Abstract
Bacterial pathogens are often classified by their toxicity and invasiveness. The invasiveness of a given bacterium is determined by how capable the bacterium is at invading a broad range of tissues in its host. Of mammalian pathogens, some of the most invasive come from a group of bacteria known as the spirochetes, which cause diseases such as syphilis, Lyme disease, relapsing fever and leptospirosis. Most of the spirochetes are characterized by their distinct shapes and unique motility. They are long, thin bacteria that can be shaped like flat-waves, helices, or have more irregular morphologies. Like many other bacteria, the spirochetes use long, helical appendages known as flagella to move; however, the spirochetes enclose their flagella in the periplasm, the narrow space between the inner and outer membranes. Rotation of the flagella in the periplasm causes the entire cell body to rotate and/or undulate. These deformations of the bacterium produce the force that drives the motility of these organisms, and it is this unique motility that likely allows these bacteria to be highly invasive in mammals. This review will describe the current state of knowledge on the motility and biophysics of these organisms and provide evidence on how this knowledge can inform our understanding of spirochetal diseases.
1. Introduction
It is hard to build an all-terrain vehicle. Tires that are best on smooth roads don’t fare well on rough, rocky mountain trails and are useless for driving across deep rivers or oceans. The structural design that is best for maneuverability and aerodynamics on relatively flat surfaces is not optimized to prevent tipping on inclines and won’t float. While there are man-made vehicles that can traverse this range of environments (1), they often utilize multiple force producing mechanisms (e.g., wheels for roads and propellers for water-based travel) and are not terribly efficient in any modality.
Pathogens that invade our bodies often have to be able to survive in and, in many cases, move through a range of diverse environments. For example, many bacteria are capable of moving through fluids (swimming) and also along solid or semisolid surfaces (gliding, twitching, and swarming) (2–4). This transition from swimming to surface-associated motility can be achieved by transitioning from flagellar-based swimming to pili-driven gliding or twitching (5), much like the switch from wheels to propellers in man-made all-terrain vehicles. Likewise, Vibrio parahaemolyticus uses polar flagella to swim and lateral flagella to swarm (6). Some flagellated bacteria, though, can use their flagella to power swarming along surfaces (7). In other words, bacteria have out-engineered us by figuring out how to make a single motility mechanism work in multiple environments.
Indeed, flagellar-based motility can also move bacteria through complicated environments such as soft agar gels, where the pore-size in the gel is approximately equal to the diameter of the bacteria (8, 9). However, as the concentration of the polymer in the gel increases, the motility of most flagellated bacteria becomes greatly inhibited (9), likely due to there being few holes large enough for the bacteria to squeeze through. This, though, is not true for the spirochetes, a unique group of bacteria with some highly pathogenic members. One aspect that makes pathogenic spirochetes so capable of setting up infections in mammals is their motility. For example, Lyme disease and syphilis are caused by the spirochetes, Borrelia burgdorferi and Treponema pallidum, respectively, and these bacteria are some of the most invasive mammalian pathogens (Fig. 1)(10, 11). Both of these bacteria are able to easily move through our skin, break into and out of blood vessels, and can cross the blood-brain barrier (12). The syphilis bacterium can even cross the placental barrier, which leads to infection of the unborn fetus, known as congenital syphilis (12). Truly, these bacteria are exceptional and efficient all-terrain vehicles for traversing the mammalian body. Why are they so adept at moving through the broad range of tissues in our bodies?
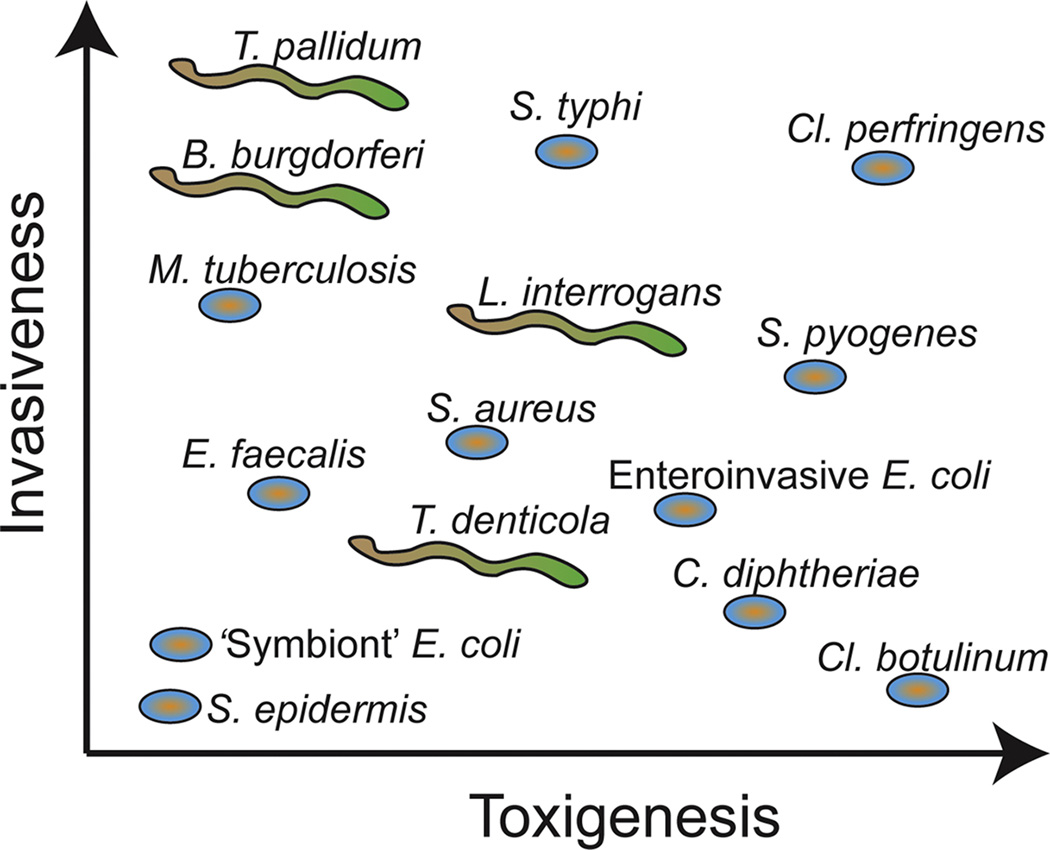
Figure 1.
Relative comparison of the invasiveness and toxin production (toxigenesis) for a number of pathogenic bacteria. The syphilis bacterium (T. pallidum) is one of the most invasive but does not release toxins into its host, whereas Cl. botulinum is highly toxic but not invasive. Schematic is based on (10).
This review focuses on describing the unique motility of the spirochetes and seeks to use our current knowledge about how these organisms move to inform some aspects of the pathogenesis of spirochetal diseases. I begin in Section 2 by describing the more prevalent diseases that are caused by spirochetes, focusing on the role of motility. Then, I discuss the current state of our knowledge about the biophysics for how these bacteria create their unique shapes (Section 3) and movements (Section 4). Though much of the research on spirochete motility has focused on the swimming of these organisms through liquid media, recent work has investigated their motility in extracellular matrix-like environments and in living mammals and ticks. Section 5 will describe how these environments affect motility. Section 6 discusses how biophysical data on motility can aid our understanding of the early stages of Lyme disease. Section 7 concludes with a discussion of where the field of spirochetal motility stands and what major outstanding questions remain.
2. Spirochetal diseases
A vast array of mammalian diseases are caused by spirochetes, including the notorious human diseases syphilis and Lyme disease. In humans, spirochetes also cause yaws (Treponema pallidum subsp. pertenue), pinta (Treponema carateum), relapsing fever (Borrelia species), leptospirosis (Leptospiraceae) and periodontal disease (Treponema species) (13, 14). In this section, I briefly review these diseases, focusing on the spirochetal behaviors that largely influence pathogenesis.
Syphilis
Relatively few diseases are as recognized and carry such a stigma as syphilis, the sexually-transmitted form of which is caused by T. pallidum subspecies pallidum. Venereal syphilis is primarily acquired either through sexual intercourse with an individual in the primary or secondary stages of the disease or congenitally, being transmitted from a mother to the unborn fetus (12). During the primary and secondary stages of the disease, mucocutaneous lesions are present, which readily enable spirochetes from the infected individual to come into contact with mucosa or skin on the uninfected partner. Surprisingly few treponemes are required to initiate syphilis: If approximately 57 organisms are inoculated onto an individual, there is a 50% chance that they will contract the disease (15). T. pallidum then rapidly disseminates, as exemplified in animal studies where treponemes were found in the blood, lymph nodes, bone marrow, spleen, and testes within 48 hr after inoculation (16, 17). T. pallidum also readily breaches the blood-brain barrier and infects the central nervous system (12). Congenital syphilis also highlights the invasiveness of T. pallidum, as very few bacteria are capable of transplacental transmission, yet treponemes can be found in fetuses as early as nine weeks (18, 19).
Syphilis begins with a 9 to 90 day incubation period, during which time the patient is asymptomatic. Replication of the spirochetes at the inoculation site induces a local inflammatory response that generates a papule, which subsequently ulcerates (12). This chancre is the defining lesion of primary syphilis. In the chancre, T. pallidum are found in the dermis in close proximity to blood vessels (20). Interestingly, the chancre is often painless, which may be due to infiltration of cutaneous sensory nerves by the bacteria (21, 22). Secondary syphilis begins four to ten weeks after primary syphilis. In secondary syphilis, the spirochetes have disseminated throughout the body. While mucocutaneous lesions are the primary manifestation of secondary syphilis, virtually any organ can be affected (23). The lesions associated with secondary syphilis typically resolve in three to twelve weeks, after which there can be periods of latency where the patient is asymptomatic (12). Periods where there are high burdens of spirochetes in the blood (spirochetemia) occur during the early years of syphilis, and at least 30% of untreated patients will develop tertiary syphilis, which include gummatous, cardiovascular and neurological complications (12).
Yaws, bejel, and pinta
Other subspecies of T. pallidum cause the related diseases of yaws (pertenue) and endemic syphilis (also known as bejel or non-venereal syphilis and caused by the subspecies endemicum) (13, 14). A related species, T. carateum, causes pinta (12). These diseases are all transmitted through non-sexual contact and include skin lesions. Endemic syphilis also includes inflammation of the leg bones and, in later stages, gummas of the nose and soft palate (24). Late stage (tertiary) yaws often includes widespread bone, joint, and soft tissue damage (25).
Periodontal disease
The other major human disease associated with treponemes is periodontal disease. There are at least 57 different species of oral Treponema, but only 10 of these species have been cultured (26). All of the cultivatable oral treponemes show some relationship with inflammatory events in periodontal disease (27). Our mouths are teeming with microorganisms, and the oral spirochetes are important members of this microbiota. However, numerous correlations link the number of spirochetes in the oral microbiota to disease (28, 29). But how is the presence of spirochetes leading to disease? A number of features of the treponemes have been suggested. For one, T. denticola and T. pectinovorum have been shown to cause proinflammatory cytokine release (30). The host immune response may then be accountable for much of the tissue and bone destruction that accompanies the disease. The oral treponemes also secrete proteases that can do damage and have been shown to alter the function of cells that are involved in periodontal homeostasis (27). Spirochete motility has also been connected to pathogenesis. A possible explanation for the role of motility is that it enables penetration of the epithelium and gingival connective tissue (27).
Lyme disease is transmitted to humans by the bite from Ixodes scapularis ticks that are infected with B. burgdorferi. During tick feeding, the bacteria replicate and a small fraction penetrate out of the tick midgut and migrate to the salivary glands, where they are then transported into the dermis of the host via the saliva (31). It takes at least 48 hours for the spirochetes to move from the gut into the dermis, and the tick remains attached to the host for approximately 4 to 5 days (31, 32). Therefore, at the end of the bloodmeal, a small inoculum of spirochetes is introduced into the dermis at the bite site. In the dermis, the spirochetes replicate and begin to disseminate both locally and hematogenously. While migration through the dermis can be fairly rapid (at speeds of a few microns per second (33)), the spirochetes also bind to extracellular matrix (ECM) proteins and can become transiently adhered to the matrix (33, 34). The tick bite along with the presence of the spirochetes in the dermis activates the innate immune response, which includes neutrophil migration to the bite site, uptake of spirochetes by immune effector cells and activation of phagocytic cells, such as macrophages (32, 35–39). The release of pro-inflammatory cytokines by macrophages leads to further recruitment of innate immune cells and T cells to the infected region (32, 36). This inflammatory cascade also causes hyperaemia in the capillaries, leading to the characteristic rash, known as erythema migrans, that is usually the first symptom of infection (31, 32). The rash can grow to be quite large (>30 cm) and can have one of three appearances, a bull’s-eye, a solid rash, or a ring-like, central clearing rash (40). During early infection, B. burgdorferi spreads through the skin and blood (41). While B. burgdorferi can invade many different tissues, the most common infection sites are the skin, nervous system, heart, and joints, where they can cause secondary rashes, neuroborreliosis, Lyme cariditis, and Lyme arthritis, respectively (41). Interestingly, in Lyme disease the burden of spirochetes in the blood is typically quite low (~ 0.1 bacterium per ml of blood).
Recent work has shown convincingly that flagellar-based motility is an essential aspect of the pathogenesis of Lyme disease. The Norris group used transposon mutagenesis to identify virulence genes in B. burgdorferi and found that 10 of 14 mutations in chemotaxis genes and 7 mutations in flagellar structure and assembly genes lead to a loss in the ability to establish infection in mice (11). In addition, B. burgdorferi mutants that lack the primary flagellar protein FlaB, the torque generating subunit of the flagellar motor MotB or the chemotaxis protein CheA2 could not infect mice or migrate out of the tick (42–44). Mutants lacking MotB also do not survive in the tick (42).
Relapsing Fever
Other species of Borrelia cause relapsing fever (e.g., B. duttonii, B. hermsii, and B. turicatae), which is transmitted to humans by either soft ticks or the body louse. Relapsing fever is characterized by the occurrence of three or more episodes of fever that are each followed by a “crisis,” which involves a period of restlessness and apprehension, then tachycardia, and finally a decline in temperature accompanied with hypotension, leucopenia, and exhaustion (45). Relapsing fever eventually resolves without specific therapy. Relapsing fever is notably different than Lyme disease. For example, the soft ticks and body lice only feed for minutes, as opposed to days, and inoculation of a single spirochete is sufficient to initiate the disease. The spirochetes multiply in the blood, and the fever episodes occur during periods of high spirochetemia (45). Relapsing fever spirochetes can also invade the central nervous system, the eye, liver, testes, and other organs and tissues. Spirochetes in these extravascular sites survive when the bacteria in the blood have been eliminated (45).
Leptospirosis is a major public health problem, especially in developing countries. The disease can range in severity from mild, flu-like symptoms to severe forms of the disease, which can include jaundice, acute renal and hepatic failure, pulmonary distress and hemorrhage (46, 47). Leptospirosis is caused by species of spirochetes from the genus Leptospira. Infection typically occurs when a mammal comes into direct contact with infected animal urine or with contaminated water. Pathogenic Leptospira can live freely in water, but will invade into mammals through cuts or abrasions in the skin or through mucous membranes of the eyes, nose or throat (46). The kidney is the primary organ where Leptospira establishes infection, as this allows the bacteria to leave the host through the urine. However, leptospirosis can be a multi-organ disease, involving the liver, heart, lungs, eyes, skin and nervous system (48). Motility of the organism is crucial for its ability to invade this wide range of tissues, and flagellar (FlaA) and motor (FliY) proteins have been shown to strongly influence the virulence of Leptospira (49, 50). The ability to adhere to host tissue via adhesion proteins is also thought to be a necessary component of leptospire pathogenicity (46).
3. The basic structure of the spirochetes
One of the most identifiable aspects of the spirochetes is their shape. Most spirochetes are long (from 1–250 µm), thin (with diameters of 0.1 – 3 µm) and have helical or flat-wave shapes (51). Like all bacteria (and cells, in general), they have an inner membrane, which separates the inside of the cell from the outside (Fig. 2). Inside this membrane is the cytoplasm, a crowded liquid environment that contains the genome along with a host of proteins, and just outside the inner membrane is the bacterial cell wall, a thin, mesh-like layer of peptidoglycan that provides the cell with its structural integrity and helps to define cell shape. The composite structure of the cell wall, inner membrane and cytoplasm is sometimes called either the cell cylinder or protoplasmic cylinder (Fig. 2) (52). Finally, there is an outer membrane that encloses this cell cylinder.
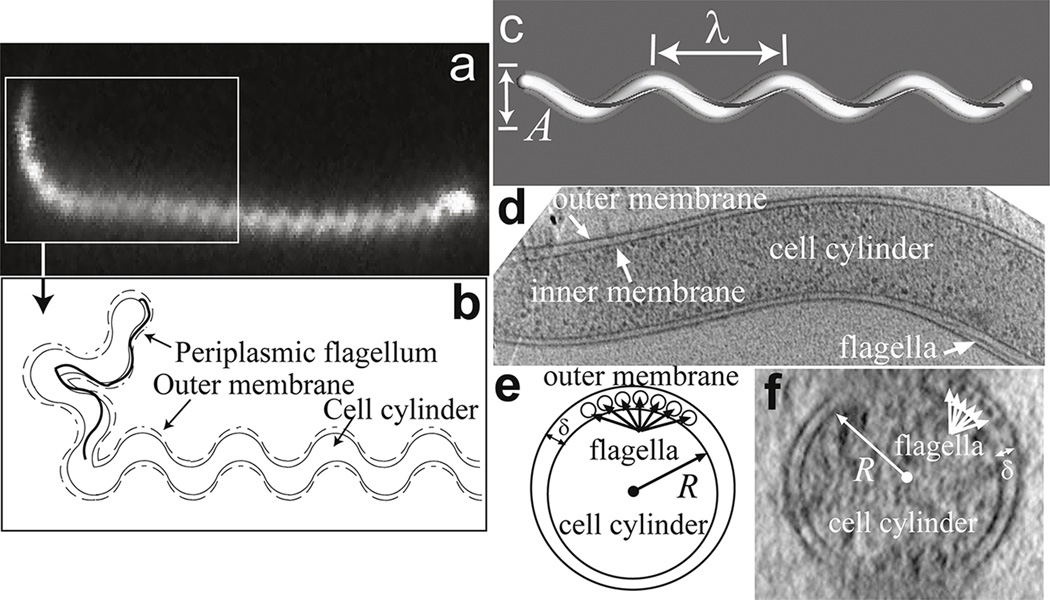
Figure 2. The basic structure of the spirochetes.
(a) Dark field image of Leptonema illini, a member of the Leptospiraceae. The boxed region highlights the hook-shaped end of the cell. Image courtesy of Stuart Goldstein. (b) Schematic diagram of the relative positon of the periplasmic flagellum to the cell body and outer membrane. Leptospira spp. Have one short flagellum at each end that is attached to a flagellar motor located at the end of the cell. The presence of the flagellum causes the cell to bend into the hook shape. (c) B. burgdorferi and some members of the Treponema species have flat-wave shapes, characterized by planar wave-like shapes with wavelength λ and amplitude A. This wave-like morphology is produced by the periplasmic flagella (dark grey) wrapping around the cylindrical cell body (white). (d) A lengthwise slice through B. burgdorferi shows the flagella located in the periplasm between the inner and outer membranes of the cell. (e,f) Cells have circular cross-sections of radius R (which in B. burgdorferi is approximately 150 nm). The periplasm is narrow with a width δ ~ 20 – 40 nm (53). (d,f) Cryo EM images courtesy of S. Goldstein and N. Charon.
In between the inner and outer membranes is a narrow space known as the periplasm that is approximately 20–40 nm thick (Fig. 2b,e,f) (53). In addition to containing the cell wall, the periplasm also contains long, thin helically-shaped organelles that are structurally and compositionally homologous to the flagella from other bacteria (54). Each of these periplasmic flagella is attached to its own flagellar motor; these motors are embedded in the inner membrane and anchored to the cell wall near each end of the bacterium. Therefore, the periplasmic flagella are attached near the ends of the bacterium and wrap inward along the cell body. Spirochetes can have anywhere from 1 to 100s of flagella anchored near each end (51). As in other bacteria (and will be discussed later), these periplasmic flagella are implicit in driving the motility of these organisms. However, in many spirochetes, the flagella also serve a skeletal function. For example, Leptospira interrogans has a helical-shaped cell body that is 6–20 µm in length (Fig. 2a,b) (55–57). The ends of these spirochetes bend into either a hook or spiral shape (58). At each end L. interrogans also have a short, tightly-coiled single flagellum that is not long enough to overlap at the center of the cell with the flagellum from the other end (59). Mutants that form uncoiled flagella or that lack flagella still have helically-shaped bodies, but the ends do not bend into hooks or spirals (59, 60). Treponema denticola cells are 6 – 16 µm in length and 0.21 – 0.25 µm in diameter; they have bundles, each containing two PFs, which overlap in the center of the cell (61–63). Most wild-type cells of T. denticola have a highly irregular (twisted) morphology along their entire length, combining both helical and planar regions in a non-distinct manner; however, a minority of cells is observed in a right-handed morphology (63). These two separate forms are relatively stable, and only rarely does one form convert to the other (63). If the outer membrane sheath is removed from the cell so that the flagella are no longer closely associated with the cell body, then an initially irregular cell takes on a right-handed helical form with a helical pitch only slightly different than those of the helical wild-type cells (63). In addition, flagella-less non-motile mutants also have this right-handed morphology, with helical parameters similar to those of the helical wild-type cells (63). B. burgdorferi and T. pallidum both have flat-wave morphologies; their cell bodies form a roughly planar, sinusoidal waveform (Fig. 2c–f)(64, 65). The flagella in both these species are long enough to overlap with flagella emanating from the other end (53, 65–68). In B. burgdorferi it has been shown that cells lacking flagella have a straight rod shape (64, 69–71).
The three examples just mentioned show that the periplasmic flagella in the spirochetes can influence cell shape; i.e., the shape of the organism depends on the flagella. But how? Possibly the simplest explanation is that the presence of the flagella in the periplasm bends the bacterium into its shape, in which case the shape represents some competition between the helical shape of the flagella and the shape of the cell cylinder. In other words, the shape is due to forces that act between the flagella and the cell body. If the flagella and cell body are elastic, then forces applied to them will cause them to bend. The flagella have to fit into the periplasm and may require being bent to fit into that space. The bent flagella would push against the cell body, consequently, causing it to bend. This physics can be converted into a mathematical model (72, 73). This model shows that this simple explanation nicely explains the hook and spiral shaped ends in L. interrogans (72). For B. burgdorferi and T. pallidum, the model also predicts the flat-wave shape but requires that flagella from either end overlap within the periplasm (73, 74).
4. The biophysics of spirochete motility
The motility of all of the spirochetes are due to the rotation of the flagella within the periplasmic space (52, 69, 75). This rotation leads to rotation of the entire cell body and often also causes the cell body to undulate. As mentioned in Sec. 2, the major spirochetal human pathogens are the Leptospiraceae, T. pallidum and B. burgdorferi. In this review, I focus on the motility of these organisms, but the general features also apply to the other spirochetes.
In regard to motility, the most studied organism is the Lyme disease bacterium, B. burgdorferi, which has 7 – 11 flagella that emanate from the ends of the bacterium and are long enough to overlap with flagella that come from the other end (Fig. 3a)(73, 74, 76). Each of these flagella is attached to its own flagellar motor, a small molecular device that rotates and produces torque, driven by the flow of charge through the motor (54, 68). Homologous flagellar motors are found in most swimming bacteria, and the biophysical behavior of these motors has been extensively studied in Escherichia coli (54). In B. burgdoferi, rotation of the flagella by the motors causes the flat-wave shape to undulate as a traveling wave (Fig. 3a)(64, 74). When the bacterium is immersed in a liquid, these wave-like deformations of the cell body produce thrust, pushing the fluid in the same direction that the wave propagates (77–79). Consequently, the bacterium is pushed in the opposite direction (Fig. 3a). In liquid media with viscosity comparable to that of water, B. burgdorferi undulates at approximately 10 Hz and swims at 6 – 7 µm/s (64, 80). The frequency of the undulation, along with the wavelength and amplitude of the cell body, dictate the speed that the organism moves through a viscous fluid, as has been shown by calculating the expected speed using low Reynolds number hydrodynamics and slenderbody theory (33, 81).
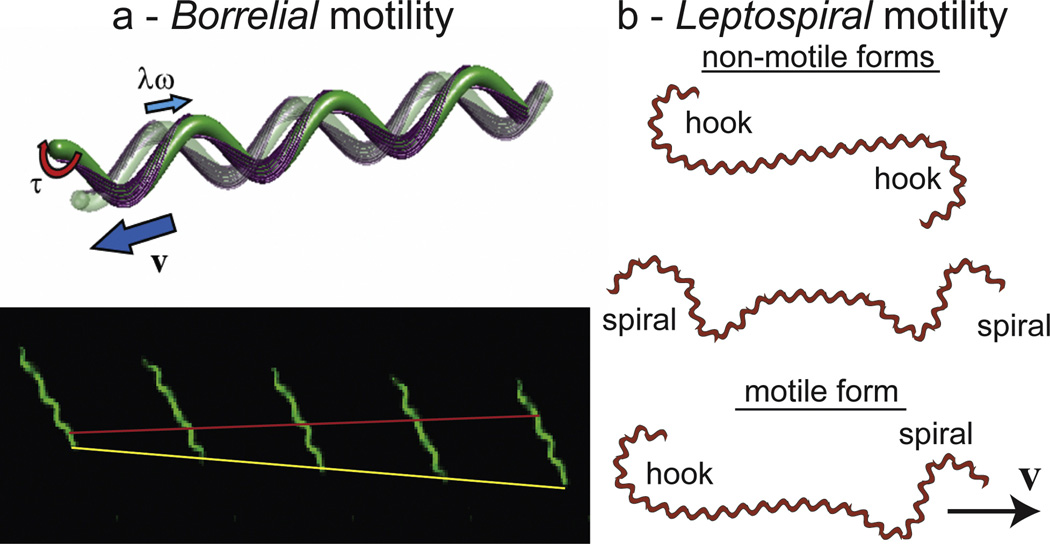
Figure 3. How spirochetes swim.
(a) The swimming of B. burgdorferi. The top panel shows a schematic of B. burgdorferi at two instants of time, with the transparent cell being at the earlier time. The outer membrane has been removed to show how the helical flagella (purple), which are anchored into flagellar motors sub-terminally from the cell poles, wrap around the cell body. A torque τ is applied to each flagellum, which causes the flagella to rotate at frequency ω and the cell body to undulate. The traveling wave undulations which move down the cell with a wave speed λω exert forces on the surrounding fluid that cause the bacterium to swim with velocity v. The bottom panel shows a time series of B. burgdorferi swimming in 1% methulcellulose. The red line denotes the backward motion of a peak in the flat-wave shape and the yellow line shows the forward progress of the bacterium. The time between frames is approximately 0.3 seconds. (b) Schematic diagram of the shapes of the Leptospiraceae. The cell bodies of spirochetes in this group are helical. Rotation of the flagella in the periplasm bends the ends into either a hook or spiral, and during swimming, the organism transitions between three forms. If both ends are in either the hook or the spiral form, then the organism does not translocate. However, if one end is spiral and the other hook-shaped, then the organism swims in the direction of the spiral-shaped end.
But why does rotation of the flagella cause the cell body to undulate as a traveling wave? As described in the previous section, the flat-wave shape of B. burgdorferi is due to the presence of the helical flagella. In fact, the cell body threads itself through the flagella in such a way that it alternates from being on the outside of the curving flagella to being on the inside. Peaks or troughs in the flat-wave shape correspond to when the cell body is on the outside curve of the flagellar helix, whereas in between these regions the flagella curve around the cell body (top panel of Fig. 3a). Rotation of the flagella about their own axes changes whether the flagella are curved towards the cell body or away from it. Therefore, as the flagella rotate, a given spot on the cell body changes from being on the outside curve of the flagella to being on the inside, and the locations of the peaks and troughs move down the cell body. Consequently, the body undulates as a traveling wave. The direction that the flagella rotate dictates the direction that the traveling wave moves along the body. Simulations of the physical interaction between the flagella and the cell body confirms that rotation of the flagella by torques from the flagellar motors can lead to traveling wave undulations of the cell body; however, if the flagella do not overlap with each other, then the cell body has a tendency to flip into a helical configuration (74). These simulations then suggest that it may be important that the flagella in B. burgdorferi are long enough for overlap with the flagella from the opposite end.
T. pallidum also appears to have a flat-wave shape; however, there are only 2 – 3 flagella per end (65). The waveform of T. pallidum is also smaller than that of B. burgdorferi, with a wavelength of 1.5 µm and an amplitude of 0.4 µm, compared to a wavelength of roughly 3.2 µm and an amplitude of 0.8 µm in B. burgdorferi (80). As in B. burgdorferi, T. pallidum swims by undulating as a traveling wave, moving at about 2 µm/s. The similarities in shape and swimming between these bacteria, as well as the fact that they are both highly invasive pathogens, has led to the suggestion that B. burgdorferi can serve as a model to study syphilis, since T. pallidum cannot be cultured (82). Recent experiments examined the effect of changing the viscosity on the swimming of these two organisms and found that they both respond to changes in viscosity in a similar way, thereby strengthening the claim for using the Lyme disease bacterium to study some aspects of the pathogenesis of syphilis (80). These same experiments were used to estimate the torque that the flagellar motors of these spirochetes exert, 2700 pN nm (B. burgdorferi) and 800 pN nm (T. pallidum), which is comparable to the stall torques measured in Caulobacter crescentus (350 pN nm) and E. coli (4500 pN nm) (83, 84). It is remarkable that simply measuring how viscosity slows down an organism allows one to measure the torque from the flagellar motors, especially since the flagella are not in direct contact with the fluid.
The motility of the Leptospiraceae is notably different from that of B. burgdorferi and T. pallidum. Swimming Leptospiraceae exhibit a number of different cell shapes (Fig. 3b). In cells that are translating, the anterior end is spiral-shaped and the posterior end is hook-shaped (57, 58). Cells readily reverse directions, with the spiral end becoming hook-shaped and the hook-shaped end becoming spiral-shaped. Non-translating forms are also seen where both ends of the cell are either hook-shaped or spiral-shaped (57, 58). Several lines of evidence indicate that counter-clockwise rotation of the periplasmic flagella (where the frame of reference is viewed along the flagella towards the insertion point on the cell cylinder) causes the spiral-shaped end morphology, and the hook-shaped end occurs when the flagellum is not rotating or is rotating in the clockwise direction. Thus, translating cells are ones where the flagella at either end are rotating in opposite directions. Taken together, the results indicate that the direction of rotation of the flagellum and its interaction with the cell cylinder determines the morphology of the end (56–58, 85, 86). Leptospira swim at about 15 µm/s, and during swimming the cell body rotates at approximately 10 Hz (87). Using hydrodynamic considerations along with the swimming speed and cell body rotation rate, the flagellar motor torque is estimated to be approximately 4000 pN nm (87).
5. Motility of the Lyme disease spirochete in host tissue and extracellular matrix-like environments
The transmission of B. burgdorferi from its arthropod vector into the mammalian host, typically the white-footed mouse Peromyscus leukopus (32), requires the bacterium to exit the tick midgut by penetrating through the epithelium and a dense basement membrane (88). They then swim through the viscous hemolymph to the salivary glands, which they penetrate in order to access the salivary stream that transports them into the dermis of the mammal. Once within the skin, the clinical symptoms of Lyme disease are a direct manifestation of the spirochetes’ ability to infiltrate and reside within the connective and soft tissues of organisms in which they incite tissue-damaging inflammatory responses.
Recent advances in imaging technology have made it possible to visualize the motility and morphology of B. burgdorferi in the mouse and tick (89, 90). For example, Moriarty, et al. used GFP-expressing B. burgdorferi and fluorescent imaging to examine spirochete motility in the ear and microvasculature of infected mice (89). In the mouse ear, B. burgdorferi was observed to undergo all the characteristic motions of translational motility that are observed in vitro and also swam at comparable speeds; however, it was often observed that cells reversed less frequently in the mouse than in vitro. In the microvasculature, spirochetes either swam or were passively carried by the blood flow, or the cells became associated with capillaries, postcapillary venules, and larger veins. When associated with postcapillary venules, B. burgdorferi cells either showed stationary attachment or transient and dragging interactions. It was also possible to observe cells that escaped from the host microvasculature. This process typically occurred through endothelial junctions and took approximately 10–11 minutes.
In the tick, B. burgdorferi encounters numerous obstacles including the basement membrane and cellular junctions of both the tick midgut and salivary glands. Intravital imaging in the tick has shown that dissemination through the tick occurs in two phases (88). During the first phase, replicating spirochetes are positioned at varying depths throughout the unfed midgut epithelium. These spirochetes are non-motile but advance toward the basolateral surface of the epithelium while adhering to differentiating and hypertrophying epithelial cells. The second phase of dissemination begins when non-motile, aggregated spirochetes deposited at the basolateral pole of the epithelial layer transition into single, motile organisms which penetrate through the basement membrane into the hemocoel and then migrate to and penetrate the salivary glands en route to the mammal. Spirochetes were observed penetrating through the basal lamina and into the hemolymph, processes that are slow compared to motility in vitro and probably represent the largest barriers to transmission to the mammalian host. Indeed, only a miniscule percentage of organisms within the midgut appear to be capable of penetrating out into the hemolymph. These intra-and extravasions are presumably driven by rotation of the periplasmic flagella and are reliant on coordinated adhesion and release from the neighboring cells and ECM.
A difficulty with intravital imaging is that host tissue is heterogeneous and cannot be controlled. In order to overcome this difficulty, an in vitro system using gelatin matrices was recently developed to mimic the ECM environment of the mammalian dermis and the basement membrane that lines the exterior of the midgut of the tick, Ixodes scapularis (33). Gelatin was selected because it is the denatured form of collagen, the primary component of the dermis, and because it forms a dense network structure reminiscent of the tick basement membrane. It was shown that in gelatin B. burgdorferi exhibits four motility states that are also observed in the dermis of the mouse. These states consisted of a non-motile state, a state where the bacteria undulate but do not translate (denoted as wriggling), a lunging state where one end of the bacterium remains fixed and the rest of the body scrunches up and then extends, and a translocating state (33). The percentage of spirochetes in each of these populations depends on the gelatin concentration. These populations were not static, a spirochete that at one time point was lunging, could at a later time be a translocator or a wriggler. The timescale for transitions between these states was determined to be on the order of 100 s (33). It is not clear whether these different motility states serve a functional role, but adhesion proteins that allow B. burgdorferi to bind to the host extracellular matrix are important for establishing infection (34). The speed that the translocating population moves through the gelatin also depends on concentration, with slower speeds as gelatin concentration is increased (33). While this decrease in speed would seem to be expected, previous work had shown that B. burgdorferi swims faster in higher concentrations of methylcellulose, which was attributed to the gel-like behavior of methylcellulose solutions (91). A somewhat startling aspect of B. burgdorferi’s motility through gelatin matrices is that for gelatin concentrations above 3%, the pore size of the gelatin network is less than 70 nm; however, the diameter of B. burgdorferi is around 300 nm (33). Transmission electron microscopy showed that the spirochetes are able to push themselves into these matrices and do not rely on degradation of the matrix or large holes to move through. While it has been suggested that the undulating wave-like motion of the bacteria can drive them into dense ECM networks, the physics of invasion remains unstudied.
6. The role of biophysics in early Lyme disease
While motility is known to be essential for the pathogenesis of Lyme disease (52, 69), does understanding the biophysics of the organism provide any insight into the disease? To begin to address this question, my group took the information that we had gained from our gelatin assays and built a mathematical model that represents a simplified view of the pathogen host interactions that occur during the first month after a mammal is bit by an infected tick (Fig. 4a)(40). We knew from our experiments that the spirochetes could either be in a translocating state or in a stationary state (non-motile, wriggling, and lunging states do not move through the matrix). In the translocating state, the spirochetes migrate at speeds ν ~ 2 – 4 µm/s and on time scales of τ ~ 100 s, translocators become stuck (33). Likewise, on about the same timescale stationary bacteria become translocators. When a stuck bacterium transitions back to being a translocator, it does not necessarily move in the same direction that it was moving in before it got stuck. Therefore, on long timescales (t >> τ), the bacteria travel along fairly straight paths, get stuck and then move off in new directions. That is, they execute random walks (92). We therefore expected that on these long timescales the bacteria in the translocating population effectively diffuse with a diffusion coefficient given by D ~ ν2τ (92). B. burgdorferi also replicates about one to two times per day. These basic features, then, define the behavior of the bacterial population.
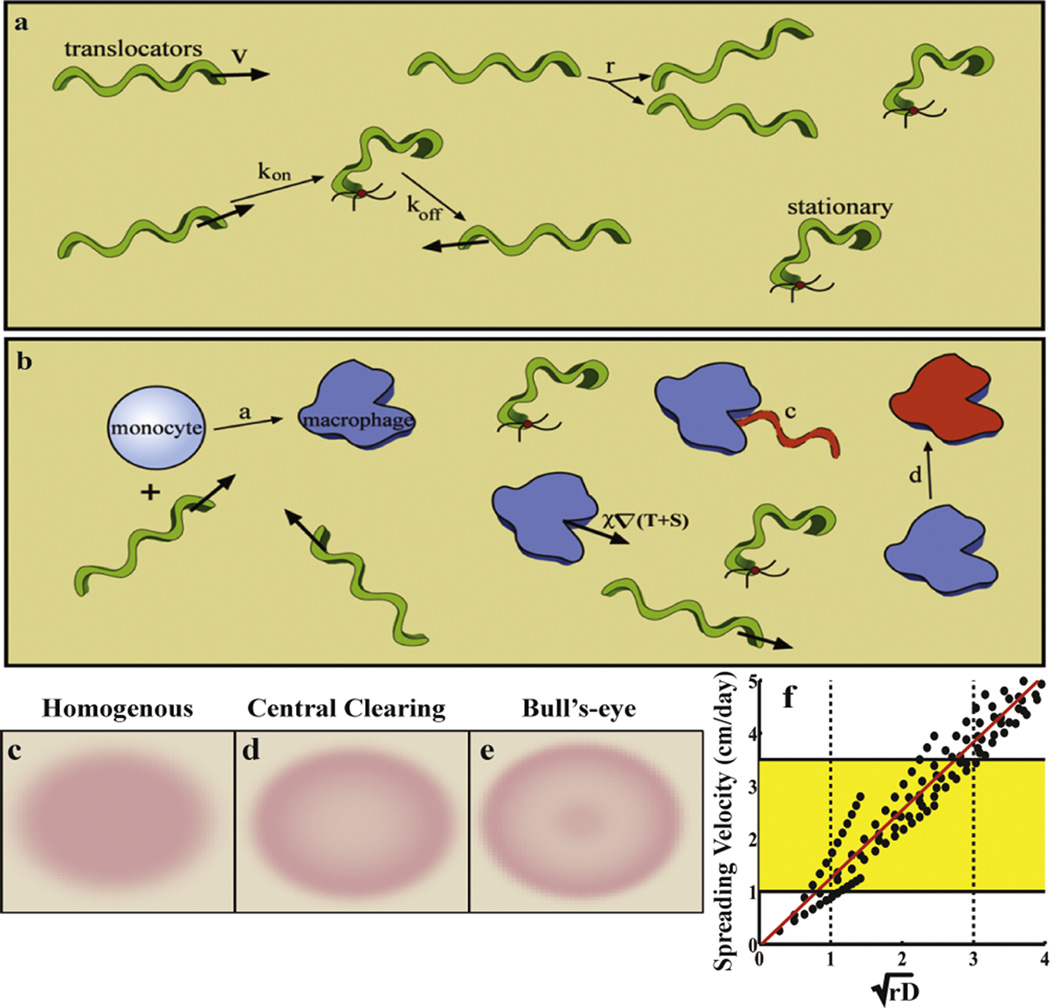
Figure 4. The role of biophysics in early Lyme disease.
(a–b) Schematic of the model. The pathogen-specific processes are shown in (a). Translocating bacteria move with velocity v, but can bind to the dermis with rate kon, thereby becoming stationary. The stationary bacteria unbind with rate koff. The bacteria replicate at rate r. (b) The innate immune response includes activation due to the presence of the bacteria (with rate a), clearing of the bacteria by macrophages with a rate constant c, and the macrophages are then cleared or die at a rate d. (c–e) This model recreates all three rash morphologies: homogeneous (or solid) rashes, central clearing rashes, and the hallmark bull’s-eye (or central erythema). (f) The model also predicts that the spreading rate is proportional to the square-root of the replication rate and the bacterial diffusion coefficient. The dotted lines show the expected values of these parameters and the yellow box highlights the clinically observed values for the spreading rate of the rash.
To model the innate immune response, we took a very simplified viewpoint (Fig. 4b). The host's innate immune system responds to invading pathogens via a complex signaling pathway that involves recognition of the pathogen and activation and inhibition of immune cells by pro- and anti-inflammatory cytokine release (93, 94). Our simplification lumped most of these affects into a single, local immune response that produces active macrophage cells, which are the first phagocytes to arrive at the site of spirochete innoculation (37, 95). The macrophages migrate toward invading spirochetes and was modeled using the Keller-Segel model for chemotaxis (96), which sets the speed that the macrophages migrate toward the bacteria as being proportional to the gradient of the bacterial concentration. Active macrophages phagocytose the spirochetes at a rate that depends on the ratio of macrophages to spirochetes (97). Finally, macrophages are cleared from the dermis on a timescale of a few days.
All the features described above were used to construct a mathematical model that described the spreading of a population of B. burgdorferi through the dermis and the subsequent innate immune response (40). We used the macrophage density as an indicator of the immune response, which would also include the inflammation of the skin that causes Erythema migrans, the hallmark rash of Lyme disease. This rash presents with one of three morphologies, the renowned bull’s-eye rash, a solid rash, or a ring-like rash known as a central clearing rash. Our mathematical model was able to reproduce all three of these morphologies with only moderate variation in the model parameters and suggests that the primary factor that distinguishes a patient with one type of rash from another is the rate that the patient’s immune system deactivates (Fig. 4c–e). The model also predicts the correct spreading rate of the rash (1 – 2 cm/day) and that this rate is set by the bacterial diffusion coefficient and the replication rate of the bacteria (Fig. 4f).
7. Conclusions
The spirochetes are an extremely interesting group of bacteria. While they use the same components to generate motility as other swimming bacteria, the method by which these are implemented is unique and produces a highly dynamic organism that is able to live and move through a wide range of different environments. It is likely that this “all-terrain” motility is the prime reason that many spirochetes have become highly effective pathogens. The motility of bacteria like B. burgdorferi and L. interrogans have fascinated researchers since their discovery. However, we are just starting to comprehend the intricate physics that enables these pathogens to be so invasive. The road to a full understanding is long with many answers still elusive. For example, B. burgdorferi expresses on its outer surface a vast number of proteins, many of which are adhesins (34, 98). Why are so many adhesion proteins needed? While adhering to the ECM may facilitate migration, being overly sticky would seem to be detrimental to evading the immune response. Is the timescale for getting stuck and breaking free from these adhesions physiologically important? And, exactly how do spirochetes like B. burgdorferi and T. pallidum invade through dense matrices like basement membranes or cross the blood-brain barrier? While the physics of these processes is undoubtedly interesting to physicists, we have shown that biophysical understanding and mathematical modeling can shed light on some aspects of disease, such as the spreading of the Erythema migrans in Lyme disease. Only time and effort will show how far biophysical investigations will take us in understanding pathogenesis in spirochetal diseases, and maybe in many other diseases, as well.
Acknowledgments
This research was supported by NIH R01 GM072004. I thank M. Harman and D. Vig for useful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WaterCar. 2015 https://www.watercar.com/index.php. [Google Scholar]
- 2.Merz AJ, Forest KT. Bacterial surface motility: slime trails, grapping hooks and nozzles. Curr. Biol. 2002;12:R297–R303. doi: 10.1016/s0960-9822(02)00806-0. [DOI] [PubMed] [Google Scholar]
- 3.Harshey RM. Bacterial motility on a surface: many ways to a common goal. Annu. Rev. Microbiol. 2003;57:249–273. doi: 10.1146/annurev.micro.57.030502.091014. [DOI] [PubMed] [Google Scholar]
- 4.Macnab RM. Flagella and Motility. In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE, editors. Cellular and Molecular Biology. Washington D.C.: ASM Press; 1996. pp. 123–145. [Google Scholar]
- 5.Rashid MH, Kornberg A. Inorganic polyphosphate is needed for swimming, swarming and twitching motility of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2000;97:4885–4890. doi: 10.1073/pnas.060030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarter LL. Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 2001;65:445–462. doi: 10.1128/MMBR.65.3.445-462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kearns DB. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 2010;8:634–644. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfe AJ, Berg HC. Migration of bacteria in semisolid agar. Proc Natl Acad Sci U S A. 1989;86:6973–6977. doi: 10.1073/pnas.86.18.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croze OA, Ferguson GP, Cates ME, Poon WCK. Migration of chemotactic bacteria in soft agar: role of gel concentration. Biophys. J. 2011;101:525–534. doi: 10.1016/j.bpj.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botkin DJ, et al. Transposon mutagenesis of infectious Borrelia burgdorferi B31: a pilot study. In: Cabello FC, Hulinska D, Godfrey HP, editors. Molecular Biology of Spirochetes. Amsterdam: IOS Press; 2006. pp. 13–23. [Google Scholar]
- 11.Lin T, Troy EB, Hu LT, Gao L, Norris SJ. Transposon mutagenesis as an approach to improved understanding of Borrelia pathogenesis and biology. Frontiers Cell Infection Microbiol. 2014;4:63. doi: 10.3389/fcimb.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radolf JD, Hazlett KRO, Lukehart SA. Pathogenesis of syphilis. In: Radolf JD, Lukehart SA, editors. Pathogenic Treponema: Molecular and Cellular Biology. Norfolk (UK): Caister Academic Press; 2006. [Google Scholar]
- 13.Schmid GP. Epidemiology and clinical similarities of human spirochetal diseases. Rev. Infect. Dis. 1989;11:S1460–S1469. doi: 10.1093/clinids/11.supplement_6.s1460. [DOI] [PubMed] [Google Scholar]
- 14.Sela MN. Role of Treponema denticola in periodontal diseases. Crit. Rev. Oral Biol. Med. 2001;12:399–413. doi: 10.1177/10454411010120050301. [DOI] [PubMed] [Google Scholar]
- 15.Magnuson HJ, et al. Inoculation syphilis in human volunteers. Medicine. 1956;35:33–82. doi: 10.1097/00005792-195602000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Stokes JH, Beerman H, Ingraham NR. Modern Clinical Syphilolology. Philadelphia: W.B. Saunders; 1944. [Google Scholar]
- 17.Brown WH, Pearce L. A note on the dissemination of Spirochaeta pallida from the primary focus of infection. Arch. Derm. Syph. 1920;2:470–472. [Google Scholar]
- 18.Benirschke K. Syphilis -- the placenta and the fetus. Am. J. Dis. Child. 1974;128:142–143. doi: 10.1001/archpedi.1974.02110270016003. [DOI] [PubMed] [Google Scholar]
- 19.Harter C, Benirschke K. Fetal syphilis in the first trimester. Am. J. Obstetr. Gynecol. 1976;124:705–711. doi: 10.1016/s0002-9378(16)33340-3. [DOI] [PubMed] [Google Scholar]
- 20.Drusin LM, Rouiller GC, Chapman GB. Electron microscopy of Treponema pallidum occuirng in a primary lesion. J. Bacteriol. 1969;97:951–955. doi: 10.1128/jb.97.2.951-955.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sell S, Salman J, Norris SJ. Reinfection of chancre-immune rabbits with Treponema pallidum. I. Light and immunofluorescence studies. Am. J. Pathol. 1985;118:248–255. [PMC free article] [PubMed] [Google Scholar]
- 22.Sell S, Salman J. Demonstration of Treponema pallidum in axons of cutaneous nerves in experimental chancres of rabbits. Sex. Transm. Dis. 1992;19:1–6. doi: 10.1097/00007435-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Lukehart SA. Syphilis. In: Brauwald E, Faucci AS, Hauser SL, Longo DL, Jameson JL, et al., editors. Harrison's principles of internal medicine. New York: McGraw Hill; 2004. [Google Scholar]
- 24.Antal GM, Lukehart SA, Meheus AZ. The endemic treponematoses. Microbes and Infection. 2002;4:83–94. doi: 10.1016/s1286-4579(01)01513-1. [DOI] [PubMed] [Google Scholar]
- 25.Mitja O, Asiedu K, Mabey D. Yaws. Lancet. 2013;381:763–773. doi: 10.1016/S0140-6736(12)62130-8. [DOI] [PubMed] [Google Scholar]
- 26.Dewhirst FE, et al. The diversity of periodonatal spirochetes by 16S rRNA analysis. Oral Microbiol. Immunol. 2000;15:196–202. doi: 10.1034/j.1399-302x.2000.150308.x. [DOI] [PubMed] [Google Scholar]
- 27.Holt SC, Ebersole JL. The Oral Spirochetes: Their ecology and role in the pathogenesis of periodontal disease. In: Radolf JD, Lukehart SA, editors. Pathogenic Treponema: Molecular and Cellular Biology. Norfolk: Caister Academic Press; 2006. [Google Scholar]
- 28.Keyes PH, Rams TE. A rational for management of periodontal disease, rapid identification of microbial "therapeutic targets" with phase-contrast microscopy. J. Am. Dent. Assoc. 1983;106:803–812. doi: 10.14219/jada.archive.1983.0436. [DOI] [PubMed] [Google Scholar]
- 29.Armitage GC, Dickenson WR, Jenderseck RJ, Levine SM, Chanbers DW. Relationship between the percentage of subgingival spirochetes and the severity of periodontal disease. J. Periodontol. 1982;53:550–556. doi: 10.1902/jop.1982.53.9.550. [DOI] [PubMed] [Google Scholar]
- 30.Deng QD, Han Y, Xia X, Kuramitsu HK. Effects of the oral spirochete T. denticola on interleukin-8 expression from epithelial cells. Oral Microbiol. Immunol. 2001;16:185–187. doi: 10.1034/j.1399-302x.2001.016003185.x. [DOI] [PubMed] [Google Scholar]
- 31.Dandache P, Nadelman RB. Erythema migrans. Infect. Dis. Clin. North Am. 2008;22:235–260. doi: 10.1016/j.idc.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harman M, et al. The heterogeneous motility of the Lyme disease spirochete in gelatin mimics dissemination through tissue. Proc. Natl. Acad. Sci. USA. 2012;109:3059–3064. doi: 10.1073/pnas.1114362109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coburn J, Fischer JR, Leong JM. Solving a sticky problem: new genetic approaches to host cell adhesion by the Lyme disease spirochete. Mol. Microbiol. 2005;57:1182–1195. doi: 10.1111/j.1365-2958.2005.04759.x. [DOI] [PubMed] [Google Scholar]
- 35.Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J. Clin. Invest. 2004;113:1093–1101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salazar JC, et al. Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J. Immunol. 2003;171:2660–2670. doi: 10.4049/jimmunol.171.5.2660. [DOI] [PubMed] [Google Scholar]
- 37.Delneste Y, et al. Interferon-gamma switches monocyte differentiation from dendritic cells to macrophages. Blood. 2003;101:143–150. doi: 10.1182/blood-2002-04-1164. [DOI] [PubMed] [Google Scholar]
- 38.CMO, et al. Local production of IFN-gamma by invariant NKT cells modulates acute Lyme carditis. J. Immunol. 2009;182:3728–3734. doi: 10.4049/jimmunol.0804111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menten-Dedoyart C, et al. Neutrophil extracellular traps entrap and kill Borrelia burgdorferi sensu stricto spirochetes and are not affected by Ixodes ricinus tick saliva. J. Immunol. 2012;189:5393–5401. doi: 10.4049/jimmunol.1103771. [DOI] [PubMed] [Google Scholar]
- 40.Vig DK, Wolgemuth CW. Spatiotemporal evolution of Erythema migrans, the hallmark rash of Lyme disease. Biophys. J. 2014;106:763–768. doi: 10.1016/j.bpj.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radolf JD, Salazar JC, Dattwyler RJ. Lyme disease in humans. In: Samuels DS, Radolf JD, editors. Borrelia: molecular biology, host interaction and pathogenesis. Norfolk, UK: Caister Academic Press; 2010. [Google Scholar]
- 42.Sultan SZ, et al. Motor rotation is essential for the formation of the periplasmic flagellar ribbon, cellular morphology, and Borrelia burgdorferi persistence within Ixodes scapularis tick and murine hosts. Infect Immun. 2015;83:1765–1777. doi: 10.1128/IAI.03097-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sultan SZ, et al. Motility is crucial for the infectious life cycle of Borrelia burgdorferi. Infect Immun. 2013;81:2012–2021. doi: 10.1128/IAI.01228-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sze CW, Zhang K, Kariu T, Pal U, Li C. Borrelia burgdorferi needs chemotaxis to establish infection in mammals and to accomplish its enzootic cycle. Infect Immun. 2012;80:2485–2492. doi: 10.1128/IAI.00145-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barbour AG, Guo BP. Pathogenesis of Relapsing Fever. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular biology, host interaction and pathogenesis. Norfolk, UK: Caister Academic Press; 2010. [Google Scholar]
- 46.Evangelista KV, Coburn J. Leptospira as an emerging pathogen: a review of its biology, pathogenesis and host immune responses. Future Microbiol. 2010;5:1413–1425. doi: 10.2217/fmb.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faine S, Adler B, Bolin C, Perolat P. Leptospira and Leptospirosis. 2nd. Australia: MediSci, Melbourne; 1999. [Google Scholar]
- 48.Levett PN. Leptospirosis. Clin. Microbiol. Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao S, et al. Inactivation of the fliY gene encoding a flagellar motor switch protein attenuates motbility and virulence of Leptospira interrogans strain Lai. BMC Microbiol. 2009;9:253. doi: 10.1186/1471-2180-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lambert A, et al. FlaA proteins in Leptospira interrogans are essential for motility and virulence but are not required for formation of the flagellum sheath. Infect Immun. 2012;80:2019–2025. doi: 10.1128/IAI.00131-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paster BJ. Phylum XV. Spirochaetes Garrity and Holt 2001. In: Krieg NR, Parte A, Ludwig W, Whitman WB, Hedlund BP, Paster BJ, Staley JT, Ward N, Brown D, editors. Bergey's Manual of Systematic Bacteriology. Vol. 4. New York: Springer; 2011. pp. 471–566. [Google Scholar]
- 52.Charon NW, et al. The unique paradigm of spirochete motility and chemotaxis. Annu. Rev. Microbiol. 2012;66:349–370. doi: 10.1146/annurev-micro-092611-150145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charon NW, et al. The flat ribbon configuration of the periplasmic flagella of Borrelia burgdorferi and its relationship to motility and morphology. J. Bacteriol. 2009;191:600–607. doi: 10.1128/JB.01288-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berg HC. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 2003;79:19–54. doi: 10.1146/annurev.biochem.72.121801.161737. [DOI] [PubMed] [Google Scholar]
- 55.Carleton O, Charon NW, Allender P, O'Brien S. Helix Handedness of Leptospira interrogans as determined by scanning electron microscopy. J. Bacteriol. 1979;137:1413–1416. doi: 10.1128/jb.137.3.1413-1416.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldstein SF, Charon NW. Motility of the spirochete Leptospira. Cell Motil. Cytoskel. 1988;9:101–110. doi: 10.1002/cm.970090202. [DOI] [PubMed] [Google Scholar]
- 57.Goldstein SF, Charon NW. Multiple-exposure photographic analysis of a motile spirochete. Proc. Natl. Acad. Sci. USA. 1990;87:4895–4899. doi: 10.1073/pnas.87.13.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berg HC, Bromley DB, Charon NW. Leptospiral Motility. Symp. Soc. Gen. Microbiol. 1978:285–294. [Google Scholar]
- 59.Bromley DB, Charon NW. Axial filament involvement in the motility of Leptospira interrogans. J. Bacteriol. 1979;137(3):1406–1412. doi: 10.1128/jb.137.3.1406-1412.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Picardeau M, Brenot A, Saint I. First evidence for gene replacement in Leptospira spp. inactivation of L. biflexa flaB results in non-motile mutants deficient in endoflagella. Mol. Microbiol. 2001;40(1):189–199. doi: 10.1046/j.1365-2958.2001.02374.x. [DOI] [PubMed] [Google Scholar]
- 61.Canale-Parola E. The spirochetes. In: Krieg NR, Holt JG, editors. Bergey's manual of systematic biology. Baltimore, MD: Williams and Wilkins; 1984. [Google Scholar]
- 62.Socransky S, Listgarten M, Hubersak C, Bial JJ, Morton HE. Morphological and biochemical differentiation of three types of small oral spirochetes. J. Bacteriol. 1969;98:878–882. doi: 10.1128/jb.98.3.878-882.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruby JD, et al. Relationship of Treponema denticola periplasmic flagella to irregular cell morphology. J. Bacteriol. 1997;179(5):1628–1635. doi: 10.1128/jb.179.5.1628-1635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldstein SF, Charon NW, Kreiling JA. Borrelia burgdorferi swims with a planar waveform similar to that of eukaryotic flagella. Proc. Natl. Acad. Sci. USA. 1994;91:3433–3437. doi: 10.1073/pnas.91.8.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Izard J, et al. Cryo-electron tomography elucidates the molecular architecture of Treponema pallidum the syphilis spirochete. J. Bacteriol. 2009;191:7566–7580. doi: 10.1128/JB.01031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goldstein SF, Buttle KF, Charon NW. Structural analysis of the Leptospiracae and Borrelia burgdorferi by high-voltage electron microscopy. J. Bacteriol. 1996;178(22):6539–6545. doi: 10.1128/jb.178.22.6539-6545.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kudryashev M, et al. Comparative cryo-electron tomography of pathogenic Lyme disease spirochetes. Mol. Microbiol. 2009;71:1415–1434. doi: 10.1111/j.1365-2958.2009.06613.x. [DOI] [PubMed] [Google Scholar]
- 68.Liu J, et al. Intact flagellar motor of Borrelia burgdorferi revealed by cryoelectron tomography: evidence for stator ring curvature and rotor/C ring assembly felxion. J. Bacteriol. 2009;191:5026–5036. doi: 10.1128/JB.00340-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Charon NW, Goldstein SF. Genetics of motility and chemotaxis of a fascinating group of bacteria: the spirochetes. Annu. Rev. Genet. 2002;36:47–73. doi: 10.1146/annurev.genet.36.041602.134359. [DOI] [PubMed] [Google Scholar]
- 70.Motaleb MA, et al. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc. Natl. Acad. Sci. USA. 2000;97(20):10899–10904. doi: 10.1073/pnas.200221797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sartakova ML, et al. Complementation of a nonmotile flaB mutant of Borrelia burgdorferi by chromosomal integration of a plasmid containing a wild-type flaB allele. J. Bacteriol. 2001;183:6558–6564. doi: 10.1128/JB.183.22.6558-6564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kan W, Wolgemuth CW. The shape and dynamics of the Leptospiraceae. Biophys. J. 2006;93:54–61. doi: 10.1529/biophysj.106.103143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dombrowski C, et al. The elastic basis for the shape of Borrelia burgdorferi. Biophys. J. 2009;96:4409–4417. doi: 10.1016/j.bpj.2009.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vig DK, Wolgemuth CW. Swimming dynamics of the Lyme disease spirochete. Phys. Rev. Lett. 2012;109:218104. doi: 10.1103/PhysRevLett.109.218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolgemuth CW, Charon NW, Goldstein SF, Goldstein RE. The flagellar cytoskeleton of the spirochetes. J. Mol. Microbiol. Biotechnol. 2006;11:221–227. doi: 10.1159/000094056. [DOI] [PubMed] [Google Scholar]
- 76.Hovind Hougen K. Ultrastructure of spirochetes isolated from Ixodes ricinus and Ixodes dammini. Yale J. Biol. Med. 1984;57:543–548. [PMC free article] [PubMed] [Google Scholar]
- 77.Taylor GI. Analysis of the swimming of microscopic organisms. Proc. R. Soc. Lond. A. 1951;209:447–461. [Google Scholar]
- 78.Taylor GI. The action of waving cylindrical tails in propelling microorganisms. Proc. R. Soc. Lond. A. 1952;211:225–239. [Google Scholar]
- 79.Lauga E, Powers TR. The hydrodynamics of swimming microorganisms. Rep. Prog. Phys. 2009;72:096601. [Google Scholar]
- 80.Harman M, Vig DK, Radolf JD, Wolgemuth CW. Viscous dynamics of Lyme disease and syphylis spirochetes reveal flagellar torque and drag. Biophys. J. 2013;105:2273–2280. doi: 10.1016/j.bpj.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chwang AT, Winet H, Wu TY. A theoretical mechanism of spirochete locomotion. J. Mechanochem. Cell Motility. 1974;3:69–76. [PubMed] [Google Scholar]
- 82.Cox DL, Radolf JD. Metabolism of the Treponema. In: Radolf JD, Lukehart SA, editors. Pathogenic Treponema: Molecular and cellular biology. Norfolk, UK: Caister Academic Press; 2006. [Google Scholar]
- 83.Li G, Tang JX. Low flagellar motor torque and high swimming efficiency of Caulobacter crescentus swarmer cells. Biophys. J. 2006;91:2726–2734. doi: 10.1529/biophysj.106.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berry RM, Berg HC. Absence of a barrier to backwards rotation of the bacterial flagellar motor demonstrated with optical tweezers. Proc. Natl. Acad. Sci. USA. 1997;94:14433–14437. doi: 10.1073/pnas.94.26.14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li C, Motaleb MA, Sal M, Goldstein SF, Charon NW. Spirochete periplasmic flagella and motility. J. Mol. Microbiol. Biotechnol. 2000;2(2):345–354. [PubMed] [Google Scholar]
- 86.Charon NW, Daughtry GR, McCuskey RS, Franz GN. Microcinematographic analysis of tethered Leptospira illini. J. Bacteriol. 1984;160:1067–1073. doi: 10.1128/jb.160.3.1067-1073.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakamura S, Leshansky A, Magariyama Y, Namba K, Kudo S. Direct measurement of helical cell motion of the spirochete Leptospira. Biophys. J. 2014;106:47–54. doi: 10.1016/j.bpj.2013.11.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dunham-Ems SM, et al. Live imaging reveals a novel, biphasic mode of dissemination of Borrelia burgdorferi within ticks. J. Clin. Invest. 2009;119:3652–3665. doi: 10.1172/JCI39401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moriarty TJ, et al. Real-time high resolution 3D imaging of the Lyme disease spirochete adhering to and escaping from the vasculature of a living host. PLOS Pathogens. 2008;4:e1000090. doi: 10.1371/journal.ppat.1000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pal U, et al. OspC facillitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Invest. 2004;113:220–230. doi: 10.1172/JCI19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kimsey RB, Spielman A. Motility of Lyme disease spirochetes in fluids as viscous as the extracellular matrix. J. Infect. Dis. 1990;162:1205–1208. doi: 10.1093/infdis/162.5.1205. [DOI] [PubMed] [Google Scholar]
- 92.Berg HC. Random Walks in Biology. Princeton, NJ: Princeton University Press; 1983. [Google Scholar]
- 93.Thakar J, Saadatpour-Moghaddam A, Harvill ET, Albert R. Constraint-based network model of pathogen-immune system interactions. J. R. Soc. Interface. 2009;6:599–612. doi: 10.1098/rsif.2008.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Campbell C, Thakar J, Albert R. Network analysis reveals cross-links of the immune pathways activated by bacteria and allergen. Phys. Rev. E. 2011;84:031929. doi: 10.1103/PhysRevE.84.031929. [DOI] [PubMed] [Google Scholar]
- 95.Montgomery RR, Lusitani D, Chevance AdB, Malawista SE. Human phagocytic cells in the early innate immune response to Borrelia burgdorferi. J. Infect. Dis. 2002;185:1773–1779. doi: 10.1086/340826. [DOI] [PubMed] [Google Scholar]
- 96.Keller EF, Segel LA. Model for chemotaxis. J. Theor. Biol. 1971;30:225–234. doi: 10.1016/0022-5193(71)90050-6. [DOI] [PubMed] [Google Scholar]
- 97.Banfi E, Cinco M, Perticarari S, Presani G. Rapid flow cytometric studies of Borrelia burgdorferi phagocytosis by human polymorphonuclear leukocytes. J. Appl. Bacteriol. 1989;67:37–45. doi: 10.1111/j.1365-2672.1989.tb04952.x. [DOI] [PubMed] [Google Scholar]
- 98.Kenedy MR, Lenhart TR, Akins DR. The role of Borrelia burgdorferi outer surface proteins. FEMS Immunol. Med. Microbiol. 2012;66:1–19. doi: 10.1111/j.1574-695X.2012.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]