14 μM, respectively. Apart from some exceptions, inhibition of the other two cell lines required higher concentrations of the tested compounds. Also, it could be noticed that the 4-chlorophenylpyrimidine derivatives 6a–h were generally less potent than their corresponding 4-unsubstitutedphenylpyri midine analogs 5a–h. Regarding the activity of the tested compounds against HT 29 and MDA-MB 231 cell lines, the most active compound against both cell lines was 5a which had no substitutions on any of the phenyl rings (IC50 = 2.49 and 3.99 μM, respectively). Activity of 5a against colon HT 29 cell line was slightly higher than doxorubicin (IC50 Dox = 2.75 μM). Substitution on the phenyl groups located on positions 3 and 5 of the pyrazoline ring led to decrease in activity. Compounds substituted with (R2) only such as compound 5f or having another substitution (R1) such as 5e, 5g and 5h were more effective against both HT 29 and MDA-MB 231 cell lines than those having only R1 substituent as in 5b–d. The pattern of activity of compounds 6a–h against HT 29 and MDA-MB 231 cell lines was different from that of compounds 5a–h. The promising compounds emerging in this series were those substituted with R2 such as 6e and 6f, in addition to the derivatives substituted with R1 only as in 6c and 6d.
Table 1.
IC50 values (μM) of the in vitro antiproliferative activity of the tested compounds against A 549 (lung), HT 29 (colon), MCF 7 and MDA-MB 231(breast) cell lines.
Regarding activity against lung (A 549) and breast (MCF 7) cell lines, the 4-unsubstitutedphenylpyrimidine analogs 5a–h were more effective than their 4-chlorophenyl analogs. Regarding the activity against the lung (A 549) cell line, the most active compound was 5c (IC50 = 1.76 μM). Compounds 5b, 5c and 5e showed activity against lung A 549 cell line comparable to or higher than doxorubicin (IC50 = 3.25, 1.76 and 2.00 μM, respectively, c.f. IC50 Dox = 3.13 μM). Good activity was also demonstrated by compound 5g (IC50 = 6.07 μM, respectively). Lower activity was observed with the other tested compounds. Considering the activity against the breast (MCF 7) cell line, only two compounds exhibited promising activity; namely 5b and 5g (IC50 = 3.28 and 4.80 μM, respectively).
Concerning the nature of substituent groups R1 and R2, there was no consistent relation that could be established between the lipophilicity and/or electronic property of these groups and the antiproliferative activity.
From this SAR analysis, it was noteworthy that some compounds exhibited promising activity against three of the cell lines such as compounds 5e (IC50 = 2.00, 4.62 and 5.10 μM against A 549, HT 29 and MDA-MB 231, respectively) and 5g (IC50 = 6.07, 9.12 and 4.80 μM against A 549, HT 29 and MCF 7, respectively). These compounds may be candidates for future lead optimization.
3. Conclusion
Two series of pyrimidine-pyrazoline hybrids, 5a–h and 6a–h, were synthesized via a cyclocondensation reaction of the precursor 2-hydrazinopyrimidines 3a,b and the appropriate propenones 4a–h. The target compounds were screened for their antiproliferative activity against four human cell lines: lung (A 549), colon (HT 29), and breast (MCF 7 and MDA-MB 231) cell lines. The two most susceptible cell lines were the colon (HT 29) and breast (MDA-MB 231). Generally the 4-unsubstitutedphenylpyrimidine analogs 5a–h were more effective than their chloro analogs 6a–h. Compounds 5e and 5g, showing high activity against three of the cell lines, were considered promising lead compounds for future optimization.
4. Experimental
4.1. Chemistry
Melting points were determined with Stuart SMP3 version 5 apparatus and were uncorrected. FT-IR spectra were recorded on Bruker FT-IR 8400S spectrophotometer using KBr cell. Unless otherwise noted, 1H NMR spectra were recorded in DMSO-d6 on Varian mercury 300BB at 300 MHz. 13C NMR spectra were run at 75.46 MHz. Chemical shifts were given in δ as parts per million (ppm) downfield from tetramethylsilane (TMS) as internal standard. The electron impact (EI) mass spectra were recorded on Finnigan Mat SSQ 7000 (70 eV) mass spectrometer. Elemental microanalysis was performed at the Regional Center for Mycology and Biotechnology, Azhar University. TLC were monitored on FLUKA silica gel TLC aluminum cards (0.2 mm thickness) with fluorescent indicator 254 nm using chloroform/methanol (9.5:0.5) as eluant to follow the course of the reactions and to check the purity of the products. All reagents and solvents were purified and dried by standard techniques.
4.1.1. 6-Oxo-4-phenyl/(4-chlorophenyl)-2-sulfanyl-1,6-dihydropyrimidine-5-carbonitriles (1a,b)
The titled compounds 1a and 1b were synthesized according to the reported methods [22].
4.1.2. 1-Methyl-2-(methylthio)-6-oxo-4-phenyl/(4-chlorophenyl)-1,6-dihydropyrimidine-5-carbonitriles (2a,b) and 2-hydrazino-1-methyl-6-oxo-4-phenyl/(4-chlorophenyl)-1,6-dihydropyrimidine-5-carbonitriles (3a,b)
The titled compounds 2a, 2b, 3a and 3b were synthesized according to the reported methods [23].
4.1.3. (E)-1-(Un)substitutedphenyl-3-(un)substitutedphenylprop-2-en-1-ones (4a–h)
Compounds 4a, 4c, 4f, 4g [24], 4b [25], 4d, 4e [26] and 4h [27] were synthesized according to the literature procedures.
4.1.4. General procedure for the preparation of compounds (5a–h) and (6a–h)
A mixture of compound 3a/3b (4 mmol), the appropriate propenone 4a–h (4 mmol) and sodium hydroxide (0.2g, 5 mmol) in absolute ethanol (30 ml) was refluxed for 72 h. The reaction mixture was poured on water, neutralized with 2 N hydrochloric acid and the residue was filtered off. The crude product obtained was crystallized from isopropanol.
4.1.4.1. 2-(3,5-Diphenyl-4,5-dihydropyrazol-1-yl)-1-methyl-6-oxo-4-phenyl-1,6-dihydropyrimidine-5-carbonitrile (5a)
M.p. 160–162 °C, yield: 75%. IR νmax/cm−1: 3057 (CH aromatic), 2950 (CH aliphatic), 2222 (CN), 1681 (C=O), 1614 (C=N). 1H NMR (CDCl3): δ 2.55 (s, 3H, N–CH3), 3.23 (dd, 1H, C4–HA pyrazoline, JAX = 6.6 Hz, JAM = 17.9 Hz), 3.58 (dd, 1H, C4–HM pyrazoline, JMX = 11.8 Hz, JMA = 17.9 Hz), 4.75 (dd, 1H, C5–HX pyrazoline, JXA = 6.6 Hz, JXM = 11.9 Hz), 7.27–7.80 (m, 15H, aromatic H). Anal. Calcd. for C27H21N5O (431.39): C, 75.16; H, 4.91; N, 16.23. Found: C, 75.22; H, 4.95; N, 16.35. Correct in all.
4.1.4.2. 2-[5-(4-Chlorophenyl)-3-phenyl-4,5-dihydropyrazol-1-yl]-1-methyl-6-oxo-4-phenyl-1,6-dihydropyrimidine-5-carbonitrile (5b)
M.p. 173–174 °C, yield: 71%. IR νmax/cm−1: 3061 (CH aromatic), 2920, 2850 (CH aliphatic), 2223 (CN), 1681 (C=O), 1622 (C=N). 1H NMR: δ 2.51 (s, 3H, N–CH3), 2.65 (dd, 1H, C4–HA pyrazoline, JAX = 6.4 Hz, JAM = 17.8 Hz), 3.11 (dd, 1H, C4–HM pyrazoline, JMX = 11.9 Hz, JMA = 17.8 Hz), 4.38 (dd, 1H, C5–HX pyrazoline, JXA = 6.4 Hz, JXM = 11.9 Hz), 7.40–7.67 (m, 14H, aromatic H). MS, m/z (%): 465.40 (M+, 0.28); 80.00 (100). Anal. Calcd. for C27H20ClN5O (465.93): C, 69.60; H, 4.33; N, 15.03. Found: C, 69.58; H, 4.37; N, 15.11.
4.1.4.3. 2-[5-(4-Methoxyphenyl)-3-phenyl-4,5-dihydropyrazol-1-yl]-1-methyl-6-oxo-4-phenyl-1,6-dihydropyrimidine-5-carbonitrile (5c)
M.p. 92–94 °C, yield: 65%. IR νmax/cm−1: 3059 (CH aromatic), 2922, 2848 (CH aliphatic), 2222 (CN), 1681 (C=O), 1608 (C=N). 1H NMR: δ 2.47 (s, 3H, N–CH3), 2.95 (dd, 1H, C4–HA pyrazoline, JAX = 6.5 Hz, JAM = 17.9 Hz), 3.28 (dd, 1H, C4–HM pyrazoline, JMX = 11.9 Hz, JMA = 17.9 Hz), 3.78 (s, 3H, OCH3), 4.24 (dd, 1H, C5–HX pyrazoline, JXA = 6.5 Hz, JXM = 11.9 Hz), 7.33–7.87 (m, 14H, aromatic H). 13C NMR: δ 27.9 (N–CH3), 40.3 (C-4 pyrazoline), 54.7 (C-5 pyrazoline), 55.9 (OCH3), 112.9–143.8 (aromatic Cs), 150.5 (C-2 pyrazoline), 160.2 (C–OCH3), 161.0 (C-2 pyrimidine), 161.3 (C=O). Anal. Calcd. for C28H23N5O2 (461.51): C, 72.87; H, 5.02; N, 15.17. Found: C, 72.89; H, 5.11; N, 15.26.
4.1.4.4. 2-[5-(4-Fluorophenyl)-3-phenyl-4,5-dihydropyrazol-1-yl]-1-methyl-6-oxo-4-phenyl-1,6-dihydropyrimidine-5-carbonitrile (5d)
M.p. 130–131 °C, yield: 68%. IR νmax/cm−1: 3061 (CH aromatic), 2920, 2848 (CH aliphatic), 2222 (CN), 1681 (C=O), 1602 (C=N). 1H NMR: δ 2.57 (s, 3H, N–CH3), 2.95 (dd, 1H, C4–HA pyrazoline, JAX = 6.4 Hz, JAM = 17.9 Hz), 3.20 (dd, 1H, C4–HM pyrazoline, JMX = 11.9 Hz, JMA = 17.9 Hz), 4.25 (dd, 1H, C5–HX pyrazoline, JXA = 6.4 Hz, JXM = 11.9 Hz), 7.15–7.95 (m, 14H, aromatic H). 13C NMR: δ 26.5 (N–CH3), 44.3 (C-4 pyrazoline), 60.6 (C-5 pyrazoline), 114.5–142.6 (aromatic Cs), 150.3 (C-2 pyrazoline), 158.3 (C-2 pyrimidine), 160.2 (C=O). Anal. Calcd. for C27H20FN5O (449.48): C, 72.15; H, 4.48; N, 15.58. Found: C, 72.21; H, 4.53; N, 15.69.
4.1.4.5. 2-[5-(4-Fluorophenyl)-3-(4-tolyl)-4,5-dihydropyrazol-1-yl]-1-methyl-6-oxo-4-phenyl-1,6-dihydropyrimidine-5-carbonitrile (5e)
M.p. 145–147 °C, yield: 62%. IR νmax/cm−1: 3059 (CH aromatic), 2920, 2850 (CH aliphatic), 2222 (CN), 1674 (C=O), 1622 (C=N). 1H NMR: δ 2.31 (s, 3H, CH3), 2.52 (s, 3H, N–CH3), 3.06 (dd, 1H, C4–HA pyrazoline, JAX = 6.5 Hz, JAM = 17.9 Hz), 3.34 (dd, 1H, C4–HM pyrazoline, JMX = 11.9 Hz, JMA = 17.9 Hz), 4.23 (dd, 1H, C5–HX pyrazoline, JXA = 6.5 Hz, JXM = 11.9 Hz), 7.22–7.85 (m, 13H, aromatic H). 13C NMR: δ 21.1 (CH3), 26.4 (N–CH3), 40.3 (C-4 pyrazoline), 57.6 (C-5 pyrazoline), 115.5–143.4 (aromatic Cs), 150.3 (C-2 pyrazoline), 159.6 (C-2 pyrimidine), 161.6 (C=O). Anal. Calcd. for C28H22FN5O (463.51): C, 72.56; H, 4.78; N, 15.11. Found: C, 72.58; H, 4.83; N, 15.25.
4.1.4.6. 2-[3-(4-Chlorophenyl)-5-phenyl-4,5-dihydropyrazol-1-yl]-1-methyl-6-oxo-4-phenyl-1,6-dihydropyrimidine-5-carbonitrile (5f)
M.p. 133–135 °C, yield: 77%. IR νmax/cm−1: 3061 (CH aromatic), 2920, 2848 (CH aliphatic), 2223 (CN), 1681 (C=O), 1616 (C=N). 1H NMR: δ 2.51 (s, 3H, N–CH3), 3.05 (dd, 1H, C4–HA pyrazoline, JAX = 6.6 Hz, JAM = 18.0 Hz), 3.42 (dd, 1H, C4–HM pyrazoline, JMX = 11.9 Hz, JMA = 18.0 Hz), 4.25 (dd, 1H, C5–HX pyrazoline, JXA = 6.6 Hz, JXM = 11.9 Hz), 7.10–7.95 (m, 14H, aromatic H). Anal. Calcd. for C27H20ClN5O (465.93): C, 69.60; H, 4.33; N, 15.03. Found: C, 69.66; H, 4.37; N, 15.14.
4.1.4.7. 2-[3-(4-Chlorophenyl)-5-(4-methoxyphenyl)-4,5-dihydropyrazol-1-yl]-1-methyl-6-oxo-4-phenyl-1,6-dihydropyrimidine-5-carbonitrile (5g)
M.p. 124–126 °C, yield: 65%. IR νmax/cm−1: 3061 (CH aromatic), 2922, 2872 (CH aliphatic), 2223 (CN), 1681 (C=O), 1621 (C=N). 1H NMR (CDCl3): δ 2.30 (s, 3H, N–CH3), 3.09 (dd, 1H, C4–HA pyrazoline, JAX = 6.4 Hz, JAM = 18.2 Hz), 3.84 (s, 3H, OCH3), 3.87 (dd, 1H, C4–HM pyrazoline, JMX = 11.9 Hz, JMA = 18.2 Hz), 4.80 (dd, 1H, C5–HX pyrazoline, JXA = 6.4 Hz, JXM = 11.9 Hz), 6.84–7.59 (m, 13H, aromatic H). 13C NMR: δ 27.5 (N–CH3), 40.4 (C-4 pyrazoline), 52.5 (C-5 pyrazoline), 55.8 (OCH3), 120.7–142.4 (aromatic Cs), 151.5 (C-2 pyrazoline), 159.3 (C–OCH3), 160.0 (C-2 pyrimidine), 162.3 (C=O). MS, m/z (%): 495.95 (M+, 2.81); 105.00 (100). Anal. Calcd. for C28H22ClN5O2 (495.96): C, 67.81; H, 4.47; N, 14.12. Found: C, 68.02; H, 4.53; N, 14.31.
4.1.4.8. 2-[3,5-Bis-(4-methoxyphenyl)-4,5-dihydropyrazol-1-yl]-1-methyl-6-oxo-4-phenyl-1,6-dihydropyrimidine-5-carbonitrile (5h)
M.p. 196–198 °C, yield: 58%. IR νmax/cm−1: 3061 (CH aromatic), 2916, 2848 (CH aliphatic), 2222 (CN), 1681 (C=O), 1600 (C=N). 1H NMR (CDCl3): δ 2.33 (s, 3H, N–CH3), 3.15 (dd, 1H, C4–HA pyrazoline, JAX = 6.4 Hz, JAM = 17.9 Hz), 3.89 (s, 6H, 2OCH3), 3.92 (dd, 1H, C4–HM pyrazoline, JMX = 11.8 Hz, JMA 17.9 Hz), 5.30 (dd, 1H, C5–HX pyrazoline, JXA = 6.4 Hz, JXM = 11.8 Hz), 6.91–7.67 (m, 13H, aromatic H). MS, m/z (%): 491.05 (M+, 23.97); 343.00 (100). Anal. Calcd. for C29H25N5O3 (491.54): C, 70.86; H, 5.13; N, 14.25. Found: C, 70.89; H, 5.17; N, 14.33.
4.1.4.9. 4-(4-Chlorophenyl)-2-(3,5-diphenyl-4,5-dihydropyrazol-1-yl)-1-methyl-6-oxo-1,6-dihydropyrimidine-5-carbonitrile (6a)
M.p. 270–272 °C, yield: 68%. IR νmax/cm−1: 3061 (CH aromatic), 2918, 2848 (CH aliphatic), 2193 (CN), 1681 (C=O), 1614 (C=N). 1H NMR (CDCl3): δ 2.34 (s, 3H, N–CH3), 3.10 (dd, 1H, C4–HA pyrazoline, JAX = 6.9 Hz, JAM = 17.1 Hz), 3.91 (dd, 1H, C4–HM pyrazoline, JMX = 12.3 Hz, JMA = 17.1 Hz), 5.23 (dd, 1H, C5–HX pyrazoline, JXA = 6.9 Hz, JXM = 12.3 Hz), 6.77–7.65 (m, 14H, aromatic H). Anal. Calcd. for C27H20ClN5O (465.93): C, 69.60; H, 4.33; N, 15.03. Found: C, 68.68; H, 4.34; N, 15.12.
4.1.4.10. 4-(4-Chlorophenyl)-2-[5-(4-chlorophenyl)-3-phenyl-4,5-dihydropyrazol-1-yl]-1-methyl-6-oxo-1,6-dihydropyrimidine-5-carbonitrile (6b)
M.p. 265–267 °C. IR νmax/cm−1: 3062 (CH aromatic), 2920, 2848 (CH aliphatic), 2193 (CN), 1662 (C=O), 1601 (C=N). 1H NMR (CDCl3): δ 2.50 (s, 3H, N–CH3), 3.05 (dd, 1H, C4–HA pyrazoline, JAX = 6.3 Hz, JAM = 17.7 Hz), 3.17 (dd, 1H, C4–HM pyrazoline, JMX = 11.9 Hz, JMA = 17.7 Hz), 3.86 (dd, 1H, C5–HX pyrazoline, JXA = 6.3 Hz, JXM = 11.9 Hz), 6.84–7.67 (m, 13H, aromatic H). 13C NMR: δ 27.4 (N–CH3), 40.4 (C-4 pyrazoline), 56.6 (C-5 pyrazo-line), 114.4–142.2 (aromatic Cs), 153.3 (C-2 pyrazoline), 158.9 (C-2 pyrimidine), 164.2 (C=O). MS, m/z (%): 500.95 (M+, 16.43); 120.00 (100). Anal. Calcd. for C27H19Cl2N5O (500.38): C, 64.81; H, 3.83; N, 14.00. Found: C, 64.86; H, 3.81; N, 14.13.
4.1.4.11. 4-(4-Chlorophenyl)-2-[5-(4-methoxyphenyl)-3-phenyl-4,5-dihydropyrazol-1-yl]-1-methyl-6-oxo-1,6-dihydropyrimidine-5-carbonitrile (6c)
M.p. 275–277 °C, yield: 64%. IR νmax/cm−1: 3064 (CH aromatic), 2920, 2848 (CH aliphatic), 2193 (CN), 1666 (C=O), 1597 (C=N). 1H NMR (CDCl3): δ 2.40 (s, 3H, N–CH3), 3.15 (dd, 1H, C4–HA pyrazoline, JAX = 6.6 Hz, JAM = 17.9 Hz), 3.89 (dd, 1H, C4–HM pyrazoline, JMX = 11.6 Hz, JMA = 17.9 Hz), 4.80 (s, 3H, OCH3), 4.23 (dd, 1H, C5–HX pyrazoline, JXA = 6.6 Hz, JXM = 11.6 Hz), 6.80–7.58 (m, 13H, aromatic H). 13C NMR: δ 26.5 (N–CH3), 44.0 (C-4 pyrazoline), 54.8 (C-5 pyrazoline), 55.3 (OCH3), 113.5–136.7 (aromatic Cs), 150.7 (C-2 pyrazoline), 159.2 (C–OCH3), 162.8 (C-2 pyrimidine), 168.25 (C=O). Anal. Calcd. for C28H22ClN5O2 (495.96): C, 67.81; H, 4.47; N, 14.12. Found: C, 67.79; H, 4.51; N, 14.23.
4.1.4.12. 4-(4-Chlorophenyl)-2-[5-(4-fluorophenyl)-3-phenyl-4,5-dihydropyrazol-1-yl]-1-methyl-6-oxo-1,6-dihydropyrimidine-5-carbonitrile (6d)
M.p. 281–282 °C, yield: 76%. IR νmax/cm−1: 3064 (CH aromatic), 2920, 2850 (CH aliphatic), 2194 (CN), 1662 (C=O), 1621 (C=N). 1H NMR: δ 2.50 (s, 3H, N–CH3), 3.18 (dd, 1H, C4–HA pyrazoline, JAX = 6.5 Hz, JAM = 17.9 Hz), 3.41 (dd, 1H, C4–HM pyrazoline, JMX = 11.9 Hz, JMA = 17.9 Hz), 4.10 (dd, 1H, C5–HX pyrazoline, JXA = 6.5 Hz, JXM = 11.9 Hz), 7.18–7.88 (m, 13H, aromatic H). MS, m/z (%): 483.10 (M+, 0.64); 105.00 (100). Anal. Calcd. for C27H19ClFN5O (483.92): C, 67.01; H, 3.96; N, 14.47. Found: C, 67.08; H, 4.01; N, 14.58.
4.1.4.13. 4-(4-Chlorophenyl)-2-[5-(4-fluorophenyl)-3-(4-tolyl)-4,5-dihydropyrazol-1-yl]-1-methyl-6-oxo-1,6-dihydropyrimidine-5-carbonitrile (6e)
M.p. 269–271 °C, yield: 66%. IR νmax/cm−1: 3061 (CH aromatic), 2922, 2850 (CH aliphatic), 2194 (CN), 1661 (C=O), 1597 (C=N). 1H NMR: δ 2.26 (s, 3H, CH3), 2.54 (s, 3H, N–CH3), 2.95 (dd, 1H, C4–HA pyrazoline, JAX = 6.3 Hz, JAM = 17.8 Hz), 3.41 (dd, 1H, C4–HM pyrazoline, JMX = 11.8 Hz, JMA = 17.8 Hz), 4.23 (dd, 1H, C5–HX pyrazoline, JXA = 6.3 Hz, JXM = 11.8 Hz), 7.13–7.88 (m, 12H, aromatic H). Anal. Calcd. for C28H21ClFN5O (497.95): C, 67.54; H, 4.25; N, 14.06. Found: C, 67.57; H, 4.26; N, 14.13.
4.1.4.14. 4-(4-Chlorophenyl)-2-[3-(4-chlorophenyl)-5-phenyl-4,5-dihydropyrazol-1-yl]-1-methyl-6-oxo-1,6-dihydropyrimidine-5-carbonitrile (6f)
M.p. 245–247 °C, yield: 75%. IR νmax/cm−1: 3061 (CH aromatic), 2920, 2850 (CH aliphatic), 2194 (CN), 1660 (C=O), 1614 (C=N). 1H NMR: δ 2.50 (s, 3H, N–CH3), 2.99 (dd, 1H, C4–HA pyrazoline, JAX = 6.5 Hz, JAM = 17.7 Hz), 3.38 (dd, 1H, C4–HM pyrazoline, JMX = 11.9 Hz, JMA = 17.7 Hz), 4.26 (dd, 1H, C5–HX pyrazoline, JXA = 6.5 Hz, JXM = 11.9 Hz), 7.23–7.95 (m, 13H, aromatic H). MS, m/z (%): 500.20 (M+, 3.46); 138.95 (100). Anal. Calcd. for C27H19Cl2N5O (500.38): C, 64.81; H, 3.83; N, 14.00. Found: C, 64.86; H, 3.88; N, 14.05.
4.1.4.15. 4-(4-Chlorophenyl)-2-[3-(4-chlorophenyl)-5-(4-methoxyphenyl)-4,5-dihydropyrazol-1-yl]-1-methyl-6-oxo-1,6-dihydropyrimidine-5-carbonitrile (6g)
M.p. 262–263 °C, yield: 72%. IR νmax/cm−1: 3061 (CH aromatic), 2922, 2848 (CH aliphatic), 2193 (CN), 1662 (C=O), 1595 (C=N). 1H NMR: δ 2.51 (s, 3H, N–CH3), 3.07 (dd, 1H, C4–HA pyrazoline, JAX = 6.3 Hz, JAM = 18.2 Hz), 3.51 (dd, 1H, C4–HM pyrazoline, JMX = 11.6 Hz, JMA = 18.2 Hz), 3.80 (s, 3H, OCH3), 4.31 (dd, 1H, C5–HX pyrazoline, JXA = 6.3 Hz, JXM = 11.6 Hz), 7.15–7.95 (m, 12H, aromatic H). 13C NMR: δ 26.5 (N–CH3), 41.2 (C-4 pyrazoline), 53.5 (C-5 pyrazoline), 55.9 (OCH3), 120.7–138.4 (aromatic Cs), 151.5 (C-2 pyrazoline), 159.8 (C–OCH3), 160.5 (C-2 pyrimidine), 162.3 (C=O). Anal. Calcd. for C28H21Cl2N5O2 (530.40): C, 63.40; H, 3.99; N, 13.20. Found: C, 63.44; H, 4.02; N, 13.31.
4.1.4.16. 4-(4-Chlorophenyl)-2-[3,5-bis-(4-methoxyphenyl)-4,5-dihydropyrazol-1-yl]-1-methyl-6-oxo-1,6-dihydropyrimidine-5-carbonitrile (6h)
M.p. >300 °C, yield: 74%. IR νmax/cm−1: 3061 (CH aromatic), 2922, 2850 (CH aliphatic), 2193 (CN), 1654 (C=O), 1598 (C=N). 1H NMR (CDCl3): δ 2.34 (s, 3H, N–CH3), 3.05 (dd, 1H, C4–HA pyrazoline, JAX = 6.8 Hz, JAM = 17.9 Hz), 3.85 (dd, 1H, C4–HM pyrazoline, JMX = 11.8 Hz, JMA = 17.9 Hz), 3.88 (s, 6H, 2 OCH3), 5.80 (dd, 1H, C5–HX pyrazoline, JXA = 6.7 Hz, JXM = 11.8 Hz), 7.10–7.91 (m, 12H, aromatic H). Anal. Calcd. for C29H24ClN5O3 (525.99): C, 66.22; H, 4.60; N, 13.31. Found: C, 66.21; H, 4.67; N, 13.35.
4.2. Antiproliferative activity
4.2.1. Cell culture
The tumor cells were obtained from ATCC. Cells were incubated under standard cell culture conditions at 37 °C in a humidified atmosphere with 5% CO2. Cells were grown in RPMI 1640 supplemented with 5% fetal bovine serum, 2 mM glutamine, 100 units/mL streptomycin, and 0.25 mg/mL amphotericin. Cells were harvested at 70 and 90% confluence with trypsin/EDTA and used immediately. Cell count and viability were determined by Trypan blue staining followed by hemocytometry. Only cultures displaying >95% cell viability were used for performing the experiments [28].
4.2.2. Growth assay
Tissue culture treated microtiter 96-well plates were seeded at a density of 5000 cells/well. The plates were incubated for 18–24 h prior to any treatment. All test compounds were solubilized in 100% DMSO and diluted with media to obtain final DMSO concentration of 0.1%. Cells were dosed. Cell viability was measured 72 h after treatment by Cell Titer Glo Assay (Promega), which is a luminescent assay that is an indicator of live cells as a function of metabolic activity and ATP content. The assay was performed according to the manufacturer's specifications. Luminescence was measured by a Perkin Elmer Victor® multi-label plate reader.
Effects of the synthesized compounds on tumor cell growth were assessed and potency was expressed in terms of the compounds IC50 values. After testing 8 concentrations for each compound with four replicates per concentration, dose–response curves were constructed and analyzed using PrismTM 4 (Graph-Pad software, San Diego, CA). IC50 values were calculated in μM from those graphs displaying the dose–survival percentage curve for each compound using a four parameter logistic equation.
Supplementary Material
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ejmech.2013.10.003.
References
- 1.Jin C, Liang Y-J, He H, Fu L. Eur. J. Med. Chem. 2011;46:429–432. doi: 10.1016/j.ejmech.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Biginelli P. Gazz. Chim. Ital. 1893;23:360–413. [Google Scholar]
- 3.Kappe CO. Eur. J. Med. Chem. 2000;35:1043–1052. doi: 10.1016/s0223-5234(00)01189-2. [DOI] [PubMed] [Google Scholar]
- 4.Barrow JC, Nantermet PG, Selnick HG, Glass KL, Rittle KE, Gilbert KF, Chang RSL, Malley SSO', Olah TV, Ellis JD, Barrish A, Kassahun K, Nagarathnam D, Forray CJ. J. Med. Chem. 2000;43:2703–2718. doi: 10.1021/jm990612y. [DOI] [PubMed] [Google Scholar]
- 5.Rovnyak GC, Kimball SD, Beyer B, Cucinotta G, Di-Marco JD, Gougoutas J, Hedberg A, Malley M, McCarthy JP, Zhang R, Moreland S. J. Med. Chem. 1995;38:119–129. doi: 10.1021/jm00001a017. [DOI] [PubMed] [Google Scholar]
- 6.Nagarathnam D, Miao SW, Harrel CM, Gluchowski C. J. Med. Chem. 1999;42:4764–4777. doi: 10.1021/jm990200p. [DOI] [PubMed] [Google Scholar]
- 7.Heys L, Moore CG, Murphy PJ. Chem. Soc. Rev. 2000;29:57–67. [Google Scholar]
- 8.Kappe CO. Acc. Chem. Res. 2000;33:879–888. doi: 10.1021/ar000048h. [DOI] [PubMed] [Google Scholar]
- 9.Kapoor TM, Mayer TU, Coughlin MH, Mitchison TJ. J. Cell Biol. 2000;150:975–988. doi: 10.1083/jcb.150.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edrees MM, Farghaly TA, El-Hag FAA, Abdalla MM. Eur. J. Med. Chem. 2010;45:5702–5707. doi: 10.1016/j.ejmech.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Manfredini S, Bazzanini R, Baraldi PG, Guarneri M, Simoni D, Marongiu ME, Pani A, Tramontano E, La Colla P. J. Med. Chem. 1992;35:917–924. doi: 10.1021/jm00083a017. [DOI] [PubMed] [Google Scholar]
- 12.Manfredini S, Bazzanini R, Baraldi PG, Bonora M, Marangoni M, Simoni D, Pani A, Scintu F, Pinna E. Anti-cancer Drug Des. 1996;11:193–204. [PubMed] [Google Scholar]
- 13.Park H-A, Lee K, Park S-J, Ahn B, Lee J-C, Cho HY, Lee K-I. Bioorg. Med. Chem. Lett. 2005;15:3307–3312. doi: 10.1016/j.bmcl.2005.03.082. [DOI] [PubMed] [Google Scholar]
- 14.Tanitame A, Oyamada Y, Ofuji K, Fujimoto M, Iwai N, Hiyama Y, Suzuki K, Ito H, Wachi M, Yamagishi J. J. Med. Chem. 2004;47:3693–3696. doi: 10.1021/jm030394f. [DOI] [PubMed] [Google Scholar]
- 15.Kucukguzel SG, Rollas S, Erdeniz H, Kiraz M, Ekinci AC, Vidin A. Eur. J. Med. Chem. 2000;35:761–771. doi: 10.1016/s0223-5234(00)90179-x. [DOI] [PubMed] [Google Scholar]
- 16.Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, Rogers RS, Rogier DJ, Yu SS, Anderson GD, Burton EG, Cogburn JN, Gregory SA, Koboldt CM, Perkins WE, Seibert K, Veenhuizen AW, Zhang YY, Isakson PC. J. Med. Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 17.Menozzi G, Mosti L, Fossa P, Mattioli F, Ghia M. J. Heterocycl. Chem. 1997;34:963–968. [Google Scholar]
- 18.Sridhar R, Perumal PT, Etti S, Shanmugam G, Ponnuswamy MN, Prabavathy VR, Mathivanan N. Bioorg. Med. Chem. Lett. 2004;14:6035–6040. doi: 10.1016/j.bmcl.2004.09.066. [DOI] [PubMed] [Google Scholar]
- 19.Bebernitz GR, Argentieri G, Battle B, Brennan C, Balkan B, Burkey BF, Eckhardt M, Gao J, Kapa P, Strohschein RJ, Schuster HF, Wilson M, Xu DD. J. Med. Chem. 2001;44:2601–2611. doi: 10.1021/jm010032c. [DOI] [PubMed] [Google Scholar]
- 20.Liu J-J, Zhang H, Sun J, Wang Z-C, Yang YS, Li D-D, Zhang F, Gong H-B, Zhu H-L. Bioorg. Med. Chem. 2012;20:6089–6096. doi: 10.1016/j.bmc.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Lu P-C, Li D-D, Li Q-S, Lu X, Xiao Z-P, Zhu H-L. Bioorg. Med. Chem. Lett. 2011;21:5374–5377. doi: 10.1016/j.bmcl.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Kambe S, Saito K, Kishi H, Sakurai A, Midorikawa H. Synthesis. 1979;4:287–289. [Google Scholar]
- 23.Modha J, Datta N, Parekh H. Il Farmaco. 2001;56:641–646. doi: 10.1016/s0014-827x(01)01118-1. [DOI] [PubMed] [Google Scholar]
- 24.Hayat F, Salahuddin A, Umar S, Azam A. Eur. J. Med. Chem. 2010;45:4669–4675. doi: 10.1016/j.ejmech.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 25.Yadav HL, Gupta P, Pawar RS, Singour PK, Patil UK. Med. Chem. Res. 2011;20:461–465. [Google Scholar]
- 26.Kanagarajan V, Thanusu J, Gopalakrishnan M. Eur. J. Med. Chem. 2010;45:1583–1589. doi: 10.1016/j.ejmech.2009.12.068. [DOI] [PubMed] [Google Scholar]
- 27.Choughary AN, Juyal V. Int. J. Pharm. Pharm. Sci. 2011;3:125–128. [Google Scholar]
- 28.Abadi AH, Abouel-Ella DA, Lehmann J, Tinsley HN, Gary BD, Piazza GA, Abdel-Fattah MA. Eur. J. Med. Chem. 2010;45:90–97. doi: 10.1016/j.ejmech.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.