Abstract
CD8+ T cells are regulatory T cells (Tregs) that suppress both alloimmunity and autoimmunity in many animal models. This class of regulatory cells includes the CD8+CD28−, CD8+CD103+, CD8+FoxP3+ and CD8+CD122+ subsets. The mechanisms of action of these regulatory cells are not fully understood; however, the secretion of immunosuppressive cytokines, such as interleukin (IL)-4, IL-10 and transforming growth factor beta (TGF-β) as well as the direct killing of target cells via Fas L/Fas and the perforin/granzyme B pathways have been demonstrated in various models. Further studies are necessary to fully understand the mechanisms underlying the suppressive effects of Tregs and to provide experimental support for potential clinical trials. We recently observed that CD8+CD122+ Tregs more potently suppressed allograft rejection compared to their CD4+CD25+ counterparts, supporting the hypothesis that CD8+ Tregs may represent a new and promising Treg family that can be targeted to prevent allograft rejection in the clinic. In this review, we summarize the progress in the field during the past 7–10 years and discuss CD8+ Treg phenotypes, mechanisms of action, and their potential clinical applications; particularly in composite tissue transplants in burn and trauma patients.
Key words: Tolerance, transplantation, CD8+ regulatory T cell, immune regulation
Introduction
Organ transplantation is the preferred treatment of choice for patients with end-stage organ failure. However, the mortality and mobility associated with broad immunosuppression in transplant patients remains a significant barrier to the actual therapeutic potential of organ transplantation. The recent emergence of composite tissue transplantation, which is the treatment of choice for patients suffering from severe burns and trauma, presents new challenges and opportunities for long-term transplant survival without drug-associated toxicities. Induction of immune tolerance is essential for avoiding pathogenic immune responses to self-antigens and alloantigens. Tolerance is also an ideal method of weaning patients off immunosuppressive drugs. It has been well-established that regulatory T cells (Tregs) are critical for maintaining immune tolerance by suppressing both autoimmunity and alloimmunity. There are multiple cell types in the immune system that exhibit immunosuppressive properties. In addition to the much publicized CD4+CD25+Foxp3+ Tregs, CD8+ Tregs have emerged as another key player in immune regulation. Early studies showed that CD8+ T cells could suppress immune responses, thereby functioning as suppressor cells,[12] and tremendous progress has occurred regarding their suppression of alloimmunity and mechanisms of action. For example, Liu et al., demonstrated that CD8+CD28− T cells inhibited T helper alloreactivity in an major histocompatibility complex (MHC) class I-restricted manner.[3] These CD8+ Tregs also induced xenoreactive CD4+ T-cell anergy. [4] Human CD8+ Tregs inhibited graft-versus-host disease (GVHD) in humanized mice.[5] Additionally, either donor-specific or induced CD8+FoxP3+ Tregs facilitated skin allograft survival in certain models.[6] Moreover, antigen-induced CD8+CD103+ Tregs have been shown to suppress alloreactive effector T cell function.[7] More importantly, several recent studies have shown that CD8+CD122+ T cells are also potent Tregs that inhibit both alloimmunity and autoimmunity.[8,19] In particular, we have recently found that in an islet transplant model, naturally occurring CD8+CD122+ Tregs in the periphery more potently suppressed allograft rejection than their CD4+CD25+ counterparts. [20] Therefore, CD8+ Tregs may represent a new and promising Treg family that can be targeted to treat autoimmune diseases and allograft rejection in the clinic. Here, we review the most recent progress in the field and discuss potential clinical applications for the induction of tolerance to composite tissue transplants.
| Access this article online | |
|---|---|
Quick Response Code:

|
Website: www.burnstrauma.com |
| DOI: 10.4103/2321-3868.126086 | |
CD8+CD28− Tregs
Previous studies have demonstrated that CD8+CD28− T cells are immunosuppressive.[18] Liu et al., originally reported the specific suppression of T helper alloreactivity by MHC class-I-restricted CD8+CD28− T cells,[3] which were generated by multiple rounds of in vitro allostimulation of peripheral blood mononucleocytes. These MHC class-I-restricted CD8+CD28− T cells induced xenoreactive human CD4+ T-cell anergy upon specific recognition of MHC class-I antigens.[4] Adoptive transfer of CD8+CD28− cells resulted in profound inhibition of corneal xenograft rejection.[21] Qa-1-restricted CD8+CD28− T cells regulated the reactivation of both T cells and natural killer T (NKT) cells.[22] Human xenospecific CD8+CD28− Tregs inhibited T helper cell responses to porcine aortic endothelial cells by suppressing necrosis factor (NF)-kappa B activity in porcine antigen-presenting cells (APCs).[23] Allospecific and induced CD8+CD28− FoxP3+ Tregs induced immunoglobulin-like transcript (ILT)3+/ILT4+ tolerogenic endothelial cells or APCs, down-regulated the expression of costimulatory (CD80/CD86) and adhesion (CD54/CD58) molecules, thereby suppressing alloimmune responses.[23–26] Although induced CD8+CD28− Tregs expressed FoxP3,[24] natural CD8+CD28− Tregs were FoxP3-negative.[27] The induced, but not natural, CD8+CD28− Tregs expressed glucocorticoid-induced tumor necrosis factor receptor-related protein (GITR) and cytotoxic T-cell antigen 4 (CTLA4), whereas both Tregs expressed CD62L[27,28] [Figure 2]. Moreover, graft function was maintained with minimal or no immunosuppression in patients that had circulatory CD8+CD28− Tregs.[29] The expansion of CD8+CD28− Tregs was associated with the reduced occurrence of acute or chronic rejection.[30] The adoptive transfer of CD8+CD28− Tregs from tolerized liver transplant recipients alleviated acute allograft rejection in rats.[31] Donor-specific T cell vaccination induced CD4+CD25+ or CD8+CD28− Tregs that promoted transplant tolerance in new recipient mice that received a cardiac allograft.[32] Taken together, mounting evidence suggests that CD8+CD28− cells are Tregs that can either suppress allograft rejection or promote allograft tolerance. CD8+CD28− Tregs are the primary effectors of transplant tolerance and act on the endothelium and antigen presenting cells (APCs) to induce a tolerogenic phenotype and inhibit CD4+ T helper cell alloreactivity.
Figure 2.
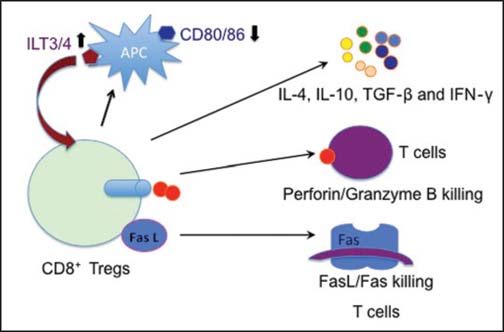
Mechanisms underlying CD8+ Treg suppression. Mechanisms responsible for CD8+ Treg suppression include immunosuppressive cytokines and the killing of target T cells via the perforin/granzyme B and Fas L/Fas pathways as well as the downregulation of CD80/CD86 but the upregulation of immunoglobulin-like transcript 3/4 (ILT3/4) that, in turn, promotes CD8+ Treg expansion antigen presenting cell (APC).
CD8+CD103+ Tregs
It has been shown that a CD8+ T cell population lacking CD44 but expressing CD103 produced transforming growth factor beta (TGF-β), inhibit CD4+ proliferation in vitro, and attenuate adoptively transferred ileitis in vivo, most likely by counteracting the proinflammatory role of the CD44high subset of T cells.[33] Therefore, these CD8+ Tregs exhibit a naive T cell phenotype (CD8+CD103+CD44lowCD62Lhigh), as shown in Figure 1. In contrast, allostimulated CD8+CD103+ Tregs displayed limited effector functions, such as cytotoxicity and interferon (IFN)-γ production. Instead, CD8+CD103+ Tregs suppressed alloreactive effector T cells.[7] Lu et al., demonstrated that CD8+CD103- T cells differentiated into CD8+CD103+FoxP3+ T cells after in vitro stimulation with either alloantigens or transforming growth factor (TGF)-β and that the number of CD8+CD103+ Tregs increased in spontaneously tolerant recipients of a liver allograft.[34] Rapamycin not only promoted the in vitro generation of CD8+CD103+ Tregs after allostimulation, but also enhanced their suppressive capacity.[35] However, a study by Feng et al., found that CD103 expression by CD8+ T cells was required for the destruction of islet allografts because CD8+CD103 T cells failed to infiltrate the islet grafts,[36] indicating that CD103 may be an effector but not a regulatory molecule. Therefore, further studies are necessary to better define the role of CD103 in alloreactive CD8+ T cell functions.
Figure 1.
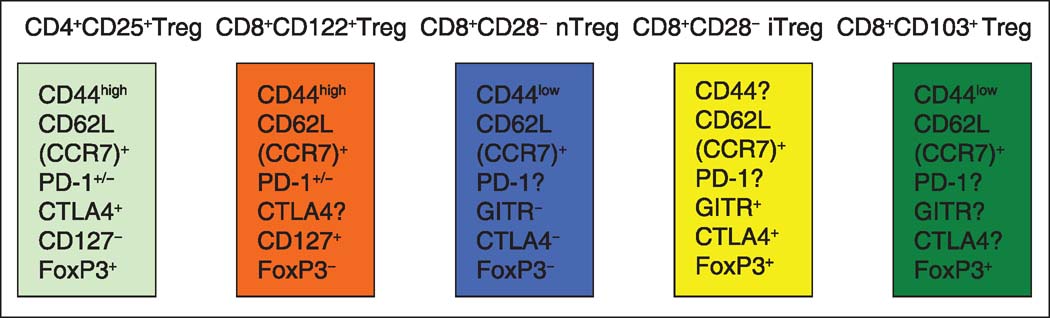
CD8+ regulatory T cell (Treg) phenotypes. Natural CD8+CD122+ Tregs are CD44highCD62L+CD127+ and partially PD-1-positive (PD-1+/−) but are FoxP3-negative. Natural CD8+CD28− Tregs (nTreg) are CD44lowCD62L+ but GITR-CTLA4-FoxP3−; whereas, induced CD8+CD28− Tregs (iTreg) are CD44highCD62L+, but GITR+CTLA4+FoxP3+. CD8+CD103+ Tregs are CD44lowCD62LhighFoxP3+. GITR = Glucocorticoid-induced tumor necrosis factor receptor-related protein, CTLA4 = cytotoxic T-cell antigen 4.
CD8+CD122+ Tregs
Recent studies have shown that naturally occurring CD8+CD122+ T cells control T cell homeostasis and play an important role in the suppression of autoimmune diseases.[8] The transfer of CD8+CD122+ Tregs significantly improved the clinical symptoms of experimental autoimmune encephalomyelitis (EAE), indicating a role for CD8+CD122+ Tregs in the recovery phase of EAE.[37] Brain DCs expressing the B7-H1 molecule recruit CD8+CD122+ Tregs to the inflammatory site, which causes a decrease in the clinical EAE peak values.[38] Notably, CD8+CD38+CD122+ Tregs inhibited effector CD4+ T cell proliferation in an antigen-nonspecific manner and reduced the clinical score of murine EAE as well as delayed disease occurrence.[39] A recent study showed that CD8+CD122+ T cells also played a regulatory role in EAE models of HLA-DR3 transgenic mice; whereas, CD8+CD122 T cells had an opposite role.[16] Furthermore, CD8+CD122+ Tregs suppressed other autoimmune diseases in many animal models. The depletion of CD8+CD122+ T cells increased the incidence of autoimmune Graves’hyperthyroidism in a mouse model,[40] suggesting that CD8+CD122+ T cells play an essential role in the inhibition of autoimmune hyperthyroidism. B6-Yaa mutant mice developed systemic lupus erythematous-like disease in association with a defect in CD8+CD122+ Treg activity, suggesting that the Treg subset may be utilized to treat systemic lupus erythematous-like autoimmune disease.[15] Therefore, in multiple experimental animal models, CD8+CD122+ Tregs play an important role in the suppression of various autoimmune diseases.
Recent studies have also demonstrated that CD8+CD122+ Tregs play a role in suppressing alloimmune responses. We originally reported that CD8+CD122+ Tregs suppressed murine allograft rejection.[17] We also found that the PD-1+ component of these Tregs were more effective than the unfractionated CD8+CD122+ Treg population;[17] whereas, antigen-specific CD8+CD122+PD-1 cells were memory T cells that could respond to a previously encountered antigen quickly and efficiently. Furthermore, we demonstrated that CD8+CD122+ Tregs were more potent in suppressing allograft rejection than their CD4+CD25+ counterparts,[20] suggesting that CD8+CD122+ Tregs may be a better target for the treatment of allograft rejection. In summary, CD8+CD122+ Tregs regulate both autoimmunity and alloimmunity and may participate in immune regulation in vivo for various diseases.
CD8+CD122+ Tregs and the classical CD4+CD25+ Tregs both express the interleukin (IL)-2 receptor. Specifically, CD122 is the β subunit of the IL-2 receptor on T cells; whereas, CD25 is the α subunit of the IL-2 receptor.[41] To identify more effective Tregs for potential clinical applications, we evaluated the efficacy of naturally arising CD8+CD122+ vs CD4+CD25+ Tregs for suppressive activities. Surprisingly, we found that CD8+CD122+ Tregs were much more efficient in the suppression of allograft rejection and underwent faster homeostatic proliferation than their CD4+CD25+ counterparts.[20] In addition, CD8+CD122+ Tregs produced significantly more IL-10 and were more effective in the suppression of in vitro T cell proliferation than their CD4+CD25+ counterparts. Importantly, adoptive transfer of CD8+CD122+ Tregs but not CD4+CD25+ Tregs, together with the infusion of recombinant IL-15, significantly delayed allograft rejection in normal wild-type mice. In contrast, the transfer of CD4+CD25+ Tregs with the administration of either recombinant IL-2 or IL-15 did not significantly prolong islet allograft survival.[20] We hypothesized that IL-2 administration promoted the expansion of both CD4+CD25+ Tregs and conventional effector T cells, which did not alter the overall immune balance; whereas, the administration of IL-15 enhanced the expansion and function of CD8+CD122+ but not CD4+CD25+ Tregs. Therefore, naturally occurring CD8+CD122+ Tregs appear to be a more promising target for suppressing allograft rejection than their CD4+CD25+ counterparts. Further studies are warranted to improve CD8+CD122+ Treg therapies and provide additional experimental data for clinical trials.
Mechanisms of action
The mechanisms underlying CD8+ Treg suppression are not fully understood. However, multiple mechanisms are likely to be involved, including the killing or direct lysis of target cells, the induction of CD4+ T cell anergy, and the secretion of immunosuppressive cytokines and molecules. Different subsets of CD8+ Tregs may utilize distinct mechanisms. Studies by Cantor’s group have demonstrated that perforin-mediated cytotoxicity is required for the suppressive activity of Qa-1-restricted CD8+ Tregs;[42] whereas, others have shown that CD11c+CD8+ Tregs can directly kill activated CD4+ T cells through the Fas ligand-Fas death pathway[43] [Figure 2]. CD8+CD28− Tregs induced xenoreactive human CD4+ T-cell anergy[4] and also downregulated expression of the costimulatory molecules CD80/CD86 on APCs,[23] which indirectly block T cell priming by APCs. TGF-β expanded CD8+CD103+ Tregs displayed cytotoxicity towards allospecific effector T cells through cell-to-cell contact.[7] Zheng et al., demonstrated that alloantigen-specific suppression by human CD8+ Tregs was partially dependent on IL-10, TGF-β, GITR and CTLA-4 expression.[44] CD8+CD28− Tregs induced ILT3+/ILT4+ expression on endothelial cells or APCs and downregulated costimulatory (CD80/CD86) and adhesion (CD54/CD58) molecules, resulting in reduced alloreactivity.[23,26] The increased ILT3+/ILT4+ expression, in turn, promoted the differentiation of CD8+CD28− FoxP3+ Tregs,[24] reinforcing their suppressive capacity. Moreover, ILT3 directly induced CD4+ Th cell anergy and suppressed the differentiation of IFN-γ-producing effector CD8+ CTL.[45]
The mechanisms of action for CD8+CD122+ Treg suppression are also not well-defined. As shown in Figure 2, IL-10 produced by CD8+CD122+ Tregs appears to be a primary mechanism responsible for suppression.[9,17,46] Endharti et al., presented the first data showing that CD8+CD122+ Tregs suppressed IFN-γ production and the proliferation of CD8+ T cells by producing IL-10 in vitro.[9] CD8+ Tregs also recognized activated T cells via the interaction of the MHC class I-αβ TCR and regulated target T cells by producing IL-10.[46] We also observed that the suppression of allograft rejection by IL-10-deficient CD8+CD122+ Tregs was largely diminished.[17] Activated CD8+CD122+ Tregs from RasGRP1(-/-) mice synthesized IL-10 and inhibited the proliferation of CD8+CD122− T cells.[10] However, IL-10 did not account for all mechanisms underlying CD8+CD122+ Treg suppression.[17] Other mechanisms, in addition to IL-10 production, may be involved in CD8+CD122+ Treg suppression. In particular, CD8+CD122+ Tregs also released IFN-γ and TGF-β that suppressed CD4+ T cell activation.[16] It remains to be determined whether the perforin/granzyme B pathway is also involved in regulating effector T cells by CD8+CD122+ Tregs. Despite these findings, further studies are required to fully identify the mechanisms responsible for the suppression of alloimmunity or autoimmunity.
Conclusion
Many recent studies have shown that the CD8+ component of T cells can also serve as Tregs in vitro and in vivo, although relatively little is known about these cells compared to the conventional CD4+CD25+ Tregs. CD8+ cells suppress both alloimmune responses and autoimmunity in many animal models. Multiple subsets of CD8+ Tregs have been identified thus far, including the CD8+CD28−, CD8+CD103+, CD8+FoxP3+ and CD8+CD122+ populations. The mechanisms of action of these cells are not well understood because of their plasticity and heterogeneity. Further investigation is necessary to fully understand the distinct mechanisms underlying their suppression as well as the mechanism by which CD8+ Tregs interact with other types of Tregs in the induction and maintenance of immune tolerance. These studies will help lay the groundwork for potential clinical trials. Most importantly, our new studies reveal that CD8+CD122+ Tregs more potently suppress allograft rejection than their CD4+CD25+ counterparts. Therefore, CD8+ Tregs likely represent a new and promising Treg family that can be targeted to suppress allograft rejection or induce long-term allograft survival or tolerance in the clinic. This will undoubtedly benefit transplant patients, including those with composite tissue transplants because of trauma and burns.
Footnotes
How to cite this article: Su J, Xie Q, Xu Y, Li XC, Dai Z. Role of CD8+ regulatory T cells in organ transplantation. Burn Trauma 2014;2:18–23.
Source of Support: Nil, Conflict of Interest: None declared.
References
- 1.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: The role of thymic lymphocytes. Immunology. 1970;18:723–37. [PMC free article] [PubMed] [Google Scholar]
- 2.Cantor H, Shen FW, Boyse EA. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: After immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976;143:1391–40. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, Tugulea S, Cortesini R, Suciu-Foca N. Specific suppression of T helper alloreactivity by allo-MHC class I-restricted CD8+CD28− T cells. Int Immunol. 1998;10:775–83. doi: 10.1093/intimm/10.6.775. [DOI] [PubMed] [Google Scholar]
- 4.Colovai AI, Liu Z, Ciubotariu R, Lederman S, Cortesini R, Suciu-Foca N. Induction of xenoreactive CD4+ T-cell anergy by suppressor CD8+CD28− T cells. Transplantation. 2000;69:1304–10. doi: 10.1097/00007890-200004150-00016. [DOI] [PubMed] [Google Scholar]
- 5.Zheng J, Liu Y, Liu M, Xiang Z, Lam KT, Lewis DB, et al. Human CD8+ regulatory T cells inhibit GVHD and preserve general immunity in humanized mice. Sci Transl Med. 2013;5:168–9. doi: 10.1186/1479-5876-11-168. [DOI] [PubMed] [Google Scholar]
- 6.Lerret NM, Houlihan JL, Kheradmand T, Pothoven KL, Zhang ZJ, Luo X. Donor-specific CD8+ Foxp3+ T cells protect skin allografts and facilitate induction of conventional CD4+ Foxp3+ regulatory T cells. Am J Transplant. 2012;12:2335–47. doi: 10.1111/j.1600-6143.2012.04120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koch SD, Uss E, van Lier RA, ten Berge IJ. Alloantigen-induced regulatory CD8+CD103+ T cells. Hum Immunol. 2008;69:737–44. doi: 10.1016/j.humimm.2008.08.281. [DOI] [PubMed] [Google Scholar]
- 8.Rifa’i M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+regulatory T cells in the maintenance of T cell homeostasis. J Exp Med. 2004;200:1123–34. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endharti AT, Rifa IM, Shi Z, Fukuoka Y, Nakahara Y, Kawamoto Y, et al. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+ T cells. J Immunol. 2005;175:7093–7. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Priatel JJ, Chow MT, Teh HS. Preferential development of CD4 and CD8 T regulatory cells in RasGRP1-deficient mice. J Immunol. 2008;180:5973–82. doi: 10.4049/jimmunol.180.9.5973. [DOI] [PubMed] [Google Scholar]
- 11.Shi Z, Rifa’i M, Lee YH, Shiku H, Isobe K, Suzuki H. Importance of CD80/CD86-CD28 interactions in the recognition of target cells by CD8+CD122+ regulatory T cells. Immunology. 2008;124:121–8. doi: 10.1111/j.1365-2567.2007.02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molloy MJ, Zhang W, Usherwood EJ. Suppressive CD8+ T cells arise in the absence of CD4 help and compromise control of persistent virus. J Immunol. 2011;186:6218–26. doi: 10.4049/jimmunol.1003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endharti AT, Okuno Y, Shi Z, Misawa N, Toyokuni S, Ito M, et al. CD8+CD122+ regulatory T cells (Tregs) and CD4+ Tregs cooperatively prevent and cure CD4+ cell-induced colitis. J Immunol. 2011;186:41–52. doi: 10.4049/jimmunol.1000800. [DOI] [PubMed] [Google Scholar]
- 14.Wang LX, Li Y, Yang G, Pang PY, Haley D, Walker EB, et al. CD122+ CD8+ Treg suppress vaccine-induced antitumor immune responses in lymphodepleted mice. Eur J Immunol. 2010;40:1375–85. doi: 10.1002/eji.200839210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HJ, Wang X, Radfar S, Sproule TJ, Roopenian DC, Cantor H. CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. Proc Natl Acad Sci U S A. 2011;108:2010–5. doi: 10.1073/pnas.1018974108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangalam AK, Luckey D, Giri S, Smart M, Pease LR, Rodriguez M, et al. Two discreet subsets of CD8 T cells modulate PLP(91–110) induced experimental autoimmune encephalomyelitis in HLA-DR3 transgenic mice. J Autoimmun. 2012;38:344–53. doi: 10.1016/j.jaut.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai H, Wan N, Zhang S, Moore Y, Wan F, Dai Z. Cutting edge: Programmed death-1 defines CD8+ CD122+ T cells as regulatory versus memory T cells. J Immunol. 2010;185:803–7. doi: 10.4049/jimmunol.1000661. [DOI] [PubMed] [Google Scholar]
- 18.Guillonneau C, Picarda E, Anegon I. CD8+ regulatory T cells in solid organ transplantation. Curr Opin Organ Transplant 2010. [DOI] [PubMed]
- 19.Suzuki M, Konya C, Goronzy JJ, Weyand CM. Inhibitory CD8+ T cells in autoimmune disease. Hum Immunol. 2008;69:781–9. doi: 10.1016/j.humimm.2008.08.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai Z, Zhang S, Xie Q, Wu S, Su J, Li S, et al. Natural CD8+CD122+ T cells are more potent in suppression of allograft rejection than CD4+CD25+ regulatory T cells. Am J Transplant. 2014;14:39–48. doi: 10.1111/ajt.12515. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Jiang S, Shi H, Lin Y, Wang X. Prolongation of corneal xenotransplant survival by T-cell vaccination-induced T-regulatory cells. Xenotransplantation. 2008;15:164–73. doi: 10.1111/j.1399-3089.2008.00471.x. [DOI] [PubMed] [Google Scholar]
- 22.Varthaman A, Khallou-Laschet J, Clement M, Fornasa G, Kim HJ, Gaston AT, et al. Control of T cell reactivation by regulatory Qa-1-restricted CD8+ T cells. J Immunol. 2010;184:6585–91. doi: 10.4049/jimmunol.0903109. [DOI] [PubMed] [Google Scholar]
- 23.Ciubotariu R, Li J, Colovai AI, Platt JL, Cortesini R, Suciu Foca Cortesini N. Human xenospecific T suppressor cells inhibit T helper cell proliferation to porcine aortic endothelial cells, and NF-kappaB activity in porcine APC. Hum Immunol. 2001;62:470–8. doi: 10.1016/S0198-8859(01)00238-5. [DOI] [PubMed] [Google Scholar]
- 24.Manavalan JS, Kim-Schulze S, Scotto L, Naiyer AJ, Vlad G, Colombo PC, et al. Alloantigen specific CD8+ CD28− FOXP3+ T suppressor cells induce ILT3+ ILT4+ tolerogenic endothelial cells, inhibiting alloreactivity. Int Immunol. 2004;16:1055–68. doi: 10.1093/intimm/dxh107. [DOI] [PubMed] [Google Scholar]
- 25.Ciubotariu R, Colovai AI, Pennesi G, Liu Z, Smith D, Berlocco P, et al. Specific suppression of human CD4+ Th cell responses to pig MHC antigens by CD8+CD28− regulatory T cells. J Immunol. 1998;161:5193–202. [PubMed] [Google Scholar]
- 26.Choi J, Enis DR, Koh KP, Shiao SL, Pober JS. T lymphocyte-endothelial cell interactions. Annu Rev Immunol. 2004;22:683–709. doi: 10.1146/annurev.immunol.22.012703.104639. [DOI] [PubMed] [Google Scholar]
- 27.Scotto L, Naiyer AJ, Galluzzo S, Rossi P, Manavalan JS, Kim-Schulze S, et al. Overlap between molecular markers expressed by naturally occurring CD4+CD25+ regulatory T cells and antigen specific CD4+CD25+ and CD8+CD28− T suppressor cells. Hum Immunol. 2004;65:1297–306. doi: 10.1016/j.humimm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Yu Y, Zitzner JR, Houlihan J, Herrera N, Xu L, Miller J, et al. Common gamma chain cytokines promote rapid in vitro expansion of allo-specific human CD8+ suppressor T cells. PLoS One. 2011;6:e28948. doi: 10.1371/journal.pone.0028948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sindhi R, Manavalan JS, Magill A, Suciu-Foca N, Zeevi A. Reduced immunosuppression in pediatric liver-intestine transplant recipients with CD8+CD28− T-suppressor cells. Hum Immunol. 2005;66:252–7. doi: 10.1016/j.humimm.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Lin YX, Yan LN, Li B, Wang LL, Wen TF, Zeng Y, et al. A significant expansion of CD8+ CD28− T-suppressor cells in adult-to-adult living donor liver transplant recipients. Transplant Proc. 2009;41:4229–31. doi: 10.1016/j.transproceed.2009.09.072. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Chen N, Chen G, You P. The protective effect of CD8+ CD28− T suppressor cells on the acute rejection responses in rat liver transplantation. Transplant Proc. 2007;39:3396–403. doi: 10.1016/j.transproceed.2007.06.089. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Zhang L, Tang J, Jiang S, Wang X. Adoptive transfer of transplantation tolerance mediated by CD4+CD25+ and CD8+CD28− regulatory T cells induced by anti-donor-specific T-cell vaccination. Transplant Proc. 2008;40:1612–7. doi: 10.1016/j.transproceed.2008.02.079. [DOI] [PubMed] [Google Scholar]
- 33.Ho J, Kurtz CC, Naganuma M, Ernst PB, Cominelli F, Rivera-Nieves J. A CD8+/CD103 high T cell subset regulates TNF-mediated chronic murine ileitis. J Immunol. 2008;180:2573–80. doi: 10.4049/jimmunol.180.4.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu L, Yu Y, Li G, Pu L, Zhang F, Zheng S, et al. CD8 (+) CD103 (+) regulatory T cells in spontaneous tolerance of liver allografts. Int Immunopharmacol. 2009;9:546–8. doi: 10.1016/j.intimp.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 35.Uss E, Yong SL, Hooibrink B, van Lier RA, ten Berge IJ. Rapamycin enhances the number of alloantigen-induced human CD103+CD8+ regulatory T cells in vitro. Transplantation. 2007;83:1098–106. doi: 10.1097/01.tp.0000259555.29762.f0. [DOI] [PubMed] [Google Scholar]
- 36.Feng Y, Wang D, Yuan R, Parker CM, Farber DL, Hadley GA. CD103 expression is required for destruction of pancreatic islet allografts by CD8 (+) T cells. J Exp Med. 2002;196:877–86. doi: 10.1084/jem.20020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee YH, Ishida Y, Rifa’i M, Shi Z, Isobe K, Suzuki H. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol. 2008;180:825–32. doi: 10.4049/jimmunol.180.2.825. [DOI] [PubMed] [Google Scholar]
- 38.Zozulya AL, Ortler S, Fabry Z, Sandor M, Wiendl H. The level of B7 homologue 1 expression on brain DC is decisive for CD8 Treg cell recruitment into the CNS during EAE. Eur J Immunol. 2009;39:1536–43. doi: 10.1002/eji.200839165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bahri R, Bollinger A, Bollinger T, Orinska Z, Bulfone-Paus S. Ectonucleotidase CD38 demarcates regulatory, memorylike CD8+ T cells with IFN-gamma-mediated suppressor activities. PLoS One. 2012;7:e45234. doi: 10.1371/journal.pone.0045234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saitoh O, Abiru N, Nakahara M, Nagayama Y. CD8+CD122+ T cells, a newly identified regulatory T subset, negatively regulate Graves’hyperthyroidism in a murine model. Endocrinology. 2007;148:6040–6. doi: 10.1210/en.2007-0300. [DOI] [PubMed] [Google Scholar]
- 41.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunological self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25)-breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 42.Lu L, Cantor H. Generation and regulation of CD8(+) regulatory T cells. Cell Mol Immunol. 2008;5:401–6. doi: 10.1038/cmi.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Z, Han Y, Gu Y, Liu Y, Jiang Z, Zhang M, et al. CD11c (high) CD8+ regulatory T cell feedback inhibits CD4 T cell immune response via Fas ligand-Fas pathway. J Immunol. 2013;190:6145–54. doi: 10.4049/jimmunol.1300060. [DOI] [PubMed] [Google Scholar]
- 44.Zheng J, Liu Y, Qin G, Chan PL, Mao H, Lam KT, et al. Efficient induction and expansion of human alloantigen-specific CD8 regulatory T cells from naive precursors by CD40-activated B cells. J Immunol. 2009;183:3742–50. doi: 10.4049/jimmunol.0901329. [DOI] [PubMed] [Google Scholar]
- 45.Vlad G, Cortesini R, Suciu-Foca N. CD8+ T suppressor cells and the ILT3 master switch. Hum Immunol. 2008;69:681–6. doi: 10.1016/j.humimm.2008.08.286. [DOI] [PubMed] [Google Scholar]
- 46.Rifa’i M, Shi Z, Zhang SY, Lee YH, Shiku H, Isobe K, et al. CD8+CD122+ regulatory T cells recognize activated T cells via conventional MHC class I-alphabetaTCR interaction and become IL-10-producing active regulatory cells. Int Immunol. 2008;20:937–47. doi: 10.1093/intimm/dxn052. [DOI] [PubMed] [Google Scholar]


