Highlights
-
•
Rhino, Influenza and Corona viruses caused ∼50% of ILI in Vietnam households.
-
•
There was no clear seasonality for ILI, or for individual viruses causing ILI.
-
•
Children did not contribute substantially to ILI burden or transmission.
-
•
Seasonality and the contribution of children were unlike temporal settings.
-
•
Seasonality and social factors may affect the involvement of children in ILI.
Abbreviations: ILI, influenza like illness; ARI, acute respiratory illness; Inf, influenza virus; Rhino, rhinoviruses; Entero, enteroviruses; Corona, coronaviruses; RSV, respiratory syncytial virus; MPV, human metapneumovirus; Boca, bocavirus; PIV, parainfluenza viruses; Adeno, adenoviruses
Keywords: Influenza-like-illness, Respiratory viruses, Household transmission, Active case finding, Cohort, Vietnam
Abstract
Background
Household studies provide opportunities to understand influenza-like-illness (ILI) transmission, but data from (sub)tropical developing countries are scarce.
Objective
To determine the viral etiology and epidemiology of ILI in households.
Study design
ILI was detected by active case finding amongst a cohort of 263 northern Vietnam households between 2008 and 2013. Health workers collected nose and throat swabs for virus detection by multiplex real-time RT-PCR.
Results
ILI was detected at least once in 219 (23.7%) of 945 household members. 271 (62.3%) of 435 nose/throat swabs were positive for at least one of the 15 viruses tested. Six viruses predominated amongst positive swabs: Rhinovirus (28%), Influenza virus (17%), Coronavirus (8%), Enterovirus (5%), Respiratory syncytial virus (3%), Metapneumovirus virus (2.5%) and Parainfluenza virus 3 (1.8%). There was no clear seasonality, but 78% of episodes occurred in Winter/Spring for Influenza compared to 32% for Rhinovirus. Participants, on average, suffered 0.49 ILI, and 0.29 virus-positive ILI episodes, with no significant effects of gender, age, or household size. In contrast to US and Australian community studies, the frequency of ILI decreased as the number of household members aged below 5 years increased (p = 0.006).
Conclusion
The findings indicate the need for tailored ILI control strategies, and for better understanding of how local childcare practices and seasonality may influence transmission and the role of children.
1. Background
Acute respiratory illnesses (ARIs) are a leading global cause of morbidity and mortality [1], commonly caused by viruses such as Influenza (Inf), Rhinoviruses (Rhino) and other Enteroviruses (Entero), Coronaviruses (Corona), Respiratory syncytial virus (RSV), Human Metapneumovirus (MPV), Parainfluenza virus (PIV) 1–4, and Adenoviruses (Adeno) [2], [3], [4], [5]. Influenza-like-illness (ILI) represents a subset of ARI patients, being variably defined as fever with at least one respiratory symptom, usually cough, which are common in patients presenting with other viral causes of ARI [3], [6], [7], and not specific for influenza [8], [9].
Household cohort studies are fundamental for understanding respiratory virus transmission [10]. There is a wealth of information regarding influenza virus transmission in households [11], [12], but relatively few studies characterize non-influenza virus transmission. In 2007, we established a household cohort in Ha Nam, northern Vietnam, and have conducted active ILI surveillance to describe influenza epidemiology [12], [13], [14]. Over 80% of swabs collected have been influenza-negative.
2. Objective
To determine the epidemiology and viral etiologies of ILI in Ha Nam households, and the effects of household and demographic factors on transmission.
3. Study design
3.1. Participants
The Ha Nam cohort was established in December 2007, and has been described previously [13]. Briefly, 270 households were selected randomly from a rural commune located 60 km from Hanoi, Vietnam. All participants provided written informed consent. Commune health workers visited houses weekly to identify ILI cases, and collected nose and throat swabs within 2 days of onset. Participants with ILI also self-presented to the commune health service. ILI was defined as an oral temperature of at least 38 °C together with cough or sore throat. The current study assessed ILI occurring between September 2008 and August 2013, but excluded the year between September of 2009 and 2010, when protocols were adjusted due to the 2009 H1N1 pandemic. 98 ILI episodes were detected during this pandemic period and 25% were H1N1 2009 pandemic strain positive.
3.2. Diagnostic testing
Internal RNA-virus control (equine arteritis virus) was added to 200 μl of combined nose and throat swab media, then RNA was extracted using MagNA Pure 96 Extraction kit (Roche, Germany). Influenza A/H1N1, A/H3N2 and B viruses were detected by real-time RT-PCR according to WHO/US CDC protocols, version 2007 for seasonal influenza and version 2009 for pandemic H1N1 (http://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf).
A four-tube real-time multiplex PCR assay developed by Jansen and colleagues was used to detect 12 respiratory viruses: respiratory syncytial viruses (RSVs) A/B; Rhinoviruses A-C; Coronaviruses OC43/HKU1 and 229E/NL63; Adenovirus, Parainfluenza viruses 1–4; human Metapneumovirus; Enteroviruses; Bocavirus; and Parechoviruses [15]. Limits of detection are between 40 and 50 copies per reaction for each target [15].
3.3. Analysis and statistics
Participants were classified as children and young children if aged below 15 and 6 years, respectively. Results are presented as means or proportions with 95% confidence intervals. Poisson log-linear regression was used to investigate factors associated with the number of ILI and virus-positive ILI episodes per participant, including age, gender, household size, and number of children per household.
4. Results
4.1. Population characteristics
924 participants from 263 households were included in this analysis after excluding 63 participants who were absent the majority of the time, because they were away for work or study, or had moved out of the commune or died, were excluded. A further six participants who had incomplete data were excluded. None had received influenza vaccine. Four reported having a pre-existing chronic condition, involving lungs (n = 2), heart (n = 1), or liver (n = 1). Households had between one and nine inhabitants, averaging 3.5 (95% CI 3.3–3.7), with 1.1 (1.0–1.2) children, and 0.5 (0.4–0.5) young children (Supplemental Fig. 1a). Numbers of children and young children per household increased with household size, such that average participant age decreased with household size (Supplementary Fig. 1a). Most households had no children (n = 102), or two children (n = 90), giving a bimodal age distribution (Supplementary Fig. 1b). Females (n = 510, 55.2%) predominated slightly over males (n = 414, 44.8%).
Panel A shows mean age by household size (circles) with error bars representing 95% confidence intervals, as well as the numbers of children per household by household size (stacked bars). The histograms in panel B show the age and gender distribution of the 924 participants studied.
4.2. ILI and virus-positive ILI detection frequencies
435 ILI episodes were detected in 219 (23.7%) participants. On average households had 1.6 (1.2–2.1) ILI episodes, but the distribution was skewed, ranging from 0 to 32, and only 120 (45.6%) households had ILI (Supplementary Fig. 2a). On average participants had 0.47 (0.40–0.54) ILI episodes, ranging from 0 to 11 over 47 months (Supplementary Fig. 2b). This equates to 0.12 episode per person per year 119 participants (13%) had a single ILI episode, while 100 (12%) had multiple episodes 271 (62.3%) swabs were virus-positive, and were from 169 (18.3%) participants from 103 (39.2%) households. On average households had 1.0 (0.8–1.3) virus-positive ILI episode, ranging from 0 to 19 (Supplementary Fig. 2a). 55 households with multiple positive episodes accounted for 82% of virus-positive episodes. On average participants had 0.29 (0.24–0.34) virus-positive ILI episodes, ranging from 0 to 6 (Supplementary Fig. 2b). 77 participants who had multiple virus-positive episodes accounted for 60% of virus-positive ILI episodes.
4.3. Relationship between ILI and household and demographics factors
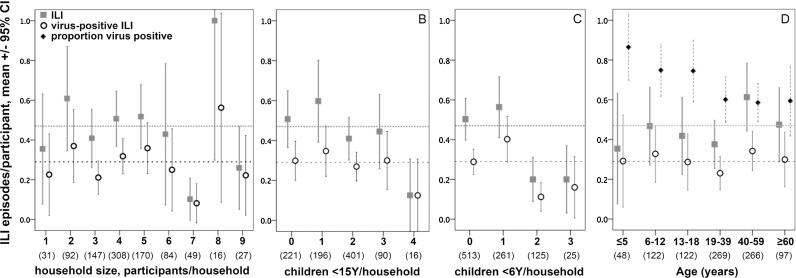
Average numbers of ILI and virus-positive ILI per participant were not detectably associated with household size (Fig. 1 a), or with the number of children in a household (Fig. 1b). Contrary to expectations, households with more young children had fewer episodes per person of ILI (Odds ratio 0.76, 95%CI 0.62–0.92, p = 0.006) and virus-positive ILI (p = 0.077) (Fig. 1c). These effects were maintained if household size was included in the analysis (ILI p = 0.038, virus positive ILI p = 0.069), or if analysis was restricted to households with at least four participants (ILI, p = 0.006; virus positive ILI p = 0.016). Average numbers of ILI or virus-positive ILI episodes per participant were not detectably associated with age (Fig. 1d). Males and females had similar frequencies of ILI (0.43, 0.33–0.54 ILI versus 0.50, 0.39–0.61) and virus-positive ILI (0.27, 0.20–0.34 versus 0.31, 0.24–0.38).
Fig. 1.
Frequency of ILI and virus-positive ILI according to characteristics of households and individuals. Results are presented as average episodes/participant in each x-axis category. Upper and lower dashed horizontal lines indicate averages for all participants combined for ILI and virus-positive ILI, respectively. The numbers of participants are shown below each category on the x-axis in parentheses.
The proportion of ILI episodes that were virus-positive was 86% for young children and declined with age to 58% amongst participants aged 40–59 years (Fig. 1d).
4.4. ILI etiology
A single viral etiology was determined for 247 (91%) of 271 virus-positive swabs (i.e. 56.5% of all swabs). Two viruses were detected in 23 swabs, and three viruses in two swabs, totalling 299 viruses detected. Rhinovirus was detected most frequently (n = 124, 28% of swabs), followed by Influenza virus (n = 72, 17%), Coronavirus (8%), Enterovirus (5%), RSV (3%), MPV (2.5%) and PIV3 (1.8%) (Fig. 2 a). Remaining viruses were detected in less than 1% of swabs. Rhinovirus-positive swabs were from 96 (10%) participants from 63 households, representing 43% of participants with ILI. Influenza virus-positive swabs were from 67 participants from 49 households, representing 31% of participants with ILI. Influenza virus B, A/H3N2 and A/H1N1 (four Brisbane/59/2007-like cases, nineteen A/Cal/07/2009-like cases) were detected in similar numbers of swabs (Fig. 2b). Seven swabs tested positive for both Rhinovirus and Enterovirus, and two swabs each contained Rhinovirus and Influenza virus B, or MPV and Bocavirus. All other virus combinations were detected in a single swab (Fig. 2b). Enterovirus was distinct in that 11 (52%) of 21 positive swabs contained additional viruses (Fig. 2b).
Fig. 2.
Respiratory viruses detected in swabs from ILI cases. Panel A shows the frequency of detection for each virus, whether detected as a single virus or in combination with others. Numbers above columns indicate the total number of episodes if there were participants who had multiple episodes. Panel B shows the composition of each swab. Numbers detected are indicated next to each virus or combination that was detected more than once.
4.5. Repeated virus detection within individuals and households
Table 1 ranks common viruses according to the proportions of affected participants and households that had multiple infections. Rhinoviruses ranked high compared to Coronaviruses. Enteroviruses affected four individuals multiple times but rarely affected other household members, whereas multiple members were affected in a quarter to a third of households with Influenza, RSV or MPV. To investigate whether clustering of these viruses reflects household transmission, episodes were classified as being temporally related if detection intervals were between 1 and 10 days [16]. Twenty-four temporally related clusters were detected in 19 households (Table 2 ). Ten Rhinovirus clusters were detected in nine households, accounting for 45% of 20 households with multiple Rhinovirus cases. Influenza cases were temporally related in seven households, accounting for 58% of households with multiple cases. RSV cases were temporally related in all households with multiple cases. The estimated household risk of transmission ranged from 7.7% for Coronavirus and Enterovirus, to 33% for RSV (Table 1).
Table 1.
Repeated virus detection in individuals and households.
| n (% of positive individuals/households) | |||
|---|---|---|---|
| individuals with >1 episode | households with >1 positive participant | households with transmission detected | |
| Rhino | 22 (23) | 20 (32) | 9 (14 a) |
| Entero | 4 (29) | 1 (7.7) | 1 (7.7 a) |
| MPV | 1 (10) | 2 (25) | 1 (12.5 a) |
| Influenza | 5 (7.4) | 12 (24) | 7 (14 a) |
| RSV | 0 (0) | 3 (33) | 3 (33 a) |
| Corona | 2 (6.4) | 3 (11.5) | 2 (7.7 a) |
household risk of transmission = number of households in which transmission occurred/number of households with a case.
Table 2.
Temporal clustering of virus detection in households.
| House | Position in family, age (years) of each case |
Intervala |
Virus | |||
|---|---|---|---|---|---|---|
| index/first | second | third | fourth | days | ||
| 1 | wife, 63 | husband, 65 | 1 | Corona | ||
| 2 b | father, 49 | son, 26 | mother, 48 | 4,5 | Corona | |
| 3 | mother, 34 | son, 11 | son, 8 | 1,1 | Inf A/H1 | |
| 4 | mother, 40 | daughter, 6 | 3 | Inf A/H1 | ||
| 5 | mother, 46 | son, 17 | 9 | Inf A/H1 | ||
| 6 | wife, 39 | husband, 49 | 1 | Inf A/H3 | ||
| 7 | son, 18 | father, 44 | 2 | Inf A/H3 | ||
| 8 | mother, 36 | son, 3 | 1 | Inf B | ||
| 9 | mother, 39 | son, 11 | 3 | Inf B | ||
| 10 | son, 6 | daughter, 12 | 3 | MPV | ||
| 11 | daughter-in-law, 21 | daughter, 12 | 1 | Rhino | ||
| 2 b | daughter-in-law, 21 | son, 26 | 1 | Rhino | ||
| 12 b | father, 43 | sister, 28 | 9 | Rhino | ||
| 13 | daughter, 16 | mother, 34 | 2 | Rhino | ||
| 14 b | son, 8 | mother, 46 | 1 | Rhino | ||
| 14 b | father, 46 | son, 7 | 2 | Rhino | ||
| 15 b | granddaughter, 13 | granddaughter, 10 | 5 | Rhino | ||
| 16 | daughter, 13 | mother, 38 | 6 | Rhino | ||
| 17 | daughter, 44 | mother, 79 | 8 | Rhino | ||
| 18 | husband, 66 | wife, 65 | 9 | Rhino | ||
| 12 b | daughter, 14 | mother, 43 | 1 | Entero | ||
| 2 b | mother, 49 | son, 27 | daughter, 28 | father, 50 | 2,7,14 | RSV |
| 15 b | granddaughter, 13 | granddaughter, 10 | 8 | RSV | ||
| 19 | grandson, 2 | grandmother, 49 | 10 | RSV | ||
The table shows members of households who developed symptoms with the same virus within 10 days of another household member.
days between ILI onset in the first case and each subsequent case in the household.
households with multiple clusters.
Five of seven Influenza virus household index cases (i.e. the first case detected in a group of related cases) were mothers, and subsequent cases were children of these mothers. In contrast, Rhinovirus index cases were a mixture of children and adults, and males and females.
4.6. Virus etiology and age
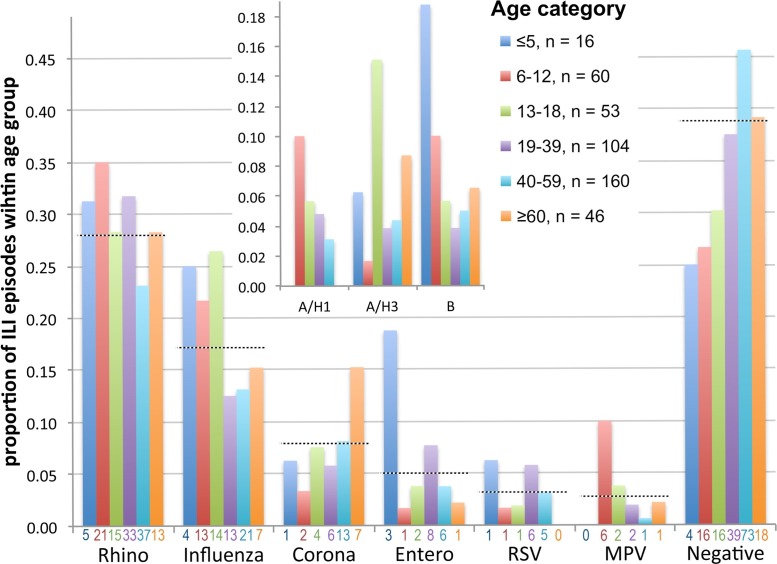
The relative contribution of the six most common viruses to ILI in different age groups is presented in Fig. 3 . Rhinoviruses accounted for the highest proportion of ILI across age groups, whereas Coronaviruses were relatively common amongst participants aged over 60 years, and average ages were 33 (CI 30–37) versus 42 (35–50) years, respectively. Influenza virus accounted for 24% (CI 17–31%) of ILI in participants aged below 18 years compared to 13% (CI 9–17%) in older participants; largely because Influenza virus B and A/H1N1 predominated in younger age groups (Fig. 3, inset). MPV accounted for 10% of ILI in 6–12 year olds (CI 2–18%), higher than for other age groups. Enteroviruses appeared to be relatively common in young children, but there were only 16 ILI episodes in young child, and all/three Enterovirus episodes involved one 2-year old child.
Fig. 3.
Viruses detected in swabs by age category. The proportion of swabs collected from each age group (see legend within the figure) that were positive for the most commonly detected viruses are shown. Dashed horizontal lines indicate the proportions of all swabs that were positive for each virus. Numerators are shown below each bar and denominators are shown in the legend. The inset shows the proportions that were positive for each influenza subtype.
4.7. Seasonality
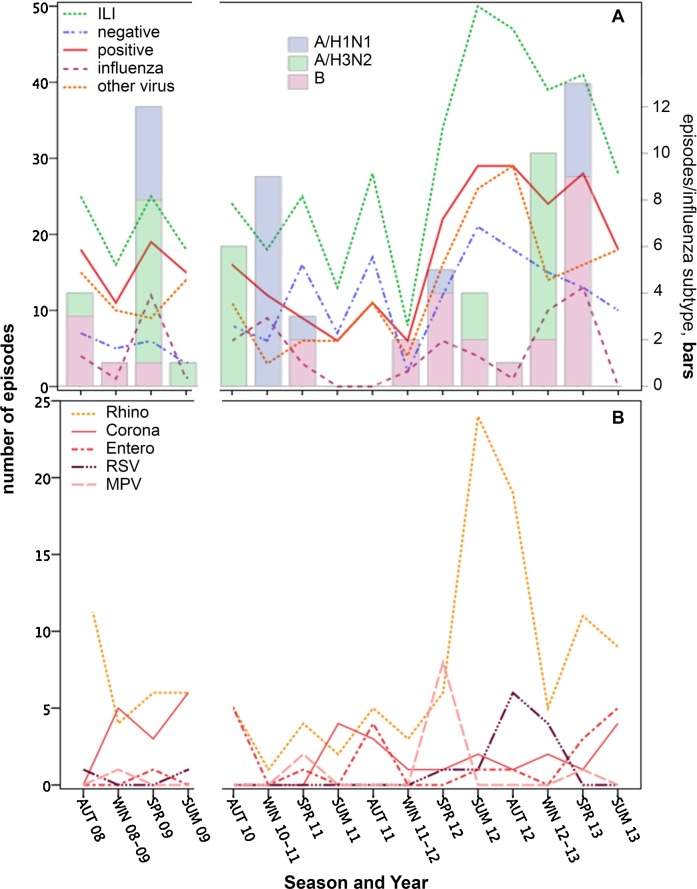
ILI was not clearly seasonal (Fig. 4 a). Substantially more ILI episodes were detected in 2012 compared to previous years, reflecting increased Rhinovirus episodes (Fig. 4a & b). Rhinoviruses were detected in all years and seasons. Influenza viruses and Coronaviruses were detected in 13 and 12 of the 16 seasons assessed, respectively. Nevertheless, 78% of Influenza virus episodes occurred in Winter/Spring compared to only 32% of Rhinovirus episodes, which predominantly occurred in Summer/Autumn (Table 3 ). A Winter/Spring bias was particularly evident for Influenza A/H1N1 and B (Table 3). A similar trend occurred for MPV, whereas RSV ILI cases occurred primarily in the Autumn and Winter.
Fig. 4.
Distribution of ILI episodes by year and season. The y-axis represents values for lines in both panels whereas the opposite axis in panel A represents values for the stacked bars indicating influenza subtypes. The break in the x-axis indicates the H1N1 pandemic period that was excluded from this analysis.
Table 3.
Virus detection over four years by season.
| n (%) of episodes for each virus |
||||||||
|---|---|---|---|---|---|---|---|---|
| Rhino | A/H1N1 | A/H3N2 | Inf B | Corona | Entero | RSV | MPV | |
| Summer | 41 (33.1) | 0 | 3 (12.0) | 2 (7.4) | 16 (48.5) | 6 (28.6) | 2 (14.3) | 0 |
| Autumn | 43 (34.7) | 0 | 7 (28.0) | 4 (14.8) | 4 (12.1) | 10 (47.6) | 7 (50.0) | 0 |
| Winter | 13 (10.5) | 9 (47.4) | 8 (32.0) | 5 (18.5) | 8 (24.2) | 0 (0) | 4 (28.6) | 1 (8.3) |
| Spring | 27 (21.8) | 10 (52.6) | 7 (28.0) | 16 (59.3) | 5 (15.2) | 5 (23.8) | 1 (7.1) | 11 (91.7) |
5. Discussion
Viral etiologies of ILI in households in northern Vietnam were similar to those for other recent household or community based studies of ILI or ARI, despite differences in case definition [2], [3], [4]. Supplementary Table 1 shows a comparison of study designs and findings. However, key epidemiological differences indicate that the factors driving transmission in this subtropical, lower middle-income setting are distinct, and that tailored control measures are needed.
Around two thirds of ILI case swabs were positive for respiratory viruses, this proportion exceeded 70% for children, and decreased with age, similar to other studies [2], [4], [17], [18], [19], [20]. Rhinovirus was detected in around half of the virus-positive ILI episodes, and was the most common virus, followed by Influenza virus or Coronavirus, RSV, MPV and PIV3, as in the recent Michigan household study [4]. Similarly, half of the virus-positive ILI cases detected amongst adults in the community in Australia were Rhinoviruses, with Influenza and Coronaviruses the next most common [3]. Interestingly, Rhinoviruses also predominated in ILI patients of all ages admitted to hospitals in Vietnam between July 2008 and June 2009 [20]. We did not have sufficient numbers to reliably describe the etiology of ILI in young children, other than to conclude that Rhinoviruses predominated, consistent with studies elsewhere [2]. Influenza virus accounted for a higher proportion of ILI amongst participants of the current study (17%), who have never received influenza vaccine, compared to studies in which more that half of the participants have been vaccinated (∼11%) [3], [4]. Furthermore, in the study of Howard et al., individual with influenza were less likely to have been vaccinated compared to the influenza virus negative group [3]. MPV was less common than in the Michigan study [4], and in community studies involving children [2] but not adults [3], and we and others found that MPV was more common in children aged 6–12 years [21], suggesting an effect of the type of household included and their age structures. Similarly, RSV detection was low in the current study compared to studies with higher proportions of young children [2], [4]. The detection of influenza virus in only 17% of ILI episodes, and the absence of seasonal trends for influenza, indicate that ILI surveillance may be of limited value for understanding influenza epidemiology if not combined with laboratory confirmation, in agreement with other studies [6], [7], [19].
Children did not contribute substantially to ILI burden or transmission in this study. In contrast, young children experienced significantly more ARI episodes than older age groups, and people from households with young children also experienced significantly more ARI episodes in the recent Michigan study [4]. Similarly, a study of adult index case households in Australia found significantly more ILI transmission if a child was present [22], and children were considered to be the main driving force for ARI transmission in the 1960/70s Tecumseh study [23]. It follows that if ILI predominates in children, they will frequently be involved in household transmission, either as index or contact cases. Differences in observations for children could reflect study design: the Michigan study only recruited households with at least four participants, and at least two children, and the case definition included milder illness. However, effects were maintained when analysis was restricted to households with at least four participants. In our previous analysis we found that influenza infection was not associated with the presence of children in the household or with caring for children if an adult [13]. Therefore differences in the role of children in household ILI may reflect differences in factors affecting transmission such as seasonality, contact patterns [24] and childcare practices. Childcare centre attendance significantly increased the rate of ILI, by 40%, amongst children in Australian communities [2]. School-based transmission may also be less intense in (sub)tropical settings because virus circulation is more constant, rather than seasonal, potentially reducing the pool of susceptible children [13]. A study of age-specific seasonality for Influenza and RSV in Hong Kong similarly concluded that none of the age groups consistently appear as the driving force for seasonal epidemics [25]. School closure has been adopted as an ILI control strategy based on studies that indicate that children are a major driving force for ILI transmission [26]. A statistical model using sentinel surveillance data from France also indicates that in this setting, where ILI predominates in children, 16–17% of cases may be prevented by school closure. The data presented in the current study indicate that school closure may have limited effect in this setting.
Several viruses were detected in sufficient numbers to investigate their epidemiology. Rhinovirus characteristics, including high case numbers, broad age range and distribution of family members who were index cases, propensity to transmit within households, and year-round detection, correspond with high infectiousness and extensive genetic diversity [27]. Influenza viruses have less extensive genetic diversity, but age-associations were consistent with greater antigenic drift for A/H3N2 compared to A/H1N1 and B [11], [13], [28], [29]. RSV exhibited high household transmission, but overall detection was low, consistent with reports that virtually all exposed, non-immunes will become infected, and with the small size of the most susceptible, early childhood age group [30]. Some households appeared to be particularly susceptible to Rhinovirus, Influenza virus or MPV because multiple members were affected, but episodes were not temporally related. Similarly, some individuals experienced repeated ILI and virus-positive ILI, suggestive of inherent susceptibility.
A limitation of this study is that virus detection by RT-PCR does not prove that the virus caused ILI. Rhinovirus and Bocavirus are often detected in swabs from asymptomatic people, whereas Coronavirus, MPV and Influenza viruses are mainly detected from symptomatic people [5]. RSV is mainly associated with symptoms in young children but not older children or adults [5]. Our ability to understand the dynamics of respiratory infections in households is also limited by restricting investigation to participants with ILI symptoms, and thus we were unable to assess the contribution of milder and asymptomatic infections to household transmission dynamics. However, viral etiologies were similar to studies that used different ARI or ILI definitions.
In summary, comparison with studies in developed countries indicates that the etiology of ILI in this subtropical, lower middle-income setting is similar despite differences in seasonality and climate as well as cultural and economic differences. However, the current study did not indicate a predominance of illness among young children or an impact of the presence of young children within households. Therefore, the strategy of targeting interventions to children needs further consideration, and understanding of effects of seasonality, childcare utilization, household structure and contact patterns.
Funding
This work was supported by the Wellcome Trust UK (grants 081613/Z/06/Z; 077078/Z/05/Z). AF was supported by the European Union FP7 project “European Management Platform for Emerging and Re-emerging Infectious Disease Entities (EMPERIE)” (no. 223498).
Competing interests
The authors do not have a commercial or other association that might pose a conflict of interest.
Ethical approval
The research was approved by the institutional review board of the National Institute of Hygiene and Epidemiology, Viet Nam, and the Oxford Tropical Research Ethics Committee, University of Oxford, UK.
Acknowledgements
We are grateful to the community of Thanh Ha Commune, Thanh Liem, Ha Nam for agreeing to participate in this study and for providing their time. We would like to thank the hamlet health workers who conducted the interviews and surveillance. We also wish to thank the Ministry of Health of Vietnam for their continuing support of the research collaboration between the Oxford University Clinical Research Unit and the National Institute for Hygiene and Epidemiology.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jcv.2016.07.014.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Organization WHO . World Health Organization; 2013. WHO Methods and Data Sources for Global Causes of Death 2000–2011. [Google Scholar]
- 2.Lambert S.B., Allen K.M., Druce J.D. Community epidemiology of human metapneumovirus, human coronavirus NL63, and other respiratory viruses in healthy preschool-aged children using parent-collected specimens. Pediatrics. 2007;120:e929–e937. doi: 10.1542/peds.2006-3703. [DOI] [PubMed] [Google Scholar]
- 3.Howard P.F., McCaw J.M., Richmond P.C. Virus detection and its association with symptoms during influenza-like illness in a sample of healthy adults enrolled in a randomised controlled vaccine trial. Influenza Other Respir. Viruses. 2013;7:330–339. doi: 10.1111/j.1750-2659.2012.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monto A.S., Malosh R.E., Petrie J.G., Thompson M.G., Ohmit S.E. Frequency of acute respiratory illnesses and circulation of respiratory viruses in households with children over 3 surveillance seasons. J. Infect. Dis. 2014;210:1792–1799. doi: 10.1093/infdis/jiu327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byington C.L., Ampofo K., Stockmann C. Community surveillance of respiratory viruses among families in the utah better identification of germs-longitudinal viral epidemiology (BIG-LoVE) study. Clin. Infect. Dis. 2015;61:1217–1224. doi: 10.1093/cid/civ486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thursky K., Cordova S.P., Smith D., Kelly H. Working towards a simple case definition for influenza surveillance. J. Clin. Virol. 2003;27:170–179. doi: 10.1016/s1386-6532(02)00172-5. [DOI] [PubMed] [Google Scholar]
- 7.Call S.A., Vollenweider M.A., Hornung C.A., Simel D.L., McKinney W.P. Does this patient have influenza. JAMA. 2005;293:987–997. doi: 10.1001/jama.293.8.987. [DOI] [PubMed] [Google Scholar]
- 8.Monto A.S., Gravenstein S., Elliott M., Colopy M., Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch. Intern. Med. 2000;160:3243–3247. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 9.Huang P.Y., Huang C.T., Tsao K.C. Syndromic recognition of influenza A infection in a low prevalence community setting. PLoS One. 2010;5:e10542. doi: 10.1371/journal.pone.0010542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klick B., Leung G.M., Cowling B.J. Optimal design of studies of influenza transmission in households. I: case-ascertained studies. Epidemiol. Infect. 2012;140:106–114. doi: 10.1017/S0950268811000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox J.P., Cooney M.K., Hall C.E., Foy H.M. Influenza virus infections in Seattle families, 1975–1979. II. Pattern of infection in invaded households and relation of age and prior antibody to occurrence of infection and related illness. Am. J. Epidemiol. 1982;116:228–242. doi: 10.1093/oxfordjournals.aje.a113408. [DOI] [PubMed] [Google Scholar]
- 12.Cauchemez S., Ferguson N.M., Fox A. Determinants of influenza transmission in South East Asia: insights from a household cohort study in Vietnam. PLoS Pathog. 2014;10:e1004310. doi: 10.1371/journal.ppat.1004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horby P., Mai le Q., Fox A. The epidemiology of interpandemic and pandemic influenza in Vietnam, 2007–2010: the Ha Nam household cohort study I. Am. J. Epidemiol. 2012;175:1062–1074. doi: 10.1093/aje/kws121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thai P.Q., Mai le Q., Welkers M.R. Pandemic H1N1 virus transmission and shedding dynamics in index case households of a prospective Vietnamese cohort. J. Infect. 2014;68:581–590. doi: 10.1016/j.jinf.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansen R.R., Schinkel J., Koekkoek S. Development and evaluation of a four-tube real time multiplex PCR assay covering fourteen respiratory viruses, and comparison to its corresponding single target counterparts. J. Clin. Virol. 2011;51:179–185. doi: 10.1016/j.jcv.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vink M.A., Bootsma M.C., Wallinga J. Serial intervals of respiratory infectious diseases: a systematic review and analysis. Am. J. Epidemiol. 2014;180:865–875. doi: 10.1093/aje/kwu209. [DOI] [PubMed] [Google Scholar]
- 17.Do A.H., van Doorn H.R., Nghiem M.N. Viral etiologies of acute respiratory infections among hospitalized Vietnamese children in Ho Chi Minh City, 2004–2008. PLoS One. 2011;6:e18176. doi: 10.1371/journal.pone.0018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peci A., Winter A.L., Gubbay J.B. Community-acquired respiratory viruses and co-infection among patients of Ontario sentinel practices, April 2009 to February 2010. Influenza Other Respir. Viruses. 2013;7:559–566. doi: 10.1111/j.1750-2659.2012.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacIntyre C.R., Ridda I., Seale H. Respiratory viruses transmission from children to adults within a household. Vaccine. 2012;30:3009–3014. doi: 10.1016/j.vaccine.2011.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wertheim H.F., Nadjm B., Thomas S. Viral and atypical bacterial aetiologies of infection in hospitalised patients admitted with clinical suspicion of influenza in Thailand, Vietnam and Indonesia. Influenza Other Respir. Viruses. 2015 doi: 10.1111/irv.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaunt E., McWilliam-Leitch E.C., Templeton K., Simmonds P. Incidence, molecular epidemiology and clinical presentations of human metapneumovirus; assessment of its importance as a diagnostic screening target. J. Clin. Virol. 2009;46:318–324. doi: 10.1016/j.jcv.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 22.McCaw J.M., Howard P.F., Richmond P.C. Household transmission of respiratory viruses—assessment of viral, individual and household characteristics in a population study of healthy Australian adults. BMC Infect. Dis. 2012;12:345. doi: 10.1186/1471-2334-12-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monto A.S., Ross H. Acute respiratory illness in the community: effect of family composition, smoking, and chronic symptoms. Br. J. Prev. Soc. Med. 1977;31:101–108. doi: 10.1136/jech.31.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horby P., Pham Q.T., Hens N. Social contact patterns in Vietnam and implications for the control of infectious diseases. PLoS One. 2011;6:e16965. doi: 10.1371/journal.pone.0016965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L., Chan K.H., Suen L.K. Age-specific epidemic waves of influenza and respiratory syncytial virus in a subtropical city. Sci. Rep. 2015;5:10390. doi: 10.1038/srep10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cauchemez S., Valleron A.J., Boelle P.Y., Flahault A., Ferguson N.M. Estimating the impact of school closure on influenza transmission from Sentinel data. Nature. 2008;452:750–754. doi: 10.1038/nature06732. [DOI] [PubMed] [Google Scholar]
- 27.Zlateva K.T., de Vries J.J., Coenjaerts F.E. Prolonged shedding of rhinovirus and re-infection in adults with respiratory tract illness. Eur. Respir. J. 2014;44:169–177. doi: 10.1183/09031936.00172113. [DOI] [PubMed] [Google Scholar]
- 28.Skowronski D.M., Hottes T.S., McElhaney J.E. Immuno-epidemiologic correlates of pandemic H1N1 surveillance observations: higher antibody and lower cell-mediated immune responses with advanced age. J. Infect. Dis. 2011;203:158–167. doi: 10.1093/infdis/jiq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox A., Mai le Q., Thanh le T. Hemagglutination inhibiting antibodies and protection against seasonal and pandemic influenza infection. J. Infect. 2015;70:187–196. doi: 10.1016/j.jinf.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson F.W., Collier A.M., Clyde W.A., Jr., Denny F.W. Respiratory-syncytial-virus infections, reinfections and immunity A prospective, longitudinal study in young children. N. Engl. J. Med. 1979;300:530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.