Abstract
New derivatives based upon the tetrahydro-β-carboline-hydantoin and tetrahydro-β-carboline-piperazinedione scaffolds were synthesized. All compounds were evaluated for their ability to inhibit PDE5 in vitro, and numerous compounds with IC50 values in the low nanomolar range were identified including compounds derived from L-tryptophan. Compounds with high potency versus PDE5 were then evaluated for inhibitory activity against other PDEs to assess isozyme selectivity. Compound 5R,11aS-5-(3,4-dichlorophenyl)-2-ethyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)dione 14 showed a selectivity index of >200 for cGMP hydrolysis by PDE5 versus PDE11. Meanwhile, 6R,12aR-6-(2,4-dichlorophenyl)-2-ethyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4dione 45 demonstrated strong potency for inhibition of PDE11 with an IC50 value of 11 nM, representing the most potent PDE11 inhibitor thus far reported. Docking experiments differentiated between active and inactive analogues and revealing the conformational, steric, and lipophilic necessities for potent PDE5 inhibition. Many derivatives, including potent PDE5 inhibitors, were able to inhibit the growth of the MDA-MB-231 breast tumor cell line with low micromolar potency.
Introduction
Cyclic adenosine monophosphate (cAMPa) and cyclic guanosine monophosphate (cGMP) are important second messengers, responsible for transducing various extracellular signals to the intracellular milieu. The intracellular levels of cyclic nucleotides are controlled by the activities of cyclases, which catalyze the formation of cyclic nucleotides from their nucleotide triphosphate precursors and phosphodiesterases (PDEs), which hydrolyze the cyclic nucleotide bond to terminate the signal.1 On the basis of sequence homology and regulatory properties, the PDEs can be classified into 11 distinct isozyme families. Often, there is more than one gene product within each family and each gene generates multiple splice variants.2 PDE5 is a cGMP specific PDE that is expressed in vascular smooth muscle and lung platelets, although recent reports demonstrate that it is also overexpressed in various carcinomas including breast, colon, lung, and bladder cancers. PDE5 has received considerable attention over the years for having been successfully developed as a therapeutic target for erectile dysfunction. PE5 inhibitors may also have benefits for pulmonary hypertension, cardioprotection, and enhancing cognitive function.3–5
Recent studies have demonstrated that PDE5 may serve as a novel target for the cancer chemopreventive drug, sulindac.6 Moreover, certain tadalafil related analogues have shown tumor cell growth inhibitory properties versus colorectal HT-29 cancer cell line that appear to be associated with their ability to inhibit PDE5.7 Other reports have revealed anticancer activities to various tetrahydro-β-carbolines through different mechanisms, including inhibition of topoisomerase II,8 cyclin dependent kinases,9 and mitotic kinesin Eg5.10 These observations suggest that tadalafil can serve as a good starting point for the discovery of novel anticancer compounds. However, traditional PDE5 inhibitors are unable to induce cGMP signaling in tumor cells and thus lack anticancer activity.
Three PDE5 inhibitors, tadalafil (Figure 1), sildenafil, and vardenafil, have received Food and Drug Administration (FDA) approval for the treatment of erectile dysfunction and pulmonary hypertension. Although these drugs share a common mechanism of action, they have different pharmacokinetic profiles, PDE isozyme selectivities, and interaction mechanism with PDE5.11–13 For example, tadalafil has a long half-life of 17.5 h and can also inhibit PDE11. The interaction of tadalafil with PDE5 involves hydrogen bonding with the conserved Gln817, which is essential for nucleotide recognition and selectivity, and π–π stacking with Phe820. Unlike sildenafil and vardenafil, the rigid chemical structure of tadalafil, which has only one rotatable bond, contributes to its binding affinity because entropy is lost upon protein binding. Additionally, tadalafil has no direct or water mediated interaction with the metal ions that are present in the binding pocket of PDE5.14–16
Figure 1.
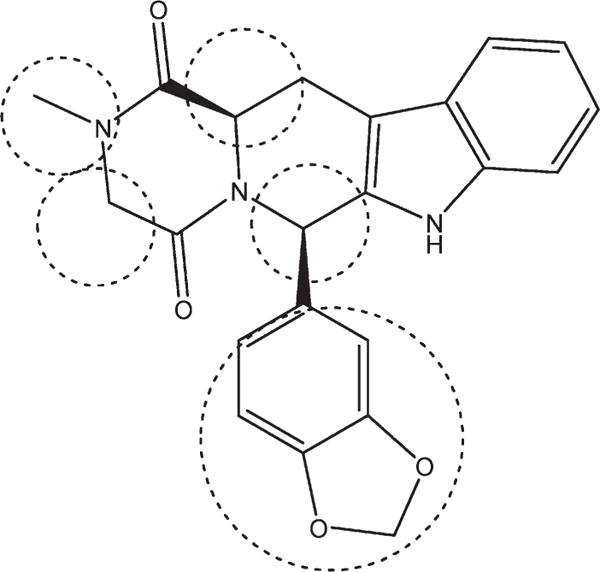
Chemical structure of tadalafil with the points of modifications encircled.
Chemically, tadalafil (Figure 1) is a tetrahydro-β-carboline-piperazinedione with two chiral carbons that are both of the R configuration, a methyl substituent on the piperazinedione nitrogen, and a pendant 1,3-benzodioxol substituent attached to C-6.
To expand the scope of structure–activity relationships with this class of compounds, herein we report the discovery of novel tadalafil analogues with the tetrahydro-β-carboline-imidazoline and the tetrahydro-β-carboline-piperazinedione scaffolds; the chiral carbons are of the R,R, R,S, S,R, or S,S configuration, the terminal ring nitrogen substituent is methyl, ethyl, butyl, or t-butyl, and the pendant aryl is 2,4-dichlorophenyl, 3,4-dichlorophenyl, or 2,6-dichlorophenyl.
To evaluate the biochemical and biological relevance of these derivatives, they were assayed for their ability to inhibit the recombinant human PDE5 enzyme and to inhibit tumor cell growth using the human MDA-MB-231 breast tumor cell line.
Chemistry
The syntheses of the 1,3-disubstituted tetrahydro-β-carbolines (Scheme 1), the tetrahydro-β-carboline-hydantoins (Scheme 2), and the tetrahydro-β-carboline-piperazinediones (Scheme 3) are shown. Both D- and L-tryptophan methyl ester were synthesized by a general synthetic procedure for amino acid esters.17 The respective ester was subjected to Pictet–Spengler reaction with 2,4-dichlorobenzaldehyde, 3,4-dichlorobenzadehyde, and 2,6-dichlorobenzaldehyde. The reaction of the ester with 2,4-dichlorobenzaldehyde and 3,4-dichlorobenzadehyde gave both the cis and trans isomers as expected. Because the cis and trans isomers are diastereomers, they were separated by column chromatography using CH2Cl2 as an eluent. The reaction of the ester with the 2,6-dichlorobenzaldehyde yielded exclusively the cis isomer. Previously reported data indicated that the trans isomer is the thermodynamically favored isomer while the cis-isomer is kinetically favored.18–21 A bulky tryptophan ester substituent, particularly benzyl, favors the formation of the corresponding trans isomer. Conversely, an allyl ester favors the formation of the cis isomer upon reaction with an aldehyde.18–21 We found that an aromatic aldehyde with bulky substituents at positions 2 and 6 of the benzene ring favors the formation of the cis isomer upon reaction with tryptophan methyl ester, probably due to restricted rotation. The Pictet–Spengler reaction was carried out at room temperature under nonstereospecific conditions. As expected, the kinetics were in favor of the cis isomer.18–21 Thus, the formation of diastereomers 3, 5, and 7 exceeded the formation of diastereomers 4, 6, and 8, respectively, with % de = 11%, 27%, and 25%, respectively, meanwhile, for the isomers 1 and 9, there was no preferentiality relative to 2 and 10.
Scheme 1. D or L-Tryptophan, D- or L-Tryptophan Methyl Ester, and 1,3-Disubstituted Tetrahydro-β-arbolinea.
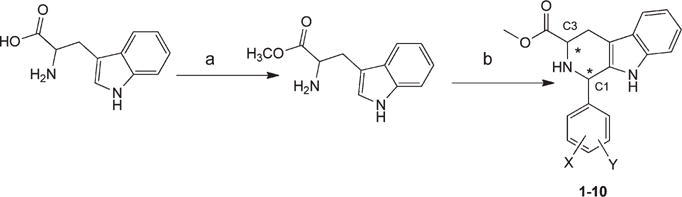
aConditions: (a) CH3COCl/methanol/reflux/6 h, basify and extract with CH2Cl2; (b) 2,4-dichlorobenzaldehyde, 3,4-dichlorobenzadehyde, or 2,6-dichlorobenzaldehyde/TDA/4 days/RT. For exact indivividual assignment, see Table 1. Stereocenters are labeled.
Scheme 2.
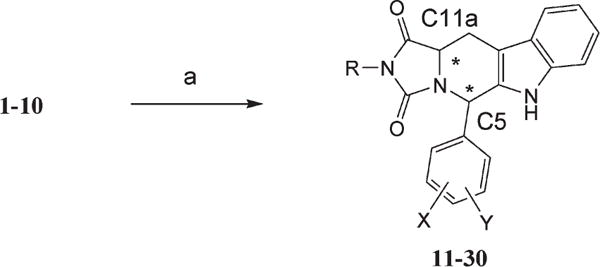
aConditions: (a) ethyl or t-butylisocyanate/2-butanone/reflux; R = –C2H5 or –C–(CH3)3, X,Y = 2,4-dichloro, 3,4-dichloro, or 2,6-dichloro. For exact individual assignment, see Table 2. Stereocenters are labeled.
Scheme 3.
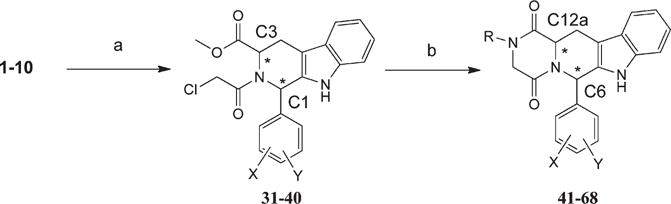
Conditions: (a) Cl-CO–CH2-Cl/chloroform/NaHCO3/RT/1 h; (b) methyl, ethyl, butyl or t-butyl amine/methanol/reflux/16hrs. For exact individual assignment, see Tables 3 and 4. Stereocenters are labeled
Reaction of the respective pure cis- or trans-isomers of 1,3-disubstituted tetrahydro-β-carboline (1–10) with commercially available ethyl- and t-butyl isocyanate produced the corresponding cis- and trans-hydantoins (11–30).
Treatment of (1–10) with chloroacetyl chloride provided the corresponding chloroacetyl derivative (31–40). The β-carboline-piperazinedione derivatives with the respective N-alkyl substituents were obtained by reaction of the respective chloroethanone derivative with different primary amines, including methylamine, ethylamine, butylamine, and t-butylamine in refluxing methanol (41–68).
The assignment of cis/trans-stereochemistry for the tetrahydro-β-carbolines (1–10) was based on a detailed study of 13C NMR spectroscopic data well established in previous literature.22,23 The 13C NMR signals for C-1 and C-3 are more shielded in the trans-isomer compared to cis-isomer with Δδ 3–4 ppm, which may be due to 1,3-diaxial spatial interactions present in the trans-isomer. The tetrahydropyridine ring exists in half-chair conformation where the ester at C-3 is equatorially located. No analogous difference was detected with C-3 in compounds (1–10). Moreover, a 500 MHz 2D NOESY experiment that was carried out for cis-1 and trans-2 showed a clear correlation between the C-1 and C3-hydrogens as expected for a cis-relationship with a coupling constant of 4.1 Hz (Supporting Information).
Moreover, a correlation exists between the Rf value on TLC and the stereochemistry of the 1,3-disubstituted tetrahydro-β-carboline (1–10) and their hydantoin analogues (11–30) where the cis-isomer is systematically less polar than the trans-isomer for the tetrahydro-β-carboline. However, in the hydantoin series, the polarity is reversed, thus the cis-isomer becomes more polar than the trans-isomer. For example, the Rf values of the 1R,3R, 1S,3R, 1S,3S, and 1R,3S isomers of the (1–4) and their corresponding N-ethylhydantoin analogues (11–14) using CH2Cl2 as the mobile phase were 0.19, 0.12, 0.19, and 0.12 and 0.16, 0.28, 0.16, and 0.28, respectively.
The 1H NMR signals for the proton at C-1 of derivatives 1–10 appeared at δ 5.2–6.2. Cyclization to the corresponding hydantoin (11–30) or piperazinedione derivatives (41–68) causes a large downfield shift to δ 5.8–7.0; this is mostly due to the electron withdrawing effect of the carbonyl in the neighboring ring.
Mass spectrometry of all derivatives showed molecular ion peaks at M+, M+ + 2, and M+ + 4 due to the isotopic nature and number of chlorine atoms in the molecule. Most of the 1,3-disubstituted-tetrahydro-β-carboline derivatives derivatives (1–10) showed the base peaks as molecular ion peaks, indicating their stable nature.
The infrared spectra of (1–10) showed bands at a stretching frequency around 3400 cm−1 for the N–H stretching and a band around 1730 cm−1 for the ester carbonyl stretching. On the other hand, the β-carboline-hydantoin derivatives (11–30) showed two carbonyl stretching bands because the hydantoin carbonyls are not equivalent as one of the carbonyls is flanked between two nitrogen atoms and the other between carbon and nitrogen.
The stretching vibrations of the two carbonyls of the piperazinedione derivatives (41–68) were generally at lower wave numbers relative to their hydantoin analogues. This may be due to the higher ring strain of the five-membered ring relative to the six-membered ring, producing higher double bond characteristics of the carbonyls in the hydantoin series.
The optical rotations of derivatives (5–8) showed that they are optically active, with those derived from D-tryptophan dextrorotatory and those derived from L-tryptophan levorotatory. The optical rotation angle of a 0.1% ethanolic solution of derivative 5 relative to 7 and compound 6 relative to 8 showed equivalent values but opposite signs, indicating their enantiomeric nature.
Results and Discussion
All of the final compounds and intermediates synthesized were screened for in vitro tumor cell growth inhibition activity using the human MDA-MB-231 breast tumor cell line and for inhibition of recombinant human PDE5 at a single concentration of 10 μM. For compounds showing >60% inhibition, the IC50 was determined by testing a range of eight concentrations with quadruple replicates per concentration, tadalafil used as a positive control. The results are shown in Tables 1–5.
Table 1.
Chemical Structure, Growth Inhibition of MDA-MB-231 Breast Tumor Cell Line, and PDE5 Inhibitory Activity of 1,3-Disubstituted-tetrahydro-β-carbolines (1–10)
| compd | stereochemistry | X,Y | % growth inhibition at 10 μMa | growth inhibition IC50 (μM) and CI at p < 0.05b | % PDE 5 inhibition at 10 μMa | PDE 5 inhibition IC50 (μM) and CI at p < 0.05b |
|---|---|---|---|---|---|---|
| 1 | 1R,3R | 2,4-dichloro | 29 | ND | 65 | 7.2 (5.51–9.01) |
| 2 | 1S,3R | 2,4-dichloro | 78 | 6.73 (2.21–12.5) | 40 | ND |
| 3 | 1S,3S | 2,4-dichloro | 50 | ND | 55 | ND |
| 4 | 1R,3S | 2,4-dichloro | 28 | ND | 82 | 0.57 (0.55–0.77) |
| 5 | 1R,3R | 3,4-dichloro | 43 | ND | 78 | 1.61 (1.30–1.98) |
| 6 | 1S,3R | 3,4-dichloro | 100 | 4.62 (4.09–5.23) | 50 | ND |
| 7 | 1S,3S | 3,4-dichloro | 62 | 6.98 (5.93–8.22) | 18 | ND |
| 8 | 1R,3S | 3,4-dichloro | 48 | ND | 24 | ND |
| 9 | 1R,3R | 2,6-dichloro | 25 | ND | 25 | ND |
| 10 | 1S,3S | 2,6-dichloro | 52 | ND | 15 | ND |
Calculated from duplicate values.
Calculated from at least 8 concentrations, each with quadruple replicates, p < 0.05.
Table 5.
PDE Isozyme Selectivity of the Most Active Tetrahydro-β-carboline-hydantoins and Piperazinedione Derivatives
| compd | PDE5A inhibition IC50 (μM, cGMP)b and CI at p < 0.05 | PDE3B inhibition IC50 (μM, cAMP)b | PDE3B inhibition IC50 (μM, cGMP)b and CI at p < 0.05 | PDE4B inhibition IC50 (μM, cAMP)b and CI at p < 0.05 | PDE11A inhibition IC (μM, cAMP)b and CI50 at p < 0.05 | PDE11A inhibition IC50, (μM, (cGMP)b and CI at p < 0.05 | selectivity indexa |
|---|---|---|---|---|---|---|---|
| 11 | 0.010 (0.007–0.013) | >50 | >50 | 34 (24.8–47.1) | 2.00 (1.4–2.90) | 0.31 (0.25–0.38) | 31.0 |
| 14 | 0.007 (0.005–0.011) | >50 | >50 | 3.60 (2.80–4.60) | 13.00 (10.9–15.6) | 1.50 (1.28–1.80) | 208.3 |
| 45 | 0.002 (0.0009–0.006) | >50 | >50 | >50 | 0.104 (0.09–0.14) | 0.011 (0.008–0.015) | 5.50 |
| tadalafil | 0.003 (0.0007–0.005) | >50 | >50 | >50 | 0.30 (0.23–0.38) | 0.05 (0.044–0.055) | 16.60 |
IC50 of PDE11A (cGMP)/IC50 of PDE5A (cGMP).
Calculated from at least 8 concentrations, each with quadruple replicates, p < 0.05.
For the 1,3-disubstituted tetrahydro-β-carbolines (1–10), most of the derivatives were inactive against PDE5 at 10 μM, with only 3 compounds (1, 4, and 5) showing activity below the screening dose. Two of the active compounds have a 2,4-dichlorophenyl substituent while one has a 3,4-dichlorophenyl substituent. Conversely, all derivatives with the 2,6-dichlorophenyl substituent (9 and 10) were inactive. This indicates that PDE5 inhibitory activity is dependent on the positional isomerization state of the compounds.
For the hydantoins (11–30), only derivatives with 2,4-dichloro or 3,4-dichloro substitution at C-5 were able to inhibit PDE5. Again, all derivatives with the 2,6-dichloro substitution were inactive, confirming the importance of positional isomers for PDE5 inhibitory activity. The most active compounds were either 5R,11aS or 5R,11aR configuration. Additionally, all compounds with the S configuration at the C-5 were inactive or less active than their R congeners, indicating that the stereochemical configuration of the carbon derived from the aldehyde and not the stereochemical aspects of the carbon derived from the tryptophan amino acid is an important determinant of PDE5 inhibitory activity. This observation expands the chemical space from which potent PDE5 inhibitors may be discovered and presents an important economic advantage, as the natural L-tryptophan is much more widely available and cheaper than the non-natural D-isomer. Additionally, the compounds derived from L-tryptophan were more active than those derived from D-tryptophan (e.g., 14 > 11, 24 > 21 and 28 > 25), further supporting these conclusions.
The nature of the alkyl side chain group on the hydantoin ring does not significantly influence the potency. It seems that a wide range of substituents on the hydantoin nitrogen appear to be tolerated without affecting PDE5 inhibitory activity.
Most of the 1,2,3-trisubstituted tetrahydro-β-carboline derivatives 31–40 were inactive as PDE5 inhibitors. However, three compounds (31, 33, and 40) did show moderate activity with IC50 values of 0.38–2.3 μM. Compounds 33 and 40 were of the S,S configuration and compound 40 was the only derivative with the pendant 2,6-dichlorophenyl that showed PDE5 inhibitory activity. This demonstrates that these derivatives may be following a different trend, which could be due to their being alkyl halides and, therefore, having an alternate means of interacting with PDE5.
We then expanded the hydantoin ring to a six-membered piperazinedione system. Again, PDE5 inhibitory activity was limited to derivatives with pendant 2,4-dichlorophenyl or 3,4-dichlorophenyl, whereas those derivatives with the 2,6-dichlorophenyl substituent were inactive, confirming that the potency for PDE5 inhibition is related to the nature of the positional isomers. Furthermore, these observations indicate that those derivatives with the 2,6-dichlorophenyl moiety may be sterically locked in a conformation that prevents binding to PDE5.
The 6R,12aR or 6R,12aS rather than the 6S,12aR or 6S,12aS were more potent for PDE5 inhibition, confirming that PDE5 inhibition is predominantly determined by the stereochemical state of C-6 rather than the chiral carbon derived from the amino acid. In the piperazinediones, we introduced four substituents on the nitrogen of the piperazinedione ring with 2,4-dichlorophenyl, viz methyl, ethyl, butyl, and tert-butyl. The order of PDE inhibitory activity was ethyl > methyl > tert-butyl > butyl as shown by compounds 41 and 44 versus 45 and 48 versus 59 and 62 versus 55 and 58, respectively. This indicates that there is an optimum size for the N substitution and steric parameters are of importance in this regard.
Compounds 11, 14, and 45 were the most potent of these derivatives and showed comparable potency to tadalafil for PDE5 inhibition. Therefore, we determined their isozyme selectivity by comparing their potency for PDE5 inhibition to their potency for inhibition of PDE3, PDE4, and PDE11. As summarized in Table 5, all three compounds as well as the reference compound tadalafil lacked the ability to inhibit either cAMP or cGMP hydrolysis by PDE3B at concentrations up to 50 μM. Interestingly, compounds 11 and 14 showed mild to moderate inhibition of the cAMP selective PDE4B despite tadalafil having little effect on the activity of this PDE isoform. The effects of the compounds on cAMP and cGMP hydrolysis by PDE11A showed that the piperazinedione derivative 45 and tadalafil were the most efficacious and potent for PDE11 inhibition. Furthermore, compound 45 represents the most potent PDE11 inhibitor reported to date with an IC50 value of 11 nM. The hydantoin derivatives 11 and 14 were more selective for inhibition of cGMP hydrolysis by PDE5 compared to inhibition of cGMP hydrolysis by PDE11A with selectivity indices of 31 and 208, respectively. Conversely, tadalafil possessed a selectivity index of just 16.6 for PDE5 versus PDE11, suggesting that compounds 11 and 14 possess a lower potential for side effects due to their reduced effect upon PDE11. Additionally, these studies demonstrate that the PDE5/PDE11 selectivity of these compounds is primarily influenced by the size and interactions of the tetracycle terminal ring and not the pendant aryl.
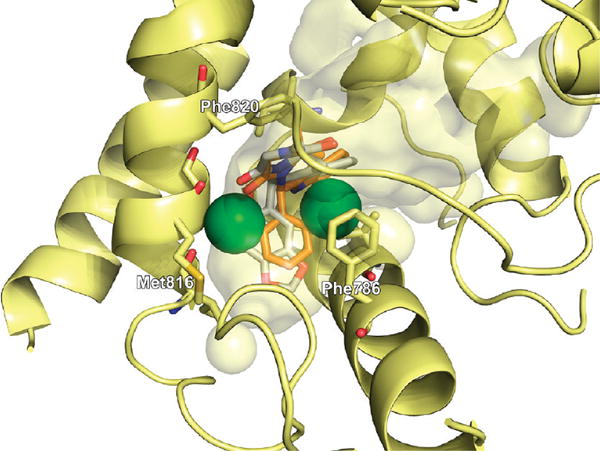
We performed in silico docking studies in order to elucidate the structural basis for the binding of the compounds. Tadalafil has been cocrystallized with human PDE5 (PDB 1UDU) at a low resolution of 2.8 Ǻ, while sildenafil has been cocrystallized with human PDE5 (PDB 2H42) at a better resolution of 2.3 Å. Analysis of the crystal structures showed that some residues in close proximity to the binding site are not resolved in 1UDU. Superimposing the two crystal structures highlights the difference between 1UDU and 2H42: following Tyr664, which is a part of the flexible loop, Ile665 is present in 2H42 but not in 1UDU. From this structural comparison, it seems reasonable to assume that this isoleucine residue could form substantial hydrophobic interactions with the N-alkyl substituent of tadalafil or its analogues as shown in Figure 2.
Figure 2.
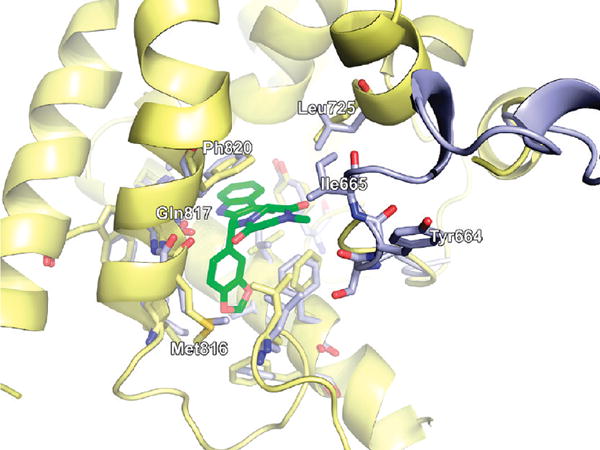
Side view of the crystal structure 1UDU with 2.83 Å resolution (yellow) containing a tadalafil molecule (green) in the active side. Another PDE5 structure from the PDB with a higher resolution of 2.30 Å that was cocrystallized with sildenafil, 2H42 (light blue), is superposed onto 1UDU to account for unresolved amino acids in the flexible loop region of 1UDU.
Compound 11, which was found to inhibit PDE5 with low nanomolar affinity, docked into the PDE5 active site in 1UDU in a very similar manner to that of tadalafil as shown in Figure 3. However, the effects of the hydrophobic side chains at the five-membered ring can not be reproduced due to the missing hydrophobic loop region containing Ile665 (Figure 4). Compound 11 has the same stereo configuration as tadalafil at the carbon where the dichlorobenzene ring is attached, leading to a rather close match of the docked pose with the central scaffold of tadalafil. The 4-chloro substituent is buried deeply within the central hydrophobic cavity. The geometry of the hydrogen bond with Gln817 as well as the π-stacking interaction with Phe820, as observed in tadalafil, is also conserved. The interaction with Gln817 is believed to restrict the rotational freedom of the side chain of Gln817 which is critical for cGMP binding.24
Figure 3.
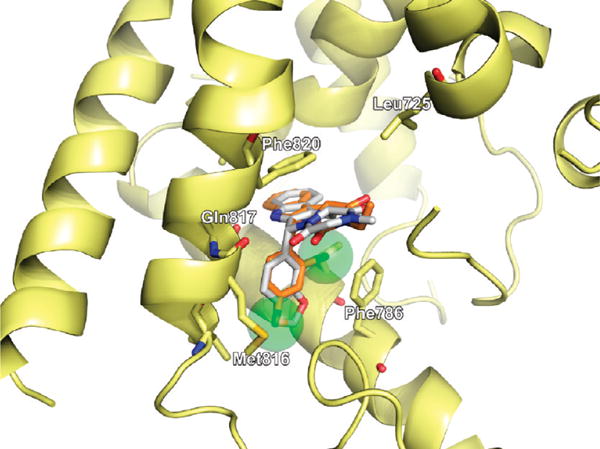
Docked pose of compound 11 (orange) in the cavity of 1UDU superimposed on tadalafil, white, featuring van der Waals spheres on the chlorine atoms illustrating the steric requirements of 11.
Figure 4.
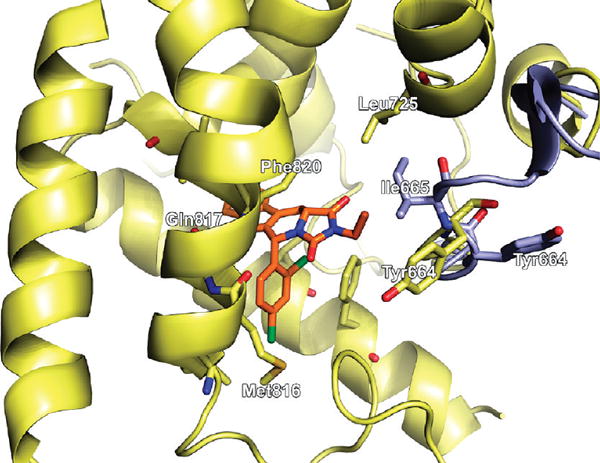
Docked pose of compound 11 (orange) in the cavity of 1UDU (yellow). The flexible loop from 2H42 containing Ile665 is superimposed for comparison (light blue).
Compound 12 differs from 11 only in one stereocenter. The inversion of the 5R center to 5S induces a 180° flip of the central scaffold. This has consequences both on the proposed binding mode (i.e., the docking pose possessing the optimal consensus score) and on the measured affinity. The hydrogen bond between Gln817 and the NH-group of the indole moiety present in both tadalafil and all potent novel compounds of R configuration is lost. Additionally, the added carbonyl group clashes with the carbonyl of Gln817 (red spheres), leading to significantly reduced binding affinity. However, the π–π stacking interaction with Phe820 is conserved as shown in Figure 5.
Figure 5.
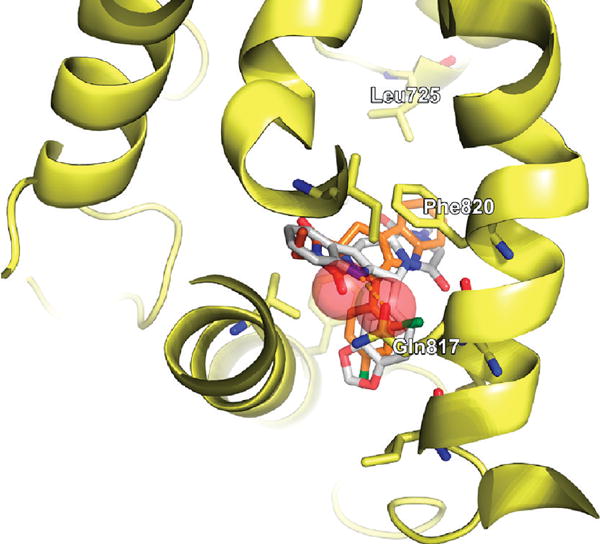
Docked pose of compound 12, orange, superimposed on tadalafil, white.
The biological data revealed the 2,6-dichloro analogues to be substantially impaired PDE5 inhibitors. The docked pose of compound 53 demonstrates that the steric requirements of the compound (chloro substituents shown as green spheres) exceed the size of the binding pocket (shown as a transparent surface). These steric hindrances likely reduce binding affinity. Additionally, the missing 4-chloro substituent in 53 leaves the deep hydrophobic cavity unaddressed, as shown in Figure 6.
Figure 6.
Effects of 2,6-dichloro substitution at the aromatic ring system exemplified by the docked pose of 53 (orange) superimposed on tadalafil, white.
Because of previous reports that PDE5 may serve as a novel target for the discovery of new anticancer drugs, the tumor cell growth inhibitory activity of these derivatives was measured versus the human MDA-MB-231 breast tumor cell line, a cell line that has been shown to rely on PDE5 for growth and survival.6 Among the 1,3-disubstituted tetrahydro-β-carbolines 1–10, only compounds 2, 6, and 7 showed IC50 values of <10 μM. The three compounds share the 1S chiral carbon, and they all possess 2,4-dichlorophenyl or 3,4-dichlorophenyl pendant aryls. This stereochemistry preference is opposite to that observed with PDE5 inhibition, whereas the pendant aryl positional isomer preference is the same.
Only seven compounds of the hydantoin derivatives 11–30 showed growth inhibitory activity, with IC50 values of 0.64–5.37 μM. Again, all the active compounds had either 2,4-dichlorophenyl or 3,4-dichlorophenyl substitution but none of them are 2,6-dichlorophenyl derivatives. This supports the dependence of the growth inhibitory activity on the substitution patterns at the aromatic ring. Conversely, the compounds were of variable stereochemistry at the chiral carbons, indicating insensitivity to chirality issues within this series of compounds. Interestingly, all growth inhibitory compounds in this series were also PDE5 inhibitors, although potency for PDE5 inhibition did not correlate with growth inhibition and may reflect additional factors when comparing activities within cells compared with effect observed in isolated enzymes.
For the chloroethanone derivatives 31–40, only three compounds showed appreciable growth inhibitory effect, namely 31, 32, and 34. Compound 32 differs from 31 by one stereocenter, and its growth inhibitory potency is almost 10 times that of 31. This indicates that the potency of these compounds for growth inhibition is related to the stereochemistry of the C-1 derived from the aldehyde. Compound 32 was the most active among all of the compounds presented here for growth inhibition with a submicromolar IC50 value. Again, none of the active compounds were of the 2,6-dichlorophenyl derivatives. In addition to PDE5 inhibitors, this series of compounds were alkylating agents and this may contribute to their activity.
For the piperazinedione derivatives 41–68, seven compounds showed growth inhibitory activity, with IC50 values in the range of 1.2–7.1 μM. As with PDE5 inhibition, none of the active growth inhibitors were of 2,6-dichlorphenyl substituent, confirming that such an isomer is deleterious to both PDE5 and growth inhibitory activity. Additionally, all active compounds were of the S configuration at the C-6 position, indicating stereochemical preferentiality for this series of compounds. The active derivatives had different substituents on the terminal piperazinedione N, demonstrating that a wide variety of substituents can be tolerated at this residue. Finally, all of the active derivatives demonstrated appreciable potency for PDE5 inhibition. Together, these data suggest that modifications to the terminal ring of the tetracycle in tetrahydro-β-carboline-hydantoin and tetrahydro-β-carboline-piperazinedione skeletons improves the PDE5 selectivity and tumor cell growth inhibitory properties of this group of compounds.
Conclusions
The present structure–activity relationship study revealed the following first-time essential findings for interaction between tetrahydro-β-carboline-hydantoins and tetrahydro-β-carboline-piperazinedione derivatives and PDE5 including: the interaction between the alkyl side chain on the N of the terminal ring and Ile665 and hydrophobic interaction of the pendant aryl with Met816, this being in addition to the known H-bonding of the indole NH with Gln817 and π–π stacking between the indole ring and the phenyl of Phe820. The stereochemistry of the chiral carbon derived from the aldehyde, not the one derived from the amino acid, is the most crucial feature for PDE5 inhibition; this expands the chemical space from which novel PDE5 inhibitors might be explored. The size and lipophilicity of the aryl attached to the stereo-carbon derived from the aldehyde modulates activity as well. The cross interaction with PDE11 can be modulated by the nature of the terminal ring; this may evoke new studies to improve PDE5/PDE11 selectivity or to bend toward one of them. Evaluation of the new 68 derivatives in a cancer cell line screening showed that the antiproliferative activity is not necessarily parallel to the PDE5 inhibition; however, the latter may be among the contributing factors to the antiproliferative activity and thus it is important to strike the right balance between selectivity/nonselectivity of cGMP-PDEs inhibition. The new emerging indications for PDE5 inhibitors confirm the need to the discovery of more inhibitors of PDE5 for novel indications.
Experimental Section
Chemistry
Mass spectra were measured using a Jeol MStation, double-focusing magnetic sector (MS 700). Melting points were determined on a Buchi melting point apparatus and are uncorrected. IR spectra were recorded on Perkin–Elmer Paragon 1000 using KBr–KCl plates. 1H NMR and 13C NMR spectra were recorded on Jeol Eclipse, 400 and 500 MHz device using CDCl3 as a solvent and TMS as a standard, chemical shifts (δ) were reported in parts per million (ppm) downfield from TMS, and J is expressed in Hz. The following abbreviations are used for multiplicity of NMR signals: s = singlet, d = doublet, q = quartet, m = multiplet, dd = doublet of doublet, br = broad. Elemental analyses were performed by the Microanalytical Unit, Faculty of Science, Cairo University; found values were within ±0.4% of the theoretical ones unless otherwise indicated. Column chromatography was performed using silica-gel 70–230 mesh. Reaction progress was monitored by TLC, performed on precoated silica gel plates (ALUGRAM SIL G/UV254), and detection of the components was made by short UV light. All reactions were carried out under nitrogen or argon. All chemicals and solvents were obtained from Sigma-Aldrich and were used without further purification. All the described compounds had >95% purity as determined by GC/MS and the areas under the peaks.
Procedure for Preparation of D- and L-Tryptophan Methyl Ester.13
A 250 mL round-bottomed flask containing methanol (100 mL) was cooled in an ice–water bath. Acetyl chloride are (8 mL) was then added dropwise to the flask over a period of 8 min using a graduated dropping funnel while the flask was still in the ice bath. The solution was stirred for 5 min before adding D or L-tryptophan (15 g, 68.77 mmol) in one portion. The solution was then heated to reflux and left for 5 h. It was then left to cool to room temperature, and the solvent was removed under reduced pressure to give tryptophan methyl ester hydrochloride. The free base was obtained by adding dilute NH4OH and extracting the base using CH2Cl2 (5 × 50 mL). The organic layer was dried over anhydrous Na2SO4 and put under reduced pressure to give a yellowish solid. This solid was then used without further purification.
General Procedures for the Preparation of Methyl 1-(Aryl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate
The appropriate tryptophan methyl ester (15.52 g, 60.93 mmol) was added to the appropriate dichlorobenzaldehyde (11.72 g, 66.95 mmol) and dissolved in CH2Cl2 (100 mL). The solution was cooled to 0 °C in an ice bath. Trifluoroacetic acid (3 mL) was then added to the solution dropwise while still in the ice bath, and the mixture was left to stir for 4 days at room temperature and under N2 atmosphere. The reaction mixture was made basic by adding dilute NH4OH solution and then extracted with CH2Cl2 (3 × 100 mL). The organic layer was washed with water and brine and dried over anhydrous Na2SO4. It was then filtered and evaporated under reduced pressure.
The residue was purified and the isomers were separated by column chromatography on silica gel eluting with CH2Cl2 to give the respective cis-isomer first followed by the trans-isomer.
(1R,3R)Methyl-1-(2,4-dichlorophenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (1)
Yield 35%; yellow powder; mp 88–92 °C; Rf = 0.19 (CH2Cl2). IR (cm−1): 3406 (–NH–), 1734 (–CO–). 1H NMR: δ 7.48 (s, 1H, NH), 7.51 (d, 1H, J = 7.0 Hz, Ar), 7.35 (d, 1H, J = 7.0 Hz, Ar), 7.26–7.15 (m, 4H, Ar + NH), 7.04–6.93 (m, 2H, Ar), 5.82 (s, 1H, CHPh), 4.03–4.00 (m, 1H, CHCOOCH3), 3.83 (s, 3H, OCH3), 3.25 (dd, 1H, J = 4.3/7.0 Hz, CHaHb), 3.23–3.04 (m, 1H, CHaHb). 13C NMR: δ 172.82, 136.16, 135.01, 134.11, 132.10, 131.23, 130.02, 128.06, 126.81, 123.40, 122.26, 119.83, 118.28, 110.97, 109.33, 56.55 (C1), 54.53, 52.40 (C3), 25.28. MS: m/z 378 (M+ + 4), m/z 376 (M+ +2), m/z 374 (M+), m/z 217 (100%). Elemental analysis: calculated for (C19H16Cl2N2O2) C, H, N.
(1S,3R)Methyl-1-(2,4-Dichlorophenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (2)
Yield 34%; yellow powder; mp 130–133 °C. Rf = 0.12 (CH2Cl2). IR (cm−1): 3388 (–NH–), 1735 (–CO–). 1H NMR: δ7.64(s, 1H, NH), 7.56(d, 1H, J = 7.0 Hz, Ar), 7.47 (s, 1H, NH), 7.00 (d, 1H, J = 7.0 Hz, Ar), 7.27–7.12 (m, 5H, Ar), 5.97 (s, 1H, CHPh), 3.92–3.88 (m, 1H, CHCOO–CH3), 3.74 (s, 3H, OCH3), 3.30 (dd, 1H, J = 4.6/7.6 Hz, CHaHb), 3.17–3.15 (m, 1H, CHaHb). 13C NMR: δ 173.09, 137.15, 136.29, 134.73, 134.51, 131.16, 130.83, 129.78, 127.27, 126.63, 122.48, 119.82, 118.38, 111.08, 109.43, 52.40(C1), 52.27, 51.15(C3), 24.39. MS: m/z 378 (M+ + 4), m/z 376 (M+ + 2), m/z 374 (M+, 100%). Elemental analysis: calculated for (C19H16Cl2N2O2) C, H, N.
(1S,3S)Methyl-1-(2,4-Dichlorophenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (3)
Yield 45%; white powder; mp 90–92 °C; Rf = 0.19 (CH2Cl2). IR (cm−1): 3433 (–NH–), 1728 (–CO–). 1H NMR: δ 7.90 (s, 1H, NH), 7.54–7.43 (m, 2H, Ar), 7.38 (d, 1H, J = 7.2 Hz, Ar), 7.26–7.10 (m, 5H, Ar NH), 5.8 (s, 1H, CHPh), 3.90–3.87 (m, 1H, CHCOOCH3),+3.82 (s, 3H, OCH3), 3.25 (dd, 1H, J = 4.3/7.6 Hz, CHaHb), 3.01–2.90 (m, 1H, CHaHb). 13C NMR: δ 172.90, 136.15, 135.10, 134.65, 133.15, 132.12, 131.45. 130.0, 129.38, 128.04, 126.85, 122.01, 119.8, 115.92, 110.96, 109.37, 56.57 (C1), 51.36 (C3), 25.38. MS: m/z 378 (M+ + 4), m/z 376 (M+ + 2), m/z 374 (M+, 100%). Elemental analysis: calculated for (C19H16Cl2N2O2) C, H, N.
(1R,3S)Methyl-1-(2,4-Dichlorophenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (4)
Yield 30%; white powder; mp 128–130 °C; Rf = 0.12 (CH2Cl2). IR (cm−1): 3396 (–NH–), 1739 (–CO–). 1H NMR: δ 7.80 (s, 1H, NH), 7.49 (s, 1H, NH), 7.46 (d, 1H, J = 7.2 Hz, Ar), 7.19–7.11 (m, 5H, Ar), 6.94 (d, 1H, J = 7.0 Hz, Ar), 5.30 (s, 1H, CHPh), 3.74 (s, 3H, OCH3), 3.68–3.62 (m, 1H, CHCOOCH3), 3.30 (dd, 1H, J = 4.6/7.6 Hz, CHaHb), 3.16–3.14 (m, 1H, CHaHb). 13C NMR: δ 173.17, 136.27, 134.70, 134.50, 131.12. 131.0, 129.77, 127.27, 126.65, 122.46, 120.01, 119.82, 118.39, 111.06, 109.46, 52.38 (C1), 51.28 (C3), 51.14. MS: m/z 378 (M+ + 4), m/z 376 (M+ + 2), m/z 374 (M+, 100%). Elemental analysis: calculated for (C19H16Cl2N2O2) C, H, N.
(1R,3R)Methyl-1-(3,4-dichlorophenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (5)
Yield 35%; yellow powder; mp 90–92 °C; Rf = 0.15 (CH2Cl2). IR (cm−1): 3396 (–NH–), 1734 (–CO–). 1H NMR: δ 9.0 (s, 1H, NH), 7.51–7.44 (m, 5H, Ar + NH), 7.25 (d, 1H, J = 7.2 Hz, Ar), 7.20–7.10 (m, 2H, Ar), 5.23 (s, 1H, CHPh), 3.96 (dd, J = 4.0/7.6 Hz, 1H, CHaHb,), 3.82 (s, 3H, OCH3), 3.05–3.01 (m, 1H, CHCOOCH3), 3.00–2.95 (m, 1H, CHaHb). 13C NMR: δ 172.88, 140.98, 136.25, 133.30, 133.12, 132.74, 130.92, 130.58, 127.94, 126.90, 122.35, 119.89, 118.37, 111.03, 109.34, 57.73 (C1), 56.64, 52.42 (C3), 25.43. MS: m/z 378 (M+ + 4), m/z 376 (M+ + 2), m/z 374 (M+, 100%). Elemental analysis: calculated for (C19H16Cl2N2O2), C, H, N. [α]20D = +58.35 (c = 0.1, EtOH)
(1S,3R)Methyl-1-(3,4-dichlorophenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (6)
Yield 20%; yellow powder; mp 140–143 °C; Rf = 0.10 (CH2Cl2). IR (cm−1):3345 (–NH–), 1710 (–CO–). 1HNMR: δ 7.55 (d, 1H, J = 7.2 Hz,, Ar), 7.50–7.02 (m, 3H, Ar), 7.20–7.10 (m, 5H, Ar), 5.49 (s, 1H, CHPh), 3.98–3.95 (m, 1H, CHCOOCH3), 3.73 (s, 3H, OCH3), 3.37 (d, 1H, J = 8.0 Hz, CHaHb), 3.29 (dd, 1H, J = 4.6/8.0 Hz, CHaHb,). 13C NMR: δ 172.87, 148.02, 136.27, 135.02, 132.95, 132.55, 130.72, 130.57, 128.02, 126.69, 122.51, 119.85, 118.45, 111.09, 108.57, 53.94 (C1), 52.59 (C3), 52.40, 24.17. MS: m/z 378 (M+ +4), m/z 376 (M+ + 2), m/z 374 (M+, 100%). Elemental analysis: calculated for (C19H16Cl2N2O2) C, H, N. [R]20D = +69.5 (c = 0.1, EtOH).
(1S,3S)Methyl-1-(3,4-dichlorophenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (7)
Yield 38%; yellow powder; mp 88–91 °C; Rf = 0.15 (CH2Cl2). IR (cm−1): 3395 (–NH–), 1734 (–CO–). 1H NMR: δ 7.51–7.44 (m, 5H, Ar NH), 7.25 (d, 1H, J = 7.2 Hz, Ar), 7.20–7.10 (m, 2H, Ar), 5.24 (s, + 1H, CHPh), 3.95 (dd, 1H, J = 4.0/8.0 Hz, CHCOOCH3), 3.82 (s, 3H, OCH3), 3.25 (dd, 1H, J = 4.6/7.6 Hz, CHaHb), 3.05–2.95 (m, 1H, CHaHb). 13C NMR: δ 172.78, 140.81, 136.27, 133.16, 133.12, 132.78, 130.919, 130.62, 127.99, 127.51, 126.88, 122.37, 119.90, 118.37, 111.04, 109.32, 57.70 (C1), 52.42 (C3), 25.36. MS: m/z 378 (M+ + 4), m/z 376 (M+ + 2), m/z 374 (M+, 100%). Elemental analysis: calculated for (C19H16Cl2N2O2) C, H, N. [α]20D =−58.35 (c = 0.1, EtOH)
(1R,3S)Methyl-1-(3,4-dichlorophenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (8)
Yield 25%; yellow powder; mp 139–142 °C; Rf = 0.10 (CH2Cl2). IR (cm−1): 3386 (–NH–), 1731 (–CO–). 1H NMR: δ 7.55 (d, 2H, J = 7.0 Hz, Ar NH), 7.40–7.35 (m, 3H, Ar + NH), 7.27 (d, 2H, J = 7.0 Hz, + Ar), 7.21–7.10 (m, 2H, Ar), 5.45 (s, 1H, CHPh), 3.97–3.93 (m, 1H, CHCOOCH3), 3.73 (s, 3H, OCH3), 3.31–2.26 (m, 1H, CHaHb), 3.18 (dd, 1H, J = 4.6/8.0 Hz, CH H 13 a b,). 13C NMR: δ 173.13, 139.96, 136.04, 135.80, 132.57, 130.45, 130.22, 129.07, 128.84, 126.98, 124.06, 121.91, 119.77, 118.01, 110.99, 108.63, 53.20 (C1), 52.36 (C3), 25.72. MS: m/z 378 (M+ + 4), m/z 376 (M+ + 2), m/z 374 (M+, 100%). Elemental analysis: calculated for (C19H16Cl2N2O2) C, H, N. [R]20D = −69.5 (c = 0.1, EtOH)
(1R,3R)Methyl-1-(2,6-dichlorophenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3 carboxylate (9)
Yield 55%; red powder; mp 102–105 °C; Rf = 0.13 (100% CH2Cl2). IR (cm−1): 3389 (–NH–), 1729 (–CO–). 1H NMR: δ 7.58–7.52 (m, 3H, Ar NH), 7.28–7.27 (m, 3H, Ar + NH), 7.26–7.25 (m, 3H, Ar),+6.29 (s, 1H, CHPh), 4.14–4.06 (m, 1H, CHCOOCH3), 3.85 (s, 3H, OCH3), 3.32 (dd, 1H, J = 4.0/8.0 Hz, CHaHb), 3.03–2.99 (m, 1H, CHaHb). 13C NMR: δ 172.25, 138.07, 136.20, 135.88, 134.37, 130.47, 129.40, 127.84, 126.60, 121.82, 119.66, 118.07, 112.06, 111.02, 56.83 (C1), 54.07, 52.21 (C3), 37.04, 27.15. MS: m/z 378 (M+ +4), m/z 376 (M+ + 2), m/z 374 (M+), m/z 217 (100%). Elemental analysis: calculated for (C19H16Cl2N2O2) C, H, N.
(1S,3S)Methyl-1-(2,6-dichlorophenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (10)
Yield 57%; red powder; mp 101–103 °C; Rf = 0.13 (100% CH2Cl2). IR (cm−1): 3386 (–NH–), 1730 (–CO–). 1H NMR: δ 7.51–7.50 (m, 3H, Ar NH), 7.43 (d, 1H, J = 7.2 Hz, Ar), 7.26–7.25 (m, 3H, Ar NH),+7.13–7.11 (m, 2H, Ar), 6.21 (s, 1H, CHPh), 4.07–4.04 (m,+1H, CHCOOCH3), 3.83 (s, 3H, OCH3), 3.25 (dd, 1H, J = 4.0/8.0 Hz, CHaHb), 2.99–2.96 (m, 1H, CHaHb). 13C NMR: δ 173.13, 136.96, 135.80, 134.3, 132.57, 130.46, 129.08, 128.84, 126.98, 121.91, 119.77, 118.01, 110.99, 108.64, 57.21 (C1), 54.21, 52.36 (C3), 36.88, 25.72. MS: m/z 378 (M+ + 4), m/z 376 (M+ + 2), m/z 374 (M+, 100%). Elemental analysis: calculated for (C19H16Cl2N2O2) C, H, N.
General Procedures for the Preparation of 5-(Aryl)-2-alkyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione
An appropriate amount of 1,3-disubstituted tetrahydro-β-carbolines 1–10 (0.38 g, 1 mmol) was dissolved in methyl ethyl ketone (10 mL) and stirred. Excess amount of ethyl or tert-butyl isocyanate (1.6 mmol) was added, and the mixture was then stirred and left to reflux under N2 atmosphere for 16 h. The solvent was evaporated under reduced pressure, and the residue was purified by column chromatography on silica gel eluting with CH2Cl2. The solution containing the desired product from the column was evaporated under reduced pressure to give the pure powder of the desired product.
(5R,11aR) 5-(2,4-Dichlorophenyl)-2-ethyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (11)
Yield 58%; yellow powder; mp 223–226 °C; Rf = 0.16 (100% CH2Cl2). IR (cm−1): 3321 (–NH–), 1764, 1705 (–CO–). 1H NMR: δ 7.79 (s, 1H, NH), 7.57 (d, 1H, J = 7.0 Hz, Ar), 7.50–7.49 (m, 1H, Ar), 7.30–7.12 (m, 5H, Ar), 6.45 (s, 1H, CHPh), 4.40 (dd, 1H, J = 4.3/7.0 Hz, CHC(O)N), 3.58–3.55 (m, 3H, NCH2 + CHaHb), 3.08–3.05 (m, 1H, CHaHb), 1.23 (t, 3H, J = 7.2 Hz, CH3). 13C NMR: δ 171.29, 154.39, 136.67, 135.06, 134.68, 133.98, 132.21, 129.44, 129.37, 128.16, 125.95, 123.14, 120.37, 118.50, 111.31, 107.61, 57.93 (C1), 51.46 (C3), 33.76, 22.46, 13.46. MS: m/z 417 (M+ + 4), m/z 415 (M+ +2), m/z 413 (M+), m/z 252 (100%). Elemental analysis: calculated for (C21H17Cl2N3O2) C, H, N.
(5S,11aR) 5-(2,4-Dichlorophenyl)-2-ethyl-5,6,11,11a-tetrahydro-1H-imidazo [1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (12)
Yield 57%; yellow powder; mp 126–129 °C; Rf = 0.28 (CH2Cl2). IR (cm−1): 3312 (–NH–), 1767, 1710 (–CO–). 1H NMR: δ 7.87 (s, 1H, NH), 7.53 (d, 1H, J = 7.0 Hz, Ar), 7.31–7.12 (m, 6H, Ar), 6.68 (s, 1H, CHPh), 4.51 (dd, 1H, J = 4.3/7.6 Hz, CHCO)N), 3.63–3.61 (m, 3H, NCH2 + CHaHb), 2.92–2.89 (m, 1H, CHaHb), 1.24 (t, 3H, J = 7.2 Hz, CH3). 13C NMR: δ 172.51, 155.24, 136.56, 135.80, 135.28, 133.57, 130.29, 130.17, 129.37, 128.10, 125.92, 123.11, 120.34, 118.46, 111.31, 107.61, 54.71 (C1), 49.13 (C3), 33.76, 22.46, 13.46. MS: m/z 417 (M+ +4), m/z 415 (M+ + 2), m/z 413 (M+), m/z 252 (100%). Elemental analysis: calculated for (C21H17Cl2N3O2) C, H, N.
(5S,11aS) 5-(2,4-Dichlorophenyl)-2-ethyl-5,6,11,11a-tetrahydro-1H-imidazo [1′,5′:1,6] pyrido[3,4-b]indole-1,3(2H)-dione (13)
Yield 68%; yellow powder; mp 226–228 °C; Rf = 0.16 (CH2Cl2). IR (cm−1): 3331 (–NH–), 1706 (–CO–), 1624 (–CO–). 1H NMR: δ 7.90 (s, 1H, NH), 7.57 (d, 2H, J = 7.8 Hz, Ar), 7.25–7.18 (m, 5H, Ar), 6.41 (s, 1H, CHPh), 4.38 (dd, 1H, J = 4.6/7.8 Hz, CHC(O)N), 3.56–3.49 (m, 3H, NCH2 + CHaH (m, b), 3.07–3.04 1H, CHaHb), 1.20 (t, 3H, J = 7.2 Hz, CH3). 13C NMR: δ 171.27, 154.33, 136.65, 135.04, 134.62, 133.98, 132.17, 129.37, 129.35, 128.10, 125.30, 123.08, 120.31, 118.45, 111.30, 107.54, 57.89 (C1), 51.41 (C3), 33.72, 22.42, 13.42. MS: m/z 418 (M+ +4), m/z 416 (M+ + 2), m/z 414 (M+, 100%). Elemental analysis:+ calculated for (C21H17Cl2N3O2) C, H, N.
(5R,11aS) 5-(2,4-Dichlorophenyl)-2-ethyl-5,6,11,11a-tetrahydro-1H-imidazo [1′,5′:1,6] pyrido[3,4-b]indole-1,3(2H)-dione (14)
Yield 45%; yellow powder; mp 125–128 °C; Rf = 0.28 (CH2Cl2). IR (cm−1): 3331 (–NH–), 1704 (–CO–), 1623 (–CO–). 1H NMR: δ 7.96 (s, 1H, NH), 7.51 (d, 2H, 8.0 J = Hz, Ar), 7.26–7.12 (m, 5H, Ar), 6.21 (s, 1H, CHPh), 4.51–4.48 (m, 1H, CHC(O)N), 3.63–3.60 (m, 3H, NCH2 + CHaHb (m,), 2.92–2.88 1H, CHaHb), 1.24 (t, 3H, J = 7.2 Hz, CH3). 13C NMR: δ 172.50, 155.20, 136.55, 135.78, 135.23, 133.57, 130.22, 130.17, 130.13, 128.03, 125.89, 123.06, 120.28, 118.42, 111.27, 107.08, 54.64 (C1), 49.09 (C3), 33.91, 23.33, 13.46. MS: m/z 418 (M+ +4), m/z 416 (M+ + 2), m/z 414 (M+), m/z 378 (100%). Elemental+ analysis: calculated for C21H17Cl2N3O2 C, H, N.
(5R,11aR) 5-(3,4-Dichlorophenyl)-2-ethyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (15)
Yield 52%; white powder; mp 229–232 °C; Rf = 0.18 (CH2Cl2). IR (cm−1): 3332 (–NH–), 1706 (–CO–), 1623 (–CO–). 1H NMR: δ 7.88 (s, 1H, NH), 7.58 (d, 1H, J = 7.0 Hz, Ar), 7.38 (s, 1H, Ar), 7.26–7.12 (m, 5H, Ar), 5.77 (s, 1H, CHPh), 4.35 (dd, 1H, J = 4.6/9.7 Hz, CHC(O)N), 3.57–3.50 (m, 3H, NCH2 + CHaHb), 3.08–3.06 (m, 1H, CHaHb), 1.15 (t, 3H, J = 7.2 Hz, CH3). 13C NMR: δ 171.19, 154.79, 139.06, 136.86, 132.99, 132.73, 132.19, 130.88, 129.72, 127.13, 126.04, 123.16, 120.38, 118.61, 111.32, 107.52, 57.90 (C1), 55.60, 53.45 (C3), 33.74, 13.46. MS: m/z 418 (M+ + 4), m/z 416 (M+ + 2), m/z 414 (M+, 100%). Elemental analysis: calculated for (C21H17Cl2N3O2) C, H, N.
(5S,11aR) 5-(3,4-Dichlorophenyl)-2-ethyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (16)
Yield 49%; yellow powder; mp 100–102 °C; Rf = 0.36 (CH2Cl2). IR (cm−1): 3389 (–NH–), 1704 (–CO–), 1624 (–CO–). 1H NMR: δ 7.94 (s, 1H, NH), 7.57 (d, 1H, J = 8.0 Hz, Ar), 7.44–7.41 (m, 2H, Ar), 7.30 (d, 1H, J = 8.0 Hz, Ar), 7.26–7.21 (m, 4H, Ar), 6.26 (s, 1H, CHPh), 4.28–4.23 (m, 1H, CHC(O)N), 3.93 (q, 2H, J = 7.2 Hz, NCH2), 3.61−3.58 (m, 1H, CHaHb), 2.92–2.89 (m, 1H, CHaHb), 1.14 (t, 3H, J = 7.2 Hz, CH3). 13C NMR: δ 172.28, 154.84, 139.21, 136.68, 133.42, 133.25, 131.15, 130.00, 129.12, 127.62, 126.20, 125.98, 123.28, 120.40, 118.58, 111.35, 108.62, 53.11 (C1), 50.93 (C3), 33.88, 13.51. MS: m/z 418 (M+ + 4), m/z 416 (M+ + 2), m/z 414 (M+, 100%). Elemental analysis: calculated for (C21H17Cl2N3O2) C, H, N.
(5S,11aS) 5-(3,4-Dichlorophenyl)-2-ethyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (17)
Yield 67%; yellow powder; mp 230–234 °C; Rf = 0.17 (CH2Cl2). IR (cm−1): 3363 (–NH–), 1708 (–CO–), 1699(–CO–). 1H NMR: δ 7.74 (s, 1H, NH), 7.58 (dd, 1H, J = 2.0/8.0 Hz, Ar), 7.38 (d, 1H, 8.0 Hz, Ar), 7.26–7.19 (m, 5H, Ar), 5.75 (s, 1H, CHPh), 4.36 (dd, 1H, J = 4.3/6.8 Hz, CHC(O)N), 3.55–3.50 (m, 3H, NCH2 + CHaHb), 3.09–3.02 (m, 1H, CHaHb), 1.21 (t, 3H, J = 7.2 Hz, CH3). 13C NMR: δ 171.14, 154.78, 139.00, 136.82, 133.02, 132.75, 132.16, 130.89, 129.67, 127.10, 126.03, 123.16, 120.39, 118.61, 111.32, 107.52, 57.90(C1), 55.60(C3), 33.74, 22.379, 13.46. MS: m/z 418 (M+ + 4), m/z 416 (M+ + 2), m/z 414 (M+, 100%). Elemental analysis: calculated for (C21H17Cl2N3O2) C, H, N.
(5R,11aS) 5-(3,4-Dichlorophenyl)-2-ethyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (18)
Yield 50%; yellow powder; mp 105–107 °C; Rf = 0.36 (CH2Cl2). IR (cm−1): 3389 (–NH–), 1702 (–CO–), 1624 (–CO–). 1H NMR: δ 8.10 (s, 1H, NH), 7.54 (d, 1H, J = 7.4 Hz, Ar), 7.42 (d, 1H, J = 7.4 Hz, Ar), 7.32–7.31 (m, 1H, Ar), 7.26–7.22 (m, 4H, Ar), 6.26 (s, 1H, CHPh), 4.27–4.25 (m, 1H, CHC(O)N), 3.93–3.93 (m, 3H, NCH2 + CHaHb), 2.92–2.88 (m, 1H, CHaHb), 1.21 (t, 3H, J = 7.2 Hz, CH3). 13C NMR: δ 172.30, 154.84, 139.28, 136.71, 133.39, 131.12, 129.99, 129.15, 127.61, 126.03, 125.98, 123.23, 120.35, 118.56, 111.36, 108.54, 53.10 (C1), 50.92 (C3), 33.88, 22.36, 13.50. MS: m/z 418 (M+ + 4), m/z 416 (M+ + 2), m/z 414 (M+), m/z 213 (100%). Elemental analysis: calculated for (C21H17Cl2N3O2) C, H, N.
(5R,11aR) 5-(2,6-Dichlorophenyl)-2-ethyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (19)
Yield 56%; yellow powder; mp 84–86 °C; Rf = 0.21 (CH2Cl2). IR (cm−1): 3338 (–NH–), 1768, 1707 (–CO–). 1H NMR: δ 7.65 (s, 1H, NH), 7.68 (d, 1H, J = 8.0 Hz, Ar), 7.48–7.47 (m, 1H, Ar), 7.30–7.17 (m, 5H, Ar), 6.84 (s, 1H, CHPh), 4.43 (dd, 1H, J = 4.0/8.4 Hz, CHC(O)N), 3.96–3.92 (m, 2H, NCH2), 3.57–3.54 (m, 1H, CHaHb) 3.11–3.07 (m, 1H, CHaHb), 1.22 (t, 3H, J = 7.2 Hz, CH3). 13C NMR: δ 171.61, 153.90, 136.48, 135.47, 134.88, 132.00, 130.27, 129.92, 129.19, 128.75, 125.88, 122.86, 120.24, 118.48, 111.14, 108.49, 57.78 (C1), 51.56 (C3), 33.63, 21.82, 13.48. MS: m/z 417 (M+ + 4), m/z 415 (M+ + 2), m/z 413 (M+), m/z 214 (100%). Elemental analysis: calculated for (C21H17Cl2N3O2) C, H, N.
(5S,11aS) 5-(2,6-Dichlorophenyl)-2-ethyl-5,6,11,11a-tetrahydro1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (20)
Yield 52%; yellow powder; mp 78–80 °C; Rf = 0.22 (CH2Cl2). IR (cm−1): 3338 (–NH–), 1768, 1707 (–CO–). 1H NMR: δ 7.63 (s, 1H, NH), 7.56 (d, 1H, J = 8.0 Hz, Ar), 7.48–7.47 (m, 1H, Ar), 7.30–7.14 (m, 5H, Ar), 6.84 (s, 1H, CHPh), 4.42 (dd, 1H, J = 4.0/8.6 Hz, CHC(O)N), 3.96–3.93 (m, 2H, NCH2), 3.57–3.54 (m, 1H, CHaHb) 3.10–3.07(m, 1H, CHaHb), 1.23 (t, 3H, J = 7.2 Hz, CH3). 13C NMR: δ 171.61, 153.90, 136.48, 135.48, 134.88, 132.01, 130.27, 129.93, 129.19, 128.75, 125.87, 122.85, 120.24, 118.48, 111.15, 108.49, 57.78 (C1), 51.57 (C3), 33.63, 21.82, 13.48. MS: m/z 417 (M+ + 4), m/z 415 (M+ + 2), m/z 413 (M+), m/z 214 (100%). Elemental analysis: calculated for (C21H17Cl2N3O2) C, H, N.
(5R,11aR) 5-(2,4-Dichlorophenyl)-2-tert-butyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (21)
Yield 50%; yellowish powder; mp 280–282 °C; Rf = 0.41(CH2Cl2). IR (cm−1): 3308 (–NH–), 1756, 1699 (–CO–). 1H NMR: δ 7.80 (s, 1H, NH), 7.53 (d, 1H, J = 8.0 Hz, Ar), 7.47 (s, 1H, Ar), 7.30–7.17 (m, 5H, Ar), 6.37 (s, 1H, CHPh), 4.24 (dd, 1H, J = 4.3/7.0 Hz, CHC(O)N), 3.43 (dd, 1H, J = 4.2/7.0 Hz, CHaHb), 2.98–2.97 (m, 1H, CHaHb), 1.58 (s, 9H, C(CH3)3). 13C NMR: δ 172.34, 155.35, 136.58, 135.94, 134.27, 132.91, 132.54, 131.23, 129.58, 128.72, 128.18, 126.02, 123.04, 120.30, 118.53, 111.26, 107.40, 58.26, 57.26 (C1), 51.33 (C3), 29.70, 28.74, 22.53. MS: m/z 445 (M+ + 4), m/z 443 (M+ + 2), m/z 441 (M+), m/z 252 (100%). Elemental analysis: calculated (C23H21Cl2N3O2) C, H, N.
(5S,11aR) 5-(2,4-Dichlorophenyl)-2-tert-butyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (22)
Yield 47%; yellowish powder; mp 205–208 °C; Rf = 0.60 (CH2Cl2). IR (cm−1): 3312 (–NH–), 1764, 1698 (–CO–). 1H NMR: δ 7.86 (s, 1H, NH), 7.53–7.50 (m, 2H, Ar), 7.28–7.15 (m, 5H, Ar), 6.64 (s, 1H, CHPh), 4.39 (dd, 1H, J = 4.3/7.0 Hz, CHC(O)N), 3.51 (dd, 1H, J = 4.3/7.0 Hz, CHaHb), 2.88–2.85 (m, 1H, CHaHb), 1.61 (s, 9H, C(CH3)3). 13C NMR: δ 173.67, 156.09, 136.13, 135.08, 133.35, 131.36, 130.40, 130.20, 130.07, 128.10, 125.97, 122.97, 120.24, 118.44, 111.21, 106.96, 58.27, 54.14 (C1), 48.94 (C3), 28.68, 23.61. MS: m/z 445 (M+ + 4), m/z 443 (M+ + 2), m/z 441 (M+), m/z 57.1 (100%). Elemental analysis: calculated (C23H21Cl2N3O2) C, H, N.
(5S,11aS) 5-(2,4-Dichlorophenyl)-2-tert-butyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (23)
Yield 58%; white powder; mp 288–290 °C; Rf = 0.42 (CH2Cl2). IR (cm−1): 3309 (–NH–), 1702 (–CO–), 1624 (–CO–). 1H NMR: δ 7.81 (s, 1H, NH), 7.53 (d, 1H, J = 7.2 Hz, Ar), 7.45 (d, 1H, J = 7.2 Hz, Ar), 7.25–7.16 (m, 4H, Ar), 6.99–7.01 (m, 1H, Ar), 6.42 (s, 1H, CHPh), 4.24 (q, 1H, J = 7.6 Hz, CHC(O)N), 3.43 (dd, 1H, J = 4.0/7.6 Hz, CHaHb), 2.99–2.98 (m, 1H, CHaHb), 1.63 (s, 9H, C(CH3)3). 13C NMR: δ 172.32, 155.32, 136.58, 135.93, 134.22, 132.93, 132.51, 131.5, 129.53, 128.71, 128.15, 125.98, 122.99, 121.7, 120.26, 118.49, 111.25, 107.36, 58.23, 57.23 (C1), 51.31 (C3), 28.72, 22.50. MS: m/z 445 (M+ + 4), m/z 443 (M+ + 2), m/z 441 (M+, 100%). Elemental analysis: calculated (C23H21Cl2N3O2) C, H, N.
(5R,11aS) 5-(2,4-Dichlorophenyl)-2-tert-butyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (24)
Yield 54%, white powder; mp 204–207 °C; Rf = 0.59 (CH2Cl2). IR (cm−1): 3311 (–NH–), 1698, 1622 (–CO–). 1H NMR: δ 7.92 (s, 1H, NH), 7.52 (d, 1H, J = 7.2 Hz, Ar), 7.47 (d, 1H, J = 7.2 Hz, Ar), 7.25 (d, 1H, J = 7.0 Hz, Ar), 7.25–7.13 (m, 4H, Ar), 6.71 (s, 1H, CHPh), 4.37 (q, 1H, J = 7.6 Hz, CHC(O)N), 3.49 (dd, 1H, 4.0/7.6 Hz, CHaHb), 2.87–2.84 (m, 1H, CHaHb), 1.62 (s, 9H, C(CH) 13 3 3). 13C NMR: δ 73.67, 156.06, 136.51, 136.11, 135.05, 133.37, 130.37, 130.16, 130.09, 128.06, 125.96, 122.94, 120.21, 118.42, 111.21, 106.94, 58.26, 57.23, 54.09 (C1), 48.92 (C3), 28.67, 23.60. MS: m/z 445 (M+ + 4), m/z 443 (M+ + 2), m/z 441 (M+), 442 (100%). Elemental analysis: calculated for (C23H21Cl2N3O2) C, H, N.
(5R,11aR) 5-(3,4-Dichlorophenyl)-2-tert-butyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (25)
Yield 53%; yellow powder; mp 175–177 °C; Rf = 0.38 (CH2Cl2). IR (cm−1): 3278 (–NH–), 1706 (–CO–), 1647 (–CO–). 1H NMR: δ 8.88 (s, 1H, NH), 7.54–7.43 (m, 2H, Ar), 7.26–7.17 (m, 5H, Ar), 6.42 (s, 1H, CHPh), 4.25 (dd, 1H, J = 3.6/7.6 Hz, CHC(O)N), 3.56–3.53 (m, 1H, CHaHb), 3.48–3.41 (m, 1H, CHaHb), 1.63 (s, 9H, C(CH3)3). 13C NMR: δ 173.06, 157.86, 143.22, 136.94, 133.83, 132.57, 132.33, 131.60, 128.31, 126.21, 126.58, 125.60, 122.54, 119.89, 118.60, 111.10, 105.99, 56.61 (C1), 54.64, 52.37, 51.36(C3), 28.69, 23.37. MS: m/z 445 (M+ + 4), m/z 443 (M+ + 2), m/z 441 (M+), m/z 442 (100%). Elemental analysis: calculated for (C23H21Cl2N3O2) C, H, N.
(5S,11aR) 5-(3,4-Dichlorophenyl)-2-tert-butyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (26)
Yield 56%; yellow powder; mp 275–277 °C; Rf = 0.66 (CH2Cl2). IR (cm−1): 3329 (–NH–), 1701 (–CO–), 1624 (–CO–). 1H NMR: δ 7.94 (s, 1H, NH), 7.58 (d, 2H, J = 8.0 Hz, Ar), 7.42–7.37 (m, 4H, Ar), 7.29–7.21 (m, 1H, Ar), 6.22 (s, 1H, CHPh), 4.13 (q, 1H, J = 7.6 Hz, CHC(O)N), 3.47–3.43 (m, 1H, CHaHb), 2.87–2.84 (m, 1H, CHaHb), 1.59 (s, 9H, C(CH3)3). 13C NMR: δ 173.47, 155.63, 139.41, 136.64, 133.33, 133.09, 131.10, 129.98, 129.41, 127.65, 126.05, 123.14, 120.30, 118.58, 111.28, 108.52, 58.21, 53.44, 52.40 (C1), 50.68 (C3), 29.65, 28.68, 23.52. MS: m/z 445 (M+ + 4), m/z 443 (M+ + 2), m/z 441 (M+, 100%). Elemental analysis: calculated (C23H21Cl2N3O2) C, H, N.
(5S,11aS)-5-(3,4-Dichlorophenyl)-2-t-butyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (27)
Yield 48%; yellow powder; mp 174–175 °C; Rf = 0.36 (CH2Cl2). IR (cm−1):3361 (–NH–), 1704 (–CO–), 1624 (–CO–). 1H NMR: δ 8.89 (s, 1H, NH), 7.61–7.36 (m, 4H, Ar), 7.26–7.22 (m, 3H, Ar), 6.32 (s, 1H, CHPh), 4.21 (dd, 1H, J = 3.6/7.6 Hz, CHC(O)N), 3.43 (dd, 1H, J = 3.6/7.8 Hz, CHaHb), 3.03–3.00 (m, 1H, CHaHb), 1.58 (s, 9H, C(CH3)3). 13C NMR: δ 172.21, 155.78, 139.73, 136.80, 133.06, 132.42, 130.97, 129.12, 126.58, 126.12, 123.06, 120.33, 118.64, 111.24, 107.55, 58.26 (C1), 57.21, 55.55, 53.42 (C3), 52.37, 29.68, 28.71, 23.37. MS: m/z 445 (M+ + 4), m/z 443 (M+ + 2), m/z 441 (M+), m/z 442 (100%). Elemental analysis: calculated for (C23H21Cl2N3O2) C, H, N.
(5R,11aS) 5-(3,4-Dichlorophenyl)-2-tert-butyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (28)
Yield 55%; yellow powder; mp 273–275 °C; Rf = 0.68 (CH2Cl2). IR (cm−1): 3320 (–NH–), 1702 (–CO–), 1624 (–CO–). 1H NMR: δ 7.80 (s, 1H, NH), 7.59 (d, 2H, J = 8.2 Hz, Ar), 7.44–7.39 (m, 4H, Ar), 7.29–7.20 (m, 1H, Ar), 6.30 (s, 1H, CHPh), 4.14 (q, 1H, J = 8.0 Hz, CHC(O)N), 3.48–3.44 (m, 1H, CHaHb), 2.88–2.85 (m, 1H, CHaHb), 1.61 (s, 9H, C(CH3)3). 13C NMR: δ 173.43, 155.61, 139.36, 136.61, 133.35, 133.12, 131.12, 129.99, 129.40, 127.67, 126.03, 123.16, 120.33, 118.57, 111.26 108.59, 58.19, 52.40 (C1), 50.70 (C3), 28.66, 23.51.; MS: m/z 445 (M+ + 4), m/z 443 (M+ + 2), m/z 441 (M+, 100%), m/z 442 (100%). Elemental analysis: calculated for (C23H21Cl2N3O2) C, H, N.
(5R,11aR) 5-(2,6-Dichlorophenyl)-2-tert-butyl-5,6,11,11a-tetrahydro-1H-imidazo [1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (29)
Yield 48%; orange-yellow powder; mp 245–248 °C; Rf = 0.46 (CH2Cl2). IR (cm−1): 3356 (–NH–), 1762, 1698 (–CO–). 1H NMR: δ 7.62 (s, 1H, NH), 7.55 (d, 1H, J = 8.2 Hz, Ar), 7.48–7.47 (m, 1H, Ar), 7.26–7.19 (m, 5H, Ar), 6.79 (s, 1H, CHPh), 4.27 (dd, 1H, J = 3.6/7.6 Hz, CHC(O)N), 3.42–3.41 (dd, J = 3.6/7.6 Hz, 1H, CHaHb), 3.08–3.05 (m, 1H, CHaHb), 1.61 (s, 9H, C(CH3)3). 13C NMR: δ 172.68, 154.73, 136.49, 134.80, 134.39, 132.58, 131.45, 130.29, 129.56, 129.28, 128.88, 125.93, 122.80, 120.16, 118.54, 111.09, 108.54, 58.03, 56.93 (C1), 53.44, 51.30 (C3), 28.76, 21.85. MS: m/z 445 (M+ + 4), m/z 443 (M+ + 2), m/z 441 (M+), m/z 385 (100%). Elemental analysis: calculated for (C23H21Cl2N3O2) C, H, N.
(5S,11aS) 5-(2,6-Dichlorophenyl)-2-tert-butyl-5,6,11,11a-tetrahydro-1Himidazo [1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (30)
Yield 45%; yellowish powder; mp 243–245 °C; Rf = 0.44 (CH2Cl2). 1H NMR: δ 7.64 (s, 1H, NH), 7.56 (d, 1H, J = 8.2 Hz, Ar), 7.49–7.48 (m, 1H, Ar), 7.30–7.17 (m, 5H, Ar), 6.80 (s, 1H, CHPh), 4.27 (dd, 1H, J = 3.6/7.6 Hz, CHC(O)N), 3.41 (dd, 1H, J = 3.6/7.6 Hz, CHaHb), 3.09–3.06 (m, 1H, CHaHb), 1.62 (s, 9H, C(CH3)3). 13C NMR: δ 172.67, 154.73, 136.47, 135.43, 134.79, 134.38, 132.58, 131.47, 130.29, 129.57, 129.29, 128.89, 122.81, 120.17, 118.54, 111.09, 108.55, 58.03, 56.93 (C1), 51.30 (C3), 29.04, 28.75, 21.84. MS: m/z 445 (M+ + 4), m/z 443 (M+ + 2), m/z 441 (M+), m/z 385 (100%). Elemental analysis: valculated for (C23H21Cl2N3O2) C, H, N.
General Procedures for the Preparation of Methyl 1-(2,6-Dichlorophenyl)-2-(2-chloroacetyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate
Chloroacetyl chloride (6.41 mmol) was added dropwise to a well stirred solution of an appropriate amount of β-carboline 1–10 (2.67 mmol) and NaHCO3 (0.271 g, 3.23 mmol) in CHCl3 (40 mL) under ice cooling. The mixture was then left to stir under nitrogen atmosphere at room temperature for 1 h. The mixture was diluted with CH2Cl2, washed with a solution of NaHCO3 and brine, dried over Na2SO4, and evaporated under reduced pressure.
(1R,3R)Methyl 1-(2,4-Dichlorophenyl)-2-(2-chloroacetyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (31)
Yield 78%; orange powder; mp 215–218 °C; Rf = 0.53 (CH2Cl2). IR (cm−1): 3294 (–NH–), 1731, 1666 (–CO–). 1H NMR: δ 7.50 (s, 1H, NH), 7.30–7.16 (m, 7H, Ar), 6.63 (s, 1H, CHPh), 4.53 (s, 1H, CHCOOCH3), 4.20–4.15 (m, 2H, COCH2Cl), 3.64 (s, 3H, OCH3), 3.43–3.39 (m, 2H, CH2). MS: m/z 454 (M+ + 4), m/z 452 (M+ + 2), m/z 450 (M+), m/z 373 (100%). Elemental analysis: calculated for (C21H17Cl3N2O3) C, H, N.
(1S,3R)Methyl 1-(2,4-Dichlorophenyl)-2-(2-chloroacetyl)-2,3,4, 9-tetrahydro-1H-β-carboline-3-carboxylate (32)
Yield 80%; green powder; mp 106–109 °C; Rf = 0.37 (100% CH2Cl2). IR (cm−1): 3370 (–NH–), 1739, 1668 (–CO–). 1H NMR: δ 8.08 (s, 1H, NH), 7.51 (d, 1H, J = 7.2, Ar), 7.28–7.11 (m, 6H, Ar), 6.57 (s, 1H, CHPh), 5.30 (br s, 1H, CHCOOCH3), 4.18–4.10 (m, 2H, COCH2Cl), 3.64 (s, 3H, OCH3), 3.53–3.49 (m, 2H, CH2). MS: m/z 454 (M+ + 4), m/z 452 (M+ + 2), m/z 450 (M+), m/z 373 (100%). Elemental analysis: calculated for (C21H17Cl3N2O3) C, H, N.
(1S,3S)Methyl 1-(2,4-Dichlorophenyl)-2-(2-chloroacetyl)-2,3,4, 9-tetrahydro-1H-β-carboline-3-carboxylate (33)
Yield 88%; white powder; mp 215–218 °C; Rf = 0.52 (CH2Cl2). IR (cm−1): 3315 (–NH–), 1737 (–CO–), 1664 (–CO–). 1H NMR: δ 7.50 (brs, 1H, NH), 7.26–7.15 (m, 7H, Ar), 6.57 (s, 1H, CHPh), 4.52 (s, 1H, CHCOOCH3), 4.20–4.15 (m, 2H, COCH2Cl), 3.63 (br s, 3H, OCH3), 3.49–3.48 (m, 2H, CH2). MS: m/z 454 (M+ + 4), m/z 452 (M+ + 2), m/z 450 (M+, 100%). Elemental analysis: calculated for (C21H17Cl3N2O3) C, H, N.
(1R,3S)Methyl 1-(2,4-Dichlorophenyl)-2-(2-chloroacetyl)-2,3,4, 9-tetrahydro-1H-β-carboline-3-carboxylate (34)
Yield 75%; white powder; mp 109–112 °C; Rf = 0.37 (CH2Cl2). IR (cm−1): 3347 (–NH–), 1739 (–CO–), 1668 (–CO–). 1H NMR: δ 7.80 (brs, 1H, NH), 7.39 (d, 1H, J = 8.0 Hz, Ar), 7.18–7.12 (m, 6H, Ar), 6.54 (s, 1H, CHPh), 5.56 (d, 1H, J = 7.0 Hz, CHCOOCH3), 3.95–3.93 (m, 2H, COCH2Cl), 3.72 (s, 3H, OCH3), 3.31 (dd, 1H, J = 3.6/7.6 Hz, CHaCHb), 3.18 (dd, 1H, J = 3.6/7.6 Hz, CHaCHb). MS: m/z 454 (M+ + 4), m/z 452 (M+ + 2), m/z 450 (M+), m/z 373 (100%). Elemental analysis: calculated for (C21H17Cl3N2O3) C, H, N.
(1R,3R)Methyl 1-(3,4-Dichlorophenyl)-2-(2-chloroacetyl)-2,3,4, 9-tetrahydro-1H-β-carboline-3-carboxylate (35)
Yield 86%; white powder; mp 283–285 °C; Rf = 0.47 (CH2Cl2). IR (cm−1): 3283 (–NH–), 1737 (–CO–), 1662 (–CO–). 1H NMR: δ 8.9 (s, 1H, NH), 7.60 (d, 2H, J = 7.6 Hz, Ar), 7.32–7.16 (m, 5H, Ar), 6.83 (s, 1H, CHPh), 4.99 (d, 1H, J = 7.0 Hz, CHCOOCH3), 4.35 (m, COCH2Cl), 4.22 (dd, 1H, J = 3.6/7.6 Hz, CHaCHb), 3.70 (dd, 1H, J = 3.6/7.6 Hz, CHaCHb), 3.49 (s, 3H, OCH3). MS: m/z 454 (M+ + 4), m/z 452 (M+ + 2), m/z 450 (M+, 100%). Elemental analysis: calculated for (C21H17Cl3N2O3) C, H, N.
(1S,3R)Methyl 1-(3,4-Dichlorophenyl)-2-(2-chloroacetyl)-2,3,4, 9-tetrahydro-1H-β-carboline-3-carboxylate (36)
Yield 79%; white powder; mp 163–165 °C; Rf = 0.22 (CH2Cl2). IR (cm−1): 3387 (–NH–), 1734(–CO–), 1663 (–CO–). 1H NMR: δ 8.9 (s, 1H, NH), 7.77 (d, 2H, J = 8.2 Hz, Ar), 7.62–7.42 (m, 2H, Ar), 7.26–7.23 (m, 3H, Ar), 6.72 (s, 1H, CHPh), 4.99–4.97 (m, 1H, CHC–OOCH3), 4.36–4.34 (m, 2H, COCH2Cl), 4.10 (s, 1H, CHaCHb), 3.75 (dd, 1H, J = 3.6/7.6 Hz, CHaCHb), 3.49 (s, 3H, OCH3). MS: m/z 454 (M+ + 4), m/z 452 (M+ + 2), m/z 450 (M+, 100%). Elemental analysis: calculated for (C21H17Cl3N2O3) C, H, N.
(1S,3S)Methyl 1-(3,4-Dichlorophenyl)-2-(2chloroacetyl)-2,3,4, 9-tetrahydro-1H-β-carboline-3-carboxylate (37)
Yield 84%; white powder; mp 283–284 °C; Rf = 0.45 (CH2Cl2). IR (cm−1): 3281 (–NH–), 1736 (–CO–), 1662 (–CO–). 1H NMR: δ 8.9 (s, 1H, NH), 7.77 (d, 2H, J = 8.0 Hz, Ar), 7.61 (d, 2H, J = 8.0 Hz, Ar), 7.32–7.17 (m, 3H, Ar), 6.83 (s, 1H, CHPh), 4.98 (d, 1H, J = 7.0 Hz, CHCOOCH3), 4.36–4.34 (m, 2H, COCH2Cl), 4.21 (d, 1H, J = 3.4/7.6Hz, CHaCHb), 3.70 (dd, 1H, J = 3.4/7.6 Hz, CHaCHb), 3.46 (s, 3H, OCH3). MS: m/z 454 (M+ + 4), m/z 452 (M+ + 2), m/z 450 (M+, 100%). Elemental analysis: calculated for (C21H17Cl3N2O3) C, H, N.
(1R,3S)Methyl 1-(3,4-Dichlorophenyl)-2(2-chloroacetyl)-2,3,4, 9-tetrahydro-1H-β-carboline-3-carboxylate (38)
Yield 77%; white powder; mp 162–165 °C; Rf = 0.24 (CH2Cl2). IR (cm−1): 3275 (–NH–), 1738, 1663 (–CO–). 1H NMR: δ 7.9 (s, 1H, NH), 7.75 (d, 2H, J = 7.6 Hz, Ar), 7.22–7.12 (m, 5H, Ar), 6.03 (s, 1H, CHPh), 4.16 (d, 1H, J = 7.0 Hz, CHCOOCH3), 4.13–4.06 (m, 2H, COCH2Cl), 3.61−3.58 (m, 2H, CH2), 3.47 (s, 3H, OCH3). MS: m/z 454 (M+ + 4), m/z 452 (M+ + 2), m/z 450 (M+, 100%). Elemental analysis: calculated for (C21H17Cl3N2O3) C, H, N.
(1R,3R)Methyl 1-(2,6-Dichlorophenyl)-2-(2-chloroacetyl)-2,3,4, 9-tetrahydro-1H-β-carboline-3-carboxylate (39)
Yield 76%; brown powder; mp 147–150 °C; Rf = 0.44 (100% CH2Cl2). IR (cm−1): 3304 (–NH–), 1739, 1666 (–CO–). 1H NMR: δ 8.98 (s, 1H, NH), 7.75–7.15 (m, 7H, Ar), 6.90 (s, 1H, CHPh), 4.99 (s 1H, CHCOOCH3), 4.25–4.22 (m, 2H, COCH2Cl), 3.67 (s, 3H, OCH3), 3.37–3.16 (m, 2H, CH2). MS: m/z 454 (M+ + 4), m/z 452 (M+ + 2), m/z 450 (M+), m/z 375 (100%). Elemental analysis: calculated for (C21H17Cl3N2O3) C, H, N.
(1S,3S)Methyl 1-(2,6-Dichlorophenyl)-2-(2-chloroacetyl)-2,3,4, 9-tetrahydro-1H-β-carboline-3-carboxyl (40)
78%; brown powder; mp 153–156 °C. Rf = 0.42 (100% CH2Cl2). IR (cm−1): 3309 (–NH–), 1737, 1666 (–CO–). 1H NMR: δ 8.98 (s, 1H, NH), 7.30–7.15 (m, 7H, Ar), 6.90 (s, 1H, CHPh), 4.99 (s, 1H, CHCOOCH3), 4.25–4.21 (m, 2H, COCH2Cl), 3.68 (s, 3H, OCH3), 3.22–3.17 (m, 2H, CH2). MS: m/z 454 (M+ + 4), m/z 452 (M+ + 2), m/z 450 (M+), m/z 375 (100%). Elemental + analysis: calculated for (C21H17Cl3N2O3) C, H, N.
General Procedures for the Preparation of 2-Alkyl 6-(Aryl)-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione
An appropriate amount of methyl amine (2.8 mmol) was added to a solution of the appropriate amount of chloroacetyl derivative (1.4 mmol) in methanol (25 mL). The mixture was heated to reflux under nitrogen atmosphere for 16 h. It was then cooled to room temperature and evaporated to dryness under reduced pressure. The residue was then dissolved in CH2Cl2, the organic layer was washed with water and dried over Na2SO4, filtered, and evaporated under reduced pressure. The crude product was then purified using column chromatography.
(6R,12aR) 6-(2,4-Dichlorophenyl)-2-methyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (41)
Yield 38%; white powder; mp 222–225 °C; Rf = 0.24 (CH2Cl2/MeOH 98:2). IR (cm−1): 3307 (–NH–), 1665, 1624 (–CO–). 1H NMR: δ 8.07 (s, 1H, NH), 7.58–7.17 (m, 7H, Ar), 6.64 (s, 1H, CHPh), 4.25 (dd, 1H, J = 3.6/7.6 Hz, CHC(O)N), 4.23–4.07 (m, 3H, CH2C(O)N + CHaHb), 3.47 (dd, 1H, J = 3.4/7.6 Hz, CHaHb), 3.01 (s,3H, NCH3). 13C NMR: δ 165.86, 162.96, 136.31, 135.61, 135.01, 131.76, 130.25, 129.05, 127.16, 126.14, 123.08, 120.31, 118.43, 111.33, 109.24, 56.45 (C1), 52.94, 51.39 (C3), 49.33, 33.34, 27.16. MS: m/z 417 (M+ + 4), m/z 415 (M+ + 2), m/z 413 (M+), m/z 57 (100%). Elemental analysis: calculated for (C21H17Cl2N3O2) C, H, N.
(6S,12aR) 6-(2,4-Dichlorophenyl)-2-methyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (42)
Yield 35%; white powder; mp 305–308 °C; Rf = 0.24 (CH2Cl2/MeOH 98:2). IR (cm−1): 3305 (–NH–), 1665, 1624 (–CO–). 1H NMR: δ 8.27 (s, 1H, NH), 7.51–7.06 (m, 7H, Ar), 6.75 (s, 1H, CHPh), 4.23 (dd, 1H, J = 3.4/7.2 Hz, CHC(O)N), 4.13–4.01 (m, 3H, CH2C(O)N + CHaHb), 3.47 (dd, 1H, J = 3.4/7.2 Hz, CHaHb), 3.00 (s,3H, NCH3). 13C NMR: δ 165.88, 162.99, 136.35, 135.59, 135.00, 133.71, 131.79, 130.21, 129.06, 127.15, 123.03, 120.26, 118.41, 111.37, 109.14, 53. 44, 52.92 (C1), 51.38, 49.37 (C3), 33.33, 27.17. MS: m/z 417 (M+ + 4), m/z 415 (M+ 2), m/z 413 (M+), m/z 57 (100%). Elemental analysis: calculated for (C21H17Cl2N3O2) C, H, N.
(6S,12aS) 6-(2,4-Dichlorophenyl)-2-methyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (43)
Yield 33%; white powder; mp 223–225 °C; Rf = 0.22 (CH2Cl2/MeOH 98:2). IR (cm−1): 3314 (–NH–), 1702 (–CO–), 1664 (–CO–). 1H NMR: δ 8.12 (s, 1H, NH), 7.55 (d, 2H, J = 7 Hz, Ar), 7.40 (d, 2H, J = 7 Hz, Ar), 7.28–7.10 (m, 3H, Ar), 6.34 (s, 1H, CHPh), 4.09–4.02 (m, 1H, CHC(O)N), 4.12–3.94 (m, 2H, CH2C(O)N), 3.81 (dd, 1H, J = 3.4/7.2 Hz, CHaHb), 3.25–3.22 (m, 1H, CHaHb), 3.09 (s,3H, NCH3). 13C NMR δ 166.13, 165.99, 138.84, 136.30, 133.61, 131.27, 129.43, 128.25, 127.24, 125.84, 122.87, 120.23, 118.59, 111.33, 111.26, 106.62, 56.23 (C1), 53.63, 53.45 (C3), 51.89, 33.76. MS: m/z 417 (M+ + 4), m/z 415 (M+ + 2), m/z 413 (M+).
(6R,12aS) 6-(2,4-Dichlorophenyl)-2-methyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (44)
Yield 30%; white powder; mp 301–303 °C; Rf = 0.23 (CH2Cl2/MeOH 98:2). IR (cm−1): 3305 (–NH–), 1665 (–CO–), 1624 (–CO–). 1H NMR: δ 8.09 (s, 1H, NH), 7.54 (d, 2H, J = 8.0 Hz, Ar), 7.31 (d, 2H, J = 8.0 Hz, Ar), 7.26–7.08 (m, 3H, Ar), 6.32 (s, 1H, CHPh), 4.24 (dd, 1H, J = 3.6/7.2 Hz, CHaHb), 4.14 (d, 1H, J = 7 Hz, CHC(O)N), 4.07 (d, 2H, J = 7 Hz, CH2C(O)N), 3.47 (dd, 1H, J = 3.6/7.2 Hz, CHaHb), 3.01 (s,3H, NCH3). 13C NMR: δ 165.86, 162.97, 135.61, 135.01, 133.70, 131.75, 130.25, 129.04, 128.15, 127.15, 126.14, 123.08, 120.31, 118.43, 111.33, 109.23, 52.95 (C1), 51.39, 49.34 (C3), 33.76, 27.16. MS: m/z 417 (M++ 4), m/z 415 (M+ + 2), m/z 413 (M+), m/z 414 (100%). Elemental analysis: calculated for (C21H17Cl2N3O2) C, H, N.
(6R,12aR) 6-(2,4-Dichlorophenyl)-2-ethyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (45)
Yield 34%; white powder; mp 203–207 °C; Rf = 0.36 (CH2Cl2/MeOH 98:2). IR (cm−1): 3444 (–NH–), 1663, 1628 (–CO–). 1H NMR: δ 8.12 (s, 1H, NH), 7.59–7.11 (m, 7H, Ar), 6.66 (s, 1H, CHPh), 4.38 (dd, 1H, J = 3.6/7.2 Hz, CHC(O)N), 4.27–3.78 (m, 3H, CH2C(O)N + CHaHb), 3.44–3.40 (m, 1H, CHaHb), 3.25–3.23 (m, 2H, NCH2), 1.23 (t, 3H, J = 7.2 Hz, CH3). 13C NMR: δ 166.57, 165.53, 165.35, 138.83, 136.26, 133.61, 132.04, 129.42, 128.25, 127.20, 122.83, 120.21, 118.56, 111.30, 106.58, 56.27 (C1), 53.42 (C3), 49.56, 41.30, 27.00, 24.12, 12.00. MS: m/z 431 (M+ + 4), m/z 429 (M+ + 2), m/z 427 (M+), m/z 57 (100%). Elemental analysis: calculated for (C22H19Cl2N3O2) C, H, N.
(6S,12aR) 6-(2,4-Dichlorophenyl)-2-ethyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (46)
Yield 33%; white powder; mp 227–230 °C; Rf = 0.37 (CH2Cl2/MeOH 98:2). IR (cm−1): 3394(–NH–), 1663, 1627 (–CO–). 1H NMR: δ 8.32 (s, 1H, NH), 7.54–7.09 (m, 7H, Ar), 6.72(s, 1H, CHPh), 4.21 (dd, 1H, J = 3.6/7.2 Hz, CHC(O)N), 4.12–3.99 (m, 3H, CH2C(O)N + CHaHb), 3.57–3.49 (m, 1H, CHaHb), 3.47–3.37 (m, 2H, NCH2), 1.22 (t, 3H, J = 7.2 Hz, CH3). 13C NMR: δ 165.39, 163.36, 136.36, 135.57, 135.03, 133.68, 131.84, 130.20, 129.12, 127.14, 126.15, 122.99, 120.23, 118.40, 111.38, 109.11, 52.99 (C1), 49.38 (C3), 48.80, 40.87, 27.04, 11.71. MS: m/z 431 (M+ + 4), m/z 429 (M+ + 2), m/z 427 (M+), m/z 57 (100%). Elemental analysis: calculated for (C22H19Cl2N3O2) C, H, N.
(6S,12aS) 6-(2,4-Dichlorophenyl)-2-ethyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (47)
Yield 33%; white powder; mp 205–208 °C; Rf = 0.35 (CH2Cl2/MeOH 98:2). IR (cm−1): 3316 (–NH–), 1670 (–CO–), 1663 (–CO–). 1H NMR: δ 8.21 (s, 1H, NH), 7.57 (d, 2H, J = 7.6 Hz, Ar), 7.52 (d, 2H, J = 7.6 Hz, Ar), 7.39–7.10 (m, 3H, Ar), 6.62 (s, 1H, CHPh), 4.37 (dd, 1H, J = 3.6/7.2 Hz, CHC(O)N), 4.11–4.04 (m, 3H, CH2C(O)N CHaHb), 3.41–3.39 (m, 1H, CHaHb), 2.99–2.96 (m, 2H, NC+H2), 1.22 (t, 3H, J = 7.2 Hz, CH3). 13C NMR: δ 165.56, 163.36, 156.59, 131.84, 131.28, 130.21, 129.42, 128.26, 127.24, 122.83, 120.25, 120.21, 118.57, 111.37, 109.16, 106.59, 56.29 (C1), 53.57 (C3), 40.96, 30.95, 27.02. MS: m/z 430 (M+ + 4), m/z 428 (M+ + 2, 100%), m/z 426 (M+). Elemental analysis: calculated for (C22H19Cl2N3O2) C, H, N.
(6R,12aS) 6-(2,4-Dichlorophenyl)-2-ethyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (48)
Yield 30%; white powder; mp 225–228 °C; Rf = 0.34 (CH2Cl2/MeOH 98:2). IR (cm−1): 3287 (–NH–), 1665 (–CO–), 1658 (–CO–). 1H NMR: δ 8.34 (s, 1H, NH), 7.52 (d, 2H, J = 7.6 Hz, Ar), 7.43 (d, 2H, J = 7.6 Hz, Ar), 7.30–7.16 (m, 3H, Ar), 6.72 (s, 1H, CHPh), 4.21 (dd, 1H, J = 3.6/7.2 Hz, CHC(O)N), 4.07–3.99 (m, 3H, CH2C(O)N + CHaHb), 3.48–3.43 (m, 1H, CHaHb), 2.99–2.96 (m, 2H, NCH2), 1.18 (t, 3H, J = 7.2 Hz, CH3). 13C NMR: δ 165.38, 136.35, 136.34, 135.57, 135.02, 133.66, 131.82, 130.19, 129.10, 127.13, 122.99, 121.30, 120.23, 118.40, 111.36, 109.09, 52.98 (C1), 49.37 (C3), 48.80, 33.80, 27.03. MS: m/z 430 (M+ + 4), m/z 428 (M+ + 2, 100%), m/z 426 (M+). Elemental analysis: calculated for (C22H19Cl2N3O2) C, H, N.
(6R,12aR) 6-(3,4-Dichlorophenyl)-2-ethyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (49)
Yield 35%; white powder; mp 298–300 °C; Rf = 0.33 (CH2Cl2/MeOH 98:2). IR (cm−1):3317 (–NH–), 1673 (–CO–), 1662 (–CO–). 1H NMR: δ 7.93 (s, 1H, NH), 7.57–7.54 (m, 2H, Ar), 7.42–7.38 (m, 2H, Ar), 7.27–7.16 (m, 3H, Ar), 7.01 (s, 1H, CHPh), 4.26 (dd, 1H, J = 3.6/7.2 Hz, CHC(O)N), 4.12–3.98 (m, 3H, CH2C(O)N + CHaHb), 3.60 (dd, 1H, J = 3.6/7.2 Hz, CHaHb), 3.01–2.96 (m, 2H, NCH2), 1.20 (t, 3H, J = 7.2 Hz, CH3). MS: m/z 430 (M+ + 4), m/z 428 (M+ + 2, 100%), m/z 426 (M+). Elemental analysis: calculated (C22H19Cl2N3O2) C, H, N.
(6S,12aR) 6-(3,4-Dichlorophenyl)-2-ethyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (50)
Yield 28%; yellow powder; mp 92–95 °C; Rf = 0.33 (CH2Cl2/MeOH 98:2). IR (cm−1): 3330 (–NH–), 1659 (–CO–), 1663 (–CO–).1H NMR: δ 8.52 (s, 1H, NH), 7.57–7.55 (m, 2H, Ar), 7.43–7.40 (m, 2H, Ar), 7.28–7.12 (m, 3H, Ar), 6.72 (s, 1H, CHPh), 3.97–3.93 (m, 3H, CH2C(O)N + CHaHb), 3.25 (dd, 1H, J = 3.6/7.2 Hz, CHC(O)N), 3.17 (dd, 1H, J = 3.6/7.2 Hz, CHaHb), 3.01–2.96 (m, 2H, NCH2), 1.24 (t, 3H, J = 7.2 Hz, CH3). MS: m/z 430 (M+ + 4), m/z 428 (M+ + 2, 100%), m/z 426 (M+). Elemental analysis: calculated for (C22H19Cl2N3O2) C, H, N.
(6S,12aS) 6-(3,4-Dichlorophenyl)-2-ethyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (51)
Yield 28%; yellow powder; mp 298–301 °C; Rf = 0.34 (CH2Cl2/MeOH 98.2). IR (cm−1):3325 (–NH–), 1712 (–CO–), 1663 (–CO–). 1H NMR: δ 8.19 (brs, 1H, NH), 7.54–7.53 (m, 2H, Ar), 7.40–7.32 (m, 2H, Ar), 7.26–7.19 (m, 3H, Ar), 6.70 (s, 1H, CHPh), 4.24 (dd, 1H, J = 3.6/7.6 Hz, CHC(O)N), 4.16–3.98 (m, 3H, CH2C(O)N + CHaHb), 3.55 (dd, 1H, J = 3.6/7.6 Hz, CHaHb), 2.95–2.94 (m, 2H, NCH2), 1.19 (t, 3H, J = 7.2 Hz, CH3). MS: m/z 430 (M+ + 4), m/z 428 (M+ + 2, 100%), m/z 426 (M+). Elemental analysis: calculated for (C22H19Cl2N3O2) C, H, N.
(6R,12aS) 6-(3,4-Dichlorophenyl)-2-ethyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (52)
Yield 34%; white powder; mp 94–96 °C; Rf = 0.35 (CH2Cl2/MeOH 98:2). IR (cm−1): 3337 (–NH–), 1710 (–CO–), 1665 (–CO–). 1H NMR: δ 7.93 (s, 1H, NH), 7.57–7.54 (m, 2H, Ar), 7.42–7.38 (m, 2H, Ar), 7.28–7.19 (m, 3H, Ar), 7.01 (s, 1H, CHPh), 4.26 (dd, 1H, J = 3.6/7.6 Hz, CHC(O)N), 4.12–3.98 (m, 3H, CH2C(O)N + CHaHb), 3.55 (dd, 1H, J = 3.6/7.6 Hz, CHaHb), 3.01–2.96 (m, 2H, NCH2), 1.22 (t, 3H, J = 7.2 Hz, CH3). MS: m/z 430 (M+ + 4), m/z 428 (M+ + 2, 100%), m/z 426 (M+). Elemental analysis: calculated for (C22H19Cl2N3O2) C, H, N.
(6R,12aR) 6-(2,6-Dichlorophenyl)-2-ethyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (53)
Yield 30%; yellowish white powder; mp 110–112 °C; Rf = 0.36 (CH2Cl2/MeOH 98:2). IR (cm−1): 3446 (–NH–), 1670, 1630(–CO–). 1H NMR: δ 9.00 (s, 1H, NH), 7.57–7.37 (m, 7H, Ar), 6.97 (s, 1H, CHPh), 4.22 (dd, 1H, J = 3.6/7.6 Hz, CHC(O)N), 3.78–3.65 (m, 3H, CH2C(O)N + CHaHb), 3.59–3.55 (m, 1H, CHaHb), 3.25–3.23 (m, 2H, NCH2), 1.25 (t, 3H, J = 7.2 Hz, CH3). MS: m/z 431 (M+ + 4), m/z 429 (M+ + 2), m/z 427 (M+), m/z 57 (100%). Elemental analysis: calculated for (C22H19Cl2N3O2) C, H, N.
(6S,12aS) 6-(2,6-Dichlorophenyl)-2-ethyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (54)
Yield 28%; yellowish white powder; mp 112–115 °C; Rf = 0.34 (CH2Cl2/MeOH 98:2). 1H NMR: δ 8.99 (s, 1H, NH), 7.52–7.12 (m,7H, Ar), 6.20 (s, 1H, CHPh), 4.18–4.15 (m, 1H, CHC(O)N), 4.07–4.02 (m, 3H, CH2C(O)N + CHaHb), 3.83–3.73 (m, 1H, CHaHb), 3.28–3.24 (m, 2H, NCH2), 1.32 (t, 3H, J = 7.2 Hz, CH3). MS: m/z 431 (M+ + 4), m/z 429 (M+ + 2), m/z 427 (M+), m/z 57 (100%). Elemental analysis: calculated for (C22H19Cl2N3O2) C, H, N.
(6R,12aR) 6-(2,4-Dichlorophenyl)-2-butyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (55)
Yield 37%; white powder; mp 140–142 °C; Rf = 0.52 (CH2Cl2/MeOH 98:2). IR (cm−1): 3285 (–NH–), 1662, 1654 (–CO–). 1H NMR: δ 8.15 (s, 1H, NH), 7.54–7.08 (m, 7H, Ar), 6.73 (s, 1H, CHPh), 4.23–4.21 (dd, 1H, CHC(O)N), 4.12–4.04 (m, 2H, CH2C(O)N), 3.48–3.46 (dd, 1H, CHaHb), 3.29–3.25 (m, 1H, CHaHb), 2.99–2.96 (t, 2H, NCH2), 1.59–1.55 (m, 2H, CH2CH2CH3), 1.38–1.34 (m, 2H, CH2CH3), 0.97–0.94 (t, 3H, CH3). 13C NMR: δ 165.58, 163.39, 136.32, 135.87, 135.04, 133.66, 131.82, 130.24, 129.14, 127.16, 126.17, 123.05, 120.28, 118.42, 111.34, 109.23, 56.44 (C1), 53.07 (C3), 49.36, 45.74, 28.58, 27.07, 19.93, 13.79. MS: m/z 459 (M+ + 4), m/z 457 (M+ 2), m/z 455 (M+), m/z 57 (100%). Elemental analysis: calculated for (C24H23Cl2N3O2) C, H, N.
(6S,12aR) 6-(2,4-Dichlorophenyl)-2-butyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (56)
Yield 38%; white powder; mp 203–206 °C; Rf = 0.52 (CH2Cl2/MeOH 98:2). IR (cm−1): 3326 (–NH–), 1668, 1651 (–CO–). 1H NMR: δ 8.28 (s, 1H, NH), 7.52–7.07 (m, 7H, Ar), 6.73 (s, 1H, CHPh), 4.22–4.20 (dd, 1H, CHC(O)N), 4.11–4.03 (m, 2H, CH2C(O)N), 3.45–3.43 (dd, 1H, CHaHb), 3.28–3.24 (m, 1H, CHaHb), 2.99–2.96 (t, 2H, NCH2), 1.59–1.51 (m, 2H, CH2CH2CH3), 1.37–1.32 (m, 2H, CH2CH3), 0.97–0.94 (t, 3H, CH3). 13C NMR: δ 165.57, 163.37, 136.31, 135.57, 135.01, 133.64, 131.81, 130.20, 129.11, 127.13, 126.14, 123.00, 120.24, 118.39, 111.35, 109.16, 53.03 (C1), 49.37 (C3), 49.33, 45.72, 28.55, 27.05, 19.90, 13.77. MS: m/z 459 (M+ + 4), m/z 457 (M+ + 2), m/z 455 (M+), m/z 57.1 (100%). Elemental analysis: calculated for (C24H23Cl2N3O2) C, H, N.
(6S,12aS) 6-(2,4-Dichlorophenyl)-2-butyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (57)
Yield 34%; white powder; mp 140–143 °C; Rf = 0.51 (CH2Cl2/MeOH 98:2). IR (cm−1): 3366 (–NH–), 1654 (–CO–), 1633 (–CO–). 1H NMR: δ 8.07 (s, 1H, NH), 7.57–7.55 (m, 2H, Ar), 7.32–7.13 (m, 5H, Ar), 6.87 (s, 1H, CHPh), 4.30–4.25 (d, 1H, CHC(O)N), 3.55–3.53 (m, 2H, CH2C(O)N), 3.32–3.29 (dd, 1H, CHaHb), 2.91–2.87 (m, 1H, CHaHb), 2.17–2.16 (t, 2H, NCH2), 1.50–1.46 (m, 2H, CH2CH2CH3), 1.36–1.30 (m, 2H, CH2CH3), 0.93–0.89 (t, 3H, CH3). 13C NMR: δ 171.22, 169.44, 136.34, 135.77, 132.78, 130.58, 130.50, 128.06, 126.82, 122.61, 121.70, 119.89, 118.89, 111.11, 110.60, 57.44 (C1), 54.44 (C3), 52.29, 39.02, 31.55, 30.95, 24.61, 20.10, 13.77. MS: m/z 458 (M+ 4), m/z 456 (M+ + 2, 100%), m/z 454 (M+). Elemental analysis: calculated for (C24H23Cl2N3O2) C, H, N.
(6R,12aS) 6-(2,4-Dichlorophenyl)-2-butyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (58)
Yield 35%; yellow powder; mp 207–210 °C; Rf = 0.51 (CH2Cl2/MeOH 98:2). IR (cm−1): 3399 (–NH–), 1699 (–CO–), 1653 (–CO–). 1H NMR: δ 8.44–8.41 (d, 1H, NH), 7.52–7.50 (d, 2H, Ar), 7.42–7.45 (d, 1H, Ar), 7.35–7.12 (m, 3H, Ar), 7.06–7.01 (d, 1H, Ar), 6.70 (d, 1H, CHPh), 5.31 (s, 1H, CHC(O)N), 4.21–4.19 (dd, 1H, CHaHb), 4.08–3.98 (m, 2H, CH2C(O)N), 2.99–2.95 (m, 1H, CHaHb), 2.17–2.16 (t, 2H, NCH2), 1.57–1.54 (m, 2H, CH2CH2CH3), 1.36–1.32 (m, 2H, CH2CH3), 0.97–0.93 (t, 3H, CH3). 13C NMR: δ 171.21, 165.59, 136.35, 135.00, 133.66, 131.84, 130.18, 129.13, 127.13, 126.15, 122.96, 120.20, 118.39, 111.37, 109.09, 53.45, 53.02 (C1), 49.39 (C3), 49.34, 45.73, 30.95, 29.70, 19.93, 13.79. MS: m/z 458 (M+ + 4), m/z 456 (M+ + 2, 100%), m/z 454 (M+). Elemental analysis: calculated for (C24H23Cl2N3O2) C, H, N.
(6R,12aR) 6-(2,4-Dichlorophenyl)-2-tert-butyl-2,3,6,7,12,12a-hexahydropyrazino [1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (59)
Yield 32%; white powder; mp 140–142 °C; Rf = 0.65 (CH2Cl2/MeOH 98:2). IR (cm−1): 3309 (–NH–), 1724, 1666(–CO–). 1H NMR: δ 8.15 (s, 1H, NH), 7.54–7.12 (m, 7H, Ar), 6.62 (s, 1H, CHPh), 4.29–4.25 (dd, 1H, CHC(O)N), 4.21–3.96 (m, 3H, CH2C(O)N + CHaHb), 3.71–3.69 (dd, 1H, CHaHb), 1.52 (s,9H, C(CH3)3). 13C NMR: δ 167.55, 167.12, 164.12, 138.84, 136.00, 133.66, 129.43, 128.28, 127.35, 127.15, 122.79, 120.24, 118.53, 111.32, 109.39, 106.67, 58.15 (C1), 57.12, 53.90 (C3), 53.25, 47.19, 27.96, 26.82, 24.11. MS: m/z 459 (M+ + 4), m/z 457 (M+ + 2), m/z 455 (M+), m/z 57 (100%). Elemental analysis: calculated for (C24H23Cl2N3O2) C, H, N.
(6S,12aR) 6-(2,4-Dichlorophenyl)-2-tert-butyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (60)
Yield 30%; white powder; mp 200–202 °C; Rf = 0.64 (CH2Cl2/MeOH 98:2). IR (cm−1): 3399 (–NH–), 1741, 1663 (–CO–). 1H NMR: δ 8.27(s, 1H, NH), 7.52–7.06 (m, 7H, Ar), 6.70 (s, 1H, CHPh), 4.24–4.20 (m, 1H, CHC(O)N), 4.15–3.97 (m, 3H, CH2C(O)N + CHaHb), 3.44–3.40 (dd, 1H, CHaHb), 1.51 (s,9H, C(CH3)3). 13C NMR: δ 166.73, 164.12, 136.36, 135.54, 133.65, 131.81, 130.19, 129.11, 127.14, 126.22, 122.95, 120.20, 118.40, 111.35, 109.30, 55.05(C1), 53.89, 53.45 (C3), 52.84, 49.25, 46.63, 27.96, 27.68, 26.83. MS: m/z 459 (M+ + 4), m/z 457 (M+ + 2), m/z 455 (M+), m/z 57 (100%). Elemental + analysis: calculated for (C24H23Cl2N3O2) C, H, N.
(6S,12aS) 6-(2,4-Dichlorophenyl)-2-tert-butyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (61)
Yield 32%; yellow powder; mp 145–148 °C; Rf = 0.62 (CH2Cl2/MeOH 98:2). IR (cm−1): 3320 (–NH–), 1710 (–CO–), 1665 (–CO–). 1H NMR: δ 8.95 (s, 1H, NH), 7.53–7.51 (m, 1H, Ar), 7.46–7.42 (d, 2H, Ar), 7.27–7.12 (m, 4H, Ar), 6.62 (s, 1H, CHPh), 4.31–4.25 (dd, 1H, CHC(O)N), 3.99–3.95 (dd, 1H, CHaHb), 3.46–3.42 (m, 2H, CH2C(O)N), 2.96–2.92 (m, 1H, CHaHb), 1.48 (s, 9H, C(CH3)3). 13C NMR: δ 167.55, 167.12, 166.72, 164.12, 131.81, 130.21, 129.42, 128.27, 127.14, 120.23, 118.52, 118.40, 111.25, 109.36, 106.67, 58.15 (C1), 57.12, 53.89 (C3), 53.26, 47.19, 46.63, 27.96, 24.10. MS: m/z 458 (M+ + 4), m/z 456 (M+ + 2, 100%), m/z 454 (M+). Elemental analysis: calculated for (C24H23Cl2N3O2) C, H, N.
(6R,12aS) 6-(2,4-Dichlorophenyl)-2-tert-butyl-2,3,6,7,12,12a exahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (62)
Yield 25%; yellow powder; mp 198–200 °C; Rf = 0.61 (CH2Cl2/MeOH 99.5:0.5). IR (cm−1): 3387 (–NH–), 1741 (–CO–), 1663 (–CO–). 1H NMR: δ 8.11–8.07 (d, 1H, NH), 7.51–7.49 (m, 2H, Ar), 7.27–7.09 (m, 5H, Ar), 6.58 (s, 1H, CHPh), 5.29–5.28 (dd, 1H, CHC(O)N), 3.66–3.61 (dd, 1H, CHaHb), 3.45–3.42 (m, 2H, CH2C(O)N), 2.96–2.91 (dd, 1H, CHaHb), 1.07 (s, 9H, C(CH3)3). 13C NMR: δ 173.39, 171.76, 167.34, 131.56, 129.61, 129.42, 128.93, 128.88, 128.01, 127.04, 125.92, 120.05, 119.91, 118.32, 111.24, 105.97, 104.65, 55.95 (C1), 53.44 (C3), 50.51, 46.00, 28.63, 24.05. MS: m/z 458 (M+ + 4), m/z 456 (M+ + 2, 100%), m/z 454 (M+). Elemental analysis: calculated for (C24H23Cl2N3O2) C, H, N.
(6R,12aR) 6-(3,4-Dichlorophenyl)-2-tert-butyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido [3,4-b]indole-1,4-dione (63)
Yield 36%; white powder; mp 299–303 °C; Rf = 0.62 (CH2Cl2/MeOH 98:2). IR (cm−1):3387 (–NH–), 1695 (–CO–), 1652 (–CO–). 1H NMR: δ 7.96 (s, 1H, NH), 7.55–7.53 (d, 2H, Ar), 7.43–7.31 (d, 2H, Ar), 7.28–7.00 (m, 3H, Ar), 6.71 (s, 1H, CHPh), 4.35–4.28 (dd, 1H, CHC(O)N), 4.66–4.60 (dd, 1H, CHaHb), 4.14–4.00 (m, 3H, CH2C(O)N + CHaHb), 1.27 (s, 9H, C(CH3)3). MS: m/z 459 (M+ + 4), m/z 457 (M+ + 2), m/z 455 (M+, 100%). Elemental analysis: calculated for (C24H23Cl2N3O2) C, H, N.
(6S,12aR) 6-(3,4-Dichlorophenyl)-2-tert-butyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (64)
Yield 35%; yellow powder; mp 93–96 °C; Rf = 0.62 (CH2Cl2/MeOH 98:2). IR (cm−1):3377 (–NH–), 1662 (–CO–), 1653 (–CO–). 1H NMR: δ 8.01 (s, 1H, NH), 7.57–7.55 (d, 2H, Ar), 7.43–7.41 (d, 2H, Ar), 7.29–7.14 (m, 3H, Ar), 6.78 (s, 1H, CHPh), 3.97–3.93 (dd, 1H, CHC(O)N), 3.73–3.26 (m, 3H, CH2C(O)N + CHaHb), 3.26–3.25 (dd, 1H, CHaHb), 1.27 (s, 9H, C(CH3)3). MS: m/z 459 (M+ + 4), m/z 457 (M+ + 2), m/z 455 (M+, 100%). Elemental analysis: calculated for (C24H23Cl2N3O2) C, H, N.
(6S,12aS) 6-(3,4-Dichlorophenyl)-2-tert-butyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (65)
Yield 38%; yellow powder; mp 298–301 °C; Rf = 0.62 (CH2Cl2/MeOH 98:2). IR (cm−1): 3322 (–NH–), 1715, 1663 (–CO–). 1H NMR: δ 7.87 (s, 1H, NH), 7.55–7.54 (d, 2H, Ar), 7.40–7.33 (d, 2H, Ar), 7.27–7.01 (m, 3H, Ar), 6.61 (s, 1H, CHPh), 4.30–4.25 (dd, 1H, CHC(O)N), 4.14–4.00 (m, 3H, CH2C(O)N + CHaHb), 4.60–4.57 (dd, 1H, CHaHb), 1.29 (s, 9H, C(CH3)3). MS: m/z 459 (M+ + 4), m/z 457 (M+ + 2), m/z 455 (M+), m/z 375 (100%). Elemental analysis: calculated for (C24H23Cl2N3O2) C, H, N.
(6R,12aS) 6-(3,4-Dichlorophenyl)-2-tert-butyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (66)
Yield 36%; yellow powder; mp 95–97 °C; Rf = 0.61 (CH2Cl2/MeOH 98:2). IR (cm−1): 3300 (–NH–), 1695 (–CO–), 1662 (–CO–). 1H NMR: δ 8.89 (s, 1H, NH), 7.70–7.65 (d, 1H, Ar), 7.45–7.42 (d, 1H, Ar), 7.29–7.14 (m, 5H, Ar), 6.80 (s, 1H, CHPh), 3.97–3.93 (dd, 1H, CHC(O)N), 3.73–3.26 (m, 3H, CH2C(O)N + CHaHb), 3.26–3.25 (dd, 1H, CHaHb), 1.26 (s, 9H, C(CH3)3). MS: m/z 459 (M+ + 4), m/z 457 (M+ + 2), m/z 455 (M+), m/z 375 (100%). Elemental analysis: calculated for (C24H23Cl2N3O2) C, H, N.
(6R,12aR) 6-(2,6-Dichlorophenyl)-2-tert-butyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (67)
Yield 26%; yellowish white powder; mp 99–103 °C; Rf = 0.61 (CH2Cl2/MeOH 98:2). IR (cm−1): 3317 (–NH–), 1730, 1685 (–CO–). 1H NMR: δ 8.99 (s, 1H, NH), 7.58–7.15 (m, 7H, Ar), 6.31 (s, 1H, CHPh), 4.32–4.31 (m, 1H, CHC(O)N), 4.17–4.07 (m, 3H, CH2C(O)N + CHaHb), 3.92–3.85 (dd, 1H, CHaHb), 1.27 (s,9H, C(CH3)3). MS: m/z 459 (M+ + 4), m/z 457 (M+ + 2), m/z 455 (M+), m/z 57 (100%). Elemental analysis: calculated for (C24H23Cl2N3O2) C, H, N.
(6S,12aS) 6-(2,6-Dichlorophenyl)-2-tert-butyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione (68)
Yield 23%; yellowish–white powder; mp 90–92 °C; Rf = 0.63 (CH2Cl2/MeOH 98:2). IR (cm−1): 3320 (–NH–), 1731, 1690 (–CO–). 1H NMR: δ 8.99 (s, 1H, NH), 7.60–7.13 (m, 7H, Ar), 6.24 (s, 1H, CHPh), 4.11–4.09 (m, 1H, CHC(O)N), 4.07–3.80 (m, 3H, CH2C(O)N + CHaHb), 3.39–3.32 (dd, 1H, CHaHb), 1.26 (s,9H, C(CH3)3). MS: m/z 459 (M+ + 4), m/z 457 (M+ + 2), m/z 455 (M+), m/z 57 (100%). Elemental analysis: calculated for (C24H23Cl2N3O2) C, H, N.
Biology
Phosphodiesterase Assay
Human recombinant PDE 5A (12.5 units/mL), 10 units/mL PDE3B, 400 units/mL PDE4B, or 50 units/mL PDE11A (BPS Biosciences) were added to the wells of black 96-well nonbinding plates. Immediately, the protein was treated with compound or vehicle control. Enzyme was incubated with drug for 30 min prior to addition of 50 nM TAMRA-cGMP and/or 50 nM fluorescein-cAMP (Molecular Devices) were added to each assay well. The plates were incubated for 1.5 h at 30 °C. After incubation, immobilized metal affinity for phosphochemicals (IMAP)-based fluorescence polarization (FP) phosphodiesterase evaluation assay (Molecular Devices) binding reagent was added to each well, and the plates were incubated for an additional 30 min at 30 °C. FP was measured according to manufacturer’s specifications using a Biotek Synergy 4 plate reader.
Cell Cultures
MDA-MB-231 breast tumor cells were obtained from ATCC. They were grown under standard cell culture conditions at 37 °C in a humidified atmosphere with 5% CO2. Cells were grown in RPMI 1640 supplemented with 5% fetal bovine serum, 2 mM glutamine, 100 units/mL penicillin, 100 units/mL streptomycin, and 0.25 μg/mL amphotericin. Cells were harvested at 70–90% confluence with trypsin/EDTA and used immediately. Cell count and viability was determined by Trypan blue staining followed by hemocytometry. Only cultures displaying >95% cell viability were used for experiments.
Growth Assays
Tissue culture treated microtiter 96-well plates were seeded at a density of 5000 cells/well. The plates were incubated for 18–24 h prior to any treatment. All test compounds were solubilized in 100% DMSO and diluted with media to obtain a final DMSO concentration of 0.1%. Cells were dosed, incubated at 37 °C, and cell viability measured 72 h after treatment by the Cell Titer Glo Assay (Promega), which is a luminescent assay that is an indicator of live cells as a function of metabolic activity and ATP content. The assay was performed according to manufacturer’s specifications. Luminescence was measured by a Perkin-Elmer Victor multilabel plate reader.
Experimental Design and Data Analysis
Drug effects on tumor cell growth and PDE activity were measured and potency was expressed by an IC50 value (50% inhibitory concentration). For growth assays, the IC50 value was determined by testing a range of eight concentrations with quadruple replicates per concentration. For enzyme assays, the IC50 value was determined by testing a range of 10 concentrations with quadruple replicates per concentration. Dose response curves were analyzed using PrismTM 4 software (GraphPad) to calculate IC50 values using a four parameter logistic equation.
Molecular Modeling
The crystal structure of tadalafil in complex with PDE5 (PDB 1UDU) was prepared for docking using Molecular Operating Environment software (MOE) 2009.10 (CCG). Addition of hydrogens and assignment of correct protonation states of all residues at physiological pH = 7.4 was performed using the Protonate3D module in MOE. The compound structures were drawn using ChemSketch. We employed a Pipeline Pilot (SciTegic Inc.) workflow to systematically name and ionize the compounds (pH = 7.4). In the same workflow, we set a chiral flag for each compound using the ChemAxon Standardizer component to keep the molecules in their correct stereoconfigurations during later steps. We then employed the wash and minimization procedures in MOE to prepare the final compound library for docking.
The docking procedure was performed with GOLD v3.2 (CCDC). For all docking runs, we used default parameters. The genetic algorithm was set to automatic mode at 200% search effiency. The binding site was defined by specifying the coordinates of the cocrystallized ligand tadalafil. The active site radius setting remained at the default value of 10 Å with cavity detection enabled.
Three independent docking experiments were done for all compounds, employing the three implemented scoring functions in GOLD (GoldScore, ChemScore, and ASP) to drive the genetic algorithm (GA). In each docking experiment, a total of 10 GA runs per compound were conducted, resulting in 10 binding poses for each compound per experiment. Thus, we obtained a total of 30 poses per compound.
All poses generated with one scoring function were then rescored in GOLD utilizing the remaining two scoring functions. Further rescoring of all poses was performed with DrugScoreCSD and DrugScorePDB using the DrugScore Online service v0.9 (http://pc1664.pharmazie.uni-marburg.de/drugscore/). Finally, a consensus score was calculated amounting to the arithmetic mean of the normalized Goldscore, Chemscore, ASP, DrugScoreCSD, and DrugScorePDB values. The depicted binding modes represent the docking pose with the optimal consensus score.
Supplementary Material
Table 2.
Chemical Structure, Growth Inhibition of MDA-MB-231 Breast Tumor Cell Line, and PDE5 Inhibitory Activity of Tetrahydro-β-carboline-hydantoins (11–30)
| compd | stereochemistry | X,Y | R | % growth inhibition at 10 μMa | growth inhibition IC50 (μM) and CI at p < 0.05b | % PDE 5 inhibition at 10 μMa | PDE 5 inhibition IC50 (μM) and CI at p < 0.05b |
|---|---|---|---|---|---|---|---|
| 11 | 5R,11aR | 2,4-dichloro | –C2H5 | 39 | ND | 95 | 0.010 (0.005–0.024) |
| 12 | 5S,11aR | 2,4-dichloro | –C2H5 | 54 | ND | 90 | 0.090 (0.08–0.11) |
| 13 | 5S,11aS | 2,4-dichloro | –C2H5 | 29 | ND | 85 | 0.85 (0.67–1.07) |
| 14 | 5R,11aS | 2,4-dichloro | –C2H5 | 68 | 5.37 (4.71–6.11) | 95 | 0.007 (0.004–0.009) |
| 15 | 5R,11aR | 3,4-dichloro | –C2H5 | 50 | ND | 95 | 0.15 (0.10–0.24) |
| 16 | 5S,11aR | 3,4-dichloro | –C2H5 | 60 | ND | 78 | 2.86 (2.40–3.40) |
| 17 | 5S,11aS | 3,4-dichloro | –C2H5 | 78 | 4.78 (1.03–12.12) | 60 | 8.75 (6.90–10.64) |
| 18 | 5R,11aS | 3,4-dichloro | –C2H5 | 18 | ND | 90 | 0.30 (0.23–0.40) |
| 19 | 5R,11aR | 2,6-dichloro | –C2H5 | 55 | ND | 18 | ND |
| 20 | 5S,11aS | 2,6-dichloro | –C2H5 | 52 | ND | 37 | ND |
| 21 | 5R,11aR | 2,4-dichloro | –C-(CH3)3 | 68 | 2.26 (1.52–3.33) | 80 | 0.59 (0.41–0.85) |
| 22 | 5S,11aR | 2,4-dichloro | –C-(CH3)3 | 65 | 0.64 (0.50–0.80) | 73 | 1.48 (1.19–1.84) |
| 23 | 5S,11aS | 2,4-dichloro | –C-(CH3)3 | 22 | ND | 75 | 4.14 (3.27–5.23) |
| 24 | 5R,11aS | 2,4-dichloro | –C-(CH3)3 | 69 | 1.26 (1.04–1.53) | 95 | 0.070 (0.06–0.08) |
| 25 | 5R,11aR | 3,4-dichloro | –C-(CH3)3 | 99 | 3.25 (1.46–4.0) | 82 | 1.87 (1.53–2.28) |
| 26 | 5S,11aR | 3,4-dichloro | –C-(CH3)3 | 58 | ND | 65 | 6.38 (5.01–8.12) |
| 27 | 5S,11aS | 3,4-dichloro | –C-(CH3)3 | 70 | 1.43 (0.94–3.79) | 80 | 2.31 (1.79–2.97) |
| 28 | 5R,11aS | 3,4-dichloro | –C-(CH3)3 | 60 | ND | 88 | 0.012 (0.005–0.024) |
| 29 | 5R,11aR | 2,6-dichloro | –C-(CH3)3 | 38 | ND | 52 | ND |
| 30 | 5S,11aS | 2,6-dichloro | –C-(CH3)3 | 15 | ND | 25 | ND |
Calculated from duplicate values.
Calculated from at least 8 concentrations, each with quadruple replicates, p < 0.05.
Table 3.
Chemical Structure, Growth Inhibition of MDA-MB-231 Breast Tumor Cell Line, and PDE5 Inhibitory Activity of 1,2,3-Trisubstituted-tetrahydro-β-carbolines (31–40)
| compd | stereochemistry | X,Y | % growth inhibition at 10 μMa | growth inhibition IC50 (μM) and CI at p < 0.05b | % PDE 5 inhibition at 10 μMa | PDE 5 inhibition IC50 (μM) and CI at p < 0.05b |
|---|---|---|---|---|---|---|
| 31 | 1R,3R | 2,4-dichloro | 98 | 0.56 (0.52–0.61) | 66 | 2.30 (1.71–3.08) |
| 32 | 1S,3R | 2,4-dichloro | 98 | 0.07 (0.05–0.11) | 25 | ND |
| 33 | 1S,3S | 2,4-dichloro | 55 | ND | 99 | 0.38 (0.25–0.44) |
| 34 | 1R,3S | 2,4-dichloro | 98 | 1.24 (1.05–1.48) | 38 | ND |
| 35 | 1R,3R | 3,4-dichloro | 40 | ND | 40 | ND |
| 36 | 1S,3R | 3,4-dichloro | 50 | ND | 30 | ND |
| 37 | 1S,3S | 3,4-dichloro | 43 | ND | 20 | ND |
| 38 | 1R,3S | 3,4-dichloro | 55 | ND | 40 | ND |
| 39 | 1R,3R | 2,6-dichloro | 15 | ND | 27 | ND |
| 40 | 1S,3S | 2,6-dichloro | 30 | ND | 67 | 0.73 (0.64–0.83) |
Calculated from duplicate values.
Calculated from at least 8 concentrations, each with quadruple replicates, p < 0.05.
Table 4.
Chemical Structure, Growth Inhibition of MDA-MB-231 Breast Tumor Cell Line, and PDE5 Inhibitory Activity of Tetrahydro-β-carboline-piperazinedione Derivatives (41–68)
| compd | stereochemistry | X,Y | R | % growth inhibition at 10 μMa | growth inhibition IC50 (μM)b and CI at p < 0.05 | % PDE 5 inhibition at 10 μMa | PDE 5 inhibition IC50 (μM)b and CI at p < 0.05 |
|---|---|---|---|---|---|---|---|
| 41 | 6R,12aR | 2,4-dichloro | –CH3 | 10 | ND | 95 | 0.050 (0.03–0.068) |
| 42 | 6S,12aR | 2,4-dichloro | –CH3 | 65 | 3.26 (2.52–4.20) | 90 | 1.20 (0.82–2.34) |
| 43 | 6S,12aS | 2,4-dichloro | –CH3 | 70 | 7.10 (4.70–9.33) | 82 | 0.85 (0.70–1.03) |
| 44 | 6R,12aS | 2,4-dichloro | –CH3 | 23 | ND | 95 | 0.050 (0.041–0.067) |
| 45 | 6R,12aR | 2,4-dichloro | –C2H5 | 30 | ND | 91 | 0.002 (0.0006–0.008) |
| 46 | 6S,12aR | 2,4-dichloro | –C2H5 | 66 | 1.54 (1.21–1.95) | 90 | 0.26 (0.14–0.48) |
| 47 | 6S,12aS | 2,4-dichloro | –C2H5 | 52 | ND | 85 | 0.99 (0.87–1.13) |
| 48 | 6R,12aS | 2,4-dichloro | –C2H5 | 20 | ND | 95 | 0.010 (0.006–0.022) |
| 49 | 6R,12aR | 3,4-dichloro | –C2H5 | 30 | ND | 80 | 1.34 (0.60–2.87) |
| 50 | 6S,12aR | 3,4-dichloro | –C2H5 | 44 | ND | 45 | ND |
| 51 | 6S,12aS | 3,4-dichloro | –C2H5 | 35 | ND | 67 | 3.30 (1.11–4.60) |
| 52 | 6R,12aS | 3,4-dichloro | –C2H5 | 50 | ND | 78 | 2.04 (1.86–2.22) |
| 53 | 6R12aR | 2,6-dichloro | –C2H5 | 31 | ND | 40 | ND |
| 54 | 6S,12aS | 2,6-dichloro | –C2H5 | 24 | ND | 28 | ND |
| 55 | 6R,12aR | 2,4-dichloro | –C4H9 | 45 | ND | 95 | 0.27 (0.19–0.37) |
| 56 | 6S,12aR | 2,4-dichloro | –C4H9 | 65 | 1.20 (0.66–2.18) | 69 | 1.11 (0.56–2.21) |
| 57 | 6S,12aS | 2,4-dichloro | –C4H9 | 82 | 3.28 (2.75–3.86) | 78 | 4.46 (3.70–5.36) |
| 58 | 6R,12aS | 2,4-dichloro | –C4H9 | 42 | ND | 89 | 0.29 (0.23–0.35) |
| 59 | 6R,12aR | 2,4-dichloro | –C-(CH3)3 | 15 | ND | 94 | 0.19 (0.09–0.43) |
| 60 | 6S,12aR | 2,4-dichloro | –C-(CH3)3 | 70 | 2.92 (1.61–5.29) | 70 | 1.26 (0.70–2.35) |
| 61 | 6S,12aS | 2,4-dichloro | –C-(CH3)3 | 71 | 2.59 (1.48–4.56) | 78 | 1.35 (1.08–1.69) |
| 62 | 6R,12aS | 2,4-dichloro | –C-(CH3)3 | 30 | ND | 70 | 0.26 (0.20–0.32) |
| 63 | 6R,12aR | 3,4-dichloro | –C-(CH3)3 | 36 | ND | 35 | ND |
| 64 | 6S,12aR | 3,4-dichloro | –C-(CH3)3 | 40 | ND | 30 | ND |
| 65 | 6S,12aS | 3,4-dichloro | –C-(CH3)3 | 58 | ND | 35 | ND |
| 66 | 6R,12aS | 3,4-dichloro | –C-(CH3)3 | 50 | ND | 85 | 1.27 (1.11–1.45) |
| 67 | 6R,12aR | 2,6-dichloro | –C-(CH3)3 | 16 | ND | 21 | ND |
| 68 | 6S,12aS | 2,6-dichloro | –C-(CH3)3 | 20 | ND | 21 | ND |
Calculated from duplicate values.
Calculated from at least 8 concentrations, each with quadruple replicates, p < 0.05.
Footnotes
Abbreviations: cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; PDEs, phosphodiesterases; PDE5, phosphodiesterase5; PDE3B, phosphodiesterase3B; PDE4, phospho-diesterase4; FDA, Food and Drug Administration; t-butyl, tertiary-butyl; CH2Cl2, methylene chloride; NOESY, nuclear Overhauser effect spectroscopy; Rf, retardation factor; μM, micromolar; PDB, Protein Data Bank; TMS, tetramethylsilane; TLC, thin layer chromatography; IMAP, immobilized metal affinity for phosphochemicals; FP, fluorescence polarization; TAMRA-cGMP, tetramethylrhodamine-cGMP.
Supporting Information Available: Results from elemental analyses for compounds 1–68 and the NOESY spectra for compounds 1 and 2. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 2.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;16:309–327. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 3.Ghofrani HA, Osterloh IH, Grimminger F. Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Nature Rev Drug Discovery. 2006;5:689–702. doi: 10.1038/nrd2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masson P, Lambert SM, Brown M, Shabsigh R. PDE-5 inhibitors: current status and future trends. Urol Clin North Am. 2005;32:511–525. doi: 10.1016/j.ucl.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe H, Ohashi K, Takeuchi K, Yamashita K, Yokoyama T, Tran QK, Satoh H, Terada H, Ohashi H, Hayashi H. Sildenafil for primary and secondary pulmonary hypertension. Clin Pharmacol Ther. 2002;71:398–402. doi: 10.1067/mcp.2002.123554. [DOI] [PubMed] [Google Scholar]
- 6.Tinsley HN, Gary BD, Keeton AB, Zhang W, Abadi AH, Reynolds RC, Piazza GA. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP and activation of protein kinase G. Mol Cancer Ther. 2009;8:3331–3340. doi: 10.1158/1535-7163.MCT-09-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abadi AH, Abouel-Ella DA, Ahmed NS, Gary BD, Thaiparambil JT, Tinsley HN, Keeton AB, Piazza GA. Synthesis of novel tadalafil analogues and their evaluation as phosphodiesterase inhibitors and anticancer agents. Arzneim Forsch. 2009;59:415–421. doi: 10.1055/s-0031-1296417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins PR, Wilson J, Emmerson D, Garcia MD, Smith MR, Gray SJ, Britton RG, Mahale S, Chaudhuri B. Design, synthesis and biological evaluation of new tryptamine and tetrahydro-beta-carboline-based selective inhibitors of CDK4. Bioorg Med Chem. 2008;16:7728–7739. doi: 10.1016/j.bmc.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Leteurtre F, Madalengoitia J, Orr A, Guzi TJ, Lehnert E, Macdonald T, Pommier Y. Rational design and molecular effects of a new topoisomerase II inhibitor, azatoxin. Cancer Res. 1992;52:4478–4483. [PubMed] [Google Scholar]
- 10.Sunder-Plassmann N, Sarli V, Gartner M, Utz M, Seiler J, Huemmer S, Mayer TU, Surrey T, Giannis A. Synthesis and biological evaluation of new tetrahydro-beta-carbolines as inhibitors of the mitotic kinesin Eg5. Bioorg Med Chem. 2005;13:6094–6111. doi: 10.1016/j.bmc.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 11.Doggrell SA. Comparison of clinical trials with sildenafil, vardenafil and tadalafil in erectile dysfunction. Expert Opin Pharmacother. 2005;6:75–84. doi: 10.1517/14656566.6.1.75. [DOI] [PubMed] [Google Scholar]
- 12.Duan H, Zheng J, Lai Q, Liu Z, Tian G, Wang Z, Li J, Shen J. 2-Phenylquinazolin-4(3H)-one, a class of potent PDE5 inhibitors with high selectivity versus PDE6. Bioorg Med Chem Lett. 2009;19:2777–2779. doi: 10.1016/j.bmcl.2009.03.125. [DOI] [PubMed] [Google Scholar]
- 13.Al-Shaiji TF, Brock GB. Phosphodiesterase type 5 inhibitors for the management of erectile dysfunction: preference and adherence to treatment. Curr Pharm Des. 2009;15:3486–3495. doi: 10.2174/138161209789206944. [DOI] [PubMed] [Google Scholar]
- 14.Sung BJ, Hwang KY, Jeon YH, Lee JI, Heo YS, Kim JH, Moon J, Yoon JM, Hyun YL, Kim E, Eum SJ, Park SY, Lee JO, Lee TG, Ro S, Cho JM. Structure of the catalytic domain of human phosphodiesterase 5 with bound drug molecules. Nature. 2003;425:98–102. doi: 10.1038/nature01914. [DOI] [PubMed] [Google Scholar]
- 15.Ke H, Wang H. Crystal structures of phosphodiesterases and implications on substrate specificity and inhibitor selectivity. Curr Top Med Chem. 2007;7:391–403. doi: 10.2174/156802607779941242. [DOI] [PubMed] [Google Scholar]
- 16.Zoraghi R, Francis SH, Corbin JD. Critical amino acids in phosphodiesterase-5 catalytic site that provide for high-affinity interaction with cyclic guanosine monophosphate and inhibitors. Biochemistry. 2007;46:13554–13563. doi: 10.1021/bi7010702. [DOI] [PubMed] [Google Scholar]
- 17.Whaley WM, Govindachari TR. The Pictet–Spengler synthesis of tetrahydroisoquinolines and related compounds. Org React. 1951;6:151. [Google Scholar]
- 18.Ungemach F, DiPierro M, Weber R, Cook JM. Stereospecific Synthesis of trans-1,3-Disubstituted-l,2,3,4-tetrahydro-β-carbolines. J Org Chem. 1981;46:164–168. [Google Scholar]
- 19.Cox ED, Cook JM. The Pictet–Spengler condensation: a new direction for an old reaction. Chem Rev. 1995;95:1797–1842. [Google Scholar]
- 20.Czerwinski K, Deng L, Cook J. Mechanism driven trans stereospecificity in the Pictet–Spengler reaction. Stereospecific fomation of trans-1,2,3-trisubstituted-tetrahydro-β-carbolines by condensation of Nb-diphenymethyl tryptophan isopropyl esters with aldehydes. Tetrahedron Lett. 1992;48:4721–4724. [Google Scholar]
- 21.Pulka K, Kulis P, Tymecka D, Frankiewicz L, Wilczek M, Kozminski M, Misicka A. Diastereoselective Pictete–Spengler condensation of tryptophan with α-amino aldehydes as chiral carbonyl components. Tetrahedron Lett. 2008;64:1506–1514. [Google Scholar]
- 22.Sandrin J, Soerens D, Cook JM. Carbon-13 NMR of 1,3-Disubstituted 1,2,3,4-Tetrahydro-β-Carbolines. Heterocycles. 1976;4:1249–1255. [Google Scholar]
- 23.Ungemach F, Soerens D, Weber R, DiPierro M, Campos O, Mokry P, Cook JM, Silverton JV. General Method for the Assignment of Stereochemistry of 1,3-Disubstituted 1,2,3,4-Tetrahydro-β-Carbolines by Carbon-13 Spectroscopy. J Am Chem Soc. 1980;102:6976–6984. [Google Scholar]
- 24.Zoraghi R, Corbin JD, Francis SH. Phosphodiesterase-5 Gln817 is critical for cGMP, vardenafil, or sildenafil affinity: its orientation impacts cGMP but not cAMP affinity. J Biol Chem. 2006;281:5553–5558. doi: 10.1074/jbc.M510372200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


