Abstract
Objective
Assess the utility of second-course ophthalmic artery chemosurgery (OAC) for patients with intra-ocular retinoblastoma that recurred following prior ophthalmic artery chemosurgery. This study evaluates the efficacy and toxicity of second-course OAC.
Design
Single-arm, retrospective study of 29 eyes in 30 patients treated with second-course OAC at Memorial Sloan-Kettering Cancer Center between May 2006 and July 2013, with a median 25.9 months follow-up.
Participants
Retinoblastoma patients who underwent a course of OAC, with a minimum of 2 months of progression-free follow-up at monthly examinations, but who subsequently received additional OAC for recurrent tumor.
Methods
Efficacy- Kaplan-Meier survival estimates were generated and the Mantel-Cox test was used to compare curves. Toxicity- Electroretinogram (ERG) amplitudes were measured in response to 30-Hz photopic flicker stimulation before and after OAC treatment; systemic adverse events were graded according to CTCAE 4.0.
Main Outcome Measure
Efficacy- Ocular progression free survival, ocular event (enucleation, external beam radiation or intravitreal melphalan) free survival and ocular survival. Toxicity- Peak-to-peak comparisons between ERG studies before and after OAC treatment; CTCAE 4.0 graded systemic adverse events.
Results
50% of all recurrences were within 4.4 months and 90% were within 16 months of completion of first course OAC. The 2-year Kaplan-Meier ocular survival, event free survival and progression free survival estimates following second-course OAC were 82.8% (95% confidence interval [CI], 60.1–93.2%), 57.3% (95%CI, 36.1–73.7%) and 26.5% (95% confidence interval [CI], 11.0–45.0%), respectively. All eyes without vitreous seeding are progression free, while eyes with vitreous seeding were significantly associated with worse ocular survival following second- course OAC (p=0.03). Following second-course OAC, 90% of eyes had stable or improved electroretinogram responses. Of all evaluable cases, there was no increased risk of systemic toxicity during second course compared to initial course OAC.
Conclusions
Retinoblastoma eyes requiring second-course OAC following initial OAC treatment have good salvage rates and the treatment has an acceptable ocular and systemic toxicity profile. However, these eyes often require additional (third or fourth-course) OAC or other treatment modalities due to progression of disease after second-line OAC, particularly if vitreous seeds are present at the time of initial OAC failure.
Precis
Eyes with recurrent tumor following initial ophthalmic artery chemosurgery can be salvaged with second-course ophthalmic artery chemosurgery, although ocular survival is worse for eyes with vitreous seeds.
Introduction
There is limited information regarding outcomes following retreatment of recurrent retinoblastoma using the same modality that was initially successful. One study cites a salvage rate of 2.2% of eyes with second-course external beam radiation1; however, there is a dearth of published material on second-course systemic chemotherapy. Likewise for chemotherapy delivered via the ophthalmic artery, our knowledge extends only to secondary OAC given after failure of the initial non-OAC therapy (typically systemic chemotherapy or radiation)2–4.
In this study, we consider a critical question, which has yet to be addressed: Is OAC an option for eyes with recurrent disease following initially successful OAC treatment? The efficacy of second-line chemotherapy is an important question for both patients and their physicians. What are the benefits and drawbacks of subjecting a patient to the time, resources and effort of additional chemotherapy? The answer is different depending on the tumor type and the regimen used. Some tumors such as gastric cancer and other solid tumors have responded unsatisfactorily to second-line treatment5, while other tumors (or the same tumors subjected to different regimens) have shown promise6,7. This study will review the cases that required second course OAC and determine the response and associated burden of ocular and systemic toxicity.
Methods
This retrospective, single institution, Institutional Review Board (IRB)-approved study included all eyes of retinoblastoma patients meeting the inclusion criteria treated at Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College from May 30 2006 to July 31 2013. Informed consent was obtained for each patient. This study is HIPAA-compliant and adhered to tenets of the Declaration of Helsinki. The study included patients who underwent a course of OAC that was discontinued due to the clinical impression of disease control, with a minimum of 2 months of progression-free follow-up at monthly examinations, but who subsequently had recurrent tumor and were treated with a second course of OAC. Patient data included age, sex, laterality, weight at start of initial course OAC and second course OAC, prior treatment status (naïve vs. prior treatment involving systemic chemotherapy or radiation therapy), age at initial OAC, and follow-up time from beginning of second-course OAC. Treatment data included time from initial OAC completion to recurrence, number of infusions, drug types and doses during initial and second-course OAC. Tumor data included Reese-Ellsworth (RE) classification, International Classification (IC), absence/presence of vitreous seeds, and response to treatment. A complete blood count (CBC) was requested (or perhaps “ordered”, since they were not always truly “obtained”) after each infusion of OAC and considered evaluable if performed within 7 to 14 days following the previous OAC or if grade 3 or 4 toxicity was noted at any time point. The standard Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 was used to grade hematologic toxicity.
Eyes were initially examined under anesthesia at three to four week intervals. Evaluation consisted of visual assessment, motility and pupillary responses, indirect ophthalmoscopy, fundus photography with RetCam (Massie Industries, Dublin, CA), ophthalmic ultrasonography (OTI ophthalmic technologies, Inc. Toronto, Canada) and and electroretinography (Espion ColorBurst, Diagnosys LLC, Lowell, MA). OAC was performed every three or four weeks in a manner that has previously been described in detail8,9. Focal treatment (TTT or cryotherapy) was performed if indicated.
Reported here are the response amplitudes to 30-Hz photopic flicker stimulation, which are representative of the full ERG protocol. As previously described10, ERG amplitudes were classified according to the following scale: < 0.1 µV : undetectable; 0.1–25 µV: poor; 25.1–50 µV: fair; 50.1–75µV: good; 75.1 – 100µV: very good; >100 µV: excellent. A change in 30-Hz response amplitude of 25µV was considered clinically meaningful, based on statistical analysis of ERGs during examination under anesthesia (EUA) of normal eyes (unpublished data). In the absence of scotopic data, photopic responses to single ISCEV standard light-adapted 3.0 flashes were also analyzed. ERG responses were analyzed in two groups: measurements prior to and one month following completion of 1. initial course OAC and 2. second course OAC. 25 eyes had evaluable ERGs for the initial course OAC and 29 eyes had evaluable ERGs for the second course OAC. Inevaluable ERGs were the result of having no baseline measurements due to the absence of an electrophysiologist.
Outcome measurements included ocular progression free survival, ocular event free survival and ocular survival. Progression of disease was defined as instances of recurrent disease that required additional OAC, external beam radiation, plaque brachytherapy, intravitreal melphalan or enucleation. For event free survival, an event was defined as enucleation, external beam radiation or treatment by injection of intra-vitreal melphalan, but excluded further courses of OAC. This analysis was intended to isolate the potential of salvage OAC from the potentially confounding effect of intra-vitreal chemotherapy. Statistical analysis was performed using Prism (GraphPad Software, Inc. La Jolla, CA). Kaplan-Meier survival data with log rank test was used to evaluate ocular and progression-free and the Mantel-Cox test was used to compare curves. Survival estimates were compared for data that evaluated disease, treatment and drug dose features. Disease features included vitreous seed status (the presence or absence of vitreous seeds at the time of initial OAC failure) and latency of relapse (less than or greater or equal than the median interval of 4.4 mos). Treatment features are shown in Table 2.
Table 2.
Factors related to efficacy of second-course ophthalmic artery chemosurgery
| VARIABLE | COMPARISON | p-value | ||
|---|---|---|---|---|
| n | PFS | OS | ||
| Vitreous seeds | Presence vs. absence of vitreous seeds | 29 | 0.35 | 0.03 |
| Prior treatment status | Prior treated vs naïve | 29 | 0.40 | 0.86 |
| Latency of relapse | Interval btwn initial OAC end & relapse <4.4mos vs >=4.4mos | 29 | 0.22 | 0.34 |
| Age at initial OAC | Age <1yr or <= 1 yr during initial OAC | 29 | 0.45 | 0.15 |
| No. of initial infusions | <3 infusions vs >= 3 infusions during initial OAC | 29 | 0.56 | 0.23 |
| Addition of new drug | Absence vs addition of new drug during 2nd course | 29 | 0.93 | 0.23 |
| No. of 2nd infusions | 1 vs >1 infusion during 2nd course | 29 | 0.37 | 1.00 |
| Max melphalan | Max melphalan <5mg vs >= 5mg during 2nd course | 29 | 0.86 | 0.49 |
| Max melphalan/Kg | Max melphalan/Kg <0.4mg/Kg vs >=0.4mg/Kg during 2nd course | 29 | 0.43 | 0.66 |
| Cumltv melphalan | Cumltv melphalan <8mg vs >= 8mg during 2nd course | 29 | 0.56 | 0.21 |
| Cumltv melphalan/Kg | Cumltv melphalan/Kg <0.6mg/Kg vs >= 0.6mg/Kg during 2nd course | 29 | 0.83 | 0.50 |
| Increasing max melphalan | Increasing vs decreasing max melphalan during 2nd course | 28 | 0.88 | 0.63 |
| Increasing max melphalan/Kg | Increasing vs decreasing max melphalan/Kg during 2nd course | 28 | 0.22 | 0.13 |
| Increasing max topotecan/Kg | Increasing vs decreasing max topotecan/Kg during 2nd course | 20 | 0.04 | 0.28 |
| Increasing max carboplatin/Kg | Increasing vs decreasing max carboplatin/Kg during 2nd course | 12 | 0.42 | 1.00 |
| Increasing max dose any drug | Increasing vs decreasing max dose any drug during 2nd course | 29 | 0.67 | 0.75 |
| Increasing max dose/Kg any drug | Increasing vs decreasing max dose/Kg any drug during 2nd course | 29 | 0.07 | 0.75 |
| Increasing cumltv melphalan | Increasing vs decreasing cumltv melphalan during 2nd course | 29 | 0.85 | 0.40 |
| Increasing cumltv melphalan/Kg | Increasing vs decreasing cumltv melphalan/Kg during 2nd course | 29 | 0.39 | 0.83 |
| Increasing cumltv topotecan/Kg | Increasing vs decreasing cumltv topotecan/Kg during 2nd course | 19 | 0.09 | 0.25 |
| Increasing cumltv carboplatin/Kg | Increasing vs decreasing cumltv carboplatin/Kg during 2nd course | 12 | 0.99 | 1.00 |
| Increasing cumltv any drug | Increasing vs decreasing cumltv dose any drug during 2nd course | 29 | 0.86 | 0.45 |
| Increasing cumtv dose/Kg any drug | Increasing vs decreasing cumtv dose/Kg any drug during 2nd course | 29 | 0.14 | 0.62 |
| Increasing max dose/Kg any drug/new drug | Increasing max dose/Kg any drug or new drug vs. not during 2nd course | 29 | 0.24 | 0.79 |
PFS= progression free survival, OS = ocular survival, cumtv= cumulative, max= maximum, OAC = ophthalmic artery chemosurgery, yr = year, no. = number, vs = versus, cumltv = cumulative, btwn = between, mos= months
Results
Patient/eye characteristics
29 eyes of 28 patients fulfilled the inclusion criteria and were included in this study, with a median follow up of 25.9 mos (range: 1 to 66 mos) from the beginning of second-course OAC. Table 1 summarizes patient, eye, treatment and outcome characteristics, including details of 3rd, 4th or 5th line therapy. Eyes received a median of 3.0 infusions (range 1 to 6) during their initial OAC course and a median of 2 infusions (range 1 to 4) during their second-course. They developed progression of disease and necessity for second-course OAC at a median of 4.4 mos (range 2.8 to 42.5 mos) following completion of initial OAC. 90% (n= 26 of 29) were within 16 months.
Table 1.
Characteristics of patients, eyes and treatment
| Patient | Eye | Age | Sex | Laterality | RE | IC | Prior Tx |
No. initial OAC infusions |
Cumulative drug dose (mg) for initial OAC* |
Latency (mos) |
No. 2nd course infusions |
Cumulative drug dose (mg) for 2nd course OAC* |
3rd, 4th, 5th line tx |
# addtl OAC courses |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | OD | 11.0 | F | B | VA | D | Yes | 3 | M5.5, T0.9, C25 | 7.0 | 3 | M8, C60, T0.8 | EBR | |
| 2 | OD | 6.3 | F | U | VB | D | No | 4 | M11 | 10.2 | 1 | M4,T0.3 | enu | |
| 3 | OS | 197.9 | F | B | VB | E | Yes | 3 | M21, T1.2, C120 | 15.2 | 2 | M8, T0.5, C110 | None | |
| 4 | OD | 3.1 | F | B | IIIA | B | No | 1 | M2 | 20.0 | 3 | M12, T.9 | OAC | 1 |
| 5 | OS | 14.0 | F | B | VB | D | Yes | 3 | M8, T0.3 | 7.0 | 2 | M11, T.9, C70 | OAC, enu | 1 |
| 6 | OD | 24.9 | M | U | VB | D | No | 3 | M15 | 4.3 | 3 | C95 | enu | |
| 7 | OS | 32.5 | M | B | VB | D | Yes | 3 | M12, T0.9 | 13.3 | 1 | M6, T0.4, C30 | Ivit, enu | |
| 8 | OD | 24.5 | M | B | IVA | B | Yes | 1 | M4 | 4.9 | 2 | M9, T0.4 | OAC | 1 |
| 9 | OS | 14.0 | M | B | VB | D | Yes | 1 | M3 | 14.7 | 4 | M25, T1.6 | OAC, enu | 1 |
| 10 | OD | 15.6 | F | B | VB | D | Yes | 3 | M9.5 | 3.3 | 1 | C30 | OAC | 1 |
| 11 | OD | 5.2 | M | U | VA | D | No | 3 | M10, T1 | 3.0 | 1 | M4 | None | |
| 12 | OD | 9.9 | M | U | IVA | C | Yes | 3 | M8, T2, C40 | 3.7 | 2 | M10, T3, C100 | None | |
| 13 | OS | 28.6 | M | B | VB | E | No | 6 | M37.5, T2.5, C250 | 14.5 | 1 | M7.5, T0.5, C50 | plq, Ivit | |
| 14 | OS | 6.9 | M | B | IIA | B | Yes | 3 | M8 | 38.7 | 1 | M7.5, T.5 | OAC | 2 |
| 15 | OD | 7.9 | M | B | IB | B | Yes | 1 | M2.5 | 3.1 | 1 | M3, T.2 | None | |
| 16 | OD | 7.2 | F | B | VB | D | Yes | 3 | M7.5, T.3 | 13.5 | 1 | M4, T.5 | OAC, Ivit | 2 |
| 17 | OD | 25.3 | M | B | VB | D | Yes | 5 | M29, T1.3 | 4.6 | 1 | T.4, C40 | OAC | 1 |
| 18 | OD | 8.9 | F | B | VA | D | No | 3 | M4, T.5, C80 | 3.5 | 2 | M9, T2, C110 | None | |
| 19 | OD | 4.4 | F | B | VA | E | Yes | 3 | M6, T.9, C30 | 2.8 | 1 | M3, T.3, C30 | OAC, Ivit | 1 |
| 20 | OS | 15.7 | M | U | VB | D | No | 4 | M16, T1.3, C30 | 2.8 | 2 | M10, T1.5, C80 | OAC, plq, Ivit |
2 |
| 21 | OD | 33.3 | M | U | VB | D | No | 3 | M15, T..4 | 3.7 | 3 | M15, T1.2 | enu | |
| 22 | OD | 10.6 | F | B | IB | B | No | 1 | M3, T.3 | 4.2 | 1 | M3, T.3 | OAC | |
| OS | 6.7 | B | VB | D | No | 2 | T.8, C60 | 3.0 | 1 | T.3, C30 | None | 2 | ||
| 23 | OD | 63.5 | F | U | VB | D | No | 5 | M28, T1.8, C30 | 7.5 | 2 | M15, T2.5, C30 | OAC, plq | 2 |
| 24 | OD | 8.6 | M | B | VB | D | No | 6 | M21, T1.6, C30 | 3.0 | 2 | M9, T.7, C55 | Ivit | |
| 25 | OD | 53.0 | F | B | IIA | D | Yes | 3 | M15, T.8, C60 | 4.4 | 1 | M6, T.5, C50 | OAC, Ivit | 1 |
| 26 | OS | 10.0 | M | U | VB | D | No | 3 | C150 | 3.5 | 2 | M8, T4 | None | |
| 27 | OS | 96.6 | M | B | VB | D | Yes | 4 | M13 | 8.4 | 2 | M8, T.3 | enu | |
| 28 | OS | 57.3 | F | U | VB | D | No | 4 | M19, T4 | 42.5 | 3 | M18, T.5, C100 | None | |
RE= Reese-Ellsworth at diganosis, IC= International Classification at diagnosis, Tx = treatment, OAC= ophthalmic artery chemosurgery, addtl = additional, OD= right eye, OS= left eye, F = female, M= male, B= bilateral, U= unilateral, EBR= external beam radiation, enu= enucleation, Ivit= intraivtreal melphalan, plq= plaque brachytherapy, *M= melphalan, T= topotecan, C= carboplatin and number refers to dose of drug in mg.
Treatment
55% of eyes (n= 16 of 29) had no increase in maximum melphalan dose/Kg and 62% of eyes (n=18 of 29) had no increase in the maximum dose/Kg of any drug during the second course compared to initial course OAC. Furthermore, 6 eyes had no increase in the maximum drug dose/Kg of any drug, nor addition of a new drug during their second course compared to initial course OAC.
Ocular survival/Event Free survival/Progression Free survival
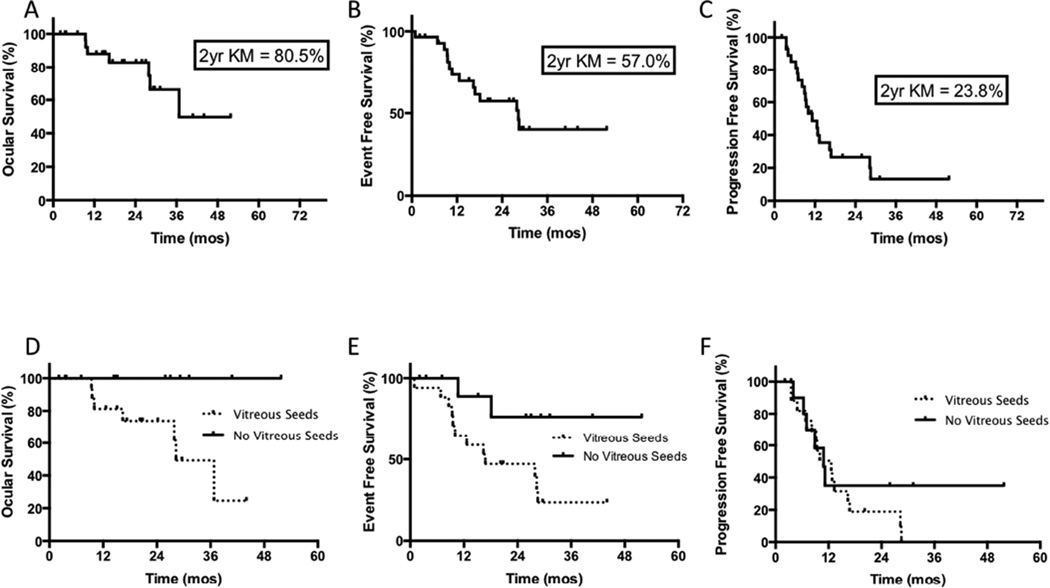
As shown in Figure 1, the 2-year Kaplan-Meier ocular survival, event free survival and progression free survival estimates following second-course OAC were 82.8% (95% confidence interval [CI], 60.1–93.2%), 57.3% (95%CI, 36.1–73.7%) and 26.5% (95% confidence interval [CI], 11.0–45.0%), respectively. Vitreous seeds (Fig. 4) were present in 17 (58%) of eyes requiring second-course OAC and were significantly associated with worse ocular survival and event free survival following second-course OAC (p=0.03 and 0.03, respectively), but not with progression of disease. Kaplan-Meier ocular survival estimates at 2-years following second-course OAC were 100% for eyes without vitreous seeds and 73.9% (95% CI 44.2–89.4%) for eyes with vitreous seeds, and are depicted in Figure 1. Kaplan-Meier event free survival estimates at 2-years following second-course OAC were 76.2% (95%CI 33.2–93.5%) for eyes without vitreous seeds and 47.1% (95% CI 23.0–68.0%) for eyes with vitreous seeds, and are also depicted in Figure 1. During second course OAC, eyes without vitreous seeding received a mean of 1.5 OAC infusions while eyes with vitreous seeding received a mean of 2 OAC infusions. Eight eyes with vitreous seeds were salvaged and five of these (63%) went on to receive intravitreal melphalan at some point in their treatment course. A total of seven eyes received intravitreal melphalan during their treatment course. Representative cases of eyes with and without vitreous seeding at the time of recurrence are shown in Figures 2 and 3.
Figure 1.
Kaplan Meier survival curves for (A) Ocular survival of all eyes, (B) Event free survival of all eyes, (C) Progression free survival of all eyes, (D) Ocular survival comparing eyes with and without vitreous seeds, (E) Event free survival comparing eyes with and without vitreous seeds, (F) Progression free survival of eyes with and without vitreous seeds. Note that ocular survival is significantly worse for eyes with vitreous seeds at time of recurrence.
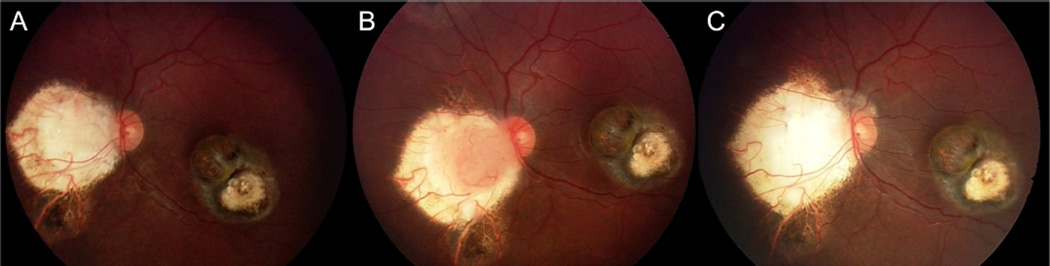
Figure 2.
Representative case without vitreous seeds. Following completion of first course OAC, the tumors have regressed (A). However, the peripapillary tumor recurs (dark arrow) 7 months later (B) and successfully responds to second course OAC (C).
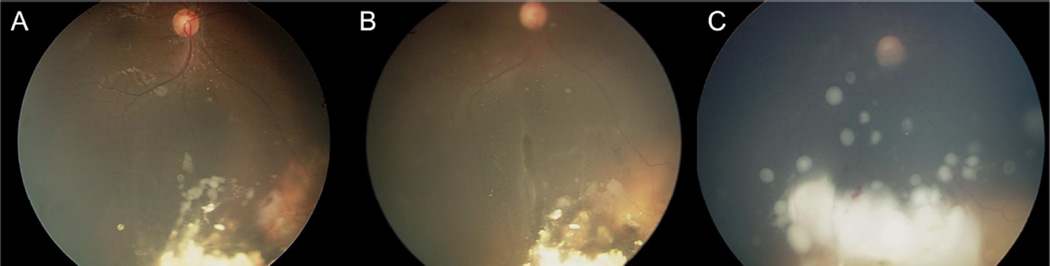
Figure 3.
Representative case with vitreous seeds. Following successful completion of first course OAC, the tumor recurs with vitreous disease (yellow arrows) (A). Recurrent tumor responds to second-course OAC (B) but vitreous disease (yellow arrows) worsens and the eye was enucleated (C).
The other significant factor included significantly worse (p= 0.04) progression free survival for eyes that had an increasing maximum topotecan dose/Kg from initial to second course OAC versus those that did not: 1-year estimates 44.4% (95% CI, 10.3–74.8%) and 54.5 % (95% CI, 22.8–77.9%), respectively. 91% of eyes receiving higher maximum topotecan dose/Kg of any drug during second course OAC had vitreous seeds; compared to 0% of eyes that did not increase maximum dose/Kg. Other factors shown in Table 2 were not significantly associated with progression free survival or ocular survival (p>0.05).
Toxicity
ERG measurements prior to, and following second course OAC were stable in 83%, decreased in 10% and increased in 7% of eyes. This outcome was similar to ERG measurements prior to and following initial course OAC, which were stable in 68%, increased in 12% and decreased in 20% of eyes. The mean change in ERG measurement from baseline was −3.5µV and −4.7µV following initial and second course OAC, respectively.
65.2% (60 of 92) and 70.0% (35 of 50) of CBCs were evaluable during initial and second course OAC, respectively. 25.0% (15 of 60) and 14.2% (5 of 35) of evaluable OAC infusions had grade 3 or 4 hemotologic toxic events during initial and second course OAC, respectively. More specifically, during initial course OAC, there were fourteen events of grade 3 or 4 neutropenia, two events of grade 3 anemia and no grade 3 or 4 thrombocytopenia (a case of neutropenia and anemia occurred together in one instance). During second course OAC, there were five events of grade 3 or 4 neutropenia and no grade 3 or 4 anemia or thrombocytopenia.
Discussion
This study examines the unexplored question of safety and efficacy of second-course OAC in eyes previously treated with initial course OAC. We demonstrate 2 year ocular survival estimates of 82.8% for all eyes, which is notable given over half the eyes received the same or lower weight-corrected maximum dose of drugs during the second-course; and of those eyes, only half were given a new drug during the second-course OAC. Two-year event free survival until enucleation, EBR or intravitreal melphalan was 57% for all eyes and was significantly better for eyes without vitreous seeds compared to those with vitreous disease. This demonstrates that more than half the eyes can be salvaged with multi-line OAC in the absence of intravitreal melphalan or EBR. We also show that ocular and hematologic toxicity is no worse during second-course compared to initial course OAC, despite the higher drug doses for some eyes. For instance, following second-course OAC, 90% of eyes maintain stable or had improved ERG recordings (compared with 80% following initial course OAC). Moreover, 25% of initial course OAC infusions were associated with grade 3 or 4 hematologic toxicity, compared with 14% of second course OAC infusions.
It is striking that all eyes without vitreous seeds could be saved. Eyes with vitreous seeding did not do as well and had significantly worse ocular survival. The dismal progression free survival (26.5% at 2 years for all eyes) is another noticeable trend for eyes receiving second-course OAC, which often require third- and sometimes fourth-course treatment in the form of additional OAC, radiation or intravitreal melphalan. It is to be noted that the majority of these eyes were treated in the pre-intravitreal melphalan era, and with the use of this relatively novel treatment, it is possible that more of the eyes with vitreous seeding could have been saved11–13 (and even spared second-line or multi-line OAC). For instance, in this study the majority (63%) of salvaged eyes with vitreous seeding received intravitreal melphalan at some point following second course OAC.
Another significant factor associated with worse ocular survival was maximum dose of topotecan/Kg during the second course compared to initial course OAC. More eyes receiving higher weight-corrected maximum topotecan doses had vitreous seeding (91% versus 0%), and therefore it is likely that the inferior ocular survival estimates may be reflective of eyes with more advanced tumors and vitreous disease status.
In other fields of oncology, there are established features that predict the success of second-line chemotherapy. For instance, in neuroblastoma, a shorter latency until recurrence predicts poor response to second-line chemotherapy6. In our cohort none of these factors appeared to have had an impact: there was no significant difference between prior treated and naïve eyes, or children receiving initial OAC at an age less than 1 year compared to greater than a year of age, or eyes with a latency to recurrence less than compared to greater than the median of 4.4 mos. There is no clear explanation for this. There may be something about retinoblastoma as a disease or the delivery method of ophthalmic artery chemosurgery that defies the trends of second-line chemotherapy that are put forth by other areas of oncology.
This paper is limited by its retrospective design and the relatively small sample size and should be interpreted with these limitations in mind. While the median follow up is 26 months, it is possible that with a longer duration of follow up, additional recurrences or complications may occur.
In conclusion, eyes with recurrent tumor following initially successful OAC can have good salvage rates following second-course OAC, particularly eyes without vitreous seeding. Second course OAC offers an acceptable ocular and systemic toxicity profile that is no worse than initial course OAC, despite higher drug doses for some eyes. However, these eyes often require additional (third or fourth-course) OAC or other treatment modalities (intravitreal melphalan, plaque) due to progression of disease after second-line OAC. Ocular survival is worse if vitreous seeds are present at the time of initial OAC failure. However, the increased use of intravitreal chemotherapy may improve the outlook for these eyes.
Acknowledgments
Funding: This work was supported by Fund for Ophthalmic Knowledge. The funding organization had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previously presented at The Association for Research in Vision Ophthalmology, May 2014.
Conflict of Interest: No conflicting relationship exists for any author.
References
- 1.Abramson DH, Ellsworth RM, Rosenblatt M, et al. Retreatment of retinoblastoma with external beam irradiation. Arch Ophthalmol. 1982;100:1257–1260. doi: 10.1001/archopht.1982.01030040235004. [DOI] [PubMed] [Google Scholar]
- 2.Bracco S, Leonini S, De Francesco S, et al. Intra-arterial chemotherapy with melphalan for intraocular retinoblastoma. Br J Ophthalmol. 2013;97:1219–1221. doi: 10.1136/bjophthalmol-2013-303267. [DOI] [PubMed] [Google Scholar]
- 3.Shields CL, Manjandavida FP, Lally SE, et al. Intra-arterial chemotherapy for retinoblastoma in 70 eyes: outcomes based on the international classification of retinoblastoma. Ophthalmology. 2014;121:1453–1460. doi: 10.1016/j.ophtha.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 4.Shields CL, Kaliki S, Al-Dahmash S, et al. Management of advanced retinoblastoma with intravenous chemotherapy then intra-arterial chemotherapy as alternative to enucleation. Retina (Philadelphia, Pa) 2013;33:2103–2109. doi: 10.1097/IAE.0b013e318295f783. [DOI] [PubMed] [Google Scholar]
- 5.Wesolowski R, Lee C, Kim R. Is there a role for second-line chemotherapy in advanced gastric cancer? Lancet Oncol. 2009;10:903–912. doi: 10.1016/S1470-2045(09)70136-6. [DOI] [PubMed] [Google Scholar]
- 6.Donfrancesco A, Jenkner A, Castellano A, et al. Ifosfamide/carboplatin/etoposide (ICE) as front-line, topotecan/cyclophosphamide as second-line and oral temozolomide as third-line treatment for advanced neuroblastoma over one year of age. Acta Paediatr Suppl. 2004;93:6–11. doi: 10.1111/j.1651-2227.2004.tb03048.x. [DOI] [PubMed] [Google Scholar]
- 7.Rino Y, Yukawa N, Sato T, et al. Phase II study on the combination of irinotecan plus cisplatin as a second-line therapy in patients with advanced or recurrent gastric cancer. Mol Clin Oncol. 2013;1:749–752. doi: 10.3892/mco.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gobin YP, Dunkel IJ, Marr BP, et al. Intra-arterial chemotherapy for the management of retinoblastoma: four-year experience. Arch Ophthalmol. 2011;129:732–737. doi: 10.1001/archophthalmol.2011.5. [DOI] [PubMed] [Google Scholar]
- 9.Gobin YP, Dunkel IJ, Marr BP, et al. Combined, sequential intravenous and intra-arterial chemotherapy (bridge chemotherapy) for young infants with retinoblastoma. PLoS ONE. 2012;7:e44322. doi: 10.1371/journal.pone.0044322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis JH, Abramson DH, Gobin YP, et al. Electroretinogram monitoring of dose-dependent toxicity after ophthalmic artery chemosurgery in retinoblastoma eyes: six year review. PLoS ONE. 2014;9:e84247. doi: 10.1371/journal.pone.0084247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munier FL, Gaillard M-C, Balmer A, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012 doi: 10.1136/bjophthalmol-2011-301450. [DOI] [PubMed] [Google Scholar]
- 12.Shields CL, Manjandavida FP, Arepalli S, et al. Intravitreal Melphalan for Persistent or Recurrent Retinoblastoma Vitreous Seeds: Preliminary Results. JAMA Ophthalmol. 2014 doi: 10.1001/jamaophthalmol.2013.7666. [DOI] [PubMed] [Google Scholar]
- 13.Francis JH, Schaiquevich P, buitrago E, et al. Local and Systemic Toxicity of Intravitreal Melphalan for Vitreous Seeding in Retinoblastoma: A Preclinical and Clinical Study. Ophthalmology. 2014 doi: 10.1016/j.ophtha.2014.03.028. [DOI] [PubMed] [Google Scholar]