Abstract
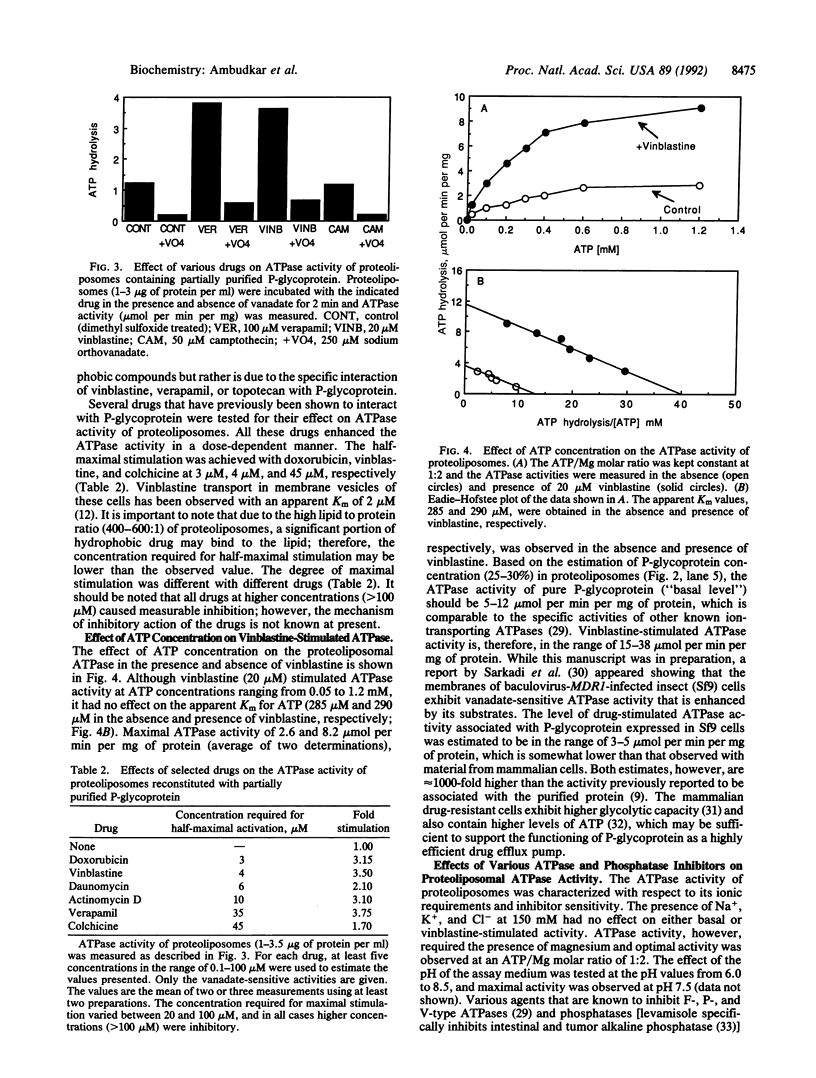
Multidrug-resistant human tumor cells overexpress the MDR1 gene product P-glycoprotein, which is believed to function as an ATP-dependent efflux pump. In this study we demonstrate that the partially purified P-glycoprotein, when reconstituted in an artificial membrane, catalyzes drug-stimulated ATP hydrolysis. Plasma membrane proteins of a human multidrug-resistant cell line, KB-V1, were solubilized with 1.4% (wt/vol) octyl beta-D-glucopyranoside in the presence of 0.4% phospholipid and 20% (vol/vol) glycerol, and the crude detergent extract was chromatographed on DEAE-Sepharose CL-6B. The 0.1 M NaCl fraction, enriched in P-glycoprotein but devoid of Na,K-ATPase, was reconstituted by the detergent-dilution method. P-glycoprotein constituted 25-30% of the reconstituted protein in proteoliposomes. ATP hydrolysis by proteoliposomes was stimulated 3.5-fold by the addition of vinblastine but was unaffected by the hydrophobic antitumor agent camptothecin, which is not transported by P-glycoprotein. The stimulatory effect of vinblastine was observed only if the protein was reconstituted in proteoliposomes, suggesting that either the substrate binding site(s) was masked by detergent or that the conformation of the soluble P-glycoprotein might not be suitable for substrate-induced activation. Several other drugs that are known to be transported by P-glycoprotein enhanced the ATPase activity in a dose-dependent manner with relative potencies as follows: doxorubicin = vinblastine greater than daunomycin greater than actinomycin D greater than verapamil greater than colchicine. The basal and vinblastine-stimulated ATPase activities were inhibited by vanadate (50% inhibition observed at 7-10 microM) but were not affected by agents that inhibit other ATPases and phosphatases. These data indicate that the P-glycoprotein, similar to other ion-transporting ATPases, exhibits a high level of ATP hydrolysis (5-12 mumol per min per mg of protein).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambudkar S. V., Maloney P. C. Anion exchange in bacteria: reconstitution of phosphate: hexose 6-phosphate antiport from Streptococcus lactis. Methods Enzymol. 1986;125:558–563. doi: 10.1016/s0076-6879(86)25045-4. [DOI] [PubMed] [Google Scholar]
- Ambudkar S. V., Maloney P. C. Bacterial anion exchange. Use of osmolytes during solubilization and reconstitution of phosphate-linked antiport from Streptococcus lactis. J Biol Chem. 1986 Aug 5;261(22):10079–10086. [PubMed] [Google Scholar]
- Ames G. F., Nikaido K., Groarke J., Petithory J. Reconstitution of periplasmic transport in inside-out membrane vesicles. Energization by ATP. J Biol Chem. 1989 Mar 5;264(7):3998–4002. [PubMed] [Google Scholar]
- Azzaria M., Schurr E., Gros P. Discrete mutations introduced in the predicted nucleotide-binding sites of the mdr1 gene abolish its ability to confer multidrug resistance. Mol Cell Biol. 1989 Dec;9(12):5289–5297. doi: 10.1128/mcb.9.12.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop L., Agbayani R., Jr, Ambudkar S. V., Maloney P. C., Ames G. F. Reconstitution of a bacterial periplasmic permease in proteoliposomes and demonstration of ATP hydrolysis concomitant with transport. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6953–6957. doi: 10.1073/pnas.86.18.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A. Y., Yu C., Potmesil M., Wall M. E., Wani M. C., Liu L. F. Camptothecin overcomes MDR1-mediated resistance in human KB carcinoma cells. Cancer Res. 1991 Nov 15;51(22):6039–6044. [PubMed] [Google Scholar]
- Chen C. C., Wilson T. H. The phospholipid requirement for activity of the lactose carrier of Escherichia coli. J Biol Chem. 1984 Aug 25;259(16):10150–10158. [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Cornwell M. M., Tsuruo T., Gottesman M. M., Pastan I. ATP-binding properties of P glycoprotein from multidrug-resistant KB cells. FASEB J. 1987 Jul;1(1):51–54. doi: 10.1096/fasebj.1.1.2886389. [DOI] [PubMed] [Google Scholar]
- D'Souza M. P., Ambudkar S. V., August J. T., Maloney P. C. Reconstitution of the lysosomal proton pump. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6980–6984. doi: 10.1073/pnas.84.20.6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A. L., Shuman H. A., Nikaido H. Mechanism of maltose transport in Escherichia coli: transmembrane signaling by periplasmic binding proteins. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2360–2364. doi: 10.1073/pnas.89.6.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. The multidrug transporter, a double-edged sword. J Biol Chem. 1988 Sep 5;263(25):12163–12166. [PubMed] [Google Scholar]
- Hamada H., Tsuruo T. Characterization of the ATPase activity of the Mr 170,000 to 180,000 membrane glycoprotein (P-glycoprotein) associated with multidrug resistance in K562/ADM cells. Cancer Res. 1988 Sep 1;48(17):4926–4932. [PubMed] [Google Scholar]
- Hamada H., Tsuruo T. Purification of the 170- to 180-kilodalton membrane glycoprotein associated with multidrug resistance. 170- to 180-kilodalton membrane glycoprotein is an ATPase. J Biol Chem. 1988 Jan 25;263(3):1454–1458. [PubMed] [Google Scholar]
- Horio M., Gottesman M. M., Pastan I. ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proc Natl Acad Sci U S A. 1988 May;85(10):3580–3584. doi: 10.1073/pnas.85.10.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde S. C., Emsley P., Hartshorn M. J., Mimmack M. M., Gileadi U., Pearce S. R., Gallagher M. P., Gill D. R., Hubbard R. E., Higgins C. F. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990 Jul 26;346(6282):362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- Kamimoto Y., Gatmaitan Z., Hsu J., Arias I. M. The function of Gp170, the multidrug resistance gene product, in rat liver canalicular membrane vesicles. J Biol Chem. 1989 Jul 15;264(20):11693–11698. [PubMed] [Google Scholar]
- Kaplan O., Jaroszewski J. W., Clarke R., Fairchild C. R., Schoenlein P., Goldenberg S., Gottesman M. M., Cohen J. S. The multidrug resistance phenotype: 31P nuclear magnetic resonance characterization and 2-deoxyglucose toxicity. Cancer Res. 1991 Mar 15;51(6):1638–1644. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lyon R. C., Cohen J. S., Faustino P. J., Megnin F., Myers C. E. Glucose metabolism in drug-sensitive and drug-resistant human breast cancer cells monitored by magnetic resonance spectroscopy. Cancer Res. 1988 Feb 15;48(4):870–877. [PubMed] [Google Scholar]
- Maloney P. C., Ambudkar S. V. Functional reconstitution of prokaryote and eukaryote membrane proteins. Arch Biochem Biophys. 1989 Feb 15;269(1):1–10. doi: 10.1016/0003-9861(89)90080-5. [DOI] [PubMed] [Google Scholar]
- Monaco J. J., Cho S., Attaya M. Transport protein genes in the murine MHC: possible implications for antigen processing. Science. 1990 Dec 21;250(4988):1723–1726. doi: 10.1126/science.2270487. [DOI] [PubMed] [Google Scholar]
- Pastan I., Gottesman M. M. Multidrug resistance. Annu Rev Med. 1991;42:277–286. doi: 10.1146/annurev.me.42.020191.001425. [DOI] [PubMed] [Google Scholar]
- Rosen B. P., Weigel U., Karkaria C., Gangola P. Molecular characterization of an anion pump. The arsA gene product is an arsenite(antimonate)-stimulated ATPase. J Biol Chem. 1988 Mar 5;263(7):3067–3070. [PubMed] [Google Scholar]
- Rothenberg M., Ling V. Multidrug resistance: molecular biology and clinical relevance. J Natl Cancer Inst. 1989 Jun 21;81(12):907–910. doi: 10.1093/jnci/81.12.907. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Price E. M., Boucher R. C., Germann U. A., Scarborough G. A. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J Biol Chem. 1992 Mar 5;267(7):4854–4858. [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Shen D. W., Cardarelli C., Hwang J., Cornwell M., Richert N., Ishii S., Pastan I., Gottesman M. M. Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J Biol Chem. 1986 Jun 15;261(17):7762–7770. [PubMed] [Google Scholar]
- Takeyasu K., Tamkun M. M., Renaud K. J., Fambrough D. M. Ouabain-sensitive (Na+ + K+)-ATPase activity expressed in mouse L cells by transfection with DNA encoding the alpha-subunit of an avian sodium pump. J Biol Chem. 1988 Mar 25;263(9):4347–4354. [PubMed] [Google Scholar]
- Tanaka S., Currier S. J., Bruggemann E. P., Ueda K., Germann U. A., Pastan I., Gottesman M. M. Use of recombinant P-glycoprotein fragments to produce antibodies to the multidrug transporter. Biochem Biophys Res Commun. 1990 Jan 15;166(1):180–186. doi: 10.1016/0006-291x(90)91928-l. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Clark D. P., Chen C. J., Roninson I. B., Gottesman M. M., Pastan I. The human multidrug resistance (mdr1) gene. cDNA cloning and transcription initiation. J Biol Chem. 1987 Jan 15;262(2):505–508. [PubMed] [Google Scholar]
- Van Belle H. Kinetics and inhibition of alkaline phosphatases from canine tissues. Biochim Biophys Acta. 1972 Nov 10;289(1):158–168. doi: 10.1016/0005-2744(72)90118-0. [DOI] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]