Abstract
Sleep disordered breathing (SDB) is reported in up to 69% of adolescents and children with sickle cell disease (SCD) [1], but data regarding the prevalence of SDB in adults with SCD are limited. To obtain a preliminary assessment of the frequency and degree of sleep-related hypoxemia and potential associations with cardiovascular function in adults with SCD, we performed overnight sleep studies, 6-minute walk tests, echocardiograms, hematologic and chemistry panels, and administered the Pittsburgh Sleep Quality Index (PSQI), fatigue and health related quality of life measurement in 20 young adults with SCD attending a sickle cell clinic for routine care. Sleep apnea, defined as an apnea-hypopnea index (AHI) >5 events/hour, was found in 50% of subjects. Traditional clinical indicators such as obesity, the presence of snoring, and reported sleep complaints did not reliably differentiate these subjects. Subjects with an AHI>5 had higher mean systolic blood pressure (p =.03), evidence of impaired left ventricular diastolic function (i.e. increased mitral valve E/A ratio, p =.05), a trend toward greater reduction in 6 minute walk distances (p =.06), and lower Health-related Quality of Life scores (p <=.01). Three of nine subjects with more severe anemia (total Hb < 9.0) demonstrated nocturnal hypoxemia in the absence of sleep apnea. As prolonged and frequent hypoxemic episodes likely increase risks for vaso-occlusive, cardiovascular, and neurologic complications of SCD, these results suggest that the prevalence and severity of SDB should be investigated further in studies of larger patient populations. If confirmed, these findings could identify opportunities to prevent or reduce nocturnal hypoxia and improve outcomes.
Keywords: sickle cell disease, sleep apnea, hypoxia, hypertension
Introduction
Sickle cell disease (SCD) is a genetic blood disease that is classified by the World Health Organization as a global health burden. Hypoxia contributes to polymerization of hemoglobin (Hb) S, which in turn leads to vaso-occlusion and other complications. Sleep disordered breathing (SDB), which is associated with transient hypoxemia and hypercapnea, has been reported in 41% of unselected children and adolescents with sickle cell disease and up to 69% of those with clinical features suggestive of SDB [1,2]. The pathophysiologic consequences of SDB, may include endothelial dysfunction with altered nitric oxide bioactivity, altered redox biology, chronic systemic inflammation, and increased expression of cell adhesion molecules [3, 4]. Each of these processes could contribute to hemolysis and vaso-occlusion. Studies in children and adolescents with SCD have demonstrated associations between nocturnal desaturations and severity of anemia [5], frequency of vaso-occlusive crises and acute chest syndrome [6] cardiac abnormalities including left ventricular hypertrophy and diastolic dysfunction [7], CNS events, impaired cognition and executive function [8, 9], priapism [3], and nocturnal enuresis [3].
Although sleep disordered breathing (SDB) is associated with morbidity in children with SCD, and occurs with increasing frequency with age in the general population, there are fewer studies evaluating SDB in adults with SCD. Sharma et al. identified obstructive sleep apnea in 44% of in 32 adult SCD patients (mean AHI 17 events/hour, (95% CI: 10–24/h) who had been referred to a sleep center based upon SDB symptoms [10], yet no studies have been undertaken to evaluate SDB in the absence of symptoms. Here we report a pilot study to evaluate the frequency and severity of SDB in clinically stable young adults with SCD with no previously identified symptoms of sleep disturbance, cardiac, or pulmonary vascular disease. We have focused this study on individuals less than 30 years of age to identify earlier stages of physiologic compromise that would be potentially modifiable with intervention.
Methods
Subjects and Procedures
Adults with SCD (HbSS, HbS-β thalassemia, or HbSC genotypes), attending a sickle cell clinic for routine care were invited to participate in this study. Exclusion criteria included hospitalization for vaso-occlusive crisis or other acute events within the prior two weeks, blood transfusion within four weeks, a prior diagnosis of pulmonary hypertension or sleep disordered breathing, and participation in a chronic transfusion program. The study was approved by the Institutional Review Board and approved and conducted at the Clinical Research Unit of Howard University Hospital.
After signing consent, all subjects underwent a complete history and physical examination and completed the Pittsburgh Sleep Quality Index (PSQI), and the SF-36. Medical records were reviewed for ascertainment of clinical data. Complete blood counts, chemistry panels, and brain natriuretic peptide (BNP) levels were obtained. Echocardiographic data obtained for clinical purposes within six months of the study was reviewed or performed, if not available.
Polysomnograms
Overnight sleep studies were performed using a Type III sleep monitor, the NOX-T3 (Carefusion, Inc.), which records snoring, abdominal and thoracic respiratory effort, derived flow (Respiratory Inductance Plethysmography Flow), nasal cannula flow, body position, actigraphy, oxygen saturation, heart rate, and a one channel EEG. Apneas were scored as a ≥ 10 second 90% reduction in flow signal. Hypopneas were scored as a ≥ 10 second 30% reduction in flow accompanied by a 3% or greater drop in saturation or arousal.
Six Minute Walk Tests
On the morning following the overnight sleep study, all subjects underwent a six minute walk test with measurement of ambulatory oximetry according to standardized protocols [11–12].
Statistical Analysis
Continuous data were presented by median (interquartile range). We compared the distribution of continuous data between two groups of patients (based on AHI) with the Kruskal-Wallis test and categorical data with Fisher’s exact test.
Results
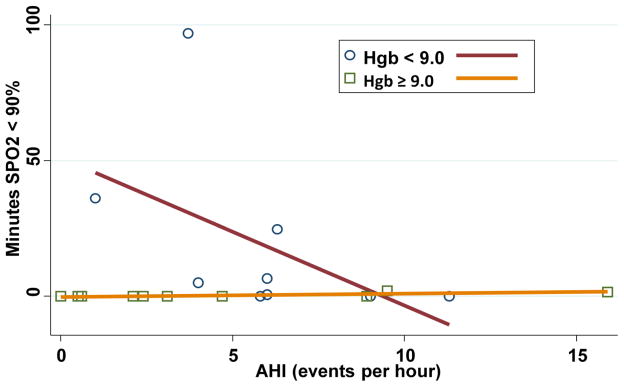
The study population and results are summarized in Table I. Ten of the twenty subjects (50%) had an AHI > 5, consistent with mild-moderate sleep apnea (mean 8.5 events/hour, range 5.8–15.9 events/hour). There were no differences in age, gender, hemoglobin genotype, hydroxyurea use, narcotic use, smoking history, or history of asthma between the two groups. Subjects with SDB had a higher BMI than those without, (median 23.8 kg/m2 vs 19.8 kg/m2, p = .049), but were not obese. A history of priapism was reported solely in subjects with sleep apnea (4 of the 7 male subjects with sleep apnea, 0 of 5 subjects without, p = .08). An abnormal PSQI (total score 5 or greater) showed modest sensitivity for sleep apnea (80%), but less specificity (40%). Three subjects with a greater magnitude of anemia, i.e. total hemoglobin (Hb) levels < 9 g/dl, demonstrated oxygen desaturations which were unrelated to AHI, as shown in Figure 1. The finding of greater duration of oxygen desaturation (oxygen saturation < 90%) with anemia was significant, (Spearman correlation coefficient = − 0.65)
Table 1.
Demographics and Results
| AHI ≤5 N=10 |
AHI>5 N=10 |
p-value | |
|---|---|---|---|
| Age (Median, Interquartile range) | 25 (23–27) | 28 (22–28) | 0.80 |
| BMI (Median, Interquartile range) | 19.8 (18.5–24.4) | 23.8 (20.7–27.6) | 0.05 |
| Female gender, n | 5 | 3 | 0.70 |
| Narcotic use, n | 5 | 1 | 0.14 |
| SS genotype, n | 8 | 8 | >0.90 |
| Sleep disorder indicated by PSQI, n | 6 | 8 | 0.60 |
| Snore index (percent of night snoring vs total time in bed) | 0.0 (0.0–0.1) | 0.8 (0.0–2.1) | 0.08 |
| HRQoL General Health Score | 50 (46–53) | 39 (31–46) | 0.01 |
| Systolic blood pressure (Median, Interquartile range) | 103 (99–120) | 114 (111–130) | 0.03 |
| 6 MW distance (meters) (Median, Interquartile range) | 401 (360–438) | 358 (322–383) | 0.06 |
| MV E/A (Median, Interquartile range) | 1.5 (1.2–1.9) | 2.0 (1.8–2.2) | 0.04 |
| BNP | 47.6 (24.0–105.9) | 42.6 (29.9–82.9) | 0.9 |
Figure 1.
Minutes of oxygen saturation < 90% and frequency of apnea and hypopnea (AHI) events per hour is shown. The Spearman correlation coefficient in patients with total hemoglobin levels < 9.0 g/dl was −0.65.
Median systolic blood pressures was 114 mm Hg in subjects with an AHI > 5 vs 103 mm Hg, (p= 0.03) in subjects with AHI < 5. All subjects had a reduced six minute walk distance for age and gender-based standards [11], however, those with an AHI > 5 demonstrated a trend toward a shorter walk distance (median 358 vs 401 meters, p = .06). Echocardiograms demonstrated that those with AHI > 5 had a higher mitral valve E/A (MV E/A) ratio, (2.0 vs 1.5, p = .04) suggestive of left ventricular diastolic dysfunction. Other echocardiographic parameters were similar between the two groups. Differences were not demonstrated between the groups in BNP levels or tricuspid regurgitant velocity.
Subjects with SCD and an elevated AHI had lower Health-related Quality of Life in the General Health (mean 50 vs 39, p = .01), Emotional (mean 50 vs 38, p = .04), Social (mean 49 vs 37, p = .01), and Mental (mean 53 vs 37, p = .02) Quality of Life domains compared to the subjects without sleep apnea.
Discussion
We prospectively examined a small group of young adults with SCD irrespective of symptoms for SDB, as an initial assessment of the scope of this condition in the young adult population. In the 20 subjects studied, 50% had an AHI > 5 events/hr even though they did not have traditional SDB risk factors. Despite the mild severity of sleep disordered breathing in this group and the small number of patients studied, it was nevertheless associated with a trend toward a reduced exercise capacity, echocardiographic abnormalities suggestive of impaired diastolic function (MV E/A) of the left ventricle, and a diminished quality of life. While blood pressures for both groups were in the normal range, it is possible the relatively higher systolic pressures in this young AHI>5 group may be a precursor for left ventricular remodeling and pulmonary hypertension over time, as some authors suggest that even minor elevations in systolic blood pressures may be deleterious in patients with SCD. [16–19]
Standard clinical screening for sleep apnea, including a history of snoring, obesity, or impaired sleep quality, would have failed to identify most individuals in the AHI >5 group. If adverse physiologic effects from subtle SDB in SCD patients are confirmed in larger studies, it suggests that a more comprehensive screening evaluation with a lower threshold for treatment may be required to identify individuals at risk in comparison to the general population.
Some subjects with more severe anemia exhibited nocturnal hypoxemia in the absence of sleep apnea, as reported in children with SCD, indicating that other mechanisms for desaturation also need to be considered in patients with SCD [5]. Cardiopulmonary complications, including pulmonary hypertension, are associated with morbidity in SCD and likely contribute to early mortality [13–19]. A growing body of data indicates that sub-clinical vascular changes are progressive in SCD. One potential mechanism for vascular dysfunction in SCD is localized tissue hypoxia, which can lead to pulmonary vasoconstriction, which can contribute to development of decompensated congestive heart failure [18].
We recognize that while these findings are suggestive, in view of the small number of subjects in this pilot study, any conclusions are preliminary. Nevertheless, these results suggest that larger studies are warranted to evaluate the prevalence and severity of SDB and nocturnal hypoxemia in adults with SCD. If confirmed, these findings could provide opportunities to intervene and potentially reduce cardiovascular complications and the frequency of acute vaso-occlusive events, and to improve the quality of life in this vulnerable population.
Highlights.
An AHI > 5 was seen in 10 of 20 subjects attending a sickle cell disease clinic.
Elevated AHI correlated with systolic BP, mitral valve E/A ratio, and QoL scores.
Hypoxemia occurred in some subjects independently from sleep apnea events.
Results suggest further study with larger subject numbers is warranted.
Acknowledgments
Supported by NIH grants P50 HL118006 and the National Center for Advancing Translational Sciences of the National Institutes of Health under the Clinical Translational Science Award UL1TR001409. The content is solely the responsibility of the authors and does not represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rogers VE, Lewin DS, Winnie GB, Geiger-Brown J. Polysomnographic characteristics of a referred sample of children with sickle cell disease. J Clin Sleep Med. 2010;6(4):374–81. [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen CL, Debaun MR, Strunk RC, et al. Obstructive sleep apnea and sickle cell anemia. Pediatrics. 2014;134(2):273–81. doi: 10.1542/peds.2013-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gileles-Hillel A, Kheirandish-Gozal L, Gozal D. Hemoglobinopathies and sleep - The road less traveled. Sleep Med Rev. 2015;24:57–70. doi: 10.1016/j.smrv.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Connes P. Obstructive sleep apnea and sickle cell disease: Towards hemorheological abnormalities and vascular dysfunction worsening. Sleep Med Rev. 2015;24:101–102. doi: 10.1016/j.smrv.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Halphen I, Elie C, Brousse V, Bouregois ML, Allali S, Bonnet D, de Montalembert M. Severe nocturnal and postexercise hypoxia in children and adolescents with sickle cell disease. PLOS One. 2014;9:1–8. doi: 10.1371/journal.pone.0097462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hargrave DR, Wade A, Evans JP, Hewes DKM, Kirkham FJ. Nocturnal oxygen saturation and painful sickle cell crises in children. Blood. 2003;101:846–848. doi: 10.1182/blood-2002-05-1392. [DOI] [PubMed] [Google Scholar]
- 7.Johnson MC, Kirkham FJ, Redline S, Rosen CL, Yan Y, Roberts I, Gruenwald J, Marek J, DeBaun MR. Left ventricular hypertrophy and diastolic dysfunction in children with sickle cell disease are related to asleep and waking oxygen desaturation. Blood; 2010 Jul 8;116(1):16–21. doi: 10.1182/blood-2009-06-227447. Epub 2010 Apr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkham FJ, Hewes DKM, Prengler P, Wade A, Lane R, et al. Nocturnal hypoxaemia and central-nervous-system events in sickle-cell disease. Lancet. 2001;357:1656–1659. doi: 10.1016/s0140-6736(00)04821-2. [DOI] [PubMed] [Google Scholar]
- 9.Hollocks MJ, Kok TB, Kirkham FJ, et al. Nocturnal oxygen desaturation and disordered sleep as a potential factor in executive dysfunction in sickle cell anemia. J Int Neuropsychol Soc. 2012 Jan;18(1):168–73. doi: 10.1017/S1355617711001469. Epub; Nov 24 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S, Efird JT, Knupp C, Kadali R, Liles D, Shiue K, Boettger P, Quan SF. Sleep disorders in adult sickle cell patients. J Clin Sleep Med. 2015;11(3):219–223. doi: 10.5664/jcsm.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 12.Gibbons WJ, Fruchter N, Sloan S, Levy RD. Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J Cardiopulm Rehabil; 2001 Mar-Apr;(2):87–93. doi: 10.1097/00008483-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Cambell A, Minitti CP, Nouraie M, et al. Prospective evaluation of haemoglobin oxygen saturation at rest and after exercise in paediatric sickle cell disease patients. Br J Haematology. 2009;149:352–359. doi: 10.1111/j.1365-2141.2009.07854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordeuk V, Minniti CP, Nouraie M, et al. Elevated tricuspid regurgitation velocity and decline in exercise capacity over 22 months of follow-up in children and adolescents with sickle cell anemia. Haematologica; 2011;96:33–40. doi: 10.3324/haematol.2010.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA. 2012;307:1254–6. doi: 10.1001/jama.2012.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordeuk VR, Sachdev V, Taylor JG, Gladwin MT, Kato G, Castro OL. Relative systemic hypertension in patients with sickle cell disease is associated with risk of pulmonary hypertension and renal insufficiency. Am J Hematol. 2008;83:15–8. doi: 10.1002/ajh.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordeuk VR, Gladwin MT, Kato G, Castro O. Prevalence of pulmonary hypertension and renal dysfunction by systemic blood pressure categories in sickle cell disease. Blood. 2005;106:885A. [Google Scholar]
- 18.Sachdev V, Machado RF, Shizukuda Y, et al. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. 2007;49:472–9. doi: 10.1016/j.jacc.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sachdev V, Kato GJ, Gibbs JS, et al. Echocardiographic Markers of elevated pulmonary pressure and left ventricular diastolic dysfunction are associated with exercise intolerance in adults and adolescents with homozygous sickle cell nnemia in the United States and United Kingdom. Circulation; 2011 Sep 27;124(13):1452–60. doi: 10.1161/CIRCULATIONAHA.111.032920. Epub 2011 Sep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]