Abstract
Introduction
Treatment non-adherence is common in heart failure and associated with poor health outcomes in this population. Recent cross-sectional work in heart failure and past work in other medical populations suggests cognitive function is a key determinant of patient’s ability to adhere to treatment recommendations. However, it is also possible that treatment adherence is an important modifier and predictor of cognitive function, though no study has examined this possibility and we sought to do so in a sample of heart failure patients.
Methods
115 patients with heart failure self-reported adherence to treatment recommendations. The Modified Mini Mental State Examination (3MS), Trail Making Test parts A and B, and the California Verbal Learning Test-II (CVLT-II) assessed cognitive function. These procedures were performed at baseline and a 12-month follow-up.
Results
Global cognition and memory abilities improved over the 12-month period. Regression analyses controlling for baseline and medical and demographic factors showed better baseline treatment adherence predicted improved 12-month performances on the 3MS and CVLT-II. Adherence to medication and diet regimens and smoking abstinence emerged as the most important contributors.
Conclusions
Better treatment adherence predicted improved cognition one-year later in HF. Prospective studies that utilize objective assessments of treatment adherence are needed to confirm our findings and examine whether improved treatment adherence preserves cognitive function in heart failure.
Keywords: Heart failure, cognitive function, treatment adherence, longitudinal
Introduction
With an annual incidence of 825,000, heart failure (HF) affects approximately >5 million American adults and prevalence rates are expected to dramatically rise over the next 15–20 years (Go et al., 2014). This pattern is alarming in light of the many health and psychosocial consequences associated with HF, including recurrent hospital readmissions, heightened mortality risk, depression, reduced functional independence, and poor quality of life (Roger et al., 2012; Gottlieb et al., 2004; Norberg, Boman, & Lofgren, 2008; Bennett et al., 2003). Indeed, there are many identified contributors to such poor outcomes in HF that include both demographic (e.g., age, gender) and medical factors (e.g., HF severity, fatigue) (Norberg et al., 2008; Bennett et al., 2003; Seo, Roberts, LaFramboise, Yates, & Yurkovich, 2011).
Cognitive impairment is also common in HF and contributes to poor outcomes in this population. Patients’ with HF are at increased risk for Alzheimer’s disease and other forms of dementia (Qiu et al., 2006). Prior to dementia, HF patients also frequently exhibit deficits on formal cognitive tasks assessing global cognition, executive function, and memory (Pressler et al., 2010). Despite this pattern, longitudinal studies examining cognitive function in HF reveal mixed findings, with evidence for both improvement and decline over time in this population (Stanek et al., 2009; Hjelm et al., 2012). Such findings suggest that cognitive impairment in HF is heterogeneous and perhaps dependent on a range of modifying factors such as disease severity, comorbid medical status (e.g., hypertension, diabetes), physical fitness, and/or demographic variables (e.g., age, education) (Pressler et al., 2010; Zuccala et al., 2005; Miller et al., 2012; Alosco et al., 2012).
Although not previously examined, treatment adherence is also likely an important modifier of cognitive function in patients with HF. Reduced treatment adherence is common in older adults with HF due to the complex treatment regimens patients are asked to follow, including medication management, symptom monitoring, and dietary and exercise recommendations (van der Wal et al., 2006; Evangelista, Berg, & Dracup, 2001; Riegal et al., 2009). Notably, up to 20% of HF patients are non-adherent to medication regimens and as many as 53% report not engaging in any form of physical activity (Riegal et al., 2009; Dunlay et al., 2011). This pattern is troubling, as treatment adherence in this population is protective against hospitalizations, deteriorating health status, and mortality risk (Fitzgerald et al., 2011; Kutzleb & Reiner, 2006; Granger et al., 2005).
Recent cross-sectional work in HF and past work among other medical populations suggests cognitive impairment is a key determinant of poor treatment adherence (Alosco et al., 2012; Hawkins et al., 2012; Andrade et al., 2012). However, it is also plausible that treatment adherence predicts cognitive function in HF. For instance, recommended health behaviors HF patients are asked to perform (e.g., medication management, dietary restrictions, exercise regimens) are all independently associated with and known to promote cognitive function (Zuccala et al., 2005; Wengreen, Neilson, Munger, & Corcoran, 2009; Alosco et al., 2013). Moreover, adherence to key treatment recommendations in other medical populations (e.g., sleep apnea) has also been shown to produce cognitive benefits (Zimmerman, Arnedt, Stanchina, Millman, & Aloia, 2006).
Despite these findings, no study to date has prospectively examined the effects of treatment adherence on cognitive function. The purpose of the current study was to examine whether adherence to key treatment recommendations predicts cognitive function at a 12-month follow-up in a sample of older adults with HF. We hypothesized that better treatment adherence at baseline would predict improved cognitive function at the 12-month follow-up.
Methods
Participants
A sample of 115 patients with HF was recruited for participation in a longitudinal National Institutes of Health funded research study examining neurocognitive function over a 12-month period in older adults with HF. The sample size was based on participants that had complete treatment adherence and cognitive data at the baseline and 12-month time points. Thus, the sample was reduced from 145 patients with HF following exclusion of participants for missing data and/or attrition. Participants excluded were not significantly different from the remaining sample in terms of age (t(142) = −0.33, p = 0.97), gender (χ2 (1, N = 145) = 0.57, p = 0.45), or HF severity (t(141) = −1.09, p = 0.28).
All participants were recruited from outpatient cardiology offices and were participating in routine clinical care for cardiac disease. For inclusion, participants must have been between the ages of 50–85 years, English speaking, and had a diagnosis of New York Heart Association (NYHA) HF class II, III, or IV at the time of enrollment. Potential participants were excluded for a history or current diagnosis of a significant neurological disorder (e.g. dementia, stroke), head injury >10 minutes loss of consciousness, severe psychiatric disorder (e.g. schizophrenia, bipolar disorder), substance abuse/dependence, and/or stage 5 chronic kidney disease.
Measures
Treatment Adherence
The Heart Failure Compliance Questionnaire (Evangelista et al., 2001) assessed treatment adherence in the current sample. This questionnaire asks participants to rate their adherence to six different health behaviors, including keeping doctor appointments, medication management, dietary recommendations, following exercise regimens, and smoking and alcohol abstinence. Participants were asked to rate their adherence to doctor appointments in the past three months, and adherence to all other behaviors in the past week. Participants rate their adherence to these behaviors on a scale with five options: 0 = none of the time, 1 = very seldom, 2 = about half of the time, 3 = most of the time, and 4 = all of the time. As a result of negative phrasing, scores for alcohol and smoking abstinence were reversed. According to standard scoring procedures for this instrument, all scores were converted to a corresponding 0 to 100 scale (i.e., 0 = 0%; 1 = 25%; 2 = 50%; 3 = 75%; and 4 = 100%) (Evangelista et al., 2001). A score of 75% or greater for the overall composite, and for each of the individual health behaviors, was indicative of adequate adherence (i.e., participants responded to adhere most of the time or all of the time) (Evangelista et al., 2001). This instrument demonstrates strong psychometric properties, including content validity (Evangelista et al., 2001).
Cognitive Function
The Modified Mini Mental State Examination (3MS) was used to assess global cognitive status. It is a well validated screening measure that taps into multiple aspects of cognition, including attention, orientation, memory, language, and calculation (Teng & Chui, 1987). Scores on this measure range from 0–100 with higher scores reflective of better performance. The 3MS is widely used to assess cognitive function in HF and is sensitive to poor outcomes (e.g., mortality) in this population (Jozwiak, Guzik, Chmielewiski, Wysocki, & Wykretowicz, 2002).
A series of other neuropsychological measures were also administered to more fully assess cognitive function across multiple domains in the current sample. Specifically, the Trail Making Test A and B (TMT A and B) (Reitan, 1958) were administered to assess attention/executive function and long delayed free recall from the California Verbal Learning Test-Second Edition (CVLT-II) examined memory abilities (Delis et al., 2000). All cognitive measures administered demonstrate strong psychometric properties, including excellent reliability and validity.
Depressive Symptomatology
Beck Depression Inventory-II (BDI-II)
The BDI-II is a widely used check-list of depressive symptoms that demonstrates good psychometric properties in persons with medical conditions (Arnau, Meagher, Norris, & Bramson, 2001; Beck, Steer, & Brown, 1996). BDI-II scores range from 0–63 with higher scores indicative of greater symptomatology.
HF Severity
The 2-minute step test (2MST) served as an estimate of HF severity in the current sample. The 2MST is a brief assessment of physical fitness that requires participants to march in place lifting his/her knees to a marked target set on the wall set at the midpoint between the kneecap and crest of the iliac for a 2-minute period (Jones & Rikli, 2002). Greater step count is associated with better physical fitness and has been correlated with metabolic equivalents derived from stress testing in a HF sample (Garcia et al., 2013). Average step count for males between the ages of 50–85 ranges from 71–115, while females range from 60–107 (Jones & Rikli, 2002). The 2MST is sensitive to neurocognitive deficits in HF populations (Alosco et al., 2013; Alosco et al., 2012)
Demographic and Medical History
Demographic and medical characteristics were ascertained through participant self-report and corroborate by medical record review.
Procedures
The Kent State University and Summa Health System Institutional Review Board (IRB) approved the study procedures and all participants provided written informed consent prior to study enrollment. During a baseline assessment, participants completed demographic and psychosocial self-report measures, including the Heart Failure Compliance Questionnaire. Participants were also administered a brief neuropsychological test battery and then completed other study procedures, including the 2MST. This protocol was repeated at 12-month follow-up to examine possible changes over time.
Statistical Analyses
To facilitate clinical interpretation, raw scores from neuropsychological measures examining attention/executive function and memory were transformed to T-scores using normative data correcting for age, as well as gender in the case of the CVLT-II. Despite this normative adjustment for age and sex, these demographic variables were still included as covariates in analyses due to the lack of a control group in this study and given the sensitivity of these variables to cognitive function and treatment adherence. The normative data may also not account for individuals in the upper end of the age range in this sample (i.e., >80 years). Simple mean imputation was used to handle four cases with missing data for left ventricular ejection fraction (LVEF). In addition, the sample size for analyses examining 2MST over time was reduced to 95 due to missing data on this variable. Repeated measures analysis of variance (ANOVA) was performed to examine change across the dependent variables over time (i.e., baseline to 12-months), including on the 3MS, TMT A and B, and the CVLT-II long delay free recall.
Multivariable hierarchical regression analyses examined the predictive validity of baseline treatment adherence on 12-month performance for the 3MS, TMT A and B, and CVLT-II long delay free recall. For each model, baseline performance of the dependent variable was entered in block 1 along with medical and demographic factors including age, sex (1 = male; 2 = female), LVEF, depressive symptomatology (as assessed by the BDI-II), history of anxiety, and diagnostic history of hypertension, diabetes, and sleep apnea. The baseline overall treatment adherence composite was entered in block 2. To explore directionality of the significant findings, the above regression models were performed with the cognitive variables as the independent variable and the treatment adherence composite as the dependent variable. These analyses were performed to confirm the predictive validity of treatment adherence on 12-month cognitive function and diminish the possibility of a bi-directional relationship.
Results
Demographic and Medical Characteristics
Refer to Table 1 for participant demographic and medical characteristics at baseline and the 12-month follow-up. Examination of participants’ medical records indicated that the sample had an average left ventricular ejection fraction of 42.87 (SD = 15.60). Performance on the 2MST fell below average for females and in the average range for males. Performance on the 2MST remained stable over time for both females (F(1,34) = 0.05, p = 0.82) and males (F(1, 60) = 1.18, p = 0.28). Comorbid medical conditions (e.g., diabetes, hypertension, sleep apnea) were also prevalent at baseline and the 12-month follow-up.
Table 1.
Demographic and Clinical Characteristics of 115 Older Adults with Heart Failure
| Demographic Characteristics | Baseline | 12-Months |
|---|---|---|
| Age, mean (SD) | 69.41 (9.51) | 70.12 (9.78) |
| Years of Education, mean (SD) | 13.65 (2.61) | -- |
| Female (%) | 37.4 | -- |
| Race (% White) | 83.5 | -- |
| Medical and Clinical Characteristics | ||
| NYHA Class (% II, III, IV) | 81.7; 13.9; 1.7 | -- |
| Diabetes (% yes) | 31.3 | 31.3 |
| Hypertension (% yes) | 70.4 | 74.8 |
| Sleep Apnea (% yes) | 24.3 | 27.8 |
| 2MST Females, mean (SD) | 59.35 (19.61) | 60.32 (23.04) |
| 2MST Males, mean (SD) | 61.28 (22.67) | 64.39 (24.50) |
| BDI-II, mean (SD) | 7.03 (6.58) | 6.92 (6.40) |
| Anxiety (% yes) | 13.0 | 13.0 |
NYHA = New York Heart Association (N = 112); 2MST = 2-minute step test; BDI-II = Beck Depression Inventory-II
Descriptive Statistics of Reported Treatment Adherence and Cognitive Function
At baseline, the sample averaged 82.25 (SD = 11.58) on the overall treatment adherence composite and 17.4% of the sample scored < 75%, thus deemed to be non-adherent. Non-adherence rates were highest for dietary (31.3%) and exercise recommendations (60.9%). Reported non-adherence to the remaining health behaviors (i.e., keeping doctor appointments, taking medications, and smoking and alcohol abstinence) was minimal. Repeated measures showed no significant changes on the treatment adherence composite over time (F(1, 114) = 1.37, p = 0.24). Refer to Table 2.
Table 2.
Descriptive Statistics of Adherence Behaviors Among 115 Older Adults with HF
| Health Behavior | Baseline Adherence, mean (SD) |
% Below 75 |
12-Month Adherence, mean (SD) |
% Below 75 |
|---|---|---|---|---|
| Doctor Appointments | 96.52 (13.20) | 1.7 | 93.48 (17.55) | 5.2 |
| Medication Management |
95.43 (13.07) | 2.6 | 93.91 (19.75) | 5.2 |
| Dietary Recommendations |
69.13 (26.76) | 31.3 | 66.30 (26.71) | 33.0 |
| Exercise Regimens | 47.61 (32.61) | 60.9 | 46.52 (30.15) | 67.0 |
| Smoking Abstinence | 94.35 (20.70) | 6.1 | 95.22 (18.99) | 6.1 |
| Alcohol Abstinence | 90.43 (24.46) | 8.7 | 90.65 (25.75) | 9.6 |
| Overall Adherence | 82.25 (11.58) | 17.4 | 81.01 (11.46) | 18.3 |
Note. A score below 75 is indicative of non-adherence.
In terms of baseline cognitive function, the sample exhibited an average 3MS of 92.59 (SD) = 5.23) and 24.3% scored below a 90. See Table 3 for a full summary of cognitive test performance at baseline and the 12-month follow-up. Relative to normative data, HF participants fell in the average range for performance on measures of attention/executive function and memory. However, when using a T-score cutoff of 35 (i.e., 1.5 SD below the mean), 15.7% of participants exhibited baseline impairments on TMT B while impairments on the other measures of cognition were less common. Repeated measures showed the sample demonstrated significant improvements on the 3MS (F(1, 114) = 10.36, p < 0.01) and the CVLT-II long delay free recall (F(1, 114) = 27.18, p < 0.001). Performance on the TMT A and B remained stable over time (p > 0.05).
Table 3.
Baseline and 12-Month Neuropsychological Test Performance among Older Adults with Heart Failure
| Baseline M(SD) |
12-month M(SD) |
|
|---|---|---|
| Global Cognition | ||
| 3MS | 92.59 (5.23) | 93.80 (5.06) |
| Attention/Executive Function (T-scores) | ||
| TMTA | 51.04 (9.31) | 50.83 (11.76) |
| TMTB | 45.61(15.66) | 45.76 (15.55) |
| Memory | ||
| CVLT LDFR | 47.74 (10.22) | 51.96 (10.42) |
Abbreviations—3MS = Modified Mini Mental State Examination; TMTA = Trail Making Test A; TMTB = Trail Making Test B; CVLT = California Verbal Learning Test; LDFR = Long Delay Free Recall
Baseline Treatment Adherence Predicts Cognitive Function
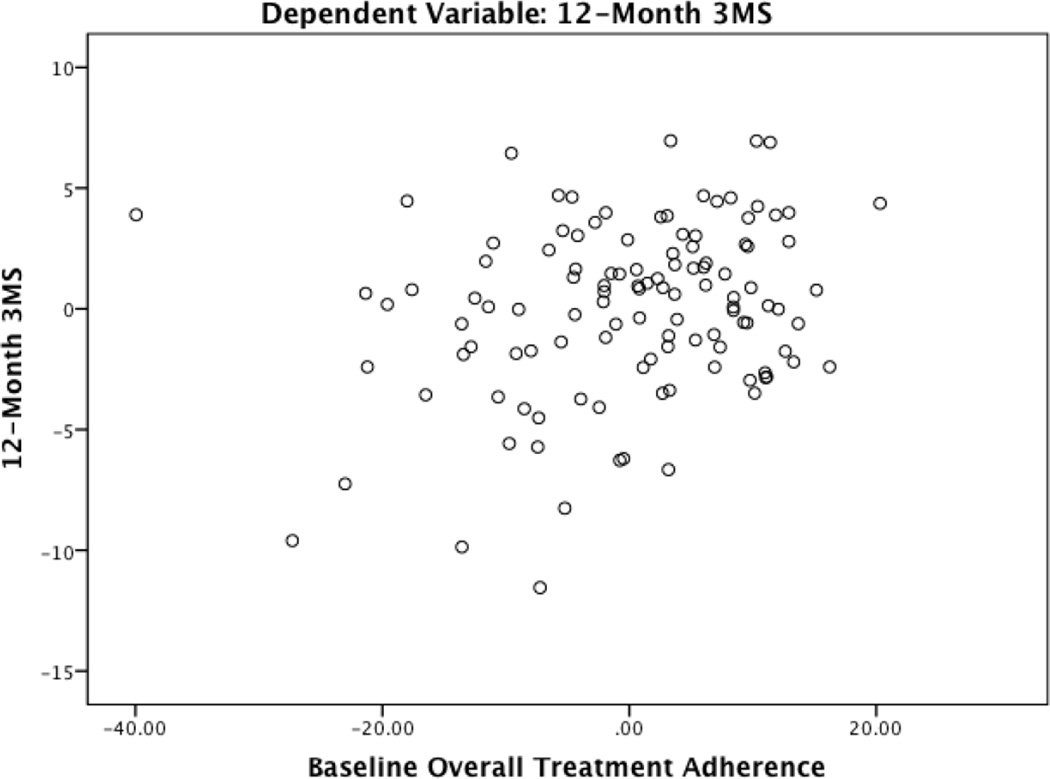
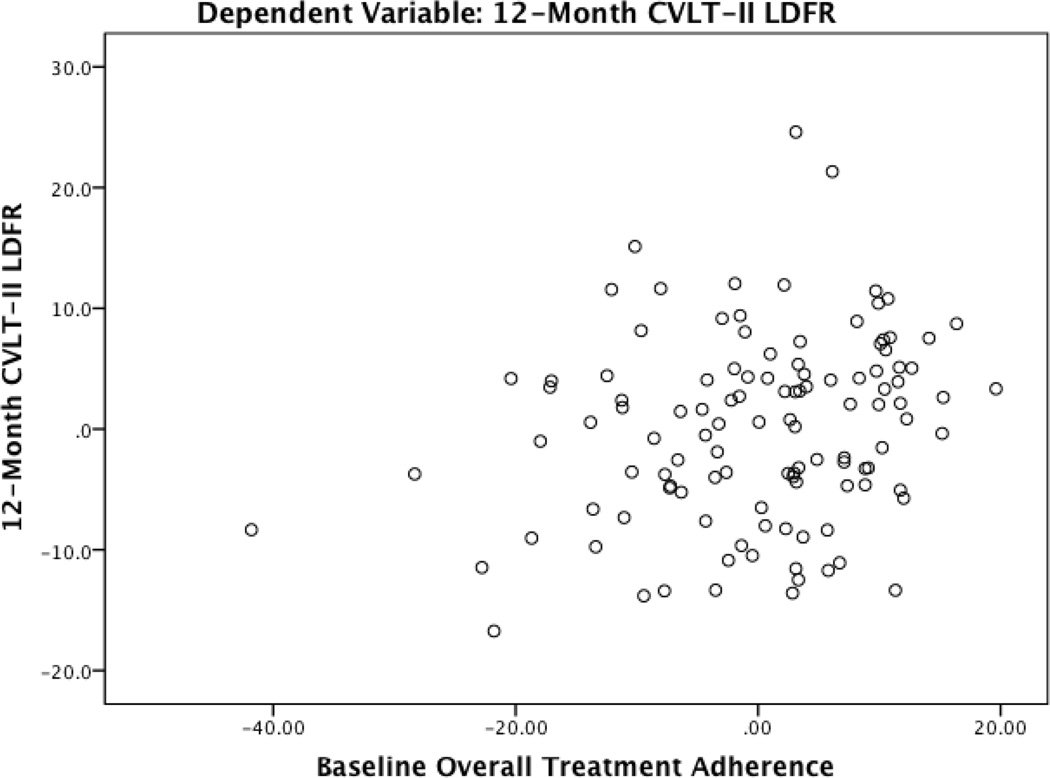
Table 4 presents a full summary of hierarchical regression analyses. For all analyses, there were no significant departures from multivariate normality as indicated by low skewness and kurtosis values for all independence and dependent variables (i.e., absolute value < 3 and 10, respectively), acceptable normal probability plots, and a Cook’s distance < 1. Block 1 with medical and demographic variables predicted 12-month performance on the 3MS (F(9, 105) = 11.69, p < 0.001), TMT A (F(9, 105) = 10.30, p < 0.001), TMT B (F(9, 105) = 15.44, p < 0.001), and the CVLT-II long delay free recall (F(9,105) = 9.79, p < 0.001). After controlling for baseline test performance, and medical and demographic characteristics, the overall treatment adherence composite demonstrated significant predictive validity for 12-month performances on the 3MS (β = 0.20, p = 0.01) and the CVLT-II long delay free recall (β = 0.19, p = 0.02). In each case, greater reported adherence at baseline predicted better cognitive function at the 12-month follow-up. See Figure 1 and 2 for partial regression plots of the relationship between the overall adherence composite and the 3MS and CVLT-II long delay free recall. This plot depicts the nature of the relationship between these variables after accounting for the other predictor variables in the model.
Table 4.
Predictive Validity of Baseline Treatment Adherence on 12-Month Cognitive Function
| 3MS | TMTA | TMTB | CVLT LDFR | |||||
|---|---|---|---|---|---|---|---|---|
| Β | SE b | β | SE b | β | SE b | β | SE b | |
| Block 1 | ||||||||
| Age | −.06 | .04 | −.01 | .10 | −.10 | .11 | .06 | .09 |
| Sex | .00 | .78 | −.05 | 1.86 | −.02 | 2.24 | −.08 | 1.67 |
| LVEF | .02 | .02 | .19* | .06 | −.04 | .07 | .12 | .05 |
| Hypertension | −.01 | .80 | −.05 | 1.95 | .04 | 2.33 | −.04 | 1.73 |
| Diabetes | −.06 | .77 | −.13 | 1.88 | .00 | 2.24 | −.01 | 1.67 |
| Sleep Apnea | −.01 | .86 | −.03 | 2.04 | −.06 | 2.43 | −.09 | 1.84 |
| BDI-II | .07 | .06 | .11 | .14 | .11 | .17 | .04 | .13 |
| Anxiety | −.13 | 1.19 | −.22** | 2.82 | −.13 | 3.39 | −.06 | 2.50 |
| Baseline Test Performance*** |
.65** | .07 | .56** | .10 | .72** | .07 | .66** | .08 |
| R2 | .50 | .47 | .57 | .46 | ||||
| F | 11.69** | 10.30** | 15.44** | 9.79** | ||||
| Block 2 | ||||||||
| Treatment Adherence |
.20* | .03 | .06 | .08 | .06 | .10 | .19* | .07 |
| R2 | .53 | .47 | .57 | .49 | ||||
| F for ΔR2 | 6.75* | .56 | .00 | 6.03* | ||||
Note.
p < 0.05;
p < 0.01;
Baseline test performance of the 12-month dependent variable was entered as a covariate
Abbreviations: 3MS = Modified Mini Mental State Examination; TMTA = Trail Making Test A; TMTB = Trail Making Test B; CVLT = California Verbal Learning Test; LDFR = Long Delay Free Recall; LVEF = Left ventricular ejection fraction
Figure 1.
Partial Regression Plot of the Relationship between Baseline Adherence and 12-Month 3MS Peformance
Note. 3MS = Modified Mini Mental State Examination
Plot depicts the nature of the relationship between baseline adherence and 12-month 3MS after accounting for the other predictor variables in the model
Figure 2.
Partial Regression Plot of the Relationship between Baseline Adherence and 12-Month Memory Performance
Note. CVLT-II LDFR = California Verbal Learning Test Long Delay Free Recall
Plot depicts the nature of the relationship between baseline adherence and 12-month memory after accounting for the other predictor variables in the model
There were no effects for TMT A or B (p > 0.05). To confirm directionality of findings, these same regression analyses were performed with the 3MS and CVLT-II long delay free recall as the independent variables and results demonstrated no predictive validity for either of these variables on the overall treatment adherence composite (p > 0.05 for all).
Follow-up partial correlation analyses adjusting for baseline cognitive test performance and medical and demographic variables revealed increased baseline adherence to medications (r(104) = 0.22, p = 0.03) and smoking abstinence (r(104) = 0.26, p = 0.01) predicted better 3MS performance at the 12-month follow-up. Increased baseline adherence to diet recommendations also predicted better 12-month performance on the CVLT-II long delay free recall (r(104) = 0.22, p = 0.02). Non-significant trends also emerged between greater baseline adherence to medications (r(104) = 0.18, p = 0.07) and smoking abstinence (r(104) = 0.17, p = 0.08) with better performance on the CVLT-II long delayed free recall. No such pattern emerged for any of the other recommended health behaviors (p > 0.05 for all).
Discussion
Previous cross-sectional studies have linked treatment adherence with cognitive function in HF and past work among other medical populations suggest cognitive impairment as a likely determinant of poor adherence (Alosco et al., 2012; Hawkins et al., 2012; Andrade et al., 2012). However, the current study extends past work by showing that greater adherence to recommended health behaviors predicted improved cognitive function at a one year follow-up in a sample of older adults with HF. Several aspects of these findings warrant further discussion.
We found that better treatment adherence predicted improved global cognitive status and memory at a one-year follow-up. Specifically, adherence to medications, dietary recommendations, and smoking abstinence emerged as the most important contributors to improved cognitive function one-year later. Adherence to these health behaviors is critical in the management of HF and each has been shown to independently promote better neurocognitive outcomes. For instance, commonly prescribed HF medications (e.g., angiotensin-converting enzyme inhibitors) have been linked with enhanced cognitive function in HF possibly stemming from improved cardiac function and subsequent increases in cerebral perfusion (Zuccala et al., 2005). In addition to complex medication regimens, HF patients are also asked to following strict dietary recommendations with restriction of sodium intake being the most important, as excessive sodium intake can lead to hospitalizations (Riegal et al., 2009; Vinson, Rich, Sperry, Shah, & McNamara, 1990; Bennett et al., 1998; Tsuyuki et al., 2001). Interestingly, sodium intake is as an important contributor to cognitive function in HF (Alosco et al., 2013) and consumption of recommended foods has also been suggested to attenuate age-related cognitive decline (Wengreen et al., 2009). Lastly, the cognitive benefits associated with smoking abstinence is not surprising in light of the vast literature demonstrating the adverse effects of smoking on cardiac function and brain integrity (Joosten et al., 2013; Debette et al., 2011). Taken together, our findings suggest the possibility that better treatment adherence in HF may preserve cognitive function in this population and future work with extended follow-ups (e.g., 2–5 years) is needed to test this possibility and examine possible mechanisms.
Despite the above, the relationship between adherence and cognitive changes appears to be complex. Indeed, the effect of adherence on only the 3MS and CVLT-II is not entirely clear, but these measures exhibited the greatest variability over time. Alternatively, domains of cognition may exhibit differential sensitivity to adherence behaviors, and global cognitive status and memory may be most affected by the medical sequalae of non-adherence. We also found that baseline cognition did not predict 12-month adherence in this study, though cognitive function has been proposed to play a key role in individual’s ability to follow complex treatment regimens. It appears likely that the largely intact cognitive functioning at baseline may limit prediction over time in the current sample. As an example, the current study used the 3MS to assess global cognitive status, which is a widely used and valid cognitive screening measure. However, recent research suggests that other cognitive screening measures (i.e., Montreal Cognitive Assessment (MoCA)) may be more sensitive to cognitive impairment in HF patients (Athilingam et al., 2011) and might better capture poor outcomes in this population. Further research with objective measures of adherence and more comprehensive cognitive batteries is clearly much needed to clarify the relationship between adherence and cognition in HF and other patient and healthy samples.
Despite the critical psychosocial and health benefits associated with treatment adherence in HF, adherence to treatment recommendations remains poor in this population. Consistent with past work, more than 30% and 60% of the current sample were non-adherent to dietary and exercise recommendations (see Riegal et al., 2009 for a review). Although better adherence to dietary recommendations predicted improved cognitive function in this study, no such pattern emerged for adherence to exercise regimens. This finding is in contrast to the extant literature that demonstrates the cognitive benefits associated with exercise and physical activity in this population (Tanne et al., 2005; Garcia et al., 2013). As a result, our findings may be due to limitations associated with self-reported exercise in this population or may even reflect low levels of physical activity in persons with HF (Alosco et al., 2012) that fall below the threshold needed to confer cognitive benefits. While self-reported exercise questionnaires typically do not account for intensity varying everyday activities, HF patients have also been shown to provide inaccurate estimates of their physical fitness levels and their responses also lack criterion validity with exercise capacity measures (Raphael et al., 2007). Indeed, objective measures of physical activity (e.g., accelerometer) have been shown to predict cognitive function in HF, which may in part be a result of increased sensitivity to physical fitness—a key determinant of neurocognitive outcomes in this population (Alosco et al., 2013; Garcia et al., 2013; Steinberg et al., 2011).
Similarly, limitations of self-reported adherence may also have played a role in the low rates of non-adherence to other health behaviors in this sample, particularly in terms of following medication regimens (e.g., only 2.7% non-adherent). Relative to objectively monitored medication adherence, past work shows that HF patients over-estimate their adherence to medication regimens (Smith, Hankins, Hodson, & George, 2010). The reason for these findings may involve several possibilities, including skewed response bias to please clinicians (Adams, Soumerai, Lomas, & Ross-Degnan, 1999), strong social support networks that assist in medication management (Evangelista et al., 2001), or even poor insight of deficits (e.g., anosognosia) (Leicht, Berwig, & Gertz, 2010). Prospective studies that employ objective assessments of treatment adherence in HF (e.g., electronic compliance monitoring, accelerometry) are much needed to confirm the benefits of treatment adherence on cognitive function.
The current study also found slight improvements in global cognitive function and memory abilities over the 12-month period. These findings are consistent with past research demonstrating improved global cognition in HF over a similar time period (Stanek et al., 2009). Although practice effects may contribute to these findings, the length of time between assessments and alternate versions used for the CVLT-II argue against this possibility. Alternatively, the examination of cognition over 12-months may not have been long enough to fully capture the extent of cognitive decline in this sample (Steinberg et al., 2011). Future studies that assess cognition across various time points over greater than a one year period are also much needed to clarify the nature of cognitive function in HF, particularly as it involves changes in adherence to treatment recommendations.
The effect sizes observed in the current study were modest and thus caution is needed in interpreting the clinical significance of our findings. Although treatment adherence is a likely contributor to cognitive function over time, it is only one factor amidst many that negatively affects poor outcomes in patients with HF. Clinicians should consider treatment adherence in the context of other medical (e.g., HF severity), demographic (e.g., age), and psychosocial (e.g., social support) variables in the evaluation of cognitive function among HF patients. For example, a majority of HF participants demonstrated good adherence including to domains that predicted 12-month cognitive function (i.e., medication adherence and smoking abstinence). It is possible that a subgroup of the least adherent HF participants (e.g., the oldest, unhealthiest, and worse psychosocial function) is driving the current analyses. In sum, while our study provides preliminary evidence for the effects of adherence on cognition over time, larger randomized control studies with several assessment time points are much needed to fully clarify the clinical implications of the current findings.
Several limitations of the current study deserve brief attention. Most notably, the use of self-report to assess treatment adherence can introduce confounding biases (e.g., subjectivity, memory errors), particularly within a cognitively impaired population. Although use of self-reported adherence measures is inexpensive and reliable (Murray et al., 2004), future work that employs objective assessments are needed to examine the effects of treatment adherence on cognitive function. Indeed, when such objective methods have been utilized higher rates of medication non-adherence (e.g., 13–20%) have been found in patients with HF (Dunlay et al., 2011; Muzzarellia et al., 2010). In addition, the mechanisms underlying the association between treatment adherence and improved cognitive function remain unclear. Cardiac dysfunction and subsequent cerebral hypoperfusion is the most commonly proposed etiological underpinning of cognitive impairment in HF (Alosco et al., 2013) and future work should determine whether adherence to recommended health behaviors attenuates this process. We also did not implement a control group and larger case-controlled prospective studies are needed to confirm our findings and examine methods for improving treatment adherence in HF (e.g., telemonitoring). Finally, although all participants were receiving routine clinical cardiology care at the time of enrollment, there are significant interindividual differences across clinicians in the delivery of treatment recommendations (e.g., written, verbally, etc). Unfortunately, the exact route and timing by which recommendations were provided prior to study entry are unknown and may be a possible confound of the current findings.
In brief sum, the current study shows that better treatment adherence predicts improved cognitive function at a one-year follow up in older adults with HF. Improved treatment adherence may help to preserve cognitive function in this population and subsequently result in better psychosocial outcomes (e.g., quality of life). Prospective studies with extended follow-ups that utilize objective assessments of treatment adherence are needed to clarify our findings and determine the long-term benefits of better treatment adherence on neurocognitive outcomes in HF.
Acknowledgments
This work was support by the National Institutes of Health grants DK075119 and HL089311.
None.
Footnotes
There are no competing interests to report.
Conflict of Interest
All authors declare no conflict of interest.
References
- Adams AS, Soumerai SB, Lomas J, Ross-Degnan D. Evidence of self-report bias inassessing adherence toguidelines. International Journal for Quality in Healthcare. 1999;11:187–192. doi: 10.1093/intqhc/11.3.187. [DOI] [PubMed] [Google Scholar]
- Alosco ML, Spitznagel MB, van Dulmen M, Raz N, Cohen R, Sweet LH, Gunstad J. The additive effects of type-2 diabetes on cognitive function in older adults with heart failure. Cardiology Research Practice. 2012;2012:348054. doi: 10.1155/2012/348054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Brickman AM, Spitznagel MB, Garcia SL, Narkhede A, Griffith EY, Gunstad J. Cerebral perfusion is associated with white matter hyperintensities in older adults with heart failure. Congest Heart Fail. 2013;19:E29–E34. doi: 10.1111/chf.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Spitznagel MB, Miller L, Raz N, Cohen R, Sweet LH, Gunstad J. Depression is associated with reduced physical activity in persons with heart failure. Health Psychology. 2012;31:754–762. doi: 10.1037/a0028711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Spitznagel MB, Raz N, Cohen R, Sweet LH, Colbert LH, Gunstad J. Dietary habits moderate the association between heart failure and cognitive impairment. Journal of Nutrition in Gerontology and Geriatrics. 2013;32:106–121. doi: 10.1080/21551197.2013.781408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Spitznagel MB, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, Gunstad J. The 2-minute step test is independently associated with cognitive function in older adults with heart failure. Aging and Clinical Experimental Research. 2012;24:468–474. doi: 10.3275/8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Brickman AM, Spitznagel MB, Griffith EY, Narkhede A, Raz N, Gunstad J. Poorer physical fitness is associated with reduced structural brain integrity in heart failure. Journal of the Neurological Sciences. 2013;328:51–57. doi: 10.1016/j.jns.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Spitznagel MB, van Dulmen M, Raz N, Cohen R, Sweet LH, Gunstad J. Cognitive function and treatment adherence in older adults with heart failure. Psychosomatic Medicine. 2012;74:965–973. doi: 10.1097/PSY.0b013e318272ef2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade AS, Deutsch R, A Celano S, Duarte NA, Marcotte TD, Umlauf A, Collier AC. Relationships among neurocognitive status, medication adherence measured by pharmacy refill records, and virologic suppression in HIV-infected persons. Journal of Acquire Immune Deficiency Syndromes. 2012;62:282–292. doi: 10.1097/QAI.0b013e31827ed678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnau R, Meagher MW, Norris MP, Bramson R. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychology. 2001;20:112–119. doi: 10.1037//0278-6133.20.2.112. [DOI] [PubMed] [Google Scholar]
- Athilingam P, et al. Montreal Cognitive Assessment and Mini-Mental Status Examination compared as cognitive screening tools in heart failure. Heart & Lung. 2011;40:521–529. doi: 10.1016/j.hrtlng.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory. 2nd. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Bennett SJ, Oldridge NB, Eckert GJ, Embree JL, Browning S, Hou N, Murray D. Comparison of quality of life measures in heart failure. Nursing Research. 2003;52:207–216. doi: 10.1097/00006199-200307000-00001. [DOI] [PubMed] [Google Scholar]
- Bennett SJ, Huster GA, Baker SL, Milgrom LB, Kirchgassner A, Birt J, Pressler ML. Characterization of the precipitants of hospitalization forheart failure decompensation. American Journal of Critical Care. 1998;7:168–174. [PubMed] [Google Scholar]
- Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D, et al. California Verbal Learning Test-Second Edition: Adult Version. Manual. San Antonio (TX): Psychological Corporation; 2000. [Google Scholar]
- Dunlay SM, Eveleth JM, Shah ND, McNallan SM, Roger VL. Medication adherence among community-dwelling patients with heart failure. Mayo Clinic Proceedings. 2011;86:273–281. doi: 10.4065/mcp.2010.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista LS, Berg J, Dracup K. Relationship between psychosocial variables and compliance in patients with heart failure. Heart Lung. 2001;30:294–301. doi: 10.1067/mhl.2001.116011. [DOI] [PubMed] [Google Scholar]
- Fitzgerald AA, Powers JD, Ho PM, Maddox TM, Peterson PN, Allen LA, Havranek P. Impact of medication nonadherence on hospitalizations and mortality in heart failure. Journal of Cardiac Failure. 2011;17:664–669. doi: 10.1016/j.cardfail.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Garcia S, Alosco ML, Spitznagel MB, Cohen R, Raz N, Sweet L, Gunstad J. Cardiovascular fitness associated with cognitive performance in heart failure patients enrolled in cardiac rehabilitation. BMC Cardiovascular Diseases. 2013;13:29. doi: 10.1186/1471-2261-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb SS, Khatta M, Friedmann E, Einbinder L, Katzen S, Baker B, Marshall J, Thomas SA. The influence of age, gender, and race on the prevalence of depression in heart failure patients. Journal of the American College of Cardiology. 2004;43:1542–1549. doi: 10.1016/j.jacc.2003.10.064. [DOI] [PubMed] [Google Scholar]
- Granger BB, Swedberg K, Ekman I, Granger CB, Olofsson B, McMurray JJ CHARM investigators. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomized, controlled clinical trial. Lancet. 2005;366:2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- Hawkins LA, Killian S, Firek A, Kashner TM, Firek CJ, Silvet H. Cognitive impairment and medication adherence in outpatients with heart failure. Heart Lung. 2012;41:572–582. doi: 10.1016/j.hrtlng.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Hjelm C, Dahl A, Brostrom A, Martensson J, Johansson B, Stromberg A. The influence of heart failure on longitudinal changes in cognition among individuals 80 years of age and older. Journal of Clinical Nursing. 2012;7–8:994–1003. doi: 10.1111/j.1365-2702.2011.03817.x. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Rikli RE. Measuring functional fitness of older adults. The Journal on Active Aging. 2002 March April; [Google Scholar]
- Joosten H, et al. Cardiovascular risk profile and cognitive function in young, middle-aged, and elderly subjects. Stroke. 2013;44:1543–1549. doi: 10.1161/STROKEAHA.111.000496. [DOI] [PubMed] [Google Scholar]
- Jozwiak A, Guzik P, Chmielewski Z, Wysocki H, Wykretowicz A. Impaired cognitive function as an independent risk factor for the in-hospital mortality in the elderly with chronic heart failure. Przegl Lek. 2002;59:276–280. [PubMed] [Google Scholar]
- Kutzleb J, Reiner D. The impact of nurse-directed patient education on quality of life and functional capacity in people with heart failure. Journal of the American Academy of Nurse Practitioners. 2006;18:116–123. doi: 10.1111/j.1745-7599.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- Leicht H, Berwig M, Gertz H. Anosognosia in Alzheimer’s disease: The role of impairment levels in assessment of insight across domains. Journal of the International Neuropsychological Society. 2010;16:463–473. doi: 10.1017/S1355617710000056. [DOI] [PubMed] [Google Scholar]
- Miller LA, Spitznagel MB, Alosco ML, Cohen RA, Raz N, Sweet LH, Gunstad J. Cognitive profiles in heart failure: a cluster analytic approach. Journal of Clinical and Experimental Neuropsychology. 2012;34:509–520. doi: 10.1080/13803395.2012.663344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MD, Morrow DG, Weiner M, Clark DO, Tu W, Deer MM, Weinberger M. Conceptual framework to study medication adherence in older adults. American Journal of Geriatric Pharmacotherapy. 2004;2:36–43. doi: 10.1016/s1543-5946(04)90005-0. [DOI] [PubMed] [Google Scholar]
- Muzzarellia S, Brunner-La Rocca H, Pfister O, Foglia P, Moschovitis G, Mombelli G, Strick H. Adherence to medical regime in patients with heart failure. European Journal of Heart Failure. 2010;12:389–396. doi: 10.1093/eurjhf/hfq015. [DOI] [PubMed] [Google Scholar]
- Norberg EB, Boman K, Lofgren B. Activites of daily living for old persons in primary health care with chronic heart failure. Scandinavian Journal of Caring Sciences. 2008;22:203–210. doi: 10.1111/j.1471-6712.2007.00514.x. [DOI] [PubMed] [Google Scholar]
- Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauve MJ, Shaw RM. Cognitive deficits in chronic heart failure. Nursing Research. 2010;59:127–139. doi: 10.1097/NNR.0b013e3181d1a747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer’s disease: a population-based cohort study. Archives of Internal Medicine. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- Raphael C, Briscoe C, Davies J, Ian Whinnett Z, Manisty C, Sutton R, Mayet J, Francis DP. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart. 2007;3:476–482. doi: 10.1136/hrt.2006.089656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual Motor Skills. 1958;8:271–276. [Google Scholar]
- Riegal B, Moser D, Anker SD, Appel LJ, Dunbar SB, Grady KL American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research. State of the science: Promoting self-care in persons with heart failure A scientific statement from the American Heart Association. Circulation. 2009;120:1141–1163. doi: 10.1161/CIRCULATIONAHA.109.192628. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y, Roberts BL, LaFramboise L, Yates BC, Yurkovich JM. Predictors of modifications in instrumental activities of daily living in persons with heart failure. Journal of Cardiovascular Nursing. 2011;26:89–98. doi: 10.1097/JCN.0b013e3181ec1352. [DOI] [PubMed] [Google Scholar]
- Smith H, Hankins M, Hodson A, George C. Measuring the adherence to medication of elderly patients with heart failure: is therea gold standard? International Journal of Cardiology. 2010;145:122–123. doi: 10.1016/j.ijcard.2009.06.031. [DOI] [PubMed] [Google Scholar]
- Stanek KM, Gunstad J, Paul RH, Poppas A, Jefferson AL, Sweet LH, Cohen RA. Longitudinal cognitive performance in older adults with cardiovascular disease: evidence for improvement in heart failure. Journal of Cardiovascular Nursing. 2009;24:192–197. doi: 10.1097/JCN.0b013e31819b54de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg G, Lossnitzer N, Schellberg D, Mueller-Tasch T, Krueger C, Haass M, Juenger J. Peak oxygen uptake and left ventricular ejection fraction, but not depressive symptoms, are associated with cognitive impairment in patients with chronic heart failure. International Journal of General Medicine. 2011;4:879–887. doi: 10.2147/IJGM.S23841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanne D, Freimark D, Poreh A, Merzeliak O, Bruck B, Schwammenthal Y, Adler Y. Cognitive functions in severe congestive heart failure before and after an exercise program. International Journal of Cardiology. 2005;103:145–149. doi: 10.1016/j.ijcard.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Teng E, Chui H. The Modified Mini-Mental State (3MS) examination. Journal of Clinical Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- Tsuyuki RT, McKelvie RS, Arnold JM, Avesum A, Jr, Barretto AC, Carvalho AC, Yusuf S. Acute precipitants of congestive heart failure exacerbations. Archives of Internal Medicine. 2001;161:2337–2342. doi: 10.1001/archinte.161.19.2337. [DOI] [PubMed] [Google Scholar]
- van der Wal MHL, Jaarsma T, Moser DK, Veeger NJ, van Gilst WH, van Veldhuisen DJ. Compliance in heart failure patients: the importance of knowledge and beliefs. European Heart Journal. 2006;27:434–440. doi: 10.1093/eurheartj/ehi603. [DOI] [PubMed] [Google Scholar]
- Vinson JM, Rich MW, Sperry JC, Shah AS, McNamara T. Early readmission of elderly patients with congestive heart failure. Journal of the American Geriatric Society. 1990;38:1290–1295. doi: 10.1111/j.1532-5415.1990.tb03450.x. [DOI] [PubMed] [Google Scholar]
- Wengreen HJ, Neilson C, Munger R, Corcoran C. Diet quality is associated with better cognitive test performance among aging men and women. Journal of Nutrition. 2009;139:1944–1949. doi: 10.3945/jn.109.106427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ME, Arnedt JT, Stanchina M, Millman RP, Aloia MS. Normalization of memory performance and positive airway pressure adherence in memory-impaired patients with obstructive sleep apnea. Chest. 2006;130:1772–1778. doi: 10.1378/chest.130.6.1772. [DOI] [PubMed] [Google Scholar]
- Zuccala G, Marzetti E, Cesari M, Lo Monaco MR, Antonica L, Cocchi A, Bernabei R. Correlates of cognitive impairment among patients with heart failure: results of a multicenter survey. American Journal of Medicine. 2005;118:496–502. doi: 10.1016/j.amjmed.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Zuccala G, Onder G, Marzetti E, Monaco MR, Cesari M, Cocchi A GIFA Study Group. Use of angiotensin-converting enzyme inhibitors and variations in cognitive performance among patients with heart failure. European Heart Journal. 2005;26:226–233. doi: 10.1093/eurheartj/ehi058. [DOI] [PubMed] [Google Scholar]