Abstract
Background
N-Acetylcysteine (NAC) improves transplant-free survival in patients with non-acetaminophen acute liver failure (ALF) when administered in early stages of hepatic encephalopathy. The mechanisms of this benefit are unknown.
Aim
To determine whether NAC improves transplant-free survival in ALF by ameliorating the surge of pro-inflammatory cytokines.
Methods
Serum samples were obtained from 78 participants of the randomized, ALF Study Group NAC Trial with grade 1 or 2 hepatic encephalopathy on randomization. Concentrations of ten cytokines, chosen to represent a wide array of inflammatory responses, were determined by multiplex ELISA.
Results
In univariate analysis, predictors of transplant-free survival included NAC administration (P=0.012), admission bilirubin (P=0.003), INR (P=0.0002), grade 1 vs. grade 2 encephalopathy (P=0.006) and lower admission interleukin (IL)-17 concentrations (P=0.011). IL-17 levels were higher in patients with grade 2 vs. 1 encephalopathy on randomization (P=0.007) and in those who progressed to grade 3 or 4 encephalopathy over the following 7 days (P≤0.01). Stepwise multivariate logistic regression analysis identified only NAC administration and lower IL-17 concentrations as independent predictors of transplant-free survival. In patients with detectable IL-17 concentrations on admission, 78% of those who received NAC vs. 44% of those who received placebo had undetectable levels by day 3-5 (P=0.042), and the mean decrease in IL-17 concentrations between admission and late samples was significantly greater in patients who received NAC vs. placebo (P=0.045).
Conclusions
NAC may improve transplant-free survival in patients with non-acetaminophen ALF by ameliorating the production of IL-17, which is associated with progression of hepatic encephalopathy and poor outcome.
Keywords: Acute liver failure, N-acetylcysteine, liver transplantation, cytokines
Introduction
Acute liver failure (ALF), the syndrome of severe liver injury resulting in coagulopathy and hepatic encephalopathy, is a rare but highly mortal condition without liver transplantation (LT) (1). The pathogenesis of the ALF syndrome remains poorly defined, although increasing experimental evidence has incriminated mediators of inflammation such as cytokines and products of endothelial cell injury (2). Pro-inflammatory cytokines mediate the systemic inflammatory response syndrome (SIRS) in ALF (3), which in turn correlates with the progression of hepatic encephalopathy, cerebral edema, and outcome (4). Early after liver injury, pro-inflammatory cytokines including tumor necrosis factor-α (TNFα), interleukin (IL)-1β, and IL-6, are proposed to lead to death by provoking multi-organ system failure (MOSF). Later after liver injury, anti-inflammatory cytokines including IL-4 and IL-10 counterbalance pro-inflammatory cytokines and may lead to a compensatory anti-inflammatory response, immunoparalysis, and death due to sepsis (2, 5).
Recently, the ALF Study Group reported that patients with non-acetaminophen-induced ALF who received NAC had increased transplant-free survival (TFS) compared to patients who received placebo (6). The benefit, however, was limited to patients with low grades of encephalopathy (grades 1 and 2). Although the mechanisms of action of NAC are well-established in patients with acetaminophen-induced ALF, mechanisms of improvement in patients with non-acetaminophen-induced ALF remain undefined. Possibilities include improving microcirculatory dysfunction and oxygen delivery in peripheral tissue beds, by an antioxidant effect, or by amelioration of the “cytokine storm” (7, 8).
The present study was undertaken to elucidate possible mechanisms of improvement in TFS in patients with non-acetaminophen ALF who were treated with NAC. We hypothesized that NAC administration might lower concentrations of pro-inflammatory cytokines more rapidly than those observed in patients who received placebo. We also sought to further define the role of cytokines in mediating the outcome of the ALF syndrome.
Patients and Methods
Patients
The study population was extracted from 116 subjects with non-acetaminophen-induced ALF and grade 1 or 2 hepatic encephalopathy on randomization. Patients with grade 3 or 4 encephalopathy on randomization were not part of the present study because NAC appeared to have no beneficial effect on outcome 6. Inclusion and exclusion criteria for the study participation have been detailed, as have demographic and clinical characteristics of the entire study population (6). Patients were randomized to receive intravenous NAC or placebo (5% dextrose in water) for an intended 72 hour infusion. The original study populations were similar in demographic characteristics, bilirubin, creatinine, and International Normalized Ratio (INR) of the prothrombin time. The NAC study was approved by Institutional Review Boards of each participating site, and the current sub-study was approved by the Ancillary Studies Committee of the ALF Study Group.
Seventy-nine of the 116 patients had serum samples available from the first 48 hours of admission (“early samples”) and a second serum sample collected between days 3-5 after the start of NAC or placebo infusion (“late samples”). Patients were included in the study only if they had late samples available in order to assess a possible dynamic effect of NAC on cytokine concentrations. One additional patient was excluded for receiving less than 1 hour of study drug because of a perceived adverse effect. Therefore, 78 patients were included in the analysis of the effects of NAC on biomarker concentrations, 39 each in the NAC and placebo groups.
The presence of the SIRS on admission was defined as two or more of the following criteria: white blood cell count (WBC) >12 or <4 × 109/L, temperature <36 or >38°C, respiratory rate of >20/minute, and pulse >90 beats/minute (9).
Laboratory methods
Serum was processed uniformly among centers according to a detailed Manual of Operations, frozen at −80° C, and stored at repositories at the University of Texas, Southwestern Medical Center (Dallas, TX) or the NIH Repository in Bethesda, MD. Serum samples were analyzed for 10 cytokines, chosen to encompass a variety of inflammatory responses, including T-helper (Th)-1 responses (IL-2, TNFα, interferon [IFN]γ), Th-2 responses (IL-4, IL-6, IL-10, IL-13), and Th-17 responses (IL-17, IL-23); IL-1β levels were also assayed.
Concentrations of biomarkers were assayed using Aushon Multiplex Protein Arrays (Aushon BioSystems, Billerica, MA). Briefly, samples were incubated for one hour on array plates pre-spotted with capture antibodies specific for each protein biomarker. Plates were decanted and washed three times before adding a cocktail of biotinylated detection antibodies to each well. After incubating with detection antibodies for 30 minutes, plates were washed three times and incubated for 30 minutes with streptavidin-horseradish peroxidase (HRP) conjugate. Incubations were done at room temperature shaking at 200 rpm. Plates were again washed before adding a chemiluminescent substrate. The luminescent signal produced from the HRP-catalyzed oxidation of the substrate was quantitated by imaging the plate using the Aushon Imaging System, which is a cooled, charge-coupled camera. Data was analyzed using Aushon Array Analyst Software, and concentrations were interpolated from a standard curve. Normal ranges for each analyte using the same assay system were determined on serum from healthy volunteers.
Statistical analysis
Data were analyzed for normality and expressed as mean [SD] or median [range], as appropriate. Demographic, clinical, and laboratory parameters were summarized by group and overall with means [SD]/median [range] for continuous measures, and with frequencies and percent for categorical measures. Continuous variables were compared with Student t tests for independent groups, and categorical variables with Chi square contingency table analyses. Cytokine concentrations were log-transformed for statistical purposes. Undetectable cytokine concentrations were assigned a value between 0 and the lower limit of detectability for the assay. Early and late biomarker concentrations were compared using Wilcoxon matched pairs signed ranks tests. Entry criteria for the stepwise multiple logistic regression model were relaxed to 10% so as not to miss a possible predictive factor. Data were analyzed using JMP® v. 9.0.2 (SAS Institute, Cary, NC) and SAS 9.1 software. Significance was defined as a P-value ≤0.05.
Results
Clinical characteristics of the study population and effects of NAC administration on outcome of ALF
The 78-patient study population consisted of 39 patients randomized to receive NAC and 39 to placebo. The mean age for the entire group was 40 years, 60% were female, and 68% were Caucasian; 58% and 42% had stage 1 or 2 hepatic encephalopathy at randomization, respectively (Table 1). The underlying etiology of ALF was idiosyncratic drug reaction in 30%, indeterminate in 30%, hepatitis B virus (HBV) in 14%, autoimmune hepatitis in 13%, hepatitis A virus (HAV) in 4%, and other etiologies in 10%. Twenty-eight percent of the cohort progressed to high-grade hepatic encephalopathy (grade 3 or 4) within 7 days of admission.
Table 1.
Demographic, clinical and laboratory predictors of outcome after acute liver failure.
| Parameter | Entire Group N = 78 | Transplant-Free Survivors N = 33 | Transplanted or Died N = 45 | P * |
|---|---|---|---|---|
|
Demographics
| ||||
| Age (y) | 40.2 [13.3] | 39.5 [13.7] | 40.7 [13.1] | 0.696 |
| Female Gender (%) | 60.0 | 51.5 | 66.7 | 0.177 |
| Caucasian race (%) | 68.0 | 69.7 | 66.7 | 0.777 |
| BMI (kg/m2) | 29.1 [7.4] | 27.4 [7.4] | 30.2 [7.2] | 0.154 |
|
Clinical Characteristics | ||||
| Etiology of ALF (%) | ||||
| Autoimmune hepatitis | 10 (13) | 3 | 7 | |
| Idiosyncratic drug | 23 (30) | 11 | 12 | |
| Hepatitis B | 11 (14) | 5 | 6 | 0.375 |
| Indeterminate | 23 (30) | 8 | 15 | |
| Hepatitis A | 3 (4) | 2 | 1 | |
| Other | 8 (10) | 4 | 4 | |
| Symptoms-encephalopathy (days) | 17 [0-153] | 13 [0-72] | 22 [0-153] | 0.031 |
| Jaundice-encephalopathy (days) | 8 [0–153] | 5 [0-22] | 13 [0-153] | 0.0002 |
| HE at randomization (% grade 1) | 57.7 | 75.8 | 44.4 | 0.006 |
| HE progression (% to grade 3/4) | 28.2 | 12.1 | 40.0 | 0.007 |
| Organ systems failed (N) | 1.2 [1.2] | 0.8 [1.2] | 1.5 [1.2] | 0.008 |
|
Laboratory Characteristics | ||||
| INR admission | 2.7 [1.1-12.3] | 2.0 [1.4-4.0] | 3.0 [1.1-12.3] | 0.0002 |
| INR peak | 3.0 [1.1-12.3] | 2.5 [1.4-7.8] | 3.8 [1.7-12.3] | <0.0001 |
| Bilirubin admission (mg/dl) | 22.2 [12.2] | 17.4 [12.2] | 25.7 [11.0] | 0.003 |
| Bilirubin peak (mg/dl) | 24.6 [12.4] | 20.6 [12.5] | 27.6 [11.6] | 0.013 |
| Creatinine admission (mg/dl) | 0.9 [0.2-7.3] | 0.9 [0.3-6.6] | 0.9 [0.2-7.3] | 0.903 |
| Creatinine peak (mg/dl) | 1.2 [0.5-9.1] | 1.1 [0.5-8.0] | 1.6 [0.5-9.1] | 0.770 |
P represents comparison of patients with transplant-free survival vs. liver transplantation/death. Continuous data are expressed as mean [SD] or median [range]. Organ systems failed refer to the number of systems failed other than hepatic.
BMI, body mass index; HE, hepatic encephalopathy.
At 21 days from admission to the study, 33 (42%) recovered without LT, 31 (40%) underwent LT, and 16 (21%) died, 2 of whom died after LT. Factors associated with outcome are depicted in Table 1. Demographic characteristics and etiology were similar in patients with TFS compared to those who underwent LT or died. In contrast, the rapidity of onset of encephalopathy (symptoms-to-encephalopathy or jaundice-toencephalopathy intervals) were shorter in patients with TFS than those who underwent LT or died (median 13 vs. 22 days, respectively; P=0.031 and median 5 vs. 16 days, respectively; P =0.0002). Progression to high-grade hepatic encephalopathy and MOSF exclusive of renal failure was more common in patients who died or underwent LT compared to those with TFS (40 vs. 12%; P <0.01, and mean of 1.5 vs. 0.8 organs failed, respectively; P <0.01). Although admission and peak INR and total bilirubin were significantly higher in those who died or underwent LT (P ≤0.01 for all comparisons), serum creatinine was not different between the two groups.
Baseline clinical characteristics on admission to the study according to treatment group are depicted in Table 2. A significantly greater proportion of female subjects were randomized to the placebo group than the NAC group (72% vs. 49%, respectively; P=0.037). Although patients randomized to receive NAC had a slightly shorter median jaundice-to-encephalopathy interval than those who received placebo (7 vs. 12 days; P=0.026), the median symptoms-to-encephalopathy interval was identical (17 days each; P=0.838). Otherwise, the study groups were similar in demographic characteristics, distribution of etiologies of ALF (P for AIH, HBV, idiosyncratic drug reaction, indeterminate, and all viral etiologies ranged from 0.69 to 1.00), encephalopathy grade, INR, creatinine, and bilirubin on admission to the study. TFS was observed in 22/39 (56%) of patients randomized to receive NAC but only 11/39 (28%) of patients randomized to receive placebo (P=0.012).
Table 2.
Clinical characteristics and outcome of patients with non-acetaminophen ALF according to treatment group.
| Parameter | NAC N = 39 | Placebo N = 39 | P |
|---|---|---|---|
| Age (y) | 39.9 [14.1] | 40.4 [12.7] | 0.886 |
| Female gender (%) | 48.7 | 71.9 | 0.037 |
| Caucasian Race (%) | 66.7 | 69.2 | 0.808 |
| BMI (kg/m2) | 30.2 [8.1] | 27.9 [6.5] | 0.231 |
| Etiology of ALF | -- | -- | 0.895 |
| Symptoms-encephalopathy (days) | 17 [2-153] | 17 [0-69] | 0.838 |
| Jaundice-encephalopathy (days) | 7 [0-153] | 12 [0-60] | 0.026 |
| Admission SIRS (% 2-4 parameters) | 66.7 | 74.4 | 0.456 |
| HE at randomization (% grade 1) | 56.4 | 59.0 | 0.819 |
| INR admission | 2.4 [1.4-8.2] | 2.9 [1.1-12.3] | 0.066 |
| Bilirubin admission (mg/dl) | 21.8 [12.9] | 22.5 [11.6] | 0.812 |
| Creatinine admission (mg/dl) | 1.4 [0.2-6.6] | 0.8 [0.5-7.3] | 0.073 |
| Outcome TFS (%) | 56.4 | 28.2 | 0.012 |
Continuous data are expressed as mean [SD] or median [range].
BMI, body mass index; HE, hepatic encephalopathy; SIRS, systemic inflammatory response syndrome; TFS, transplant-free survival.
Serum cytokine concentrations and the relationship to grade of hepatic encephalopathy and outcome of ALF
Mean cytokine concentrations are depicted in Table 3 and are compared to concentrations in normal healthy controls using the same assay system. Concentrations of all biomarkers varied widely in patients with ALF, but were generally much higher in patients than controls, many of whom had undetectable levels. The proportion of ALF patients with detectable cytokine concentrations in serum was higher than controls for IL-1β, IL-2, IL-6, IL-10, IL-13, IL-17, IFNγ, and TNFα, and lower only for the anti-inflammatory cytokine, IL-4.
Table 3.
Concentrations of serum cytokines in normal adult controls and in samples from patients with acute liver failure.
| Marker | Normal Controls N=21 | Acute Liver Failure N=78 | |||||
|---|---|---|---|---|---|---|---|
| Mean (pg/ml) | Range (pg/ml) | % Detected | Sample* | Mean (pg/ml) | Range (pg/ml) | % Detected | |
| IL-1β | 3.4 | <0.4-22.2 | 43 | Early | 6.7 | <0.4-243.6 | 76 |
| Late | 7.0 | <0.4-160.2 | 65 | ||||
| IL-2 | 18.1 | <2.0-79.1 | 62 | Early | 518.9 | <2.0-24334.3 | 95 |
| Late | 193.7 | <2.0-5236.6 | 94 | ||||
| IL-4 | 62.2 | <4.0-272.7 | 67 | Early | 3.2 | <4.0-52.3 | 33 |
| Late | 3.5 | <4.0-117.0 | 23 | ||||
| IL-6 | 4.6 | <0.4-11.9 | 91 | Early | 578.0 | 2.4-10001.0 | 100 |
| Late | 270.0 | 2.4-2282.3 | 100 | ||||
| IL-10 | 4.1 | <2.0-9.0 | 24 | Early | 43.1 | <2.0-1908.7 | 96 |
| Late | 48.5 | <2.0-2920.6 | 91 | ||||
| IL-13 | 0.9 | <0.4-1.3 | 13 | Early | 9.2 | <0.4-552.3 | 59 |
| Late | 4.7 | <0.4-209.9 | 55 | ||||
| IL-17 | 1.0 | <0.8-1.6 | 5 | Early | 72.6 | <0.8-5292.5 | 37 |
| Late | 22.3 | <0.8-1341.9 | 28 | ||||
| IL-23 | 861.5 | 4.6-3055 | 100 | Early | 8327.7 | 5.5-139921.0 | 100 |
| Late | 5637.0 | 5.4-129626.0 | 100 | ||||
| IFNγ | 32.0 | <0.8-61.9 | 10 | Early | 115.7 | <0.8-3672.9 | 53 |
| Late | 57.0 | <0.8-1813.5 | 51 | ||||
| TNFα | 45.1 | <4.7-123.6 | 14 | Early | 981.0 | <4.7-64470 | 67 |
| Late | 396.6 | <4.7-22058.9 | 65 | ||||
Early indicates serum obtained from the first 48 hours of admission; late indicates serum obtained from day 3-5 of admission.
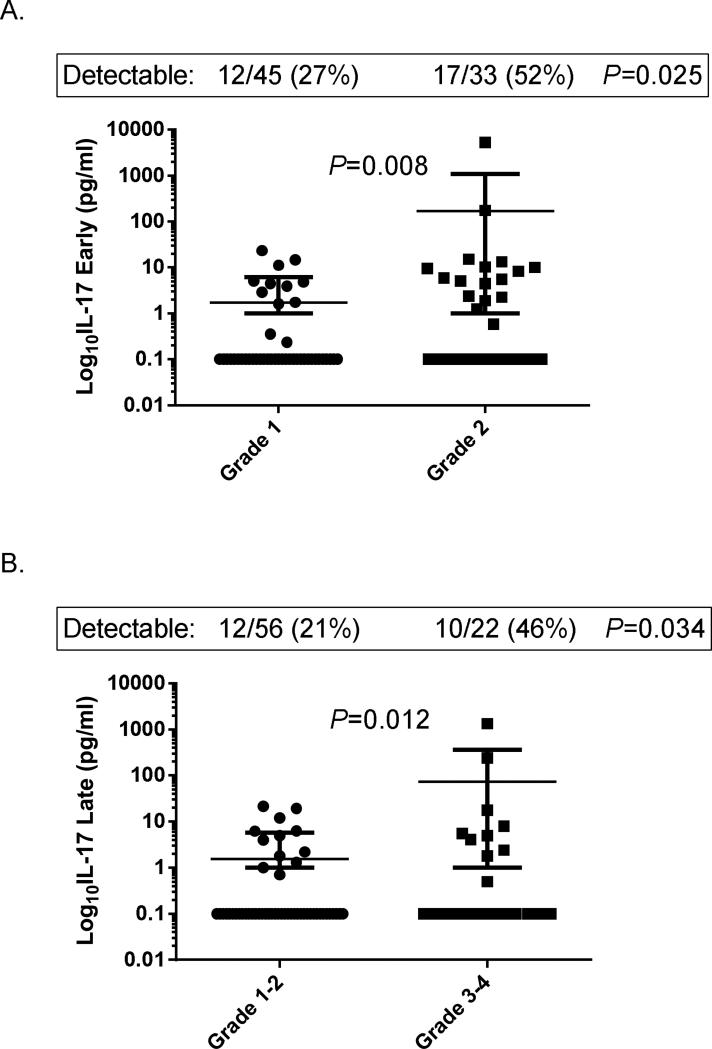
In univariate analysis of serum cytokine concentrations in patients with ALF, only concentrations of IL-17 were significantly related to encephalopathy grade and outcome. As shown in Figure 1, IL-17 concentrations in early serum samples were higher in patients with grade 2 vs. grade 1 encephalopathy on randomization to the study (P = 0.008; Fig. 1A), and were higher in late serum samples in those who progressed to grade 3 or 4 encephalopathy by day 7 than in those who remained in grade 1 or 2 encephalopathy (P = 0.012; Fig. 1B). Since IL-17 levels were frequently below the limit of detectability for the assay, data were also analyzed using detectability as a dichotomous variable. IL-17 was detectable in 52% of patients with grade 2 vs. 27% of patients with grade 1 encephalopathy on randomization (P=0.025), and in 46% who progressed to grade 3 or 4 vs. 21% who remained in grade 1 or 2 encephalopathy within the first 7 days (P=0.034).
Fig 1. Serum IL-17 concentrations according to hepatic encephalopathy grade in patients with acute liver failure.
(A). IL-17 concentrations in early serum samples according to hepatic encephalopathy grade on randomization to the study. (B). IL-17 concentrations in late serum samples according to maximal grade of hepatic encephalopathy over the first 7 days of admission.
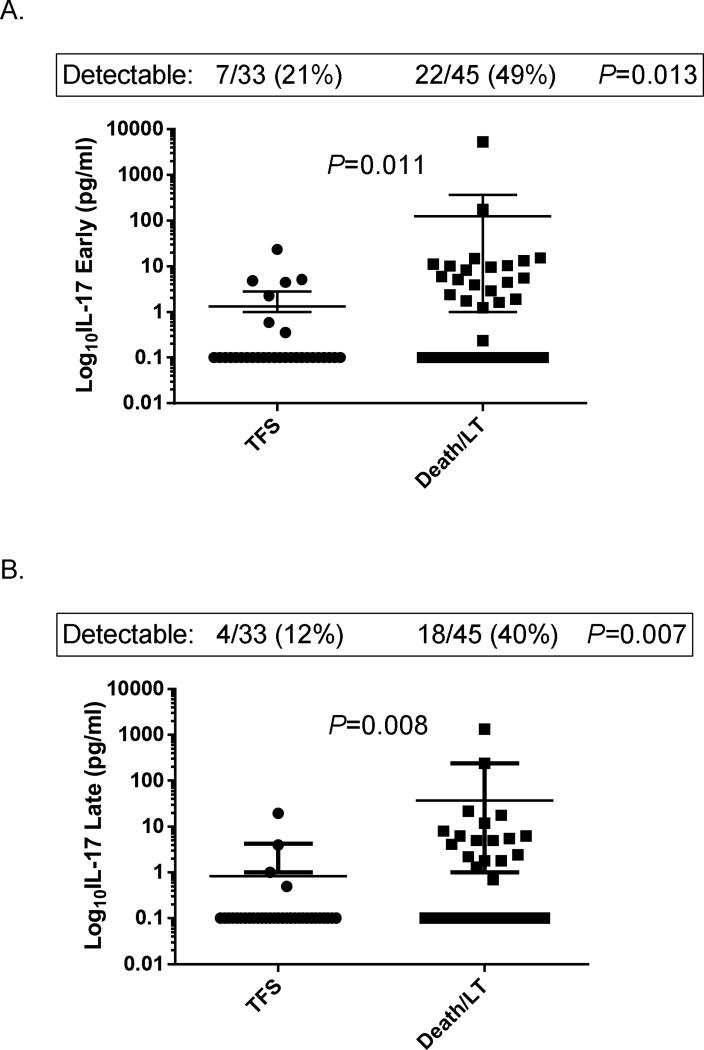
As shown in Figure 2, patients who died or underwent LT are also shown to have higher concentrations of IL-17 than transplant-free survivors. IL-17 concentrations in early serum samples were significantly higher in patients who died or underwent LT than transplant-free survivors (P = 0.011; Fig. 2A), in whom 49% vs. 21% had detectable levels, respectively (P=0.013). Similarly, IL-17 concentrations in late serum samples were significantly higher in patients who died or underwent LT than transplant-free survivors (P=0.008; Fig. 2B), in whom 40% vs. 12% had detectable levels, respectively (P=0.007).
Fig. 2. Serum IL-17 concentrations according to outcome of acute liver failure.
(A). IL-17 concentrations in early serum samples according to outcome at 21 days. (B). IL-17 concentrations in late serum samples according to outcome at 21 days.
LT, liver transplantation; TFS, transplant-free survival.
Stepwise multivariate logistic regression analysis of outcomes was next performed using cytokine concentrations and treatment group (Table 4). Cytokines were entered into multivariate models if they have been shown in the present work or previous studies to be involved in the severity of hepatic encephalopathy or the outcome of patients with ALF (IL-1b, IL-2, IL-6, IL-10, IL-17, TNFα, IFNγ). Using early cytokine concentrations in the first model, only IL-17 levels were found to be an independent predictor of death or LT (P=0.016), with a unit odds ratio (OR) of 3.46 for a 10-fold increase, and an area under the receiver operator characteristic curve (AUROC) of 0.65. The addition of treatment group to the cytokine model identified early IL-2 and IL-17 concentrations, and receiving placebo (rather than NAC) as independent predictors of death/LT (unit OR 6.15 [P=0.007] and 7.42 [P<0.001] for each 10-fold increase of IL-2 and IL-17, respectively, and 5.00 [P=0.007] for receiving placebo), yielding an AUROC of 0.82. Similar models using late serum samples also identified only IL-17 concentrations as an independent predictor of death/LT in a model of cytokine concentrations (unit OR 5.29; P=0.020; AUROC 0.66). The addition of treatment group to the cytokine model identified late IL-17 concentrations and placebo treatment group as independent predictors of death/LT (unit OR 4.78, P=0.035 and OR 3.13, P=0.035, respectively), yielding an AUROC of 0.75. IL-6, high levels of which have previously been reported to be associated with poor outcome in ALF, was weakly but not significantly associated with death/LT in the above multivariate analyses (P=0.075).
Table 4.
Multivariate logistic regression models predicting death/liver transplantation in patients with acute liver failure according to serum cytokine concentrations and treatment group.
| Model | Cytokine/Treatment Group | P | Odds Ratio | 95% CI | AUROC |
|---|---|---|---|---|---|
| Early Cytokines* | IL-17 | 0.016 | 3.46 | 1.41-10.77 | 0.65 |
| Early Cytokines* + Treatment Group | IL-2 | 0.007 | 6.15 | 1.84-26.25 | 0.82 |
| IL-17 | <0.001 | 7.42 | 2.51-27.59 | ||
| Placebo vs. NAC | 0.007 | 5.00 | 1.64-16.67 | ||
| Late Cytokines* | IL-17 | 0.020 | 5.29 | 1.64-28.97 | 0.66 |
| Late Cytokines* + Treatment Group | IL-17 | 0.035 | 4.78 | 1.44-28.02 | 0.75 |
| Placebo vs. NAC | 0.035 | 3.13 | 1.10-7.69 |
Cytokines entered into models were those associated with outcome in patients with ALF in either previous or the present study: IL-1b, IL-2, IL-6, IL-10, IL-17, TNFα, IFNγ.
Effects of NAC on serum cytokine concentrations
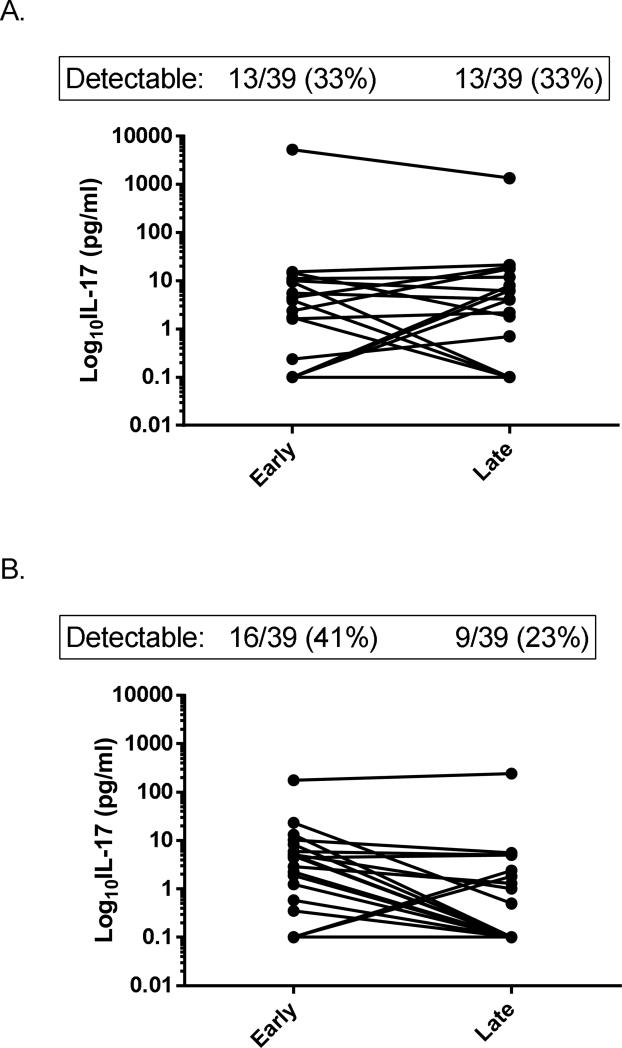
The effect of NAC administration on cytokine concentrations was also examined. Concentrations of all biomarkers in early serum samples were not significantly different between patients who received NAC vs. those who received placebo (data not shown). However, in patients with detectable IL-17 concentrations in early serum samples, the administration of NAC was associated with undetectable IL-17 concentrations in late samples in 78%, compared 44% in patients who received placebo (P=0.042; Figure 3). The mean decrease in IL-17 concentrations between early and late samples was also significantly greater in patients who received NAC than placebo (P=0.045). Changes between early and late serum samples of all other biomarkers were not significantly different between the two treatment groups.
Fig. 3. Change in individual IL-17 concentrations from early to late serum samples in patients with acute liver failure.
(A). Change in IL-17 in patients who received placebo (N = 38). (B). Change in IL-17 in patients who received NAC (N = 38). (P = 0.045 for difference in change NAC vs. placebo).
Discussion
The results of the present study implicate IL-17 in the pathogenesis of the ALF syndrome. Higher IL-17 concentrations were not only associated with higher-grade hepatic encephalopathy on admission to the study but also were predictive of progression to high grade encephalopathy within 7 days. Moreover, higher IL-17 concentrations in early and in late serum samples were associated with death or LT, and were independent predictors of adverse outcome in multivariate logistic regression analysis in addition to the administration of placebo rather than NAC. The data also suggest that the benefit of NAC may be mediated through attenuation of IL-17 secretion, since IL-17 concentrations decreased to undetectable levels more frequently in patients who received NAC than in patients who received placebo.
IL-17 is a product of effector helper Th17 cells and cells of the innate immune response, and is increasingly recognized as a key pro-inflammatory cytokine (10). Among its numerous functions, IL-17 promotes inflammatory responses by neutrophils, and high levels are associated with acute and chronic immune-mediated liver diseases, including alcoholic hepatitis (11, 12), acute and chronic hepatitis B (13, 14), primary biliary cirrhosis (15), non-alcoholic steatohepatitis (16), and autoimmune hepatitis (17, 18). In the liver, IL-17 plays a central role in positive feedback loops to potentiate the secretion of pro-inflammatory cytokines and chemokines by non-parenchymal cells (19). The current observations implicating IL-17 in the pathogenesis of the ALF syndrome support a previous study which correlated serum IL-17 levels with the severity of acute liver injury (20).
Our data also suggest that high IL-17 levels correlate with the severity and progression of hepatic encephalopathy. Other pro-inflammatory cytokines such as IL-1β, TNFα, and IL-6 have been implicated in potentiating astrocyte swelling caused by ammonia (21) and by increasing cerebral blood flow (4, 22) in patients with ALF. Disruption of the blood brain barrier (BBB) occurs in patients and experimental animals with ALF. Mechanisms of neuroinflammation include the transfer of pro-inflammatory cytokines across the BBB as well as production in situ (22). Recent studies have suggested a role for IL-17 in promoting the permiabilization of the BBB to both macromolecules (eg., cytokines) and T-lymphocytes (23), providing one plausible link between IL-17 levels and encephalopathy progression in the current study. Specifically, IL-17-induced oxidative stress has been implicated in mediating disruption of the BBB in experimental models (24). Gut bacteria-derived, Th17-associated cytokines including IL-17 and IL-23 have also recently been implicated in the pathogenesis of hepatic encephalopathy in patients with cirrhosis (25).
The mechanisms by which NAC decreases IL-17 levels cannot be determined from this study. However, we have recently observed the same study participants treated with NAC experienced a faster decline in bilirubin and ALT than those who received placebo(26), suggesting that an amelioration of liver injury may be involved. In a mouse model of non-acetaminophen ALF, the administration of NAC decreased IL-17 levels by 1.3-fold (P < 0.01) (27). Similar to our findings, the same model showed that NAC had no effect on IL-1β, IL-2, IL-6, IL-13 and TNFα, suggesting that there is specificity of the effect. NAC has also been shown to decrease IL-17-mediated inflammation in cultured human smooth muscle cells (28), but has not yet been tested in experimental models of liver injury.
We acknowledge limitations of the present study. Admission and late serum samples were not available from every patient who participated in the NAC trial, and some patients received abbreviated (<72 hour) infusions of NAC, introducing a possible source of bias. Similar to other studies, many patients had cytokine concentrations below the limit of detectability for the assay, such that undetectable levels had to be imputed, and quantification of mean concentrations were thereby less meaningful. Although our results show that NAC treatment decreased IL-17 levels and higher IL-17 levels were associated with higher grade hepatic encephalopathy, patients who received NAC were equally as likely to progress to high grade encephalopathy as those who received placebo (data not shown). Thus, although other studies suggest that NAC prevents neurological complications of ALF (27, 29), our data revealed no difference.
In conclusion, the present study suggests that IL-17 is an independent predictor of poor outcome in patients with non-acetaminophen ALF, that higher IL-17 levels are associated with progression of hepatic encephalopathy, and that NAC may improve outcome by decreasing IL-17 concentrations. These data provide a rationale to test the hypothesis that IL-17 plays a key role in perpetuating the ALF syndrome, and that NAC improves TFS by decreasing IL-17-mediated oxidative stress. Additional therapies that target IL-17 might also be considered in the future.
Acknowledgments
Financial Support. This work was supported by grant U-01 DK58369 from the National Institutes of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, W. M. Lee, Principal Investigator.
Abbreviations
- ALF
acute liver failure
- ALT
alanine aminotransferase
- AUROC
area under the receiver operator characteristic curve
- BBB
blood-brain barrier
- BMI
body mass index
- ELISA
enzyme-linked immunosorbent assay
- HAV
hepatitis A virus
- HBV
hepatitis B virus
- HE
hepatic encephalopathy
- HRP
horseradish peroxidase
- IFNγ
interferon-γ
- IL
interleukin
- INR
International Normalized Ratio of prothrombin time
- LLD
lower limit of detection
- LT
liver transplantation
- MOSF
multi-organ system failure
- NAC
N-acetylcysteine
- SIRS
systemic inflammatory response syndrome
- TFS
transplant-free survival
- Th
T-helper lymphocyte
- TNFα
tumor necrosis factor-α
Footnotes
Conflicts of Interest. The authors have no conflicts of interest to report.
Reference List
- 1.Bernal W, Auzinger G, Sizer E, Wendon J. Intensive care management of acute liver failure. Semin Liver Dis. 2008;28(2):188–200. doi: 10.1055/s-2008-1073118. [DOI] [PubMed] [Google Scholar]
- 2.Antoniades CG, Berry PA, Wendon JA, Vergani D. The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol. 2008 Nov;49(5):845–861. doi: 10.1016/j.jhep.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000 Oct;32(4 Pt 1):734–739. doi: 10.1053/jhep.2000.17687. [DOI] [PubMed] [Google Scholar]
- 4.Jalan R, Olde Damink SW, Hayes PC, Deutz NE, Lee A. Pathogenesis of intracranial hypertension in acute liver failure: inflammation, ammonia and cerebral blood flow. J Hepatol. 2004 Oct;41(4):613–620. doi: 10.1016/j.jhep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Berry PA, Antoniades CG, Hussain MJ, McPhail MJ, Bernal W, Vergani D, et al. Admission levels and early changes in serum interleukin-10 are predictive of poor outcome in acute liver failure and decompensated cirrhosis. Liver Int. 2010 May;30(5):733–740. doi: 10.1111/j.1478-3231.2010.02219.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non acetaminophen acute liver failure. Gastroenterology. 2009 Sep;137(3):856–64. 864. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison PM, Wendon JA, Gimson AE, Alexander GJ, Williams R. Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N Engl J Med. 1991 Jun 27;324(26):1852–1857. doi: 10.1056/NEJM199106273242604. [DOI] [PubMed] [Google Scholar]
- 8.Harrison P, Wendon J, Williams R. Evidence of increased guanylate cyclase activation by acetylcysteine in fulminant hepatic failure. Hepatology. 1996 May;23(5):1067–1072. doi: 10.1053/jhep.1996.v23.pm0008621135. [DOI] [PubMed] [Google Scholar]
- 9.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992 Jun;20(6):864–874. [PubMed] [Google Scholar]
- 10.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009 Aug 27;361(9):888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 11.Emanuele E, Bertona M. Interleukin-17-targeted treatment of alcoholic liver disease. Hepatology. 2009 Jul;50(1):329–330. doi: 10.1002/hep.22901. [DOI] [PubMed] [Google Scholar]
- 12.Lemmers A, Moreno C, Gustot T, Marechal R, Degre D, Demetter P, et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009 Feb;49(2):646–657. doi: 10.1002/hep.22680. [DOI] [PubMed] [Google Scholar]
- 13.Niu Y, Liu H, Yin D, Yi R, Chen T, Xue H, et al. The balance between intrahepatic IL-17(+) T cells and Foxp3(+) regulatory T cells plays an important role in HBV-related end-stage liver disease. BMC Immunol. 2011;12:47. doi: 10.1186/1471-2172-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge J, Wang K, Meng QH, Qi ZX, Meng FL, Fan YC. Implication of Th17 and Th1 cells in patients with chronic active hepatitis B. J Clin Immunol. 2010 Jan;30(1):60–67. doi: 10.1007/s10875-009-9328-2. [DOI] [PubMed] [Google Scholar]
- 15.Rong G, Zhou Y, Xiong Y, Zhou L, Geng H, Jiang T, et al. Imbalance between T helper type 17 and T regulatory cells in patients with primary biliary cirrhosis: the serum cytokine profile and peripheral cell population. Clin Exp Immunol. 2009 May;156(2):217–225. doi: 10.1111/j.1365-2249.2009.03898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Y, Bian Z, Zhao L, Liu Y, Liang S, Wang Q, et al. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin Exp Immunol. 2011 Nov;166(2):281–290. doi: 10.1111/j.1365-2249.2011.04471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao L, Tang Y, You Z, Wang Q, Liang S, Han X, et al. Interleukin-17 contributes to the pathogenesis of autoimmune hepatitis through inducing hepatic interleukin-6 expression. PLoS One. 2011;6(4):e18909. doi: 10.1371/journal.pone.0018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lafdil F, Wang H, Park O, Zhang W, Moritoki Y, Yin S, et al. Myeloid STAT3 inhibits T cell-mediated hepatitis by regulating T helper 1 cytokine and interleukin-17 production. Gastroenterology. 2009 Dec;137(6):2125–2135. doi: 10.1053/j.gastro.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lafdil F, Miller AM, Ki SH, Gao B. Th17 cells and their associated cytokines in liver diseases. Cell Mol Immunol. 2010 Jul;7(4):250–254. doi: 10.1038/cmi.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasumi Y, Takikawa Y, Endo R, Suzuki K. Interleukin-17 as a new marker of severity of acute hepatic injury. Hepatol Res. 2007 Apr;37(4):248–254. doi: 10.1111/j.1872-034X.2007.00040.x. [DOI] [PubMed] [Google Scholar]
- 21.Rama Rao KV, Jayakumar AR, Tong X, Alvarez VM, Norenberg MD. Marked potentiation of cell swelling by cytokines in ammonia-sensitized cultured astrocytes. J Neuroinflammation. 2010;7:66. doi: 10.1186/1742-2094-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butterworth RF. Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology. 2011 Apr;53(4):1372–1376. doi: 10.1002/hep.24228. [DOI] [PubMed] [Google Scholar]
- 23.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007 Oct;13(10):1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, et al. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010 Apr;24(4):1023–1034. doi: 10.1096/fj.09-141978. [DOI] [PubMed] [Google Scholar]
- 25.Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012 Jan;302(1):G168–G175. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh S, Hynan LS, Lee WM. Improvements in Hepatic Serological Biomarkers Are Associated with Clinical Benefit of Intravenous N-Acetylcysteine in Early Stage Non-Acetaminophen Acute Liver Failure. Dig Dis Sci. 2013 Jan 17; doi: 10.1007/s10620-012-2512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bemeur C, Vaquero J, Desjardins P, Butterworth RF. N-acetylcysteine attenuates cerebral complications of non-acetaminophen-induced acute liver failure in mice: antioxidant and anti-inflammatory mechanisms. Metab Brain Dis. 2010 Jun;25(2):241–249. doi: 10.1007/s11011-010-9201-2. [DOI] [PubMed] [Google Scholar]
- 28.Wuyts WA, Vanaudenaerde BM, Dupont LJ, Van Raemdonck DE, Demedts MG, Verleden GM. N-acetylcysteine inhibits interleukin-17-induced interleukin-8 production from human airway smooth muscle cells: a possible role for anti- oxidative treatment in chronic lung rejection? J Heart Lung Transplant. 2004 Jan;23(1):122–127. doi: 10.1016/s1053-2498(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 29.Butterworth RF. Neuroinflammation in acute liver failure: Mechanisms and novel therapeutic targets. Neurochem Int. 2011 Nov;59(6):830–836. doi: 10.1016/j.neuint.2011.07.014. [DOI] [PubMed] [Google Scholar]