Abstract
Imaging studies that use rodents sometimes involve intraperitoneal administration of pharmacological compounds. To facilitate such studies, the authors developed a simple and easily mastered technique for placing an intraperitoneal catheter in a conscious mouse. This technique eliminates the need to remove the animal from the scanner to administer a drug through the intraperitoneal route.
When studying how rodents respond to pharmacological compounds, researchers commonly inject drugs through the intraperitoneal (i.p.) space. Like intravenous (i.v.) injection, i.p. injection results in rapid absorption of administered compounds1, but i.p. injection is easier and quicker than i.v. injection in a small rodent1. However, i.p. injection can result in delivery of drugs to different locations in the peritoneal cavity, even when done by experienced researchers. As a result, the absorption rate and the amount of a drug that reaches the blood stream from an i.p. injection can be variable2–4. Additionally, in one strain of mice, i.p. injection resulted in increased activity of c-Fos in the brain5, suggesting that i.p. injections might induce increased stress in animals. Despite these disadvantages, i.p. injection remains a widely used technique in small animal studies6–8.
For rodent imaging studies, researchers most commonly administer drugs and contrast agents i.v.9–12 but have also used the i.p. route to administer contrast agents to conscious mice before placing them in scanners13 and to sedated rats secured in scanners10. To our knowledge, however, administration of i.p. injections to conscious rodents that are secured in scanners has not been previously reported.
In an imaging study, it is advantageous to administer a drug to an animal without having to remove the animal from the scanner between baseline and post-injection scans, because moving the animal introduces confounding factors. As small animal imaging systems and methods to scan conscious, unanesthetized animals become increasingly available14, researchers will benefit from improved techniques for administering pharmacological agents to conscious animals. This is especially important when researchers have behavioral data on the effects of i.p. injection of a drug and want to design imaging studies to assess the corresponding in vivo effects. To address these issues, we have developed a technique for placing and securing an i.p. catheter in mice. We designed this technique for pharmacological magnetic resonance imaging (MRI) studies on mice, but the technique could be modified for use with other imaging modalities, such as fluorescence imaging8.
Methods
Conditioning of mice
The Portland Veterans Affairs Medical Center IACUC approved all aspects of this study, in accordance with the United States Department of Agriculture and United States Public Health Service Guidelines. To prepare our animals for conscious scanning, we use a procedure based on a published acclimation protocol in rats14. First, we surgically attach a U-shaped plastic plate to the skull of each mouse. This plate helps to immobilize the animal's head during the animal's acclimation to MRI scanning and during actual MRI scanning. After a week of recovery, each animal undergoes four acclimation sessions (2 h per session) in a mock MRI set-up to prepare for actual scanning. These sessions are spaced throughout a 2-week period. We use no sedation and do not insert the catheter during acclimation.
Materials
Before beginning the catheterization procedure, we assemble the following equipment: non-sterile gloves, an intravenous catheter (24 gauge, 0.5 in, SURFLO I.V. Catheter, Terumo Medical, Somerset, NJ) with stylette, masking tape (0.25 in), tissue adhesive and small surgical scissors. We prepare the catheter by folding a 2-in strip of the masking tape around the catheter's Luer taper with the sticky side facing inward, to create 0.5-in ‘wings’ (Fig. 1). Because the sticky side of the tape is not exposed, the tape does not stick to the animal's fur.
Figure 1.
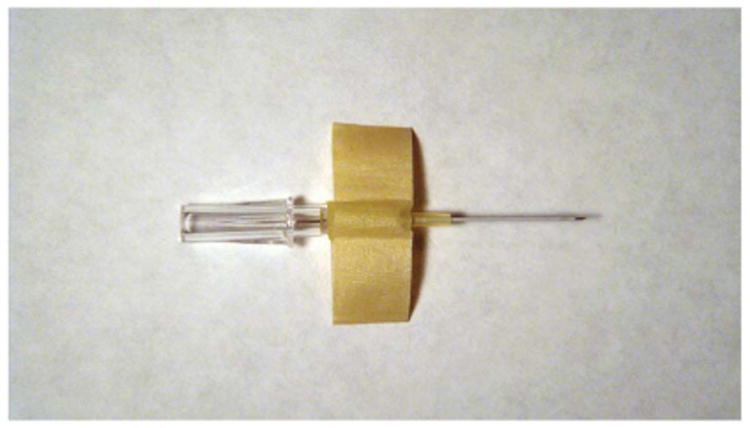
We fold tape over the catheter Luer taper, with the sticky side facing inward, to create wings on the side of the catheter that can be secure to the animal's abdomen using tissue adhesive.
Catheter insertion and positioning
We transport the animal to the MRI suite. Before starting the scanning, we place the i.p. catheter using the procedure described below. We do not carry out any special site preparation before placing the catheter.
The animal is held securely with its abdomen exposed. The catheter, with the stylus in place, is inserted at a 45° angle into the lower right quadrant of the animal's abdomen. The catheter is advanced until approximately half of the needle is inserted into the abdominal cavity, which should be sufficient to puncture the peritoneal wall. We remove the stylus, before fully inserting the catheter, to minimize injury to the mouse's internal abdominal structures, and then fully advance the flexible catheter tubing into the abdominal cavity.
We apply one or two drops of tissue adhesive to each of the catheter tape wings and secure them (in the midline position) to the animal's fur. Next, we gently compress the tape wings to the mouse's body for a few seconds, allowing the adhesive to stick (Fig. 2).
Figure 2.
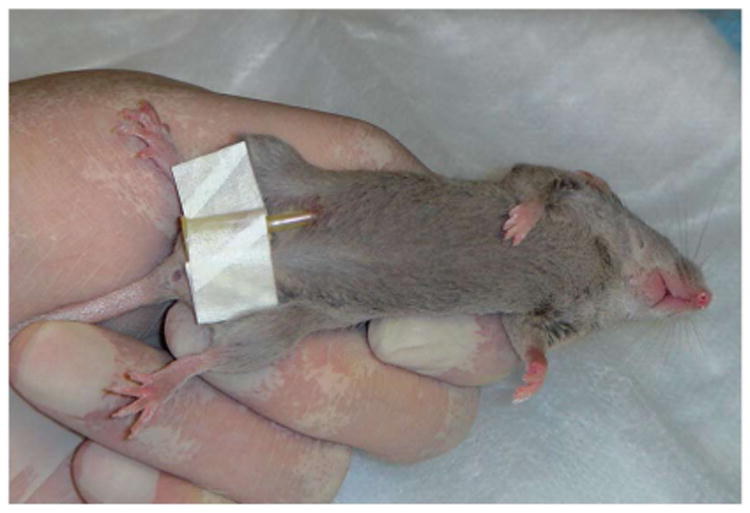
We securely hold the animal in one hand to expose the animal's ventral surface. We then insert the catheter at a 45° angle into the lower right abdominal quadrant and adhere the catheter tape wings to the fur of the abdomen with tissue adhesive.
Next, we use flexible tubing to attach the catheter tip to a drug delivery pump system. The pump should already be backfilled with the drug to be injected. For our MRI protocol, we then place the mouse into the clear, padded MRI cylinder restrainer, in a prone position (Fig. 3). The i.p. catheter and line exit the holder along the mouse's tail. We further secure the line to the MRI holder by taping the line a few inches from the exit of the restrainer. We have found that mice tolerate this procedure well. When we secure the line along the mouse's midline, the animal cannot dislodge it with its hind limbs.
Figure 3.

We secure the mouse in the MRI restraining device in the prone position. The catheter and line exit the apparatus posteriorly and in the midline, along the length of the animal's tail.
When the scanning is complete, we detach the catheter from the line, use small surgical scissors to gently clip the tape wings on either side of the catheter Luer taper and remove the line. Only a small amount of medical tape remains attached to the animal's fur on each side. The animal easily removes this residual tape during routine grooming. If we attempt to remove the secured tape pieces, we might damage the mouse's skin and increase the mouse's risk of infections.
Discussion
We have used this technique to place catheters in more than 12 animals and have found that the animals tolerate it well. Our staff veterinarian has carried out necropsies on four of these animals and found that the catheter was correctly placed in the peritoneum. The veterinarian saw no damage to internal structures, indicating that neither catheter placement nor fluid injection caused any injuries.
We recommend that researchers use this technique in conscious animals only if the animals have been acclimated to prolonged restraint as described above; animals that have not been acclimated may become agitated. Potential complications of this technique include infection at the catheter placement site and irritation from the adhesive. We use the minimum amount of adhesive required to secure the tape wings. To prevent blockage of the urinary and gastrointestinal tracts, we ensure that the adhesive does not touch the anus or the prepubital or clitoral extension. We do not carry out any special skin preparation, because we are concerned that skin irritation from the tissue adhesive touching newly shaved skin may outweigh any benefit from inserting the catheter into a shaved and disinfected site. To date, we have not seen any of the complications listed above.
Using this technique, we can complete baseline and post-injection scans without removing the animal from the scanner. With slight modifications, researchers could use similar techniques for other types of imaging studies8.
Acknowledgments
This work was supported by the Department of Veterans Affairs and NIH NIAAA grant P60 AA010760. We thank Ky Dehlinger, DVM for carrying out the necropsies.
Footnotes
Competing Interests Statement: The authors declare no competing financial interests.
References
- 1.Hayward AM, et al. Biomethodology and surgical techniques. In: Fox JG, et al., editors. The Mouse in Biomedical Research. 2nd. Vol. 3. Academic; San Diego: 2007. pp. 437–488. [Google Scholar]
- 2.Svendsen O. Ethics and animal welfare related to in vivo pharmacology and toxicology in laboratory animals. Basic Clin Pharmacol Toxicol. 2005;97:197–199. doi: 10.1111/j.1742-7843.2005.pto_letter_974.x. [DOI] [PubMed] [Google Scholar]
- 3.Miner NA, Koehler J, Greenaway L. Intraperitoneal injection of mice. Appl Microbiol. 1969;17:250–251. doi: 10.1128/am.17.2.250-251.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arioli V, Rossi E. Errors related to different techniques of intraperitoneal injection in mice. Appl Microbiol. 1970;19:704–705. doi: 10.1128/am.19.4.704-705.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryabinin AE, Wang YM, Finn DA. Different levels of Fos immunoreactivity after repeated handling and injection stress in two inbred strains of mice. Pharmacol Biochem Behav. 1999;63:143–151. doi: 10.1016/s0091-3057(98)00239-1. [DOI] [PubMed] [Google Scholar]
- 6.Schiffer WK, Mirrione MM, Dewey SL. Optimizing experimental protocols for quantitative behavioral imaging with 18F-FDG in rodents. J Nucl Med. 2007;48:277–287. [PubMed] [Google Scholar]
- 7.Meibach RC, Glick SD, Ross DA, Cox RD, Maayani S. Intraperitoneal administration and other modifcations of the 2-deoxy-D-glucose technique. Brain Res. 1980;195:167–176. doi: 10.1016/0006-8993(80)90874-4. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman RM. The multiple uses of fuorescent proteins to visualize cancer in vivo. Nat Rev Cancer. 2005;5:796–806. doi: 10.1038/nrc1717. [DOI] [PubMed] [Google Scholar]
- 9.Tipre DN, et al. PET imaging of brain 5-HT1A receptors in rat in vivo with 18F-FCWAY and improvement by successful inhibition of radioligand defuorination with miconazole. J Nucl Med. 2006;47:345–353. [PubMed] [Google Scholar]
- 10.Kida I, Rothman DL, Hyder F. Dynamics of changes in blood flow, volume, and oxygenation: implications for dynamic functional magnetic resonance imaging calibration. J Cereb Blood Flow Metab. 2007;27:690–696. doi: 10.1038/sj.jcbfm.9600409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin CL, et al. Pharmacological MRI in awake rats predicts selective binding of α4β2 nicotinic receptors. Synapse. 2008;62:159–168. doi: 10.1002/syn.20474. [DOI] [PubMed] [Google Scholar]
- 12.Bruns A, Künnecke B, Risterucci C, Moreau JL, von Kienlin M. Validation of cerebral blood perfusion imaging as a modality for quantitative pharmacological MRI in rats. Magn Reson Med. 2009;61:1451–1458. doi: 10.1002/mrm.21779. [DOI] [PubMed] [Google Scholar]
- 13.Yu X, Wadghiri YZ, Sanes DH, Turnbull DH. In vivo auditory brain mapping in mice with Mn-enhanced MRI. Nat Neurosci. 2005;8:961–968. doi: 10.1038/nn1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King JA, et al. Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Meth. 2005;148:154–160. doi: 10.1016/j.jneumeth.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


