Keywords: nerve regeneration, brain injury, cerebral ischemia/reperfusion, angiotensin II type 2 receptor, interleukin-1β, tumor necrosis factor-α;, interleukin-10, middle cerebral artery occlusion, infarct area, CGP42112, PD123319, immune and inflammatory responses, neural regeneration
Abstract
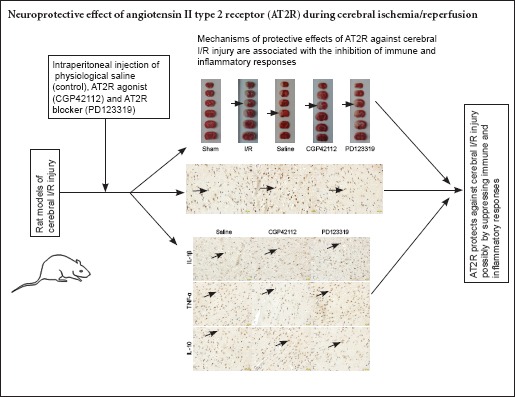
Angiotensin II type 2 receptor (AT2R) activation has been shown to protect against stroke, but its precise mechanism remains poorly understood. We investigated whether the protective effect of AT2R against ischemia/reperfusion injury is mediated by the suppression of immune and inflammatory responses. Rat models of middle cerebral artery occlusion were intraperitoneally injected with physiological saline, the AT2R agonist CGP42112 (1 mg/kg per day) or antagonist PD123319 (1 mg/kg per day). In the CGP42112 group, AT2R expression increased, the infarct area decreased, interleukin-1β and tumor necrosis factor-α expression decreased, and interleukin-10 expression increased compared with the saline group. Antagonisin AT2R using PD123319 produced the opposite effects. These results indicate that AT2R activation suppresses immune and inflammatory responses, and protects against cerebral ischemia/reperfusion injury.
Introduction
Acute inflammation promotes the development of secondary brain injury after cerebral ischemia/reperfusion (I/R) injuries. The cytokines produced in the early stage of I/R underlie the transition from ischemic injury to inflammatory damage (Saward et al., 1996). Various cytokines have broad roles in this process and their expression sequence and functions are closely balanced and regulated. Therefore, regulating the inflammatory response and antagonizing its proinflammatory effects may reduce secondary injury following cerebral ischemia.
Interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) gene expression increases markedly after cerebral I/R injury, and administration of an IL-1β or TNF-α antibody reduces infarct volume and the inflammatory response (Barone et al., 1997; Wang et al., 1997), lessening brain injury. IL-10 inhibits proinflammatory responses mediated by IL-1β and TNF-α, and plays a neuroprotective effect on brain ischemia by suppressing cytokine receptor expression and activation (Wang et al., 2007).
The angiotensin II type 2 receptor (AT2R) promotes the differentiation and regeneration of nerve cells, contributing to the recovery of neurological function (Reinecke et al., 2003). Li et al. (2005) reported that AT2R expression is up-regulated following focal cerebral ischemia, and that AT2R activation plays a protective role in stroke. However, the inhibitory effect of AT2R on inflammatory responses during ischemic injury remains controversial.
Here, we investigated changes in AT2R expression and related immune factors following cerebral I/R injury in a rat model of middle cerebral artery occlusion (MCAO) using the AT2R agonist CGP42112 and antagonist PD123319. We explored the possible mechanisms by which brain AT2R mediates inflammatory responses to provide new directions for the treatment of cerebral I/R injury.
Materials and Methods
Experimental animals
Forty-two healthy male Sprague-Dawley rats aged 12 weeks and weighing approximately 300 g were sourced from the Animal Experimental Center of Dalian Medical University of China (animal license No. SCXK (Liao) 2008-0002). All protocols were approved by the Animal Ethics Committee of Dalian Medical University of China. The rats were housed at 22°C in 12-hour reversed light/dark cycles, with free access to food and water. All surgery was performed under anesthesia, and all efforts were made to minimize the pain and distress of the animals. All experiments were carried out in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animal (NIH Publication No. 85-23, revised 1986).
Experimental design
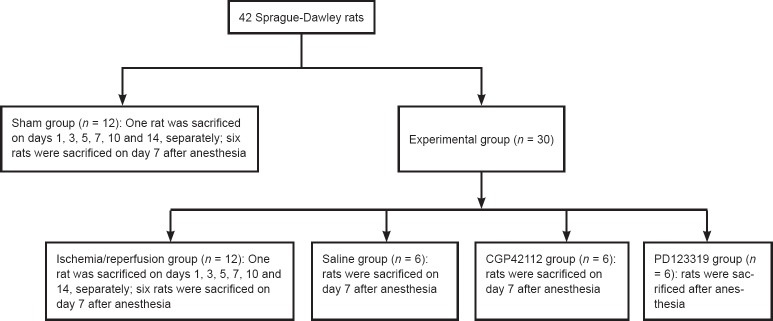
Forty-two rats were randomized into sham (n = 12) and MCAO experimental groups (n = 30). The 30 rats in the MCAO group were sub-divided to four groups: I/R (n = 12), saline (n = 6), CGP42112 (n = 6) and PD123319 (n = 6). The study design is shown in Figure 1.
Figure 1.
Experimental design.
MCAO model establishment
The rats in the experimental group were anesthetized with 10% chloral hydrate (3.5 mL/kg). After disinfection, a median incision was made on the neck. The superficial fascia between both sides of the submandibular gland was cut open to expose sternocleidomastoid muscle on one side. The gap between the sternohyoid and sternocleidomastoid muscles was bluntly dissociated to expose the vagus nerve, common carotid artery, internal carotid artery and external carotid artery. A suture (18 ± 2 mm) was inserted into the internal carotid artery along the external carotid artery to block the initial part of the middle cerebral artery. Two hours later, the suture was removed. Assessment of the establishment of a successful model of I/R was made upon regaining consciousness using standard criteria: paralysis of the contralateral limb, inability to stand and circling to one side when the tail was lifted.
Rats were scored at 24 hours after I/R in accordance with Longa’s method (Longa et al., 1989): 0 points, no nerve damage symptoms; 1 point, cannot fully extend forepaw (mild); 2 points, circling to the contralateral side (moderate); 3 points, falling to the opposite side (severe); 4 points, cannot walk by itself, loss of consciousness. Rats in the sham group suffered from ipsilateral Horner syndrome (decreased sweating on the affected side of the face, ptosis, sinking of the eyeball into the face, and a small pupil on the affected side), but did not experience limb paralysis on the contralateral side. Rats scoring 1–3 points were considered successful ischemic models (MCAO group). Rats scoring 0 and 4 points were excluded from the study and replaced.
Sham surgery
In the sham group, the common carotid artery, external carotid artery and internal carotid artery were dissociated, but the middle cerebral artery was not blocked, and the subcutaneous tissue and skin were sutured. In the I/R group, rats did not undergo any drug treatment. In the sham and I/R groups, rats were sacrificed on days 1, 3, 5, 7, 10 and 14 (one rat per day). Brain tissue was fixed in 4% formaldehyde for subsequent investigation. The remaining six rats in the sham and I/R groups were sacrificed on day 7. The comparison of AT2R expression on days 1, 3, 5, 7, 10 and 14 after reperfusion in the sham and I/R groups showed that AT2R expression peaked on days 7–10. Day 7 was selected as the most appropriate for analysis.
Drug treatments
Rats in the treatment groups were intraperitoneally injected with 1.67 mL physiological saline, the AT2R agonist CGP42112 (Tocris, Bristol, UK; 1 mg/kg per day dissolved in saline) or the AT2R antagonist PD123319 (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1 mg/kg per day dissolved in saline). An injection was administered each day until day 7 when the rats were sacrificed. A sample of each brain was fixed, frozen, and stored at −80°C. The entire brain of one rat from each group was frozen at −20°C for 30 minutes and then assayed using 2,3,5-triphenyltetrazolium chloride (TTC).
TTC assay
The frozen brain tissue was sliced coronally into six 2-mm-thick sections. Six sections were placed in 2% TTC solution (prepared with PBS) in a 37°C incubator for 15 minutes in the dark. Normal brain tissue stained red, and the infarct area remained white. The cerebral infarct area percentage was calculated as infarct area/whole brain area × 100% using IPP software (Mediacybernetics, Bethesda, MD, USA).
Immunohistochemical staining
Brain sections were washed with running water, dehydrated with a graded alcohol series, and embedded. Sections on the slice frame were removed in boiling antigen-retrieval solution in a microwave oven for 10 minutes, and cooled at room temperature. The sections were then immersed in PBS, incubated in 3% H2O2, washed with PBS, and incubated with goat serum. After removal of the serum, sections were incubated with primary antibody diluted in PBS at 4°C overnight. Sections placed on the slice frame were immersed in PBS. Details of the primary antibodies are given in Table 1. The samples were incubated with the secondary antibody biotin-labeled goat anti-rabbit/mouse IgG (Beyotime Biotechnology, Nantong, Jiangsu Province, China; 1:200), then immersed in PBS. After drying, the samples were incubated with horseradish peroxidase labeled avidin (Beyotime Biotechnology, Shanghai, China) at 37°C for 30 minutes. Sections were placed on the slice frame, immersed in PBS, visualized with 3,3′-diaminobenzidine, counterstained with hematoxylin, dehydrated, permeabilized and mounted.
Table 1.
Details of primary antibodies

Immunohistochemical analysis
Staining was observed and photographed at 400× magnification with a light microscope (Olympus, Tokyo, Japan). Five high power fields were randomly selected and the number of positive cells in each field was recorded. The integrated optical density of positive staining was calculated using Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD, USA).
Statistical analysis
The data were analyzed using SPSS 11.5 software (SPSS, Chicago, IL, USA) and expressed as mean ± SD. A paired t-test was used to compare the difference between the sham and I/R groups. One-way analysis of variance and the least significant difference tests were used to compare intergroup differences. A value of P < 0.05 was considered statistically significant.
Results
Changes in AT2R expression in brain tissue of rats with I/R injury
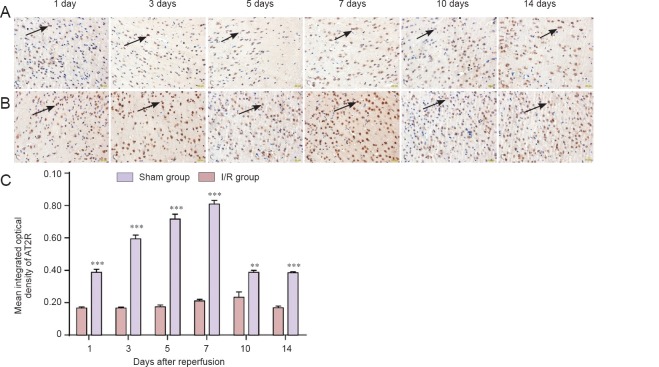
Immunohistochemical staining revealed that AT2R expression was increased in MCAO brain tissue on day 1 after reperfusion compared with the sham group (P < 0.001), peaked on days 7–10, and then gradually declined (Figure 2). AT2R expression increased in the CGP42112 group (P < 0.05), but decreased in the PD123319 group (P < 0.05) compared with the saline group (Figure 3).
Figure 2.
AT2R expression changes in brain tissue of middle cerebral artery occlusion rats over 14 days.
(A, B) Immunoreactivity of AT2R in brain tissue on days 1, 3, 5, 7, 10 and 14 in the sham group (A) and I/R group (B) (immunohistochemical staining, × 400). (C) Mean integrated optical density changes in brain tissue AT2R on days 1, 3, 5, 7, 10 and 14 in the sham group and I/R group. **P < 0.01, ***P < 0.001, vs. sham group (mean ± SD, n = 3, paired t-test). AT2R: Angiotensin II type 2 receptor; I/R: ischemia/reperfusion.
Figure 3.
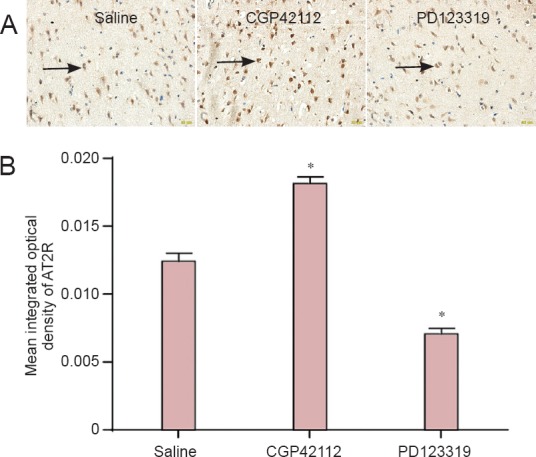
Effects of CGP42112 and PD123319 on AT2R expression in rat middle cerebral artery occlusion brain tissue.
(A) Immunohistochemical staining on day 7 following reperfusion (× 400). The number of AT2R-positive cells increased with CGP42112 treatment, and decreased with PD123319 treatment, compared with the saline group. Arrows indicate AT2R-positive cells. (B) Changes in mean integrated optical density in brain tissue AT2R. *P < 0.05, vs. saline group (mean ± SD, n = 3, one-way analysis of variance and the least significant difference test). AT2R: Angiotensin II type 2 receptor.
Changes in infarct area in rats with I/R injury
TTC staining revealed infarct foci in the I/R groups, but not in the sham group. Compared with the saline group, the infarct area was smaller in the CGP42112 group (P < 0.05), but larger in the PD123319 group (P < 0.01) (Figure 4).
Figure 4.
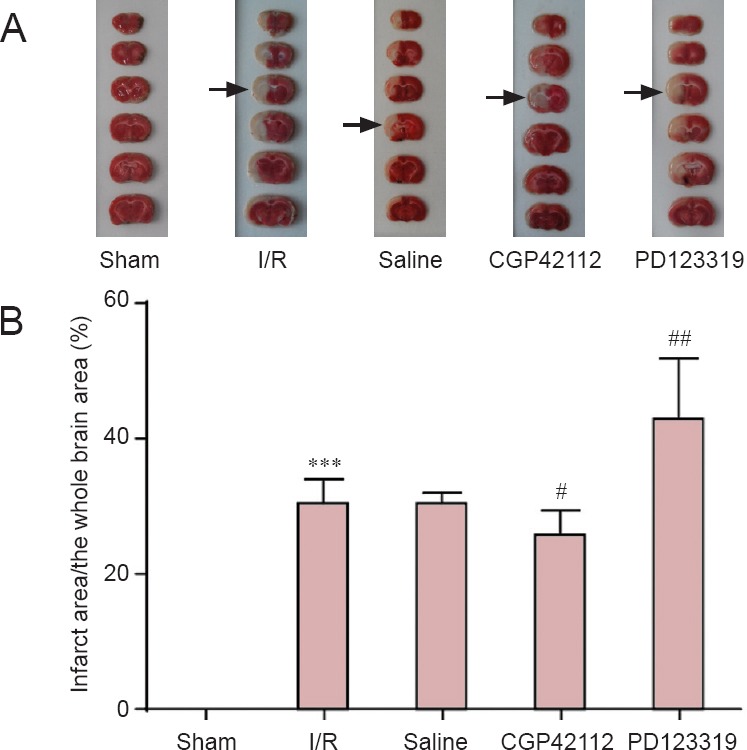
Changes in infarct area in the rats with cerebral I/R injury (TTC assay).
(A) TTC assay results in sham, I/R, saline, CGP42112 and PD123319 groups on day 7 following I/R. Arrows indicate infarcts. (B) Infarct area as a percentage of the whole brain. ***P < 0.001, vs. sham group; #P < 0.05, ##P < 0.01, vs. saline group (mean ± SD, n = 6, one-way analysis of variance and the least significant difference test). I/R: Ischemia/reperfusion; TTC: 2,3,5-triphenyltetrazolium chloride.
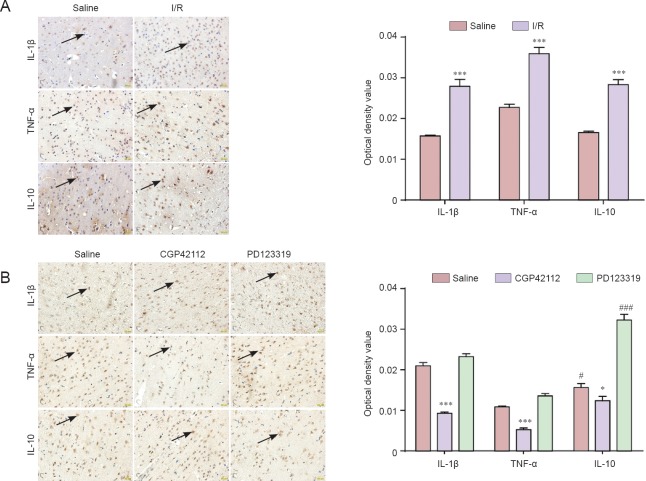
IL-1β, TNF-α and IL-10 expression in MCAO brain tissue
Immunohistochemical staining showed that IL-1β, TNF-α and IL-10 expression increased after I/R (P < 0.001). IL-1β and TNF-α expression decreased (P < 0.001), but IL-10 expression increased (P < 0.05) after treatment with CGP42112. IL-1β and TNF-α expression increased (P < 0.05), but IL-10 expression decreased (P < 0.001) following PD123319 treatment (Figure 5).
Figure 5.
IL-1β, TNF-α and IL-10 expression in brain tissue of rats with cerebral I/R injury.
(A) Immunoreactivity of IL-1β, TNF-α and IL-10 in sham and I/R groups (immunohistochemical staining, × 400). (B) Immunoreactivity of IL-1β, TNF-α and IL-10 in saline, CGP42112 and PD123319 groups on day 7 following cerebral I/R (immunohistochemical staining, × 400). (A, B) *P < 0.05, ***P < 0.001, vs. saline group and sham group, respectively. #P < 0.05, ###P < 0.001, vs. saline group (mean ± SD, n = 3, one-way analysis of variance and the least significant difference test). TNF-α: Tumor necrosis factor-α; IL: interleukin; I/R: ischemia/reperfusion.
Discussion
In this study, immunohistochemical staining demonstrated that AT2R participates in cerebral I/R. AT2R expression is high in the embryonic stage (Inagami, 1999), but rapidly decreases after birth, and remains low throughout adulthood. AT2R expression increases under certain pathological conditions, such as heart failure, myocardial infarction, wound healing, vascular injury and inflammation (Horiuchi et al., 1999). Activated AT2R dilates blood vessels, contributes to cell differentiation and proliferation, and remodels and repairs tissues (Carey et al., 2000; Zapparoli et al., 2011).
Reinecke et al. (2003) proposed that AT2R in nerve tissue promotes cell differentiation and regeneration, and the recovery of neurological function. Iwai et al. (2004) found that AT2R activation plays a protective role in stroke. Another study demonstrated that an AT2R antagonist inhibited the neuroprotective effect of AT1R following focal cerebral ischemia. AT2R activation improved the survival rate of cultured neurons and promoted axonal growth (Li et al., 2005). In our study, AT2R agonists and antagonists directly regulate AT2R expression. When directly activating or blocking AT2R, we found that AT2R expression was higher in the CGP42112 group, and lower in the PD123319 group, than in the saline group. These findings suggest that AT2R expression could be regulated by AT2R agonists and antagonists following cerebral I/R injury. McCarthy et al. (2012) demonstrated that in rats, infarct volume decreased and motor function was improved 6 hours after cerebral ischemia when treated CGP42112 was administered into the ventricle. In our study, treatment with CGP42112 and PD123319 revealed that greater AT2R expression led to a smaller infarct volume, and lower AT2R expression resulted in a larger infarct volume. These results indicate that AT2R exerts a protective effect on cerebral I/R.
Several studies have verified that immune and inflammatory responses are involved in ischemic injury (Tu et al., 2014; Wu et al., 2014; Zhou et al., 2014). The expression and concentration of IL-1β (Haqqani et al., 2005), TNF-α (Murakami et al., 2005; Offner et al., 2006) and IL-10 (Nayak et al., 2009) in plasma and brain tissue are altered following cerebral ischemia. This suggests that immune and inflammatory responses are strongly associated with cerebral ischemia, which is supported by the current study. Following middle cerebral artery occlusion, the AT2R agonist C21 has a neuroprotective effect by reducing the expression of the pro-inflammatory cytokines MCP-1 and TNF-α on the ischemic side (Min et al., 2014). Our results demonstrate that after cerebral I/R injury, AT2R may suppress immune and inflammatory responses by down-regulating IL-1β and TNF-α expression and up-regulating IL-10 expression, exerting neuroprotective effects.
In summary, AT2R protects against cerebral I/R injury probably by suppressing immune and inflammatory responses. However, this study is limited by uncertainty as to whether AT2R protects cerebral ischemia via other pathways. Future research will aim to determine this mechanism.
Acknowledgments
We are very grateful to Peng Lv from the Experimental Center of the Second Hospital of Dalian Medical University of China for his technical support.
Footnotes
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Brooks W, Yajima W, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 2.Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension. 2000;35:155–163. doi: 10.1161/01.hyp.35.1.155. [DOI] [PubMed] [Google Scholar]
- 3.Haqqani AS, Nesic M, Preston E, Baumann E, Kelly J, Stanimirovic D. Characterization of vascular protein expression patterns in cerebral ischemia/reperfusion using laser capture microdissection and ICAT-nanoLC-MS/MS. FASEB J. 2005;19:1809–1821. doi: 10.1096/fj.05-3793com. [DOI] [PubMed] [Google Scholar]
- 4.Horiuchi M, Akishita M, Dzau VJ. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999;33:613–621. doi: 10.1161/01.hyp.33.2.613. [DOI] [PubMed] [Google Scholar]
- 5.Inagami T. Molecular biology and signaling of angiotensin receptors: an overview. JASN. 1999;10(Suppl 11):S2–7. [PubMed] [Google Scholar]
- 6.Iwai M, Liu HW, Chen R, Ide A, Okamoto S, Hata R, Sakanaka M, Shiuchi T, Horiuchi M. Possible inhibition of focal cerebral ischemia by angiotensin II type 2 receptor stimulation. Circulation. 2004;110:843–848. doi: 10.1161/01.CIR.0000138848.58269.80. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Culman J, Hortnagl H, Zhao Y, Gerova N, Timm M, Blume A, Zimmermann M, Seidel K, Dirnagl U, Unger T. Angiotensin AT2 receptor protects against cerebral ischemia-induced neuronal injury. FASEB J. 2005;19:617–619. doi: 10.1096/fj.04-2960fje. [DOI] [PubMed] [Google Scholar]
- 8.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy CA, Vinh A, Broughton BR, Sobey CG, Callaway JK, Widdop RE. Angiotensin II type 2 receptor stimulation initiated after stroke causes neuroprotection in conscious rats. Hypertension. 2012;60:1531–1537. doi: 10.1161/HYPERTENSIONAHA.112.199646. [DOI] [PubMed] [Google Scholar]
- 10.Min LJ, Mogi M, Tsukuda K, Jing F, Ohshima K, Nakaoka H, Kan-No H, Wang XL, Chisaka T, Bai HY, Iwanami J, Horiuchi M. Direct stimulation of angiotensin II type 2 receptor initiated after stroke ameliorates ischemic brain damage. Am J Hypertens. 2014;27:1036–1044. doi: 10.1093/ajh/hpu015. [DOI] [PubMed] [Google Scholar]
- 11.Murakami Y, Saito K, Hara A, Zhu Y, Sudo K, Niwa M, Fujii H, Wada H, Ishiguro H, Mori H, Seishima M. Increases in tumor necrosis factor-alpha following transient global cerebral ischemia do not contribute to neuron death in mouse hippocampus. J Neurochem. 2005;93:1616–1622. doi: 10.1111/j.1471-4159.2005.03163.x. [DOI] [PubMed] [Google Scholar]
- 12.Nayak AR, Kashyap RS, Purohit HJ, Kabra D, Taori GM, Daginawala HF. Evaluation of the inflammatory response in sera from acute ischemic stroke patients by measurement of IL-2 and IL-10. Inflamm Res. 2009;58:687–691. doi: 10.1007/s00011-009-0036-4. [DOI] [PubMed] [Google Scholar]
- 13.Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 14.Reinecke K, Lucius R, Reinecke A, Rickert U, Herdegen T, Unger T. Angiotensin II accelerates functional recovery in the rat sciatic nerve in vivo: role of the AT2 receptor and the transcription factor NF-kappaB. FASEB J. 2003;17:2094–2096. doi: 10.1096/fj.02-1193fje. [DOI] [PubMed] [Google Scholar]
- 15.Saward L, Zahradka P. The angiotensin type 2 receptor mediates RNA synthesis in A10 vascular smooth muscle cells. J Mol Cell Cardiol. 1996;28:499–506. doi: 10.1006/jmcc.1996.0046. [DOI] [PubMed] [Google Scholar]
- 16.Tu QY, Cao H, Zhong W, Ding BR, Tang XQ. Atorvastatin protects against cerebral ischemia/reperfusion injury through anti-inflammatory and antioxidant effects. Neural Regen Res. 2014;9:268–275. doi: 10.4103/1673-5374.128220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Barone FC, Aiyar NV, Feuerstein GZ. Interleukin-1 receptor and receptor antagonist gene expression after focal stroke in rats. Stroke. 1997;28:155–161. doi: 10.1161/01.str.28.1.155. [DOI] [PubMed] [Google Scholar]
- 19.Wu LF, Zhang KN, Hu GZ, Yan HY, Xie C, Wu XM. Inflammatory response and neuronal necrosis in rats with cerebral ischemia. Neural Regen Res. 2014;9:1753–1762. doi: 10.4103/1673-5374.143419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zapparoli A, Figueiredo JF, Boer PA, Rocha Gontijo JA. Impaired dipsogenic and renal response to repetitive intracerebroventricular angiotensin II (AngII) injections in rats. J Renin Angiotensin Aldosterone Syst. 2011;12:161–168. doi: 10.1177/1470320310392617. [DOI] [PubMed] [Google Scholar]
- 21.Zhou F, Wang L, Liu PP, Hu WW, Zhu XD, Shen H, Yao YY. Puerarin protects brain tissue against cerebral ischemia/reperfusion injury by inhibiting the inflammatory response. Neural Regen Res. 2014;9:2074–2080. doi: 10.4103/1673-5374.147934. [DOI] [PMC free article] [PubMed] [Google Scholar]