Abstract
Aims
Ovarian endometrioid carcinomas (OEC) of low grade have characteristic morphologic features, but high-grade tumors can mimic high-grade serous and undifferentiated carcinomas. We reviewed tumors initially diagnosed as OEC to determine whether a combination of pathologic and immunohistochemical features can improve histologic subclassification.
Methods
Tumors initially diagnosed as OEC were reviewed using World Health Organization criteria. We also noted the presence of associated confirmatory endometrioid features (CEFs): i) squamous metaplasia; ii) endometriosis; iii) adenofibromatous background; and iv) borderline endometrioid or mixed Mullerian component. A tissue microarray was constructed from 27 representative tumors with CEF and 14 without CEF, and sections were stained for WT-1, p16, and p53.
Results
Of 109 tumors initially diagnosed as OEC, 76 (70%) tumors were classified as OEC. The median patient age was 55 years and 75% of patients were younger than 60 years. 92% presented with disease confined to the pelvis and 87% of tumors were unilateral. The median tumor size was 11.8 cm. Only 3% of tumors were high-grade (grade 3 out of 3). 80% of cases had at least 1 CEF and 59% had at least 2 CEFs. 11% overexpressed p16, 0% overexpressed p53 and 3% expressed WT-1. Only 10% of patients died of disease at last follow-up. Thirty-three (33) tumors, or 30% of tumors originally classified as endometrioid, were re-classified as serous carcinoma (OSC). The median patient age was 54.5 years and 59% of patients were younger than 60 years of age. Only 27% had disease confined to the pelvis at presentation, 52% of tumors were unilateral and the median tumor size was 8 cm. Associated squamous differentiation, endometrioid adenofibroma and endometrioid or mixed Mullerian borderline tumor (CEFs) were not present in any case, but 6% of patients had endometriosis. Approximately one-half of the reclassified OSC demonstrated SET-pattern morphology (combinations of glandular, cribriform, solid and transitional cell-like architecture) and were immunophenotypically indistinguishable from OSCs with papillary architecture. 60% of OSC overexpressed p16, 50% overexpressed p53 and 82% expressed WT-1. At last follow-up, 52% had died of disease. Compared to OSC, OEC patients more frequently presented aged <60 years (p=0.046); had low-stage tumors (p<0.001); were more frequently unilateral (p<0.001); more frequent synchronous endometrial endometrioid carcinomas (p<0.001); and had no evidence of disease at last follow up (p<0.001). Their tumors were of lower grade (p<0.001); had more CEFs (p<0.001); and less frequently overexpressed p16 and p53 (p=0.003 and p<0.001, respectively) and less frequently expressed WT-1 (p<0.001).
Conclusion
This analysis emphasizes the diagnostic value of CEFs, the presence of a low-grade gland-forming endometrioid component and WT-1 negativity, as valid, clinically relevant criteria for a diagnosis of OEC. Glandular and/or cribriform architecture alone may be seen in both OECs and OSCs and are therefore not informative of diagnosis. Further study is needed to elaborate the characteristics of the exceedingly rare high-grade OEC.
Keywords: diagnosis, endometrioid carcinoma, immunohistochemistry, ovary, pathology, serous carcinoma
Introduction
Primary ovarian endometrioid carcinomas (OEC) represent approximately 10% of all ovarian carcinomas and are the second or third most commonly encountered primary ovarian malignancy.1–5 They are generally associated with a good prognosis as the majority are low-grade and patients often present with stage I or II disease.6 In most instances, low-grade OEC can readily be distinguished from other subtypes of ovarian carcinoma based solely on histopathologic features. Morphologic features commonly associated with OEC (confirmatory endometrioid features, CEFs) include: squamous metaplasia; endometriosis, adenofibromatous background and associated borderline endometrioid component.1 High-grade OEC are infrequently encountered, but they are a cause of significant interobserver disagreement, as they may be confused with high-grade ovarian serous carcinoma (OSC) or mixed epithelial carcinoma.6 Recent molecular and protein studies have shown that a proportion of tumors previously classified as “high-grade endometrioid carcinomas” display similar genetic and immunophenotypic profiles to high-grade OSC 6 and a subset of these tumors demonstrate solid, pseudo-endometrioid and/or transitional-cell-like growth patterns (“SET pattern”)7, 8 that are increasingly thought to be part of the spectrum of OSC, particularly in the presence of a BRCA1 mutation.8 This highlights the diagnostic challenges faced by many pathologists in trying to subtype these tumors using morphological criteria.
Using modern diagnostic criteria we re-evaluated a large series of unselected ovarian carcinomas classified as endometrioid to determine the prevalence of high-grade endometrioid carcinoma and misclassification as high-grade endometrioid carcinoma. To do so, we synthesized a variety of morphologic. immunohistochemical, mutational and clinical data. Based on this, we make recommendations about the application of morphological and immunohistochemical features that should allow reproducible and clinically meaningful diagnosis and classification of ovarian carcinomas.
Materials and Methods
Case Selection and Review
The study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center. Institutional databases were queried for cases with a diagnosis of OEC, treated between 1998–2010 with available slides in the department archives. The cases included both in-house surgical resections for which both glass slides and blocks were available, and consultation cases that included only glass slides.
All available hematoxylin and eosin (H&E) stained slides (median 10 slides per case, range 1–36) were reviewed independently by up to 4 gynecologic pathologists who were blinded to the initial diagnoses and histologic reports, imunohistochemical findings and clinical outcomes. Sections of fallopian tube were examined if present, but none of cases were submitted for “sectioning and extensively examining the fimbriated end” (SEE-FIM).
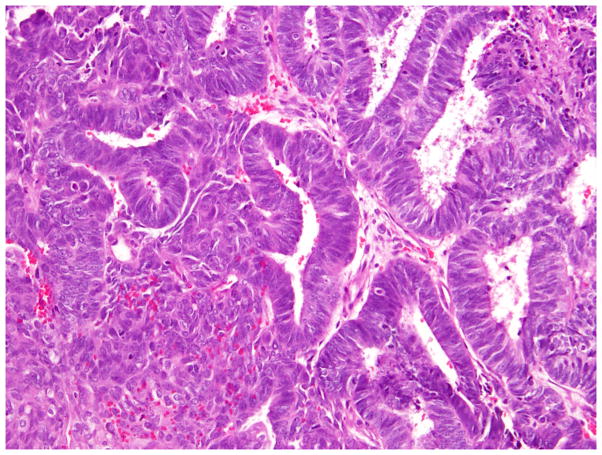
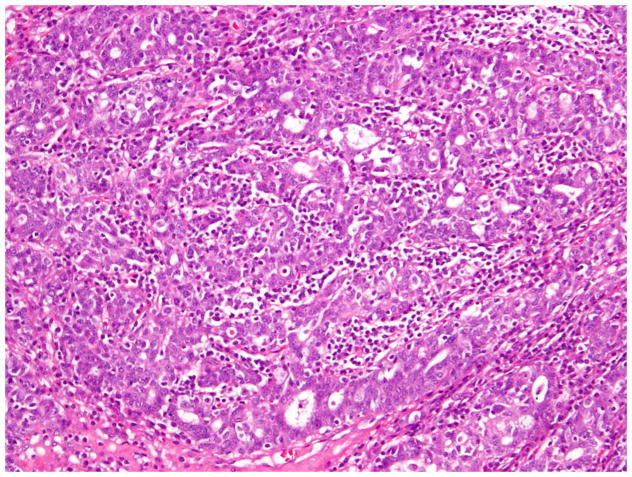
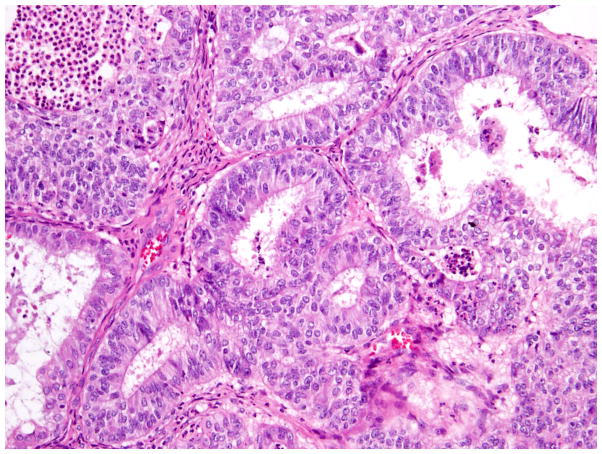
The 2014 World Health Organization (WHO) histologic criteria, briefly outlined here, were largely used in tumor classification.6 We also paid particular attention to the presence of morphologic features commonly associated with OEC (Fig. 1). These confirmatory endometrioid features (CEFs) were: i) squamous metaplasia - presence of keratinization, intercellular bridges or solid growth of cells with abundant dense eosinophilic cytoplasm and distinct cellular borders and present either at the stromal interface or as morules separating adjacent glands (Fig. 1); ii) endometriosis - presence of endometrial type glands and/or stroma, often associated with evidence of haemorrhage; iii) adenofibromatous background - presence of tubular and cystic endometrial type glands surrounded by prominent fibrous stroma; and iv) borderline endometrioid or mixed Mullerian component – this may exhibit two growth patterns, either adenofibomatous or intracystic, and is composed of glandular proliferation similar to that observed in atypical hyperplasia of the endometrium.6 Tumors lacking CEFs were still classified as endometrioid if the tumor contained components that were histologically identical to the tubules of low-grade endometrioid carcinoma of endometrium (Fig. 1) (i.e. the tubules are lined by stratified tall columnar cells with eosinophilic cytoplasm).9
Fig. 1.
Endometrioid carcinoma showing squamous morule and low-grade endometrioid glandular differentiation.
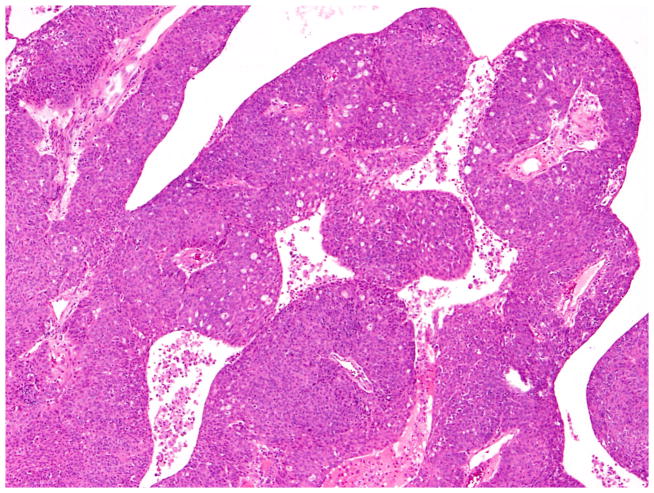
Tumors that were classified as “non-endometrioid” were further studied to specify tumor type and determine the prevalence of SET pattern high-grade serous carcinoma (OSC-SET),8 a known mimic of high-grade endometrioid and transitional cell carcinomas. OSC-SET are characterized by one or more of the following architectural patterns: solid, cribriform (pseudoendometrioid) or transitional cell carcinoma-like.8 One pathologist (RAS) examined all available slides from the non-endometrioid carcinomas, and subclassified them further as follows: OSC-SET (Figs. 2, 3, 4 and 5); high-grade papillary (conventional) serous carcinoma (OSC-P); and other/unclassified. OSC-SET are cytologically high-grade tumors that form glands and cribriform structures, with or without solid architecture, with or without blunt papillae reminiscent of transitional cell carcinoma. Many tumors have high numbers of tumor-infiltrating lymphocytes, but these were not required for diagnosis as OSC-SET in this study. Importantly, the presence of any CEF other than endometriosis was considered exclusionary, as was the presence of any component that resembled the tubules of low-grade endometrioid adenocarcinoma.
Fig. 2.
High-grade serous carcinomas with SET features (originally diagnosed as FIGO grade 3 endometrioid carcinoma) showing solid architecture.
Fig. 3.
High-grade serous carcinomas with SET features (originally diagnosed as FIGO grade 3 endometrioid carcinoma) showing pseudo-endometrioid growth pattern.
Fig. 4.
High-grade serous carcinomas with SET features (originally diagnosed as FIGO grade 3 endometrioid carcinoma) showing transitional cell-like architecture.
Fig. 5.
High-grade serous carcinomas with SET features (originally diagnosed as FIGO grade 3 endometrioid carcinoma) showing numerous tumor-infiltrating lymphocytes.
Tumors were graded using the International Federation of Gynecology and Obstetrics (FIGO) grading system 6, identical to the grading system used for endometrioid carcinomas of the uterine corpus. Tumors were also assigned a Shimizu-Silverberg grade, which makes use of tumor architecture, nuclear grade and mitotic index.10
Clinical and follow-up information was obtained from the patients’ electronic medical records. Available paraffin-embedded blocks from tumors resected at Memorial Sloan Kettering Cancer Center were used for the construction of a tissue microarray (TMA). Representative tumor areas were annotated by a gynecologic pathologist and three 0.6mm cores from different areas of each tumor were sampled for the TMA (Manual Tissue Arrayer MTA-1, Beecher Instruments, Sun Prairie WI). Immunohistochemical studies were preformed on sections from the TMA using commercially available antibodies for WT-1 (clone C-19, Santa Cruz Biotechnology, Inc., Dallas TX), p16 (INK4A, Ventana Medical Systems, Inc., Tucson AZ), and p53 (clone DO-7, Ventana Medical Systems, Inc., Tucson AZ). WT-1 was considered positive if there was diffuse reactivity throughout the tumor. p16 was considered positive if >50% of the tumor was immunoreactive without intervening single or aggregated cells lacking staining. Aberrant p53 expression was defined as nuclear positivity in >50% of tumor cells or complete absence of staining in the presence of a positive internal control.
Statistical Analysis
Statistical analysis was performed using PASW SPSS 18.0 software (IBM Corporation, Armonk, NY). Fisher exact or chi-squared tests were used to test associations of histologic type with nominal and ordinal covariates and non-parametric tests were used for comparison of medians for continuous covariates. All statistical tests were two-tailed, and a p-value of <0.05 was considered statistically significant.
Results
H&E slides were available for 118 patients, whose tumors were diagnosed over a 12-year period spanning 1998–2010. Nine cases were excluded from analysis; of these, 6 were associated with a concurrent uterine endometrioid tumor and it was unclear whether the ovarian tumors represented metastatic or synchronous disease; 1 was reclassified as an endometrioid borderline tumor; and 2 were poorly differentiated tumors that were not classifiable.
Endometrioid carcinomas
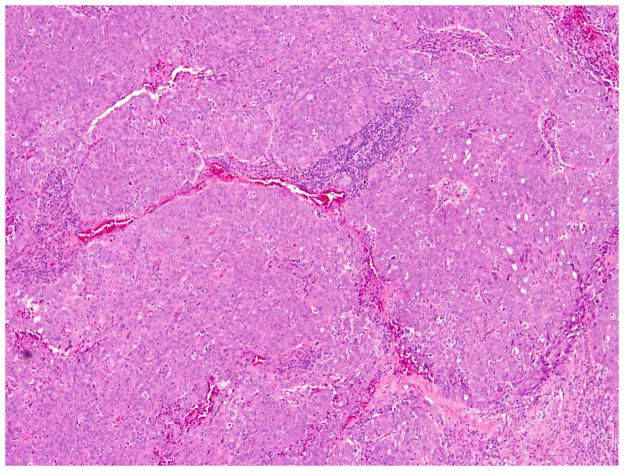
Seventy-six (76) tumors were classified as OEC (Figs 1, 6 and 7), representing 70% of tumors originally diagnosed as endometrioid. The median patient age was 55.0 years, with a range of 25–81 years; 75% of patients were younger than 60 years of age. 67 of 73 staged patients (92%) presented with disease confined to the pelvis (53 stage I and 14 stage II), while only 6 patients had extrapelvic disease at presentation (5 stage III and 1 stage IV). 3 patients were unstaged. 87% of tumors were unilateral. The median tumor size was 11.8 cm, with a range of 0.4–30 cm.
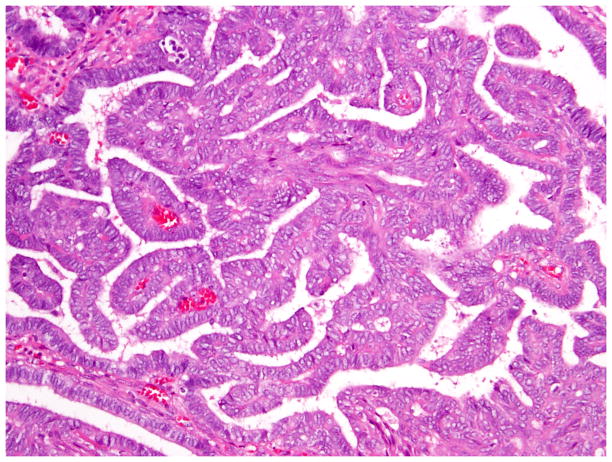
Fig. 6.
Low-grade endometrioid carcinoma exhibiting papillary architecture.
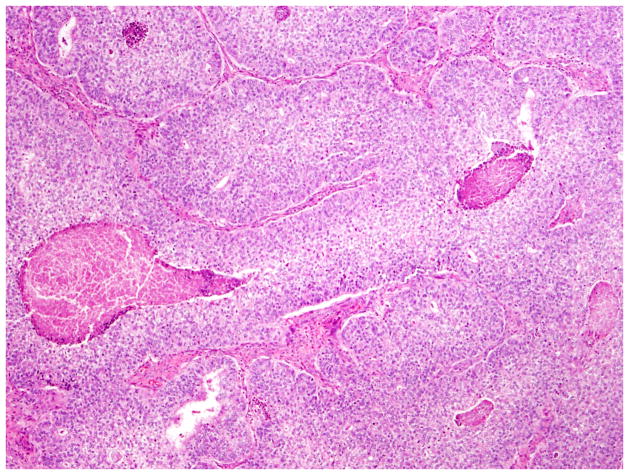
Fig. 7.
Low-grade endometrioid carcinoma exhibiting and sex cord-like pattern.
Microscopically, 54 (71%) were FIGO grade 1, 18 (24%) were grade 2, and 4 (5%) were grade 3. Using the Shimizu-Silverberg system, 64 (84%) were grade 1, 9 (12%) were grade 2 and 2 (3%) were grade 3. Therefore, between 84 and 97% of ovarian endometrioid carcinomas are “low-grade,” with the higher value grouping together grade 1 and grade 2 tumors. The median mitotic index was 1 per 10 high power fields, with a range of 1 to 24. Squamous differentiation was present in 50 cases (76%), endometriosis in 37 cases (49%), endometrioid or mixed Mullerian borderline tumor in 32 cases (42%), endometrioid adenofibroma in 16 cases (21%) and a synchronous endometrial endometrioid carcinoma in 28 cases (37%) (Table 1). 80% of cases had at least 1 CEF and 59% had at least 2 CEFs (Table 2). All cases contained histologically low-grade gland-forming endometrioid components. Detailed associations of CEFs with clinico-pathological features and tumor classification are presented in Supplementary Tables 1 and 2. For a diagnosis of OEC, the presence of any CEF yielded sensitivity, specificity, positive predictive values and negative predictive values of 80%, 94%, 97% and 67%, respectively, while the presence of more than 1 CEF yielded values of 59%, 100%, 100% and 59%, respectively (Table 3).
Table 1.
Associations of tumor type with clinico-pathological features
| Endometrioid | Non-endometrioid | P value | ||
|---|---|---|---|---|
| N | 109 | 76 | 33 | |
|
| ||||
| Age | <60 years | 60 | 20 | 0.046 |
| >= 60 years | 16 | 13 | ||
|
| ||||
| Age (median, range) | 55 (25–81) | 54.5 (37–80) | 0.22 | |
|
| ||||
| Tumor size (median, range) | 11.8 (0.4–30) | 8 (3.5–18.5) | 0.02 | |
|
| ||||
| pT stage | I | 53 | 6 | <0.001 |
| IA | 24 | 5 | ||
| IB | 2 | 0 | ||
| IC | 27 | 1 | ||
| II | 14 | 3 | ||
| IIA | 4 | 0 | ||
| IIB | 3 | 0 | ||
| IIC | 7 | 3 | ||
| III | 5 | 20 | ||
| IIIA | 1 | 3 | ||
| IIIB | 0 | 0 | ||
| IIIC | 4 | 17 | ||
| IV | 1 | 4 | ||
|
| ||||
| Laterality | Unilateral | 66 | 17 | <0.001 |
| Bilateral | 10 | 16 | ||
|
| ||||
| Status at last follow-up | NED | 59 | 12 | <0.001 |
| AWD | 3 | 3 | ||
| Dead | 7 | 16 | ||
|
| ||||
| Architectural pattern | Glandular | 56 | 5 | <0.001 |
| Papillary | 12 | 6 | ||
| Solid | 7 | 17 | ||
|
| ||||
| Mitotic rate (per 10 hpf) (median, range) | 1 (1–24) | 7.5 (1–96) | <0.001 | |
|
| ||||
| FIGO grade | 1 | 54 | 0 | <0.001 |
| 2 | 18 | 8 | ||
| 3 | 4 | 25 | ||
|
| ||||
| Shimizu-Silverberg grade | 1 | 64 | 3 | <0.001 |
| 2 | 9 | 6 | ||
| 3 | 2 | 20 | ||
|
| ||||
| Squamous differentiation | Absent | 26 | 33 | <0.001 |
| Present | 50 | 0 | ||
|
| ||||
| Endometriosis | Absent | 39 | 31 | <0.001 |
| Present | 37 | 2 | ||
|
| ||||
| Adenofibroma | Absent | 60 | 33 | 0.004 |
| Present | 16 | 0 | ||
|
| ||||
| Borderline tumor | Absent | 44 | 33 | <0.001 |
| Present | 32 | 0 | ||
|
| ||||
| Number of CEFs | 0 | 15 | 31 | <0.001 |
| 1 | 16 | 2 | ||
| 2 | 24 | 0 | ||
| 3 | 13 | 0 | ||
| 4 | 8 | 0 | ||
|
| ||||
| Concurrent endometrial tumor | Absent | 46 | 29 | <0.001 |
| Present | 28 | 1 | ||
|
| ||||
| P16 | Negative | 28 | 4 | 0.003 |
| Positive | 3 | 6 | ||
|
| ||||
| P53 | Negative | 30 | 5 | <0.001 |
| Positive | 0 | 5 | ||
|
| ||||
| WT-1 | Negative | 29 | 1 | <0.001 |
| Positive | 1 | 9 | ||
Table 2.
Associations of clinico-pathological features with presence or absence of CEFs
| Absent | Present | P value | ||
|---|---|---|---|---|
| N | 109 | 46 | 63 | |
|
| ||||
| Tumor classification | Endometrioid | 15 | 61 | <0.001 |
| Non-endometrioid | 31 | 2 | ||
|
| ||||
| Age | <60 years | 26 | 54 | 0.001 |
| >= 60 years | 20 | 9 | ||
|
| ||||
| Age (median, range) | 62.5 | 51 | 0.003 | |
| 37–81 | 25–78 | |||
|
| ||||
| Tumor size, cm (median, range) | 9 | 11.2 | 0.23 | |
| 3.5–18.5 | 0.4–30 | |||
|
| ||||
| pT stage | I | 14 | 45 | <0.001 |
| II | 7 | 10 | ||
| III | 21 | 4 | ||
| IV | 3 | 2 | ||
|
| ||||
| Laterality | Unilateral | 30 | 53 | 0.04 |
| Bilateral | 16 | 10 | ||
|
| ||||
| Status at last follow-up | NED | 23 | 48 | 0.002 |
| AWD | 3 | 3 | ||
| Dead | 17 | 6 | ||
|
| ||||
| Architectural pattern | Glandular | 15 | 46 | <0.001 |
| Papillary | 9 | 9 | ||
| Solid | 17 | 7 | ||
|
| ||||
| Nuclear grade | 1 | 1 | 14 | <0.001 |
| 2 | 15 | 44 | ||
| 3 | 25 | 4 | ||
|
| ||||
| Mitotic rate (per 10 hpf) (median, range) | 3 | 1 | <0.001 | |
| 1–96 | 1–17 | |||
|
| ||||
| FIGO grade | 1 | 8 | 46 | <0.001 |
| 2 | 13 | 13 | ||
| 3 | 25 | 4 | ||
|
| ||||
| Shimizu-Silverberg grade | 1 | 13 | 54 | <0.001 |
| 2 | 9 | 6 | ||
| 3 | 20 | 2 | ||
|
| ||||
| Squamous differentiation | Absent | 46 | 13 | <0.001 |
| Present | 0 | 50 | ||
|
| ||||
| Endometriosis | Absent | 46 | 24 | <0.001 |
| Present | 0 | 39 | ||
|
| ||||
| Adenofibroma | Absent | 46 | 47 | <0.001 |
| Present | 0 | 16 | ||
|
| ||||
| Borderline tumor | Absent | 46 | 31 | <0.001 |
| Present | 0 | 32 | ||
|
| ||||
| Concurrent endometrial tumor | Absent | 34 | 41 | 0.10 |
| Present | 8 | 21 | ||
|
| ||||
| P16 | Negative | 12 | 20 | 0.45 |
| Positive | 5 | 4 | ||
|
| ||||
| P53 | Negative | 13 | 22 | 0.14 |
| Positive | 4 | 1 | ||
|
| ||||
| WT-1 | Negative | 8 | 22 | 0.001 |
| Positive | 9 | 1 | ||
Table 3.
Number of CEFs and Tumor Classification
| CEFs | Endometrioid | Non-endometrioid | P value | Sn | Sp | PPV | NPV |
|---|---|---|---|---|---|---|---|
| 0–1 CEFs | 31 | 45 | <0.001 | 59% | 100% | 100% | 59% |
| 2–4 CEFs | 45 | 0 | |||||
|
| |||||||
| Absent | 15 | 31 | <0.001 | 80% | 94% | 97% | 67% |
| Present | 61 | 2 | |||||
Sn = sensitivity; Sp = specificity; PPV = positive predictive value; NPV = negative predictive value (Uncertain cases excluded from these calculations)
Immunohistochemistry was attempted in 31 tumors with available material. Three of 31 cases (11%) overexpressed p16, 0 of 30 cases (0%) overexpressed p53 and 1 of 30 cases (3%) expressed WT-1 (Table 1). After a median follow-up period of 65 months (range 6–180 months), 59 patients (86%) had no evidence of disease, 3 (4%) were alive with disease and 7 (10%) had died of disease (Table 1).
High-grade endometrioid carcinomas
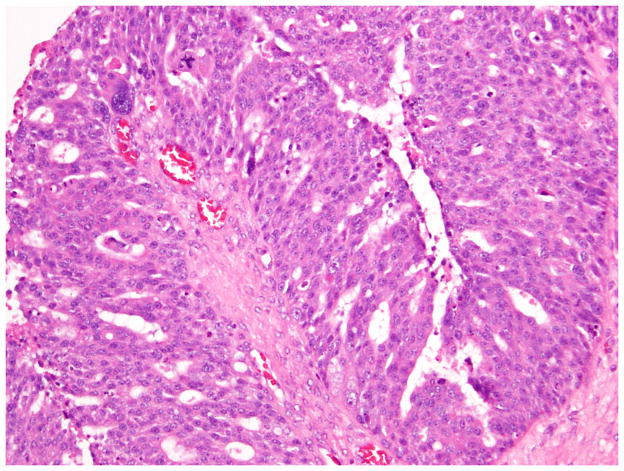
Only 2 of 76 endometrioid carcinomas (Figs. 8 and 9), details of which are presented in Table 4, were classified as Shimizu-Silverberg grade 3, or high-grade.
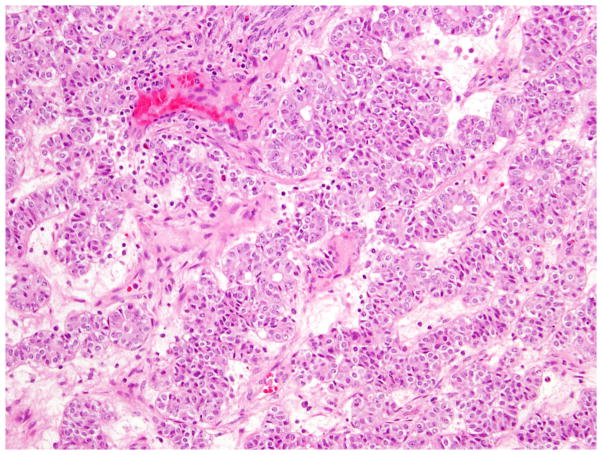
Fig. 8.
High-grade (FIGO grade 3) endometrioid carcinoma with a predominantly solid growth pattern.
Fig. 9.
High-grade (FIGO grade 3) endometrioid carcinoma with focal low-grade endometrioid glandular differentiation.
Table 4.
Characteristics of two high-grade endometrioid carcinomas
| Case 1 | Case 2 | |
|---|---|---|
| Age | 59 | 67 |
| Stage | IIC | IIIC |
| Laterality | Unilateral | Unilateral |
| CEFs | - | - |
| P16 IHC | - | NA |
| P53 IHC | - | NA |
| WT1 IHC | - | NA |
| Follow-up status (duration) | No evidence of disease (173 months) | No evidence of disease (135 months) |
Non-endometrioid carcinomas
Thirty-three (33) tumors were classified as non-endometrioid carcinoma, representing 30% of tumors originally diagnosed as endometrioid. The median patient age was 54.5 years, with a range of 37 to 80 years; 59% of patients were younger than 60 years of age. Just 9 staged patients (27%) presented with disease confined to the pelvis (6 stage I and 3 stage II), while 24 patients (73%) had extrapelvic disease at presentation (20 stage III and 4 stage IV). 52% of tumors were unilateral and 48% were bilateral. The median tumor size was 8 cm, with a range of 3.5–18.5 cm (Table 1).
Microscopically, none (0%) were FIGO grade 1, 8 (24%) were grade 2, and 25 (76%) were grade 3. Using the Shimizu-Silverberg system, 3 (10%) were grade 1, 6 (21%) were grade 2 and 20 (69%) were grade 3. The median mitotic index was 7.5 per 10 high power fields, with a range of 1 to 96. Squamous differentiation, endometrioid adenofibroma and endometrioid or mixed Mullerian borderline tumor was not present in any case; 2 patients (6%) had endometriosis. Six of 10 cases (60%) overexpressed p16, 5 of 10 cases (50%) overexpressed p53 and 9 of 11 cases (82%) expressed WT-1 (Table 1).
After a median follow-up duration of 80 months (range 18–158 months), 12 patients (38%) had no evidence of disease, 3 (10%) were alive with disease and 16 (52%) had died of disease.
Non-endometrioid subsets
One pathologist (RAS) examined all available slides from 21 non-endometrioid carcinomas, which were subclassified further as follows: high-grade papillary (conventional) serous carcinoma (OSC-P) (n=8); SET pattern high-grade serous carcinomas (OSC-SET) (n=11) (Figs. 2, 3, 4 and 5) and other/unclassified (n=2) (Table 5). There were no statistically significant clinical or immunohistochemical differences between OSC-P or OSC-SET, although there were twice as many patients under 60 years of age in the OSC-SET group and more than twice as many OSC-SET patients with bilateral tumors than patients with OSC-P. The numbers in these subgroups were small, however (Table 5).
Table 5.
Serous carcinomas – comparison of conventional and SET-associated tumors
| OSC-P | % | OSC-SET | % | P value | ||
|---|---|---|---|---|---|---|
| N | 8 | 42 | 11 | 58 | ||
|
| ||||||
| Age | <60 years | 3 | 33 | 6 | 67 | 0.65 |
| >60 years | 5 | 50 | 5 | 50 | ||
|
| ||||||
| Stage | I | 2 | 67 | 1 | 33 | 0.46 |
| II | 0 | 0 | 1 | 100 | ||
| III | 4 | 33 | 8 | 67 | ||
| IV | 2 | 67 | 1 | 33 | ||
|
| ||||||
| Laterality | Unilateral | 5 | 62 | 3 | 38 | 0.18 |
| Bilateral | 3 | 27 | 8 | 73 | ||
|
| ||||||
| P16 IHC | Negative | 2 | 50 | 2 | 50 | 1.00 |
| Positive | 3 | 50 | 3 | 50 | ||
|
| ||||||
| P53 IHC | Negative | 3 | 60 | 2 | 40 | 1.00 |
| Positive | 2 | 40 | 3 | 60 | ||
|
| ||||||
| WT1 IHC | Negative | 1 | 100 | 0 | 0 | 1.00 |
| Positive | 4 | 44 | 5 | 56 | ||
OSC-P = conventional serous carcinoma; OSC-SET = Serous, pseudoEndometrioid or Transitional cell pattern-associated serous carcinoma
Comparison of endometrioid and serous carcinomas originally diagnosed as endometrioid carcinomas
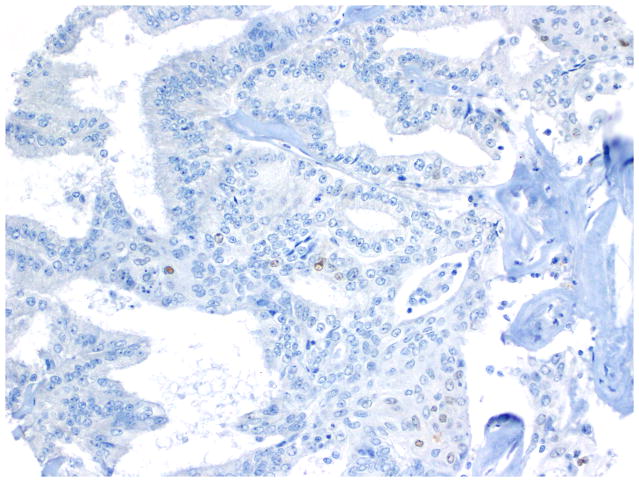
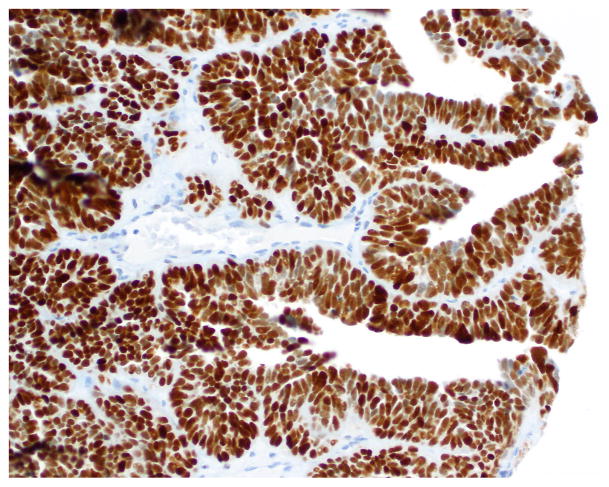
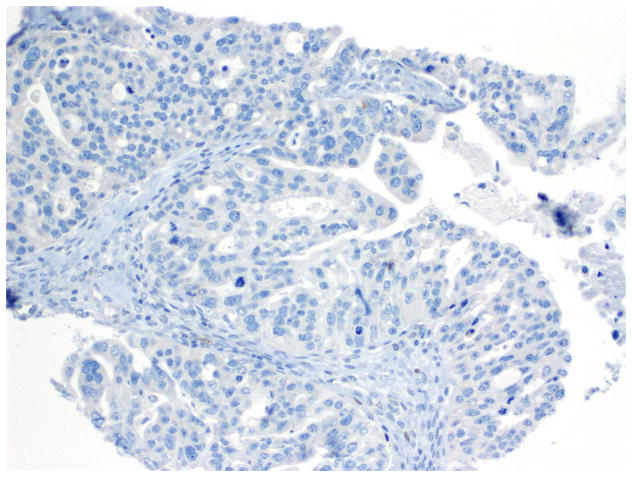
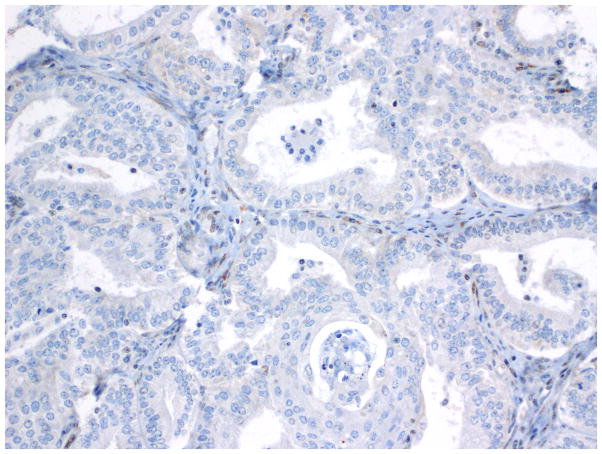
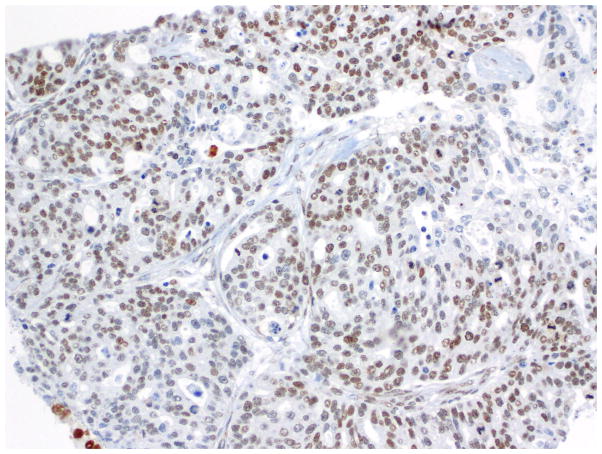
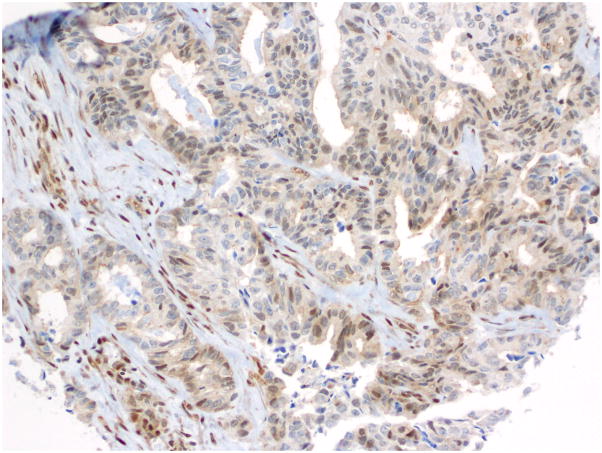
Compared to OSC patients, OEC patients more frequently presented under age 60 (p=0.046); had low-stage tumors (p<0.001); were more frequently unilateral (p<0.001); more frequent synchronous endometrial endometrioid carcinomas (p<0.001); and had no evidence of disease at last follow up (p<0.001). Their tumors were of lower grade (p<0.001); had more CEFs (p<0.001); and less frequently overexpressed p16 and p53 (p=0.003 and p<0.001, respectively) (Figs. 10, 11 and 12) and less frequently expressed WT-1 (p<0.001) (Figs. 13, 14 and 15) (Table 1). Due to the small numbers of high-grade OECs in the cohort (Table 5), it was not meaningful to statistically compare them with OSCs.
Fig. 10.
Immunohistochemistry - p53 expression in endometrioid carcinoma.
Fig. 11.
Immunohistochemistry - p53 expression in OSC-SET.
Fig. 12.
Null pattern of p53 expression in an OSC-SET. Note the rare non-neoplastic cells that display weak p53 expression; this serves as a positive internal control.
Fig. 13.
Immunohistochemistry - WT-1 expression in endometrioid carcinoma.
Fig. 14.
Immunohistochemistry - WT-1 expression in OSC-SET.
Fig. 15.
Rare WT-1 positive cells in a low-grade endometrioid carcinoma.
Discussion
Our criteria identified 2 distinct groups of patients 30% of whose tumors were reclassified as OSC despite originally being diagnosed as OEC; immunophenotyping validated this morphology-based classification. Approximately one-half of OSC exhibited SET pattern,8 a newly described pattern that has historically been considered to represent high-grade endometrioid or transitional cell carcinoma. Clinicopathological and immunohistochemical comparison to papillary serous carcinomas confirm that these are serous carcinoma variants, not high-grade endometrioid carcinomas.8 Patients with OSC-SET showed a trend towards younger ages and more frequent bilaterality of their tumors than those with OSC-P.7
In this analysis, we emphasize the diagnostic value of CEFs and presence of a low-grade gland-forming endometrioid component as central to a diagnosis of OEC. Glandular and/or cribriform architecture alone is not a valid criterion since these architectural features were found in nearly every case examined. However, 80% of OECs have at least 1 CEF, compared to 23% of OSCs (which represented only 2 patients, both of whom had endometriosis). For a diagnosis of OEC, the presence of any CEF yields high sensitivity, specificity and positive predictive values and modest negative predictive values, while the presence of more than 1 CEF yields perfect specificity and positive predictive values but only modest sensitivity and negative predictive values.
Immunohistochemical markers may serve as useful adjuncts to the classification of ovarian carcinomas.11, 12 The immunoprofile of OEC is broadly similar to its uterine counterparts and is characterized by frequent immunoreactivity for estrogen receptor (ER) and progesterone receptor (PR) and absent or focal positivity for WT-1 and p16 as well as ‘wild-type’ p53 expression.6 Low-grade tumors, particularly those with squamous morules can also exhibit nuclear beta-catenin expression.13–17 In contrast, although ER and PR are also commonly expressed in OSC, the majority demonstrates diffuse WT-1 and p16 positivity as well as aberrant p53 expression.18–21 In a study of ovarian carcinomas by Gilks et al,22 50 cases originally diagnosed as OEC were reclassified as OSC using morphologic criteria. These tumors were predominantly high-grade (16 grade 2 tumors and 33 grade 3 tumors) and 68% of these tumors were WT-1 positive, a result comparable to that observed in their cohort of OSC (78%) but significantly different from OEC (4%). Although studies have shown that WT-1-positive OEC tend to be high-grade, immunoreactivity in low-grade tumors has been reported in the literature. Madore et al23 identified WT-1 positivity in 31% (10/32) of grade 1 and grade 2 tumors. In our cohort, only rare OECs overexpressed p16, 1 was WT-1-positive and none overexpressed p53, confirming our morphologic classification scheme with immunopositivity rates that are in line with most previously published values.
Given these data, one can make the following generalizations about OEC: they typically present as large, unilateral, low-grade ovarian tumors, confined to the pelvis, with excellent clinical outcomes. This information, particularly the size and laterality information, can be used as adjuncts for differential diagnosis, not only to separate OECs and OSCs, but also to distinguish primary OECs from metastatic endometrioid carcinomas. Only 2% of tumors studied were high-grade OEC. Therefore a diagnosis of high-grade OEC should be approached with caution, particularly when SET patterns are present and CEFs are lacking. Gene expression studies have revealed that some high-grade OEC demonstrate expression profiles similar to that of high-grade OSC.14, 24 In a study of 29 OEC (10 grade 1, 11 grade 2 and 8 grade 3), Geyer et al14 observed p53 overexpression in 6 grade 3 tumor categorized as OECs with the majority lacking molecular alterations characteristic of lower grade tumors, such as beta-catenin and KRAS mutations. This difference in biology between low-grade and high-grade OEC is also reflected in their clinical behavior; high-grade tumors tend to be associated with significantly poorer clinical outcomes than low-grade tumors.14 These findings have led some investigators to hypothesize that OEC may arise via a dual pathogenic pathway or that some “high-grade OEC” may in fact represent misclassified high-grade OSC,14, 23 a conclusion that our data supports. It has been shown that the distinction of high-grade OECs from OSC is often associated with significant interobserver disagreement.25, 26 On the other hand, there is immunohistochemical and molecular evidence to support the existence of a true high-grade OEC.14, 23 Madore and colleagues 23 identified two high-grade, high-stage, WT-1-negative OEC with concurrent β-catenin and TP53 aberrations. One of the tumors was spontaneously immortalized in cell culture and gave rise to an aggressive endometrioid carcinoma cell line. Nevertheless, the true incidence of high-grade OEC is likely to be substantially lower than that reported in earlier literature.4, 27 Unfortunately, we did not have genotype data on any of the cases in our cohort, but it is clear from our findings and the literature that further research on high-grade OECs is required.
The distinction between high-grade OEC and OSC can have important consequences. For example, incorrectly classifying a high-grade OSC as OEC might deprive the patient of the opportunity to be tested for germline BRCA1/BRCA2 mutations, which may have several downstream repercussions, including (i) affecting the choice of adjuvant chemotherapeutic agents such as PARP inhibitors;28, 29 (ii) prognostic assessment; and (iii) appropriate screening and surveillance of other family members. Accurate diagnosis is also important for the correct assignment of patients to appropriate clinical trials.
Of the 30% of tumors in this cohort that were reclassified as OSCs, approximately 50% were OSC-SETs. It is understandable that these tumors, specifically, were originally classified as high-grade OECs because of the frequent combinations of glandular, cribriform and solid growth patterns. The OSC-Ps had combinations of papillary and glandular architecture with high nuclear grade, making it difficult to understand why these tumors had been classified as endometrioid; perhaps the long timeframe over which the cases were originally diagnosed (1998–2010) and subsequent refinements in diagnostic criteria are factors that contributed to their initial classification as OEC. Clinical and immunohistochemical data reported herein support that OSC-SETs are unequivocally OSCs, not OECs. Immunohistochemistry can be used as a diagnostic adjunct when a diagnosis of high-grade OEC is under consideration, with WT-1 being the most robust discriminator; all tested OSC-SETs were WT-1 positive, as compared to only 3% of OECs.
Several recent papers have reported that SET pattern tumors belong to the family of OSC. The most recent literature on the topic reports that OSC-SETs constitute a variant of OSC with association with BRCA1 mutation or promoter methylation,7, 8 presentation at younger age, better prognosis and infrequent association with serous tubal intraepithelial carcinoma.7 Although these tumors represented approximately 50% of OSCs in this cohort that was enriched for tumors with an endometrioid appearance, this almost definitely overestimates the prevalence of OSC-SET among unselected OSCs.
This study’s strengths include a large number of relatively rare tumors with good clinical annotation and follow-up, and sufficient numbers of reclassified OSCs that revealed heterogeneity within this category. However, there were insufficient numbers of bona fide high-grade OECs for statistical comparison with tumors reclassified as OSC. Many cases were consultation cases lacking tissue blocks, which limited the number of cases studied with immunohistochemistry.
Conclusions
This analysis emphasizes the diagnostic value of CEFs, the presence of a low-grade gland-forming endometrioid component and WT-1 negativity, as valid, reproducible and clinically relevant criteria for a diagnosis of OEC. Glandular and/or cribriform architecture alone may be seen in both OECs and OSCs and are therefore not informative of diagnosis. Further study is needed to elaborate the characteristics of the exceedingly rare high-grade endometrioid carcinomas of ovary.
References
- 1.Cho KR. Ovarian cancer update: lessons from morphology, molecules, and mice. Arch Pathol Lab Med. 2009;133(11):1775–81. doi: 10.5858/133.11.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long ME, Taylor HC., Jr Endometrioid Carcinoma of the Ovary. Am J Obstet Gynecol. 1964;90:936–50. doi: 10.1016/0002-9378(64)90790-2. [DOI] [PubMed] [Google Scholar]
- 3.Gray LA, Barnes ML. Endometrioid carcinoma of the ovary. Obstet Gynecol. 1967;29(5):694–701. [PubMed] [Google Scholar]
- 4.Kline RC, Wharton JT, Atkinson EN, et al. Endometrioid carcinoma of the ovary: retrospective review of 145 cases. Gynecol Oncol. 1990;39(3):337–46. doi: 10.1016/0090-8258(90)90263-k. [DOI] [PubMed] [Google Scholar]
- 5.Kurman RJ, Craig JM. Endometrioid and clear cell carcinoma of the ovary. Cancer. 1972;29(6):1653–64. doi: 10.1002/1097-0142(197206)29:6<1653::aid-cncr2820290633>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 6.Kurman RJ, Carcangiu ML, Herrington CS, et al. WHO classification of tumours of female reproductive organs. Lyon: IARC Press; 2014. [Google Scholar]
- 7.Howitt BE, Hanamornroongruang S, Lin DI, et al. Evidence for a dualistic model of high-grade serous carcinoma: BRCA mutation status, histology, and tubal intraepithelial carcinoma. Am J Surg Pathol. 2015;39(3):287–93. doi: 10.1097/PAS.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 8.Soslow RA, Han G, Park KJ, et al. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod Pathol. 2012;25(4):625–36. doi: 10.1038/modpathol.2011.183. [DOI] [PubMed] [Google Scholar]
- 9.Hoang LN, McConechy MK, Kobel M, et al. Histotype-genotype correlation in 36 high-grade endometrial carcinomas. Am J Surg Pathol. 2013;37(9):1421–32. doi: 10.1097/PAS.0b013e31828c63ed. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu Y, Kamoi S, Amada S, et al. Toward the development of a universal grading system for ovarian epithelial carcinoma: testing of a proposed system in a series of 461 patients with uniform treatment and follow-up. Cancer. 1998;82(5):893–901. doi: 10.1002/(sici)1097-0142(19980301)82:5<893::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Darvishian F, Hummer AJ, Thaler HT, et al. Serous endometrial cancers that mimic endometrioid adenocarcinomas: a clinicopathologic and immunohistochemical study of a group of problematic cases. Am J Surg Pathol. 2004;28(12):1568–78. doi: 10.1097/00000478-200412000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Yemelyanova A, Ji H, Shih Ie M, et al. Utility of p16 expression for distinction of uterine serous carcinomas from endometrial endometrioid and endocervical adenocarcinomas: immunohistochemical analysis of 201 cases. Am J Surg Pathol. 2009;33(10):1504–14. doi: 10.1097/PAS.0b013e3181ac35f5. [DOI] [PubMed] [Google Scholar]
- 13.Catasus L, Bussaglia E, Rodrguez I, et al. Molecular genetic alterations in endometrioid carcinomas of the ovary: similar frequency of beta-catenin abnormalities but lower rate of microsatellite instability and PTEN alterations than in uterine endometrioid carcinomas. Hum Pathol. 2004;35(11):1360–8. doi: 10.1016/j.humpath.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Geyer JT, Lopez-Garcia MA, Sanchez-Estevez C, et al. Pathogenetic pathways in ovarian endometrioid adenocarcinoma: a molecular study of 29 cases. Am J Surg Pathol. 2009;33(8):1157–63. doi: 10.1097/PAS.0b013e3181a902e1. [DOI] [PubMed] [Google Scholar]
- 15.Irving JA, Catasus L, Gallardo A, et al. Synchronous endometrioid carcinomas of the uterine corpus and ovary: alterations in the beta-catenin (CTNNB1) pathway are associated with independent primary tumors and favorable prognosis. Hum Pathol. 2005;36(6):605–19. doi: 10.1016/j.humpath.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Moreno-Bueno G, Gamallo C, Perez-Gallego L, et al. beta-Catenin expression pattern, beta-catenin gene mutations, and microsatellite instability in endometrioid ovarian carcinomas and synchronous endometrial carcinomas. Diagn Mol Pathol. 2001;10(2):116–22. doi: 10.1097/00019606-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Oliva E, Sarrio D, Brachtel EF, et al. High frequency of beta-catenin mutations in borderline endometrioid tumours of the ovary. J Pathol. 2006;208(5):708–13. doi: 10.1002/path.1923. [DOI] [PubMed] [Google Scholar]
- 18.Acs G, Pasha T, Zhang PJ. WT1 is differentially expressed in serous, endometrioid, clear cell, and mucinous carcinomas of the peritoneum, fallopian tube, ovary, and endometrium. Int J Gynecol Pathol. 2004;23(2):110–8. doi: 10.1097/00004347-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Hirschowitz L, Ganesan R, McCluggage WG. WT1, p53 and hormone receptor expression in uterine serous carcinoma. Histopathology. 2009;55(4):478–82. doi: 10.1111/j.1365-2559.2009.03390.x. [DOI] [PubMed] [Google Scholar]
- 20.Nofech-Mozes S, Khalifa MA, Ismiil N, et al. Immunophenotyping of serous carcinoma of the female genital tract. Mod Pathol. 2008;21(9):1147–55. doi: 10.1038/modpathol.2008.108. [DOI] [PubMed] [Google Scholar]
- 21.Waldstrom M, Grove A. Immunohistochemical expression of wilms tumor gene protein in different histologic subtypes of ovarian carcinomas. Arch Pathol Lab Med. 2005;129(1):85–8. doi: 10.5858/2005-129-85-IEOWTG. [DOI] [PubMed] [Google Scholar]
- 22.Gilks CB, Ionescu DN, Kalloger SE, et al. Tumor cell type can be reproducibly diagnosed and is of independent prognostic significance in patients with maximally debulked ovarian carcinoma. Hum Pathol. 2008;39(8):1239–51. doi: 10.1016/j.humpath.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Madore J, Ren F, Filali-Mouhim A, et al. Characterization of the molecular differences between ovarian endometrioid carcinoma and ovarian serous carcinoma. J Pathol. 2010;220(3):392–400. doi: 10.1002/path.2659. [DOI] [PubMed] [Google Scholar]
- 24.Wu R, Hendrix-Lucas N, Kuick R, et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell. 2007;11(4):321–33. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Kobel M, Kalloger SE, Baker PM, et al. Diagnosis of ovarian carcinoma cell type is highly reproducible: a transcanadian study. Am J Surg Pathol. 2010;34(7):984–93. doi: 10.1097/PAS.0b013e3181e1a3bb. [DOI] [PubMed] [Google Scholar]
- 26.Stalsberg H, Abeler V, Blom GP, et al. Observer variation in histologic classification of malignant and borderline ovarian tumors. Hum Pathol. 1988;19(9):1030–5. doi: 10.1016/s0046-8177(88)80082-0. [DOI] [PubMed] [Google Scholar]
- 27.DePriest PD, Banks ER, Powell DE, et al. Endometrioid carcinoma of the ovary and endometriosis: the association in postmenopausal women. Gynecol Oncol. 1992;47(1):71–5. doi: 10.1016/0090-8258(92)90079-x. [DOI] [PubMed] [Google Scholar]
- 28.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber V, Dantzer F, Ame JC, et al. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7(7):517–28. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]