Abstract
The epidemiology of prostate cancer has dramatically changed since the introduction of prostate-specific antigen (PSA) screening in the 1980’s. Most prostate cancers today are detected at early stages of the disease and are considered “indolent”, however some patients’ prostate cancers demonstrate a more aggressive behavior which leads to rapid progression and death. Increasing understanding of the biology underlying the heterogeneity that characterizes this disease has lead to a continuously evolving role of imaging in the management of prostate cancer. Functional and metabolic imaging techniques are gaining importance as the impact on the therapeutic paradigm has shifted from structural tumor detection alone to distinguishing patients with indolent tumors that can be managed conservatively (e.g., by active surveillance) from patients with more aggressive tumors that may require definitive treatment with surgery or radiation. In this review, we discuss advanced imaging techniques that allow direct visualization of molecular interactions relevant to prostate cancer and their potential for translation to the clinical setting in the near future. The potential use of imaging to follow molecular events during drug therapy as well as the use of imaging agents for therapeutic purposes will also be discussed.
Keywords: prostate cancer, molecular imaging, MRI, PET, optical imaging, Cerenkov imaging
INTRODUCTION
Prostate cancer is the most common cancer and the second most common cause of cancer-related death amongst men in the Americas, Europe and Australia. The role of imaging in prostate cancer is continuously evolving in parallel with increasing understanding of the underlying biological heterogeneity which characterizes the disease. Functional and metabolic imaging techniques are gaining importance as the emphasis in management has shifted from structural tumor detection to accurate risk-stratification at the time of diagnosis and post-treatment follow-up. Several imaging modalities are considered the key vehicles for translating molecular biology approaches into the clinical realm in prostate cancer. Technological advances in magnetic resonance imaging (MRI), positron-emission tomography (PET), optical imaging and Cerenkov imaging, offer the possibility to directly visualize molecular interactions, something not achievable with standard imaging techniques [1]. These advances include the use of activatable imaging agents, which comprise the most complex of all imaging probes [2]. Through interaction with their target, activatable probes undergo a transformation that leads to a change in the emitted signal (usually, it is switched “on” or “off”). Thus, in contrast to targeted agents, which provide information merely about the physical presence of the target, activatable agents (often called "smart" sensors) deliver information on the biological activity of their target [2]. Activatable agents have been devised predominantly for optical imaging applications but also for MRI. Since radioactive decay is a physical process that cannot be modified, activatable radioactive agents have long been considered impossible. However, use of the radioactive decay signal of Cerenkov light [3] may soon open the door for radiotracer-based activatable imaging agents.
Identification of a suitable molecular target
Designing imaging probes for advanced prostate cancer imaging can be challenging and involves meeting several key requirements: 1) identifying a suitable target specifically associated with prostate cancer and finding the an appropriate ligand that will bind to it with high specificity; 2) labeling this ligand with a label suitable for the preferred imaging modality, which should allow for clinical translation. To find targets and ligands, various approaches can be used, including gene expression profiling and exploration of libraries. The design of the imaging probe must also take into consideration barriers it might encounter during its journey to its target. For prostate cancer, several targets have been identified, which are discussed below.
Androgen Receptor (AR) Imaging
Androgen deprivation therapy has played a role in the management of patients with advanced prostate cancer for over half a century [4]. Most patients with metastatic prostate cancer respond to androgen deprivation (pharmacologic or surgical), however almost invariably this is followed by progression to “castration-resistant” disease which occurs due to “sensitizing” or “bypassing” of the Androgen Receptor (AR) pathway. The AR is a ligand-dependent transcription activator which plays a key role in cell differentiation and cell proliferation [5]. The treatment of castration-resistant prostate cancer has been revolutionized in recent years by the introduction of novel therapeutic agents targeting the AR (e.g. enzalutamide) [6].
The distribution of anatomic sites where the AR is overexpressed can be imaged using the PET agent 16β-[18F]fluoro-5α-dihydrotestosterone (18F-FDHT) [7–9]. The differential overexpression of AR has been shown in studies comparing 18F-FDHT and 18F-FDG in men with castration-resistant prostate cancer which have identified different “phenotypes” amongst this patients, including those with lesions demonstrating preferential tracer accumulation on FDHT PET (AR-predominant), preferential accumulation on FDG PET (glycolysis predominant) and mixed (ie. Uptake on both FDHT and FDG PET) phenotypes (Figure 1) [10; 11]. A recent study evaluated the associations between morphologic CT patterns, glycolytic activity, and AR expression on PET and found that the numbers of bone lesions on CT, FDG PET, and FDHT PET scans, as well as the intensity of FDHT uptake are significantly associated with overall survival [11].
Figure 1.
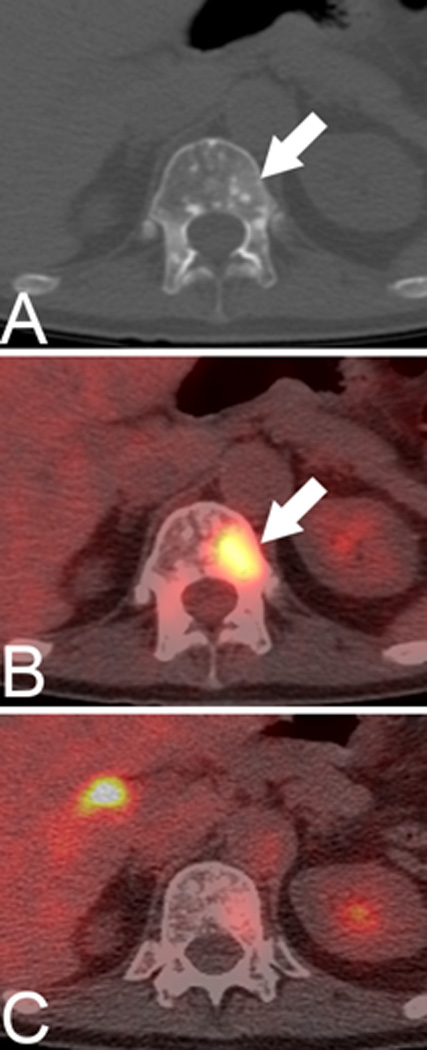
Axial CT (A), fused FDG PET/CT (B) and fused FDHT PET/CT (C) images demonstrate the biological diversity of bone metastases in patients with castration-resistant prostate cancer. Subtle groundglass and miliary density lesion in T12 vertebral body (arrows), with marked glycolytic activity but minimal androgen expression, as evidenced by uptake on FDG but not on FDHT PET.
18F-FDHT may be also have a potential role as a pharmacodynamic marker of drug targeting. One study reported that enzalutamide (AR receptor antagonist) induced 18F-FDHT uptake changes in metastatic lesions could be considered a surrogate marker for adequate “targeting” of prostate cancer metastases with AR overexpression [6].
Prostate-specific membrane antigen (PSMA)
PSMA is probably the most prominent prostate cancer-associated antigen. It is a dimeric Type II integral membrane glycoprotein, highly expressed on prostate cancer cells[12]. Higher PSMA levels are seen in metastatic and higher-grade prostate cancer [13]. High levels of PSMA expression correlate significantly with early biochemical recurrence in surgically treated prostate cancer and also with tumor stage, Gleason grade as well as preoperative PSA and HER2 expression (P<0.0001 each)[14]. It has also been shown that the level of PSMA expression associated with benign prostatic hyperplasia (BPH) is lower than that associated with normal prostate and significantly lower than that associated with prostate cancer[15; 16]. Therefore BPH is not expected to substantially interfere with any PSMA-based methods of characterizing prostatic tissue (e.g., as benign or malignant).
Conventionally, PSMA is detected using labeled monoclonal antibodies or small molecules [17; 18], which indicate the expression of PSMA protein. The most prominent of the monoclonal antibodies are 7E11 (ProstaScint®) and the more recently developed J591. In 1996, the US Food and Drug Administration approved the use of ProstaScint (indium-111 [111In]-labeled capromab pendetide or 7E11, Cytogen Corporation, Princeton NJ, USA), for single-photon emission computed tomography (SPECT) imaging of soft-tissue (but not bone) sites of metastatic prostate cancer for pre-surgical staging or evaluation of prostate-specific antigen (PSA) relapse after radical prostatectomy. 7E11 is a murine mAb specific for an epitope on the intracellular domain of PSMA. In several studies, ProstaScint imaging displayed sensitivity of 60%, specificity of 70%, positive predictive value of 60% and negative predictive value of 70% for prostate cancer soft-tissue lesions [19–21]. However, the intracellular binding site of 111In-7E11 is only accessible upon membrane disruption in dying or dead cells [22]. Therefore, probably because the number of available targets in the absence of membrane disruption is limited, SPECT with 111In-7E11 has low sensitivity for viable tumor sites (62% for lymph node metastases, 50% for prostate bed recurrence). Furthermore, 111In-capromab pendetide does not bind to viable prostate cancer sites in bone (the most common site of metastatic disease), and in contrast to PET, SPECT remains only semiquantitative in the clinical setting.
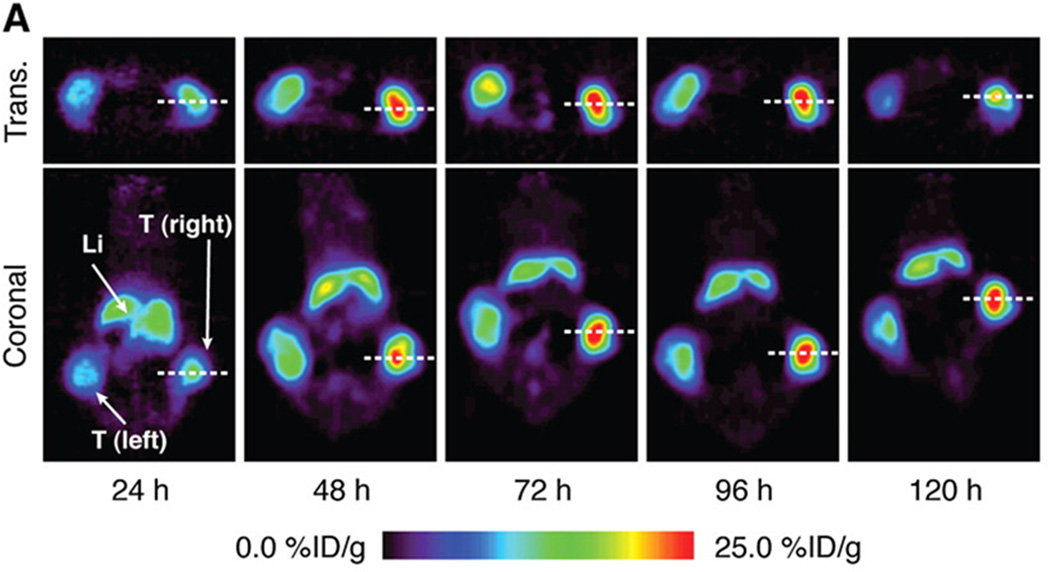
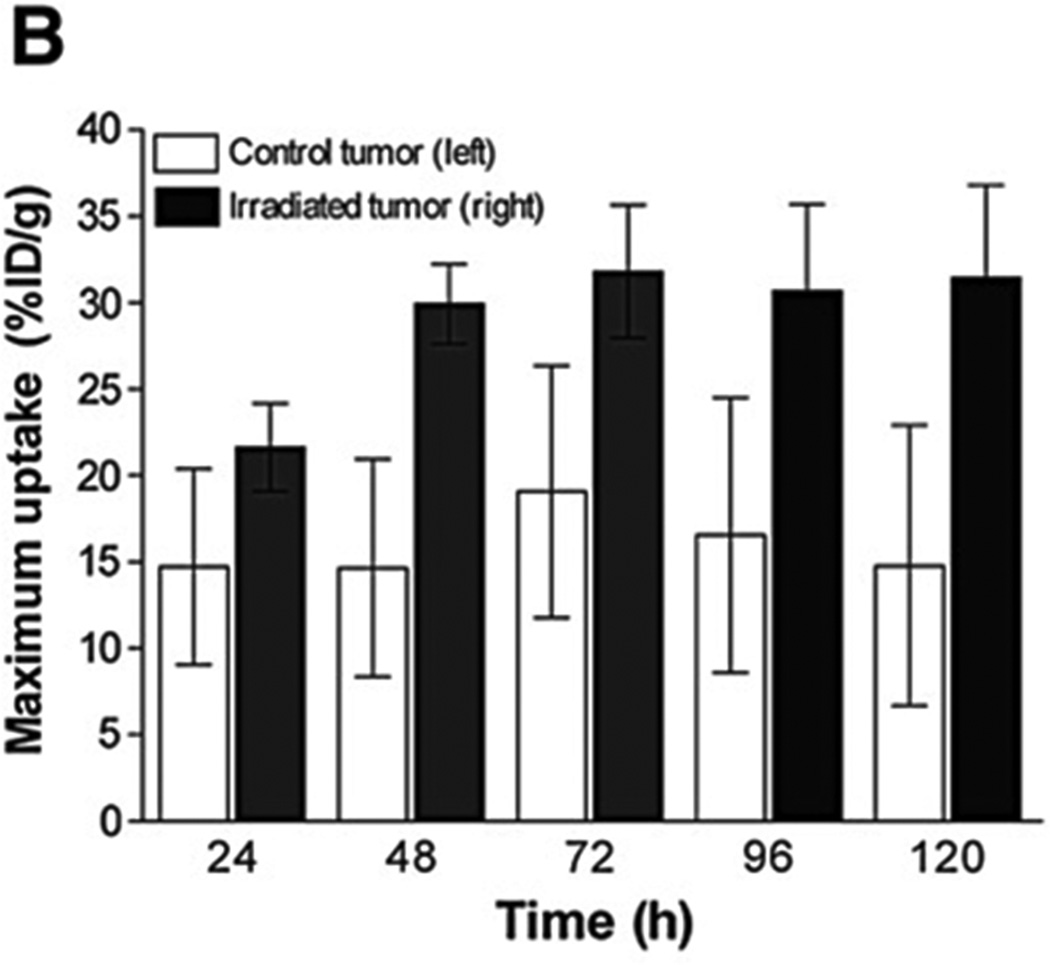
For PET, a variety of appropriate radioisotopes with different properties are available and already in clinical use. The novel PET nuclide Zirconium-89 (89Zr), with its unique physical decay properties and energy (t1/2 = 78.43 h, β+ = 22.3%, Eβ+,max = 901 keV, Eγ = 909 keV), is ideally suited for targeted imaging probes, especially antibodies. The greater half-life provides copious activity at the required circulation times for optimal targeting to disease sites [23; 24]. The longer half-life even enables imaging at later time points, including 120 hours post injection, an imaging window difficult to achieve with the shorter-lived PET nuclides 18F and 64Cu [23; 25]. Recently, a new imaging approach was described for 7E11, utilizing the perceived disadvantage that the recognized epitope only becomes accessible upon membrane disruption [24]. 89Zr was conjugated to 7E11 to provide a radiotracer for measuring the response of prostate cancer to treatment by using biodistribution studies and immunoPET imaging. It was shown that the effects of chemotherapy, chemical castration or radiotherapy could be monitored by observing 7E11 uptake, which increased with progressing cell membrane disruption resulting from treatment-induced cell death (Figure 2). The uptake of 89Zr-7E11 correlated strongly with markers of cell death and apoptosis (Figure 3). The results confirmed that 89Zr-7E11 immunoPET could potentially be used for successful non-invasive evaluation of treatment response regardless of the type of therapy applied [24].
Figure 2.
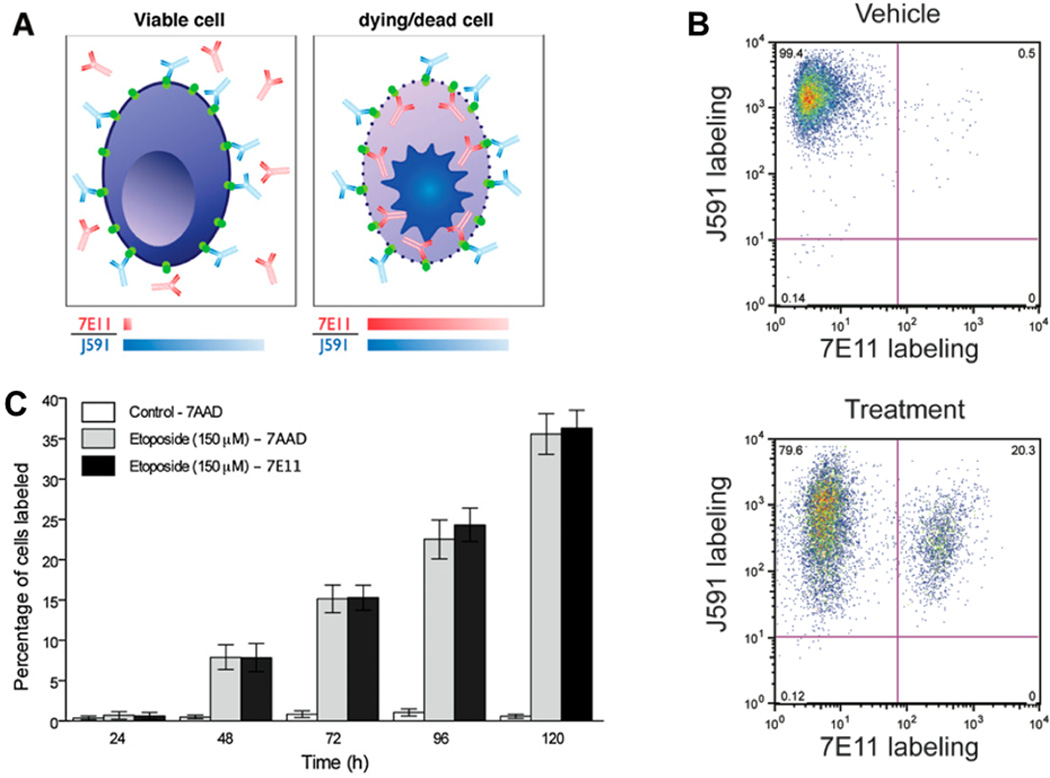
PSMA+ cells’ responses to different treatments in vitro. (A) The monoclonal antibody 7E11 binds to an intracellular epitope of PSMA, labeling apoptotic or already dead cells, whose leaky cell membrane permits access of the antibody to the intracellular domain. The monoclonal antibody J591, recognizes the extracellular domain of PSMA and thus binds to all PSMA-positive cells, regardless of their viability. (B) Higher percentage of 7AAD and 7E11 stained cells is observed over time with flow cytometry after treatment compared to control (P<0.05). (C) Corresponding staining is observed for both 7E11 and 7AAD staining, a marker of late apoptosis (and thus cell death) after treatment with etoposide (PC3/PSMA+ cells). With permission from Ruggiero A et al. (2012) J Nucl Med 52(8):1608-15
Figure 3.
In vivo imaging of therapy response with 89Zr-7E11 ImmunoPET in xenograft-bearing mice. (A) Representative transverse and coronal 89Zr-7E11 immunoPET images at different time points in a LNCaP xenograft-bearing mouse treated with selective radiation to the right side. Increased uptake of 89Zr-7E11 was observed in the selectively irradiated tumor (right) compared to the control (left). T: Tumor, Li: Liver. The dashed line represents the position of the perpendicularly oriented image. (B) 89Zr-7E11 uptake values (obtained from the PET data as maximum %ID/g) were significantly higher in irradiated than control tumors at 24 h (P=0.0376), 48 h (P=0.0009), 72 h (P=0.0086), 96 h (P=0.01), 120 h (P=0.0075). With permission from Ruggiero A et al. (2012) J Nucl Med 52(8):1608-15
In contrast to 7E11, the humanized J591 monoclonal antibody targets the extracellular epitope of PSMA [26], which is available on live and dead cells alike. J591 is therefore not significantly influenced by changes in membrane permeability. It has been radiolabeled with 89Zr for immunoPET imaging where it has demonstrated high tumor-to-background tissue ratios [23] (Figure 4). Overall, the novel radiotracers 89Zr-7E11 and -J591 represent promising candidates for translation to the clinic for noninvasively diagnosing prostate cancer and assessing its response to treatment. A clinical trial using J591 is currently underway (NCT01543659) as are several trials using J591 as a therapeutic agent. The radiolabeled J591 has also been used to perform targeted Cerenkov imaging in mouse models [3]. Compared to standard optical imaging approaches, Cerenkov imaging approaches using approved radiotracers offer the possibility of relatively rapid clinical translation [27]. Cerenkov radiation is produced when charged particles travel faster than the speed of light through a dielectric medium [27]. Cerenkov imaging exploits the light emission from commonly used diagnostic (e.g. all PET isotopes) and many therapeutic radionuclides [27]. This modality detects a lower amount of light compared to other optical imaging techniques such as fluorescence and bioluminescence, however it benefits from high signal to noise ratios resulting from lack of incident light sources [27].
Figure 4.

Temporal immunoPET images of 89Zr-DFO-J591 (10.9–11.3 MBq [295–305 mCi], 60–62 mg of mAb, in 200 mL of sterile saline) recorded in LNCaP tumor–bearing (PSMA-positive, left shoulder) (A) and PC-3 tumor– bearing (PSMA-negative, right shoulder) (B) mice between 3 and 144 h after injection. Transverse and coronal planar images intersect center of tumors, and mean tumor-to-muscle ratios derived from volume-of-interest analysis of immunoPET images are given. Upper thresholds of immunoPET have been adjusted for visual clarity, as indicated by scale bars. With permission from Holland J et al. (2012) J Nucl Med 51(8):1293-1300
Hormone therapy is often used in the management of prostate cancer. Wright and colleagues [28] have observed that hormone deprivation leads to an increase of PSMA expression in vitro while hormone replenishment suppresses PSMA expression. Recently, it was demonstrated that therapy with the new anti-androgen enzalutamide increased PSMA expression. This change in response to treatment was subsequently quantitatively measured with PET utilizing radiolabeled J591 (64Cu-J591) in prostate cancer xenograft models [29]. Similar findings have also been reported for PSA.
In addition to antibodies, several small molecules have been described as ligands to PSMA. Low-molecular-weight imaging agents have several inherent advantages over bigger ligands such as antibodies, including faster tumor uptake and increased clearance from non-target sites. Many low-molecular-weight inhibitors of PSMA have been reported [30–32]. A prominent example - now translated to the clinic – is N-[N-[(S)-1,3-dicarboxypropyl]carbamoyl]-4-(18)F-fluorobenzyl-L-cysteine (18F-DCFBC), a small molecule inhibitor of PSMA's carboxypeptidase function [32]. As an inhibitor this small molecule binds specifically and irreversibly to PSMA's active side. Since tumor uptake and blood clearance are more rapid for small molecules than for antibodies, the pharmacodynamics of 18F-DCFBC are more favorable, with higher tumor-to-background ratios; however, a moderate degree of remaining blood pool activity has been noted, possibly due to binding to serum proteins. Abnormal uptake may be noted at sites (e.g. lymph nodes) that would not be deemed metastatic based on size criteria on standard cross-sectional imaging (Figure 5). Similar agents have been labeled with 123I for SPECT imaging [33]. These compounds also demonstrate rapid distribution, blood clearance rates and tumor uptake, providing very good tumor-to-background contrast (Figure 6). The same inhibitor has also been used for a targeted, nanoparticle-based theranostic approach [34], combining imaging and drug delivery in one entity [35]. For targeting to PSMA, this particle carried the small molecule PSMA inhibitor; for therapy, it carried the enzyme bacterial cytosine deaminase (bCD) to convert the nontoxic pro-drug 5-fluorocytosine (5-FC) to cytotoxic 5-fluorouracil (5-FU) and a vector for siRNA to downregulate choline kinase, which is overexpressed in prostate cancer. The particle was labeled for imaging with the near-infrared fluorochrome Cy5.5 for optical imaging and 111In-DOTA for SPECT imaging.
Figure 5.

Clinical PSMA imaging with fluorinated small molecule. (A and B) Focal 18F-DCFBC PET uptake at aortic bifurcation (arrow, A) with correlative small LN seen on concurrent contrast-enhanced CT (arrow, B), not considered to be nodal metastasis by CT but positive by PET. (C) Retrospective review of prior contrast-enhanced CT scan obtained 1 y previously demonstrates LN in this region (arrow). With permission from Cho SY et al. (2012) J Nucl Med 53(12):1883-91
Figure 6.

Clinical PSMA imaging with iodinated small molecule. Patients with radiographically confirmed metastatic prostate cancer to whom 370 MBq (10 mCi) of 123I-MIP 1072 and 123I-MIP- 1095 were administered, and for comparative purposes, healthy volunteer to whom 370 MBq of 123I-MIP 1072 were administered. Depicted are representative transaxial (left), coronal (middle), and sagittal (right) slices from reconstructed SPECT/CT at 4 h after injection, demonstrating excellent depiction of the prostate tumor. With permission from Barrett JA et al. (2013) J Nucl Med 54(3):380-7
Multiple optical imaging agents targeting PSMA have been developed, some utilizing the J591 antibody with various light emitters, including radiotracers for Cerenkov emission [3; 36]. A few small molecule-based fluorescent agents have been developed as well, based on either phosphoramidate or urea-based peptidomimetics [17; 37]. While these agents currently remain in the pre-clinical realm (Figure 7), considerable efforts are underway to move them forward into clinical applications. A number of methods exist to perform whole-body tomography with fluorescent agents on animals [38], but at present there are no methods for applying this concept to whole body human scanning. Therefore, surface-based optical imaging (mostly intraoperative and endoscopic) is being pursued [39]. Enhanced intraoperative detection of cancerous lesions has been achieved with fluorescence-guided imaging and resection, using fluorescent dyes that target tumors [39–41]. Because of superior tissue penetration and decreased autofluorescence, dyes with emission in the near-infrared (NIR) window of the spectrum (650−900 nm) are preferred.
Figure 7.
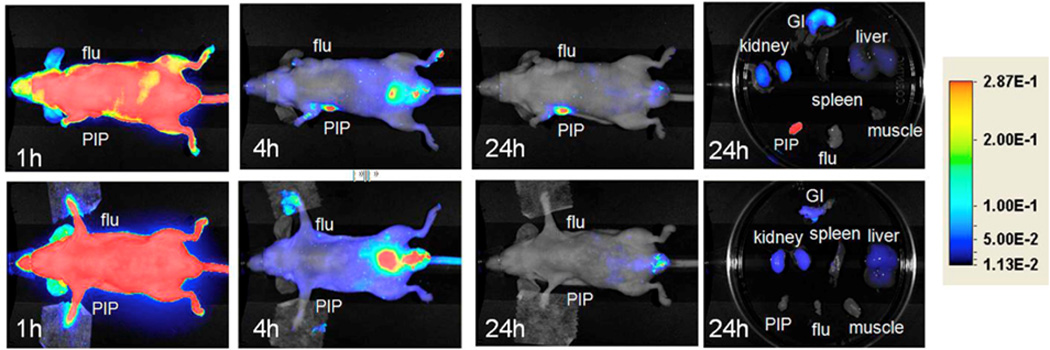
Optical imaging. Top row: Images after administration of 1 nmol Cy7-3 at (left to right) 1, 4, and 24 h postinjection, as well as images of the excised organs at 24 h postinjection. Bottom row: Image after administration of 1 nmol Cy7-3 + 1 mmol DCIBzL, a high-affinity ligand for PSMA (blocker) at same time points as above. Note lack of uptake in the mice treated with DCIBzL, indicating binding specificity. With permission from Chen Y et al. (2012) Bioconjug Chem 23(12):2377-85
Another promising ligand to detect PSMA is an aptamer. Aptamers are relatively short strands of oligonucleic molecules that bind to a specific target [42]. Aptamers are widely known as a substitute for antibodies, because these molecules overcome some weaknesses of antibodies. They offer high stability, facile production, low immunogenicity, rapid clearance and the possibility of binding to targets that are low in immunogenicity. An aptamer targeting PSMA (A10) has been developed [43] and has been coupled to a co-polymeric drug-delivering nanoparticle; a single intratumoral administration of the construct was significantly more efficacious in tumor reduction than were nontargeted particles and controls in animal experiments [44]. Similar constructs have been made with the toxin gelonin, resulting in 600-fold higher potency on PSMA-positive cells than that achieved with negative controls [45]. Polymeric nanoparticles containing cyanine dyes as fluorochromes have been devised for fluorescence imaging [46]. Iron oxide nanoparticles coated with the aptamer and intercalated doxorubicin have been used for MR imaging and targeted drug delivery [47]. Recently, A10 has been radiolabeled with 64Cu for possible PET imaging of PSMA-positive tumors [48].
Prostate-specific antigen (PSA)
Standard prostate cancer screening is partially based on the detection of abnormally increased serum PSA levels, however prostate cancers are found in up to 15% of patients with normal serum, including aggressive tumors with Gleason scores of ≥7 [49]. PSA screening therefore cannot reliably differentiate between indolent and aggressive disease [50]. Although PSA expression is tightly coupled to androgen receptor signaling and approximately 80% to 90% of prostate cancers are dependent on androgens, the ability to detect PSA in serum requires not only its expression within the cells but also its secretion and leakage into the circulation [51]. Only a very small amount of PSA is secreted into perivascular space and then ultimately into the serum [52]. Importantly, PSA is initially produced as an active protease (“free” PSA), and after its release into the perivascular space, it is quickly converted to its inactive forms (“complexed” PSA) [53]. A monoclonal antibody (5A10) recognizing an epitope near the catalytic cleft of PSA has been developed. This antibody only binds to free PSA, which remains associated with the tumor. 89Zr-labeled 5A10 has exhibited excellent tumor uptake in multiple preclinical models of prostate cancer [51]. Importantly, the androgen dependence of the PSA could be visualized with PET. In one study, therapy with increasing amounts of the anti-androgen enzalutamide demonstrated a dose-dependent depression of tumor-associated 89Zr-5A10 in primary tumors as well as bone lesions, while testosterone supplementation resulted in increased binding [51]. This study provided more evidence that therapy-dependent molecular changes of AR-targeted prostate-specific genes - the expression levels of PSA [51] as well as PSMA [29] - can be quantified non-invasively with radiolabeled antibodies and PET. Since such tracers can be translated relatively quickly for clinical use, initial clinical trials are imminent.
Other targets
Several other targets on prostate cancer cells as well as on neovasculature of prostate cancers have been described. Prostate stem cell antigen (PSCA) is a cell surface glycoprotein, which is over-expressed in the majority of prostate cancers and prostate bone metastases. Antibodies against this antigen have been raised [54] for PET imaging, although the uptake in the tumor, at less than 5%, was relatively low. The antigen has also been targeted with an antibody-nanoparticle construct for combined MR imaging and immunotherapy [55] or drug release [56]. Another promising target is bombesin. The gastrin-releasing peptide receptor (GRPR) provides a promising target for staging and monitoring prostate cancer, since it is overexpressed only in prostate cancer and not in normal prostatic tissue. Bombesin is a 14-amino-acid peptide with a high binding affinity and specificity to the GRPR [57]. Radiopharmaceuticals containing bombesin or its analogues, labeled with 99mTechnetium, have been developed to target GRPR-expressing tumors for nuclear imaging, mainly with SPECT. Limited clinical studies have shown promise in detecting primary prostate cancer, nodal and bone metastases [58; 59]. Other labeling methods to obtain agents suitable for PET imaging are currently underway [60]. Fluorescently labeled versions have been evaluated in animal models [61] and could find their way into intraoperative imaging eventually.
Conclusion
Multiple molecular imaging agents for prostate cancer are currently being evaluated – most of them in preclinical settings but a few in clinical trials. In the future, quantitative radiotracers may be used not only to characterize prostate cancer and to stage disease, but also to follow molecular events during drug therapy to gauge therapeutic effectiveness much earlier. Once approved, optical imaging agents could literally lead the surgeon to the cancer and to potential areas of invasion or metastatic lymph nodes. The result may be improved surgical resection and, hopefully, better outcomes. In addition, many of the imaging agents described have been explored for therapeutic approaches as well, either as theranostic nano-agents or for simply delivering a cytotoxic agent to the tumor. The future of molecularly-driven prostate cancer diagnosis and therapy appears promising.
KEY POINTS.
Advanced imaging techniques allow direct visualization of molecular interactions in prostate cancer
MRI/PET, optical and Cerenkov imaging facilitate the translation of molecular biology.
Multiple compounds targeting PSMA expression are currently undergoing clinical translation.
Other targets (eg PSA, Prostate-stem cell antigen, GRPR) are in development
ABBREVIATIONS
- MRI
magnetic resonance imaging
- PET
positron-emission tomography
- SPECT
single-photon emission computed tomography
- PSMA
prostate-specific membrane antigen
- PSA
prostate-specific antigen
- PSCA
Prostate stem cell antigen
- GRPR
gastrin-releasing peptide receptor
- BPH
benign prostatic hyperplasia
REFERENCES
- 1.Elias DR, Thorek DLJ, Chen AK, Czupryna J, Tsourkas A. In vivo imaging of cancer biomarkers using activatable molecular probes. Cancer Biomarkers. 2008;4:287–305. doi: 10.3233/cbm-2008-4602. [DOI] [PubMed] [Google Scholar]
- 2.Thorek DL, Grimm J. Enzymatically activatable diagnostic probes. Curr Pharm Biotechnol. 2012;13:523–536. doi: 10.2174/138920112799436339. [DOI] [PubMed] [Google Scholar]
- 3.Ruggiero A, Holland JP, Lewis JS, Grimm J. Cerenkov luminescence imaging of medical isotopes. J Nucl Med. 2010;51:1123–1130. doi: 10.2967/jnumed.110.076521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;168:9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 5.Azzouni F, Mohler J. Biology of Castration-Recurrent Prostate Cancer. Urologic Clinics of North America. 2012;39:435–452. doi: 10.1016/j.ucl.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larson SM, Morris M, Gunther I, et al. Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med. 2004;45:366–373. [PubMed] [Google Scholar]
- 8.Beattie BJ, Smith-Jones PM, Jhanwar YS, et al. Pharmacokinetic assessment of the uptake of 16beta-18F-fluoro-5alpha-dihydrotestosterone (FDHT) in prostate tumors as measured by PET. J Nucl Med. 2010;51:183–192. doi: 10.2967/jnumed.109.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanzonico PB, Finn R, Pentlow KS, et al. PET-based radiation dosimetry in man of 18F-fluorodihydrotestosterone, a new radiotracer for imaging prostate cancer. J Nucl Med. 2004;45:1966–1971. [PubMed] [Google Scholar]
- 10.Fox JJ, Autran-Blanc E, Morris MJ, et al. Practical Approach for Comparative Analysis of Multilesion Molecular Imaging Using a Semiautomated Program for PET/CT. Journal of Nuclear Medicine. 2011;52:1727–1732. doi: 10.2967/jnumed.111.089326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vargas HA, Wassberg C, Fox JJ, et al. Bone Metastases in Castration-Resistant Prostate Cancer: Associations between Morphologic CT Patterns, Glycolytic Activity, and Androgen Receptor Expression on PET and Overall Survival. Radiology. 2014;271:220–229. doi: 10.1148/radiol.13130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su SL, Huang IP, Fair WR, Powell CT, Heston WDW. Alternatively Spliced Variants of Prostate-Specific Membrane Antigen Rna - Ratio of Expression as a Potential Measurement of Progression. Cancer Research. 1995;55:1441–1443. [PubMed] [Google Scholar]
- 13.Mannweiler S, Amersdorfer P, Trajanoski S, Terrett JA, King D, Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res. 2009;15:167–172. doi: 10.1007/s12253-008-9104-2. [DOI] [PubMed] [Google Scholar]
- 14.Minner S, Wittmer C, Graefen M, et al. High level PSMA expression is associated with early PSA recurrence in surgically treated prostate cancer. Prostate. 2011;71:281–288. doi: 10.1002/pros.21241. [DOI] [PubMed] [Google Scholar]
- 15.Chikkaveeraiah BV, Bhirde A, Malhotra R, Patel V, Gutkind JS, Rusling JF. Single-wall carbon nanotube forest arrays for immunoelectrochemical measurement of four protein biomarkers for prostate cancer. Anal Chem. 2009;81:9129–9134. doi: 10.1021/ac9018022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapidus RG, Tiffany CW, Isaacs JT, Slusher BS. Prostate-specific membrane antigen (PSMA) enzyme activity is elevated in prostate cancer cells. Prostate. 2000;45:350–354. doi: 10.1002/1097-0045(20001201)45:4<350::aid-pros10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Dhara S, Banerjee SR, et al. A low molecular weight PSMA-based fluorescent imaging agent for cancer. Biochem Biophys Res Commun. 2009;390:624–629. doi: 10.1016/j.bbrc.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hillier SM, Maresca KP, Femia FJ, et al. Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res. 2009;69:6932–6940. doi: 10.1158/0008-5472.CAN-09-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haseman MK, Reed NL, Rosenthal SA. Monoclonal antibody imaging of occult prostate cancer in patients with elevated prostate-specific antigen. Positron emission tomography and biopsy correlation. Clin Nucl Med. 1996;21:704–713. doi: 10.1097/00003072-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Babaian RJ, Sayer J, Podoloff DA, Steelhammer LC, Bhadkamkar VA, Gulfo JV. Radioimmunoscintigraphy of pelvic lymph nodes with 111indium-labeled monoclonal antibody CYT-356. J Urol. 1994;152:1952–1955. doi: 10.1016/s0022-5347(17)32277-2. [DOI] [PubMed] [Google Scholar]
- 21.Polascik TJ, Manyak MJ, Haseman MK, et al. Comparison of clinical staging algorithms and 111indium-capromab pendetide immunoscintigraphy in the prediction of lymph node involvement in high risk prostate carcinoma patients. Cancer. 1999;85:1586–1592. [PubMed] [Google Scholar]
- 22.Elgamal AA, Holmes EH, Su SL, et al. Prostate-specific membrane antigen (PSMA): current benefits and future value. Semin Surg Oncol. 2000;18:10–16. doi: 10.1002/(sici)1098-2388(200001/02)18:1<10::aid-ssu3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 23.Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, Lewis JS. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J Nucl Med. 2010;51:1293–1300. doi: 10.2967/jnumed.110.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruggiero A, Holland JP, Hudolin T, et al. Targeting the internal epitope of prostate-specific membrane antigen with 89Zr-7E11 immuno-PET. J Nucl Med. 2011;52:1608–1615. doi: 10.2967/jnumed.111.092098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holland JP, Caldas-Lopes E, Divilov V, et al. Measuring the pharmacodynamic effects of a novel Hsp90 inhibitor on HER2/neu expression in mice using Zr-DFO-trastuzumab. PLoS One. 2010;5:e8859. doi: 10.1371/journal.pone.0008859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H, Moy P, Kim S, et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Research. 1997;57:3629–3634. [PubMed] [Google Scholar]
- 27.Thorek D, Robertson R, Bacchus WA, et al. Cerenkov imaging - a new modality for molecular imaging. Am J Nucl Med Mol Imaging. 2012;2:163–173. [PMC free article] [PubMed] [Google Scholar]
- 28.Wright GL, Jr, Grob BM, Haley C, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–334. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 29.Evans MJ, Smith-Jones PM, Wongvipat J, et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc Natl Acad Sci U S A. 2011;108:9578–9582. doi: 10.1073/pnas.1106383108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barinka C, Rovenska M, Mlcochova P, et al. Structural insight into the pharmacophore pocket of human glutamate carboxypeptidase II. J Med Chem. 2007;50:3267–3273. doi: 10.1021/jm070133w. [DOI] [PubMed] [Google Scholar]
- 31.Mease RC, Dusich CL, Foss CA, et al. N-[N-[(S)-1,3-Dicarboxypropyl]carbamoyl]-4-[18F]fluorobenzyl-L-cysteine, [18F]DCFBC: a new imaging probe for prostate cancer. Clin Cancer Res. 2008;14:3036–3043. doi: 10.1158/1078-0432.CCR-07-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho SY, Gage KL, Mease RC, et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J Nucl Med. 2012;53:1883–1891. doi: 10.2967/jnumed.112.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrett JA, Coleman RE, Goldsmith SJ, et al. First-in-Man Evaluation of 2 High-Affinity PSMA-Avid Small Molecules for Imaging Prostate Cancer. J Nucl Med. 2013;54:380–387. doi: 10.2967/jnumed.112.111203. [DOI] [PubMed] [Google Scholar]
- 34.Chen Z, Penet MF, Nimmagadda S, et al. PSMA-targeted theranostic nanoplex for prostate cancer therapy. ACS Nano. 2012;6:7752–7762. doi: 10.1021/nn301725w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimm J, Scheinberg DA. Will nanotechnology influence targeted cancer therapy? Semin Radiat Oncol. 2011;21:80–87. doi: 10.1016/j.semradonc.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakajima T, Mitsunaga M, Bander NH, Heston WD, Choyke PL, Kobayashi H. Targeted, activatable, in vivo fluorescence imaging of prostate-specific membrane antigen (PSMA) positive tumors using the quenched humanized J591 antibody-indocyanine green (ICG) conjugate. Bioconjug Chem. 2011;22:1700–1705. doi: 10.1021/bc2002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu T, Wu LY, Hopkins MR, Choi JK, Berkman CE. A targeted low molecular weight near-infrared fluorescent probe for prostate cancer. Bioorg Med Chem Lett. 2010;20:7124–7126. doi: 10.1016/j.bmcl.2010.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grimm J, Kirsch DG, Windsor SD, et al. Use of gene expression profiling to direct in vivo molecular imaging of lung cancer. Proc Natl Acad Sci U S A. 2005;102:14404–14409. doi: 10.1073/pnas.0503920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Dam GM, Themelis G, Crane LM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 40.Schaafsma BE, Mieog JS, Hutteman M, et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol. 2011;104:323–332. doi: 10.1002/jso.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaafsma BE, van der Vorst JR, Gaarenstroom KN, et al. Randomized comparison of near-infrared fluorescence lymphatic tracers for sentinel lymph node mapping of cervical cancer. Gynecol Oncol. 2012;127:126–130. doi: 10.1016/j.ygyno.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song KM, Lee S, Ban C. Aptamers and their biological applications. Sensors (Basel) 2012;12:612–631. doi: 10.3390/s120100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lupold SE, Hicke BJ, Lin Y, Coffey DS. Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Research. 2002;62:4029–4033. [PubMed] [Google Scholar]
- 44.Farokhzad OC, Cheng J, Teply BA, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006;103:6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chu TC, Marks JW, 3rd, Lavery LA, et al. Aptamer:toxin conjugates that specifically target prostate tumor cells. Cancer Res. 2006;66:5989–5992. doi: 10.1158/0008-5472.CAN-05-4583. [DOI] [PubMed] [Google Scholar]
- 46.Tong R, Coyle VJ, Tang L, Barger AM, Fan TM, Cheng J. Polylactide nanoparticles containing stably incorporated cyanine dyes for in vitro and in vivo imaging applications. Microsc Res Tech. 2010;73:901–909. doi: 10.1002/jemt.20824. [DOI] [PubMed] [Google Scholar]
- 47.Yu MK, Kim D, Lee IH, So JS, Jeong YY, Jon S. Image-guided prostate cancer therapy using aptamer-functionalized thermally cross-linked superparamagnetic iron oxide nanoparticles. Small. 2011;7:2241–2249. doi: 10.1002/smll.201100472. [DOI] [PubMed] [Google Scholar]
- 48.Rockey WM, Huang L, Kloepping KC, Baumhover NJ, Giangrande PH, Schultz MK. Synthesis and radiolabeling of chelator-RNA aptamer bioconjugates with copper-64 for targeted molecular imaging. Bioorg Med Chem. 2011;19:4080–4090. doi: 10.1016/j.bmc.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 50.Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. 2008;8:268–278. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 51.Ulmert D, Evans MJ, Holland JP, et al. Imaging androgen receptor signaling with a radiotracer targeting free prostate-specific antigen. Cancer Discov. 2012;2:320–327. doi: 10.1158/2159-8290.CD-11-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stege RH, Tribukait B, Carlstrom KAM, Grande M, Pousette AHL. Tissue PSA from fine-needle biopsies of prostatic carcinoma as related to serum PSA, clinical stage, cytological grade, and DNA ploidy. Prostate. 1999;38:183–188. doi: 10.1002/(sici)1097-0045(19990215)38:3<183::aid-pros2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 53.Lilja H. A kallikrein-like serine protease in prostatic fluid cleaves the predominant seminal vesicle protein. J Clin Invest. 1985;76:1899–1903. doi: 10.1172/JCI112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lepin EJ, Leyton JV, Zhou Y, et al. An affinity matured minibody for PET imaging of prostate stem cell antigen (PSCA)-expressing tumors. Eur J Nucl Med Mol Imaging. 2010;37:1529–1538. doi: 10.1007/s00259-010-1433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ren J, Wang F, Wei G, et al. MRl of prostate cancer antigen expression for diagnosis and immunotherapy. PLoS One. 2012;7:e38350. doi: 10.1371/journal.pone.0038350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao X, Luo Y, Wang Y, et al. Prostate stem cell antigen-targeted nanoparticles with dual functional properties: in vivo imaging and cancer chemotherapy. Int J Nanomedicine. 2012;7:4037–4051. doi: 10.2147/IJN.S32804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith CJ. Radiochemical investigations of gastrin-releasing peptide receptor-specific [(99m)Tc(X)(CO)3-Dpr-Ser-Ser-Ser-Gln-Trp-Ala-Val-Gly-His-Leu-Met-(NH2)] in PC-3, tumor-bearing, rodent models: syntheses, radiolabeling, and in vitro/in vivo studies where Dpr = 2,3-diaminopropionic acid and X = H2O or P(CH2OH)3. Cancer research (Baltimore) 2003;63:4082–4088. [PubMed] [Google Scholar]
- 58.De Vincentis G, Remediani S, Varvarigou AD, et al. Role of 99mTc-bombesin scan in diagnosis and staging of prostate cancer. Cancer Biother Radiopharm. 2004;19:81–84. doi: 10.1089/108497804773391711. [DOI] [PubMed] [Google Scholar]
- 59.Scopinaro F, De Vincentis G, Varvarigou AD, et al. 99mTc-bombesin detects prostate cancer and invasion of pelvic lymph nodes. Eur J Nucl Med Mol Imaging. 2003;30:1378–1382. doi: 10.1007/s00259-003-1261-7. [DOI] [PubMed] [Google Scholar]
- 60.Honer M, Mu L, Stellfeld T, et al. 18F-labeled bombesin analog for specific and effective targeting of prostate tumors expressing gastrin-releasing peptide receptors. J Nucl Med. 2011;52:270–278. doi: 10.2967/jnumed.110.081620. [DOI] [PubMed] [Google Scholar]
- 61.Cai QY, Yu P, Besch-Williford C, et al. Near-infrared fluorescence imaging of gastrin releasing peptide receptor targeting in prostate cancer lymph node metastases. Prostate. 2012 doi: 10.1002/pros.22630. 10.1002/pros.22630:n-a-n/a. [DOI] [PubMed] [Google Scholar]