Abstract
Background
Recombinant human endostatin (Endostar) has been widely used to suppress angiogenesis in carcinoma patients. Hypertrophic scar (HS) tissue, much like a carcinoma, is often associated with angiogenesis. However, there have been few studies conducted on the effects of Endostar on HS or its mechanism.
Objective
This paper investigated the effects Endostar on the HS of rabbit ears and studied the effects of Endostar on VEGF and TIMP-1 expression.
Methods
Sixteen New Zealand white rabbits were used to establish HS models. Then, rabbit ears containing HS were randomly assigned to either the Endostar group or the control group. The changes of appearance and histology were evaluated using the naked eye, hematoxylin eosin staining, and a scar elevation index. The VEGF and TIMP-1 expressions were detected by immunohistochemical staining, RT-PCR, and western blot.
Results
The thickness of the connective tissue in the Endostar group were thinner, the numbers of micro vessels and fibroblasts were fewer, and the collagen fibers were smoother. Moreover, the mRNA and protein expressions of VEGF and TIMP-1 in the Endostar group were significantly lower than those in the control group.
Conclusion
The results suggested that Endostar reduced the formation of HS by down-regulation of VEGF and TIMP-1 expressions.
Keywords: Endostar, endostatin, hypertrophic scar, vascular endothelial growth factor, tissue inhibitor of metalloproteinase-1
Introduction
A hypertrophic scar (HS), an excessive healing reaction to injury, is characterized by excessive fibrosis and collagen deposition in extracellular matrix (ECM)1. Surgical excision, intralesional injection of steroids, radio frequency, radiation, and use of lasers are the most frequently used treatments for HS2. Nevertheless, there is no consensus on which treatment is the most effective with the fewest side effects. Therefore, novel strategies for the prevention and treatment of HS are worthy of further investigation toward the goal of its occurrence being avoided entirely.
Recent research shows that HS is associated with the abnormal proliferation of fibroblasts and the overproduction of collagen and extracellular matrix1,3. In addition, angiogenesis plays an essential role in the early stages of wound healing, and micro vascular abnormality is found in pathological scars3. Moreover, research suggests that HS has increased vascular components compared to normal dermis or scars4–6. Thus, it was theorized that antiangiogenesis is the key to inhibiting the formation of HS. Endostar is a modified human endostatin with a nine amino acid sequence at the N-terminus (MGGSHHHHH). It is widely used in suppressing angiogenesis in carcinomaand is far more efficient than previous endostatins7. Although HS presents angiogenesis much like carcinoma, there are limited studies regarding the effects of Endostar on HS and its molecular mechanism.
Vascular endothelial growth factor (VEGF), a positive regulator of angiogenesis, is produced in large amounts by epidermal cells during wound healing8. The over expression of VEGF has been demonstrated in early stages of pathological scar formation9,10. Inhibition of VEGF activity with the injection of anti-VEGF antibodies has been shown to decrease capillary formation, collagen I production, and overall scar volume11. Although the over expression of VEGF has shown to be significantly correlated with the formation of scars, the relationship between Endostar and VEGF remains undetermined in HS.
The major effectors of ECM degradation and remodeling belong to a family of structurally related enzymes called matrix metalloproteinases (MMPs). Endogenous inhibitors of MMPs are tissue inhibitors of metalloproteinases (TIMPs). Increased levels of TIMP-1 have been found in patients with HS, suggesting that elevated systemic TIMP-1 concentrations might contribute to tissue fibrosis, leading to HS formation12.
Excessive expressions of TIMP-1 and VEGF have been shown to be correlated with pathological scar formation. Because HS is associated with the occurrence of angiogenesis and Endostar is an angiogenesis inhibitor, this study aimed to investigate the effects of Endostar on HS tissue in model of rabbit ears and the effects of Endostar on VEGF and TIMP-1 expressions.
Materials and methods
Ethics statement
All animals were studied according to the guidelines of the Declaration of Helsinki, and experiments were carried out with the approval of the Animal Experimentation Ethics Committee of Chongqing Medical University.
Establishment of model rabbit ears
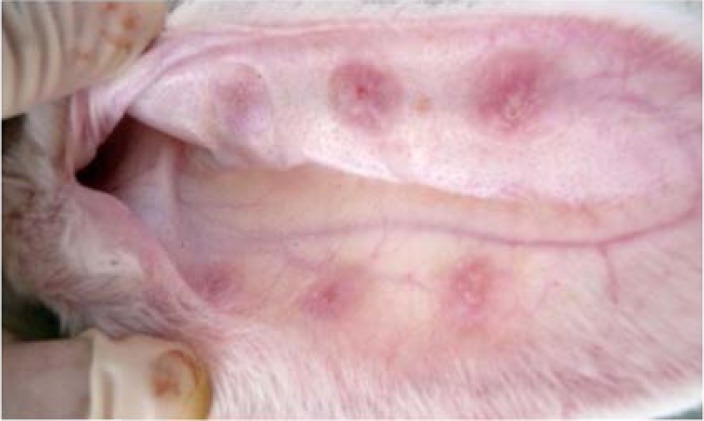
Sixteen male adult New Zealand white rabbits weighing between 2.2 and 2.5 kg were selected for this study. The rabbit HS models were established according to the previous report13. The animals were anaesthetized with 1% (10 g/L) pentobarbital sodium (1 mg/kg), and then one hundred ninety-two wounds were created on the ventral surface of each ear by means of an 11 mm pen cover (Figure 1). An operating loupe was used to ensure the removal of the epidermis, dermis, and perichondrium in each wound. Thereafter, the wounds were cleaned every day to remove the crust and stimulate the growth of granulation. After 25 days, 128 rabbit HSs had formed. Two wounds on the tail of each ear were discarded because no HS had formed according to the scar elevation index.
Figure 1.
Formation of punch defects
Treatment
Thirty-two ears, which contained a total of 128 HSs, were randomly assigned to the Endostar group and the control group. The control group was treated with saline injections, and the Endostar group was treated with Endostar injections (5 mg/mL, Simcere Pharmaceutical Group, China). Each scar was injected with 1 mL solution once every two days for a total of 5 treatments. After 30 days, the rabbits were euthanized and the scars were harvested.
Scar elevation index
Scar elevation index (SEI) is a ratio of the total height of the wound area tissue to the area of normal tissue below the scar. The height of the normal tissue is determined based on the height of the adjacent unwounded dermis. A SEI of 1 indicates that no newly hypertrophied dermis has formed, whereas an index >1 indicates the formation of HS14.
Hematoxylin - Eosin staining
The samples were fixed with 10% buffered formalin, dehydrated, embedded in paraffin, cut in 5 mm sections, and stained with haematoxylin and eosin (H&E).
Quantitative real-time RT-PCR
Twenty randomly selected tissues from the Endostar group and twenty from the control group were selected for immunohistochemistry examination and the VEGF and TIMP expressions at the RNA level were evaluated. Total RNA was extracted using Trizol reagent (Takara, Dalian, China) according to the manufacturer's instructions, and contaminating genomic DNA was removed by incubation with DNase I (Takara, Dalian, China). RNA purity and concentration were determined by spectrophotometry. PCR was carried out according to the standard protocol on a real-time PCR-system (Applied BioSystems) with SYBR Green detection. After an initial incubation of the 10 µl reaction mixture for 1 min at 95° C, 39 cycles (95° C for 20 s, 58° C for 25 s) were performed for amplification. The specificity of amplification was confirmed by melting curve analysis. Each sample was tested in triplicate and the results were normalized to the level of gene GAPDH. The sequences of each set of primers were as follow: 5′-CATATTCAAGCCTTCCTGC-3′ (sense) and 5′-GGTCTGCATTCACATTTGTTG-3′ (antisense) for VEGF; 5′-TGTTGTTGCTGTGGCTGATAG-3′ (sense) and 5′-CGCTGGTATAAGGTGGTCTGG-3′ (anti-sense) for TIMP-1; 5′-CAGCGACACCCACTCCTC-3′ (sense) and 5′-TGAGGTCCACCACCCTGT -3′ (anti-sense) for GAPDH. Relative gene expression was calculated as 2-ΔΔCT.
Immunohistochemical staining
Sixty-four tissues from the Endostar group and sixty-four from the control group were collected for immunohistochemical staining, which was performed on 5 µm paraffin tissue sections mounted on polylysine-coated slides and dried at 37°C overnight. After the slides were deparaffinized in xylene and rehydrated conventionally, the endogenous peroxidase was blocked with 3% hydrogen peroxide in methanol for 20 minutes. Each slide was incubated with normal goat serum for 20 minutes at room temperature. The sections were incubated with rabbit anti-VEGF polyclonal antibody (1:100 dilution, Boster Biological Technology, Ltd, Wuhan, China) and rabbit anti-TIMP-1 polyclonal antibody (1:100 dilution, Boster Biological Technology, Ltd, Wuhan, China) overnight at 4° C. After washing with phosphate-buffered saline, sections were incubated for 30 minutes with a horseradish peroxidase labeled polymer anti-rabbit secondary antibody (Boster Biological Technology). Chromogen 3, 3-diaminobenzidine (Boster Biological Technology) was used for 15 minutes to visualize immunolabeling, resulting in a brown precipitate. After washing, the sections were counterstained with hematoxylin. Positive and negative immunohistochemistry controls were routinely performed.
Staining intensity was given one of the following four grades: none (0), weak (1), moderate (2), or strong (3). The percentage of positive cells was also given one of the following four grades: 0 (<1%), 1 (2%–20%), 2 (21%–50%), 3 (51%–100%). The staining result was semi-quantitatively assessed by the score combined with staining intensity and the percentage of positive cells15. An express severity score less than 2 was negative (-), 2–3 was weakly positive (+), more than 3 was strongly positive (++); the results were then judged according to the score. The scores from two independent investigators were compared and disagreements were resolved by consensus.
Western blot
Twenty randomly selected tissues from the Endostar group and twenty from the control group were selected for immunohistochemistry, and the VEGF and TIMP-expressions at the protein level were evaluated. Protein samples (40 µg) were separated on precast 10% SDS polyacrylamide gels (SDS-PAGE). After electrophoresis, the proteins were transferred to PVDF membrane filters (Millipore Biotechnology, Billerica, MA, USA). The membranes were incubated overnight at 4°C with primary rabbit polyclonal VEGF-A antibodies (Immunoway Biotechnology Company, Newark, DE, USA) or primary rabbit polyclonal TIMP-1 antibodies (Immunoway Biotechnology Company, Newark, DE, USA). After washing three times in TBS-T, horseradish peroxidase (HRP)-conjugated secondary antibodies were used at a dilution of 1:5000 in TBS-T for 2 h at room temperature. After three additional washes with TBS-T, the immunoreactive bands were visualised with a chemiluminescence reagent (ECL, Millipore Biotechnology, Billerica, MA, USA) and quantified using a Bio-Rad imaging system (Bio-Rad Laboratories, Inc, Hertfordshire, UK).
Statistical analysis
SPSS software version 14.0 Sciences (SPSS Inc, USA) was used for statistical evaluation. Mann-Whitney U and Student's tests were used for comparing the groups. A probability value of P <0.05 was considered significant.
Results
Formation of hypertrophic scars on rabbit ears
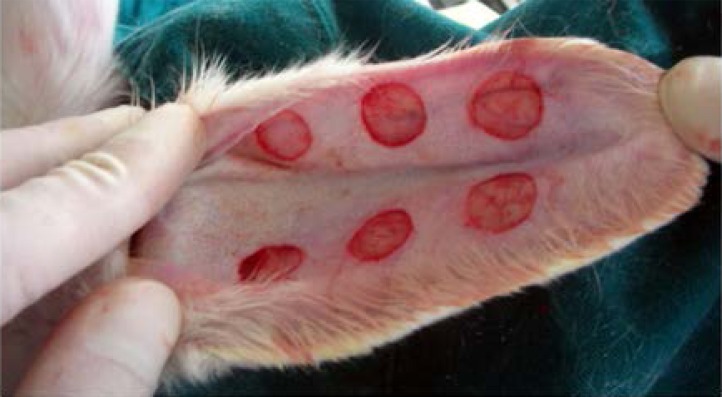
After 25 days, 128 HSs on the rabbit ears were successfully formed based on the rule of SEI >1. The HSs were erythematous, highly elevated from surrounding tissue, and stiff upon palpation (Figure 2). Afterwards, the HSs were divided into two groups and treated with either Endostar or saline.
Figure 2.
HS on rabbit ear successfully established after 25 days
Endostar improves appearance of scar
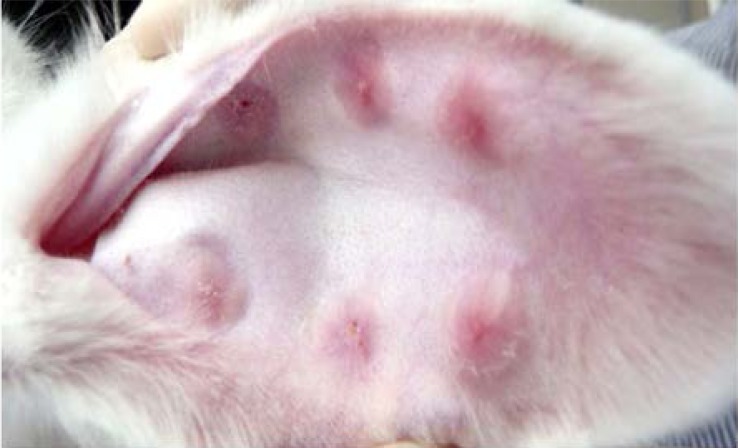
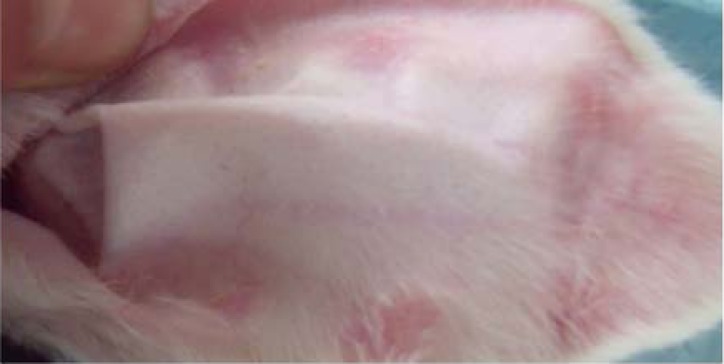
As shown in Figure 3A and Figure 3B, scars in the Endostar group were softer, less erythematous, and thinner when compared to the control group after treatment with Endostar.
Figure 3.
Endostar improved the appearance of HS: A) Appearance of scar in the Endostar group
Figure 3B.
Appearance of scar in the control group.
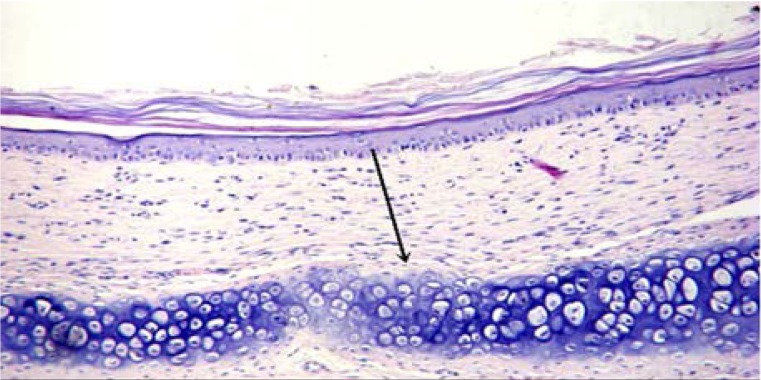
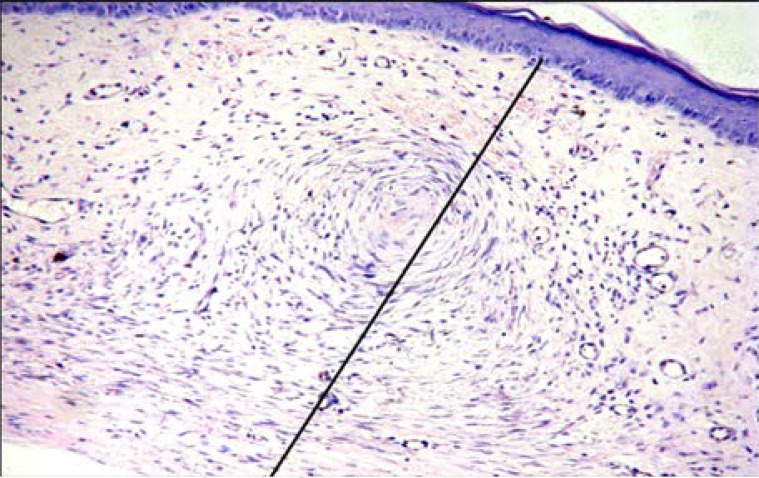
Histology changes and scar index
To investigate whether Endostar affected the histology of HS, HE staining was conducted. As shown in Figures 4A and 4B, scars in the Endostar group had thinner connective tissue and less capillary and fibroblasts than those in the control group. Moreover, the distribution of collagen fibers in the Endostar group was regular, while they were littery and circinate in the control group. To better understand these changes, the scars were harvested for SEI measurement. It was discovered that the SEI of the Endostar group was significantly lower than that of the control group. SEI in the Endostar group was 1.37 ±0.21 compared to 2.65 ±0.21 in the control group (P<0.05).
Figure 4A.
Endostar group (HE ×200).
Figure 4B.
Control group (HE ×200).
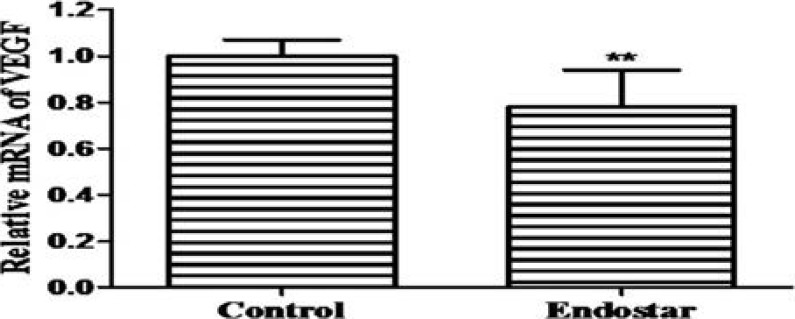
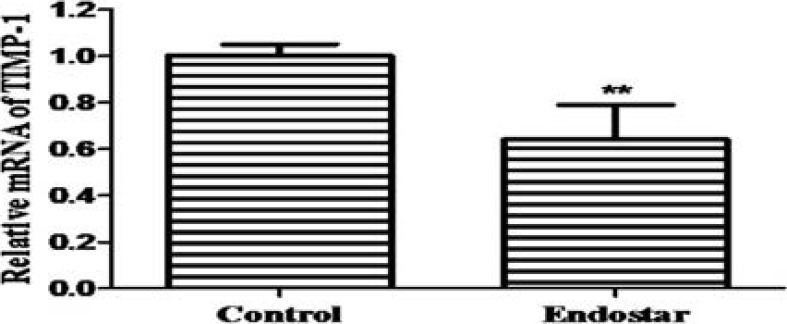
Endostar down-regulates mRNA expressions of VEGF and TIMP-1
To determine the effects of Endostar on the VEGF and TIMP-1 mRNA expressions, RT-PCR was used to analyze their levels in the scar tissues. The relative expression of VEGF was 1.00 ±0.07 and 0.78 ±0.16 in the control group and Endostar group, respectively (Figure 5A). The relative expression of TIMP-1 was 1.00 ±0.05 and 0.64 ±0.15 in the control group and Endostar group, respectively (Figure 5B). These results showed that Endostar significantly suppressed the expressions of VEGF and TIMP-1 mRNA.
Figure 5A.
mRNA expression of VEGF.
Figure 5B.
mRNA expression of TIMP-1.
Endostar down-regulated protein expressions of VEGF and TIMP-1
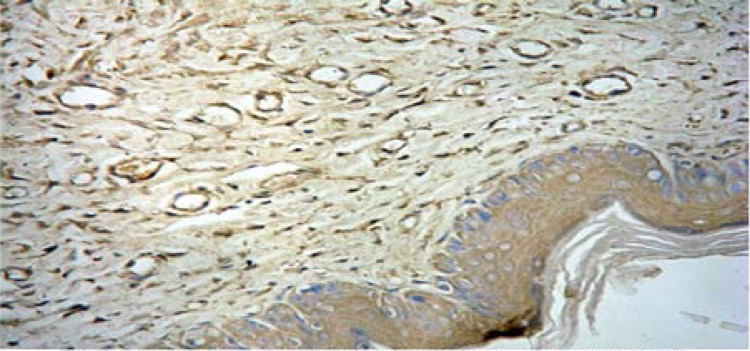
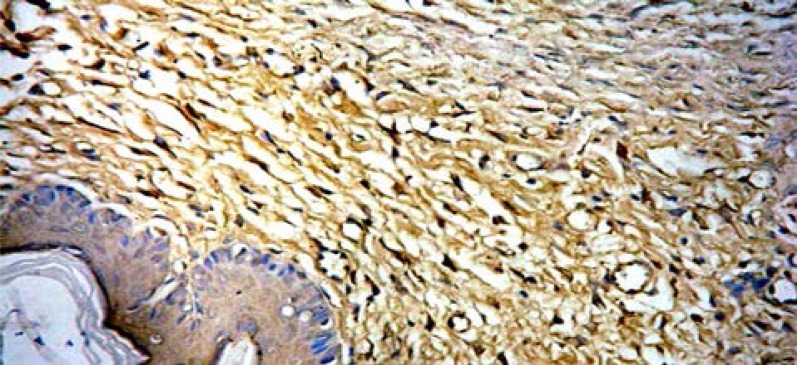
Firstly, sixty-four scars in the Endostar group and sixty-four scars in the control group were tested for VEGF and TIMP-1 expressions by immunohistochemistry with their specific antibodies. Positive staining of VEGF was observed in 92.2% (59/64) scars in the control group, while there were only 59.4% (38/64) observed in the Endostar-group (Figures 6A, 6B).
Figure 6A.
Positive staining of VEGF in control group (SAB×400).
Figure 6B.
Positive staining of VEGF in Endostar group (SAB×400).
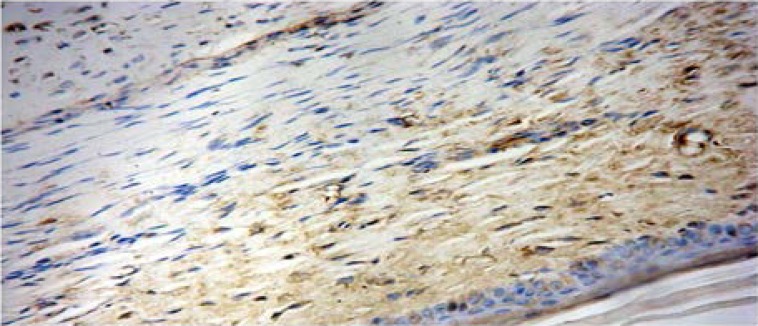
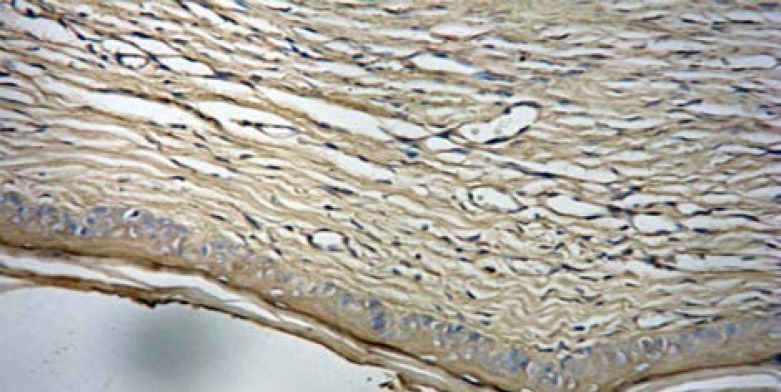
TIMP-1 was expressed in 87.5% (56/64) of the scars in the control group, and only 56.3% (36/64) of the scars in the Endostar group (Figure 6C, Figure 6D).
Figure 6C.
Positive staining of TIMP-1 in control group (SAB ×400).
Figure 6D.
Positive staining of TIMP-1 in Endostar group (SAB×400).
These results showed that the protein expressions of VEGF and TIMP-1 were significantly down-regulated by Endostar (both P<0.05) (Table 1).
Table I.
Expression of VEGF and TIMP-1 in scar
| VEGF | TIMP-1 | ||||||||
| Type | n | − | + | ++ | P value | − | + | ++ | P value |
| Endostar | 64 | 26 | 33 | 5 | <0.05 | 28 | 31 | 5 | <0.05 |
| Control | 64 | 5 | 46 | 13 | 8 | 40 | 16 | ||
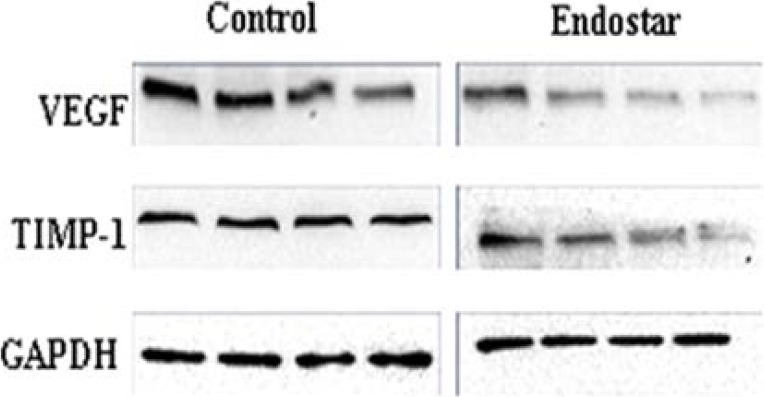
Thereafter, to verify the above results, protein expressions of VEGF and TIMP-1 were further detected by western blot. These results also suggested that Endostar significantly decreased the expressions of VEGF and TIMP-1, compared to the scars in the control group (Figure 7).
Figure 7.
Expressions of VEGF and TIMP-1 Proteins in HS
Discussion
Altered appearance and function seriously affect the lives of HS patients. In addition, no single treatment appears to be effective for all HS sufferers. However, Endostar is a potent inhibitor of angiogenesis and has been successfully used in carcinoma therapy for its anti-angiogenesis properties7. It also plays an important role in anti-fibrosis treatment16. For example, Endostar significantly decreases the expression of Collagen-I, Collagen-III, and TGF-β1 in HS of rabbit ears17–18. Endostar also decreases the expressions of α-SMA and CTGF in other models19–20. Because HS is also associated with increased angiogenesis and ECM21–23, it was chosen as the object of this study, which showed that Endostar significantly reduced the formation of HS in rabbit ears by down-regulating VEGF and TIMP-1 expressions.
In this study, Endostar was administrated to the rabbits after the formation of the scars rather than before because it was believed that Endostar could decrease the microvessel density of the granulation tissue and delay wound healing24. After treatment with Endostar, the thickness of the connective tissues in HS was thinner, the number of micro vessels and fibroblasts were fewer, and the collagen fibers were smoother than those in the control group. This suggested that Endostar had a significantly inhibitive effect on HS formation. VEGF monoclonal antibody, another anti-angiogenesis protocol, has been reported to inhibit collagen production and excessive scar growth in HS11,25. In this study, it was found that mRNA and protein expression of VEGF was significantly decreased following Endostar treatment. Therefore, it was concluded that the inhibition of HS formation with Endostar may partly result from the reduction of angiogenesis by anti-VEGF mechanism.
It is important to maintain a balance between the synthesis and degradation of ECM in wound healing26. Excessive synthesis or lessened degradation of ECM will result in hypertrophic scars23. TIMPs and MMPs play a critical role in the formation, alteration, and degradation of matrix proteins27–29. TIMP-1 can combine with most MMP active sites, thus inhibiting enzyme activity30–31. However, some research has found that the expression of TIMP-1 is significantly higher in the scar tissue of patients with HS than in scar tissues of patients with normotrophic scars32. Moreover, targeting TIMP-1 using small interfering RNA has significant therapeutic potential as an approach to treating keloids33. In this study, the expression of TIMP-1 was significantly decreased with Endostar treatments, while the group that did not receive Endostar therapy showed over expression of TIMP-1. Thus, it was thought that down-regulated TIMP-1 by Endostar also contributed to the inhibition of HS formation.
Conclusion
The findings suggested that Endostar reduced the formation of HS by down-regulation of VEGF and TIMP-1 expressions. However, whether Endostar directly targeted TIMP-1 or by another pathway requires further investigation. Therefore, further studies are needed to elucidate the more precise molecular mechanism of Endostar on the inhibition of HS.
Conflict of interest
The authors have no conflict of interest.
References
- 1.Berman B, Flores F. The treatment of hypertrophic scars and keloids. Eur J Dermatol. 1998;8(8):591–595. [PubMed] [Google Scholar]
- 2.Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars: a meta-analysis and review of the literature. Arch Facial Plast Surg. 2006;8(6):362–368. doi: 10.1001/archfaci.8.6.362. [DOI] [PubMed] [Google Scholar]
- 3.Kischer CW. The micro vessels in hypertrophic scars, keloids and related lesions: a review. J Submicrosc Cytol Pathol. 1992;24(2):281–296. [PubMed] [Google Scholar]
- 4.Amadeu T, Braune A, Mandarim-de-Lacerda C, Porto LC, Desmouliere A, Costa A. Vascularization pattern in hypertrophic scars and keloids: a stereological analysis. Pathol ResPract. 2003;199(7):469–473. doi: 10.1078/0344-0338-00447. [DOI] [PubMed] [Google Scholar]
- 5.Thomas DW, Hopkinson I, Harding KG, Shepherd JP. The pathogenesis of hypertrophic/keloid scarring. Int J Oral Maxillofac Surg. 1994;23(4):232–236. doi: 10.1016/s0901-5027(05)80377-7. [DOI] [PubMed] [Google Scholar]
- 6.Ehrlich HP, Kelley SF. Hypertrophic scar: an interruption in the remodeling of repair—a laser Doppler blood flow study. Plast Reconstr Surg. 1992;90(6):993–998. [PubMed] [Google Scholar]
- 7.Eder JP, Jr, Supko JG, Clark JW, Puchalski TA, Garcia-Carbonero R, Ryan DP, et al. Phase I clinical trial of recombinant human endostatin administered as a short intravenous infusion repeated daily. J Clin Oncol. 2002;20(18):3772–3784. doi: 10.1200/JCO.2002.02.082. [DOI] [PubMed] [Google Scholar]
- 8.Steinbrech DS, Mehrara BJ, Chau D, Rowe NM, Chin G, Lee T, et al. Hypoxia upregulates VEGF production in keloid fibroblasts. Ann Plast Surg. 1999;42(5):514–519. doi: 10.1097/00000637-199905000-00009. discussion 519–520. [DOI] [PubMed] [Google Scholar]
- 9.Bock O, Schmid-Ott G, Malewski P, Mrowietz U. Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res. 2006;297(10):433–438. doi: 10.1007/s00403-006-0651-7. [DOI] [PubMed] [Google Scholar]
- 10.Ong CT, Khoo YT, Tan EK, Mukhopadhyay A, Do DV, Han HC, et al. Epithelial-mesenchymal interactions in keloid pathogenesis modulate vascular endothelial growth factor expression and secretion. J Pathol. 2007;211(1):95–108. doi: 10.1002/path.2081. [DOI] [PubMed] [Google Scholar]
- 11.Yue YG, Jiang CW, Li PY, Zhou S. Influence of the VEGF antibody targeted vascular therapy on the expression ofcollagen type I in hyperplastic. Zhonghua Shao Shang Za Zhi. 2006;22(6):427–430. [PubMed] [Google Scholar]
- 12.Ulrich D, Noah EM, von Heimburg D, Pallua N. TIMP-1, MMP-2, MMP-9, and PIIINP as serum markers for skin fibrosis in patients following severe burn trauma. Plast Reconstr Surg. 2003;111(4):1423–1431. doi: 10.1097/01.PRS.0000049450.95669.07. [DOI] [PubMed] [Google Scholar]
- 13.Morris DE, Wu L, Zhao LL, Bolton L, Roth SI, Ladin DA, et al. Acute and chronic animal models for excessive dermal scarring: Quantitative studies. Plast Reconstr Surg. 1997;100(3):674–681. doi: 10.1097/00006534-199709000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Marcus JR, Tyrone JW, Bonomo S, Xia Y, Mustoe TA. Cellular mechanisms for diminished scarring with aging. Plast Reconstr Surg. 2000;105(5):1591–1599. doi: 10.1097/00006534-200004050-00001. [DOI] [PubMed] [Google Scholar]
- 15.Taylor CR. Quantifiable internal reference standards for immunohistochemistry: the measurement of quantity by weight. Appl Immunohistochem Mol Morphol. 2006;14(3):253–259. doi: 10.1097/00129039-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi YI, Feghali-Bostwick CA. Role of endostatin in fibroproliferative disorders.-as a candidate for anti-fibrosis therapy. Nihon Rinsho Meneki Gakkai Kaishi. 2013;36(6):452–458. doi: 10.2177/jsci.36.452. [DOI] [PubMed] [Google Scholar]
- 17.Yu Jian, Zhang Xiao-ming, Huang Xue-ying, et al. Study of the inhibitory effects of recombinant human endostatin on hypertrophic scars. Acta Universitatis Medicinalis Anhui. 2014;49(7):891–895. [Google Scholar]
- 18.Zhang Xiao-ming, Yu Jian, Huang Xue-ying, et al. Effects of recombinant human endostatin on the expression ofvascular endothelial growth factor,transforming growth factor-β1 and basic fibroblast growth factor on hypertrophic scar in rabbit ears. Acta Anatomica Sinica. 2015;46(1):101–105. [Google Scholar]
- 19.Chen J, Liu DG, Yang G, et al. Endostar, a novel human recombinant endostatin, attenuates liver fibrosis in CCl4-induced mice. Exp Biol Med. 2014;239(8):998–1006. doi: 10.1177/1535370214532595. [DOI] [PubMed] [Google Scholar]
- 20.Zhan-qing WU, Qiang MA, Yu-feng GAO. Changes of VEGF, HIF-1α, OPN and CTGF levels in serum of HCC patients after recombinant human endostatin combined with transcatheter arterial chemoembolization. Chinese Journal of Biochemical Pharmaceutics. 2015;35(6):98–101. [Google Scholar]
- 21.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 22.Leung DW, Cachianes G, Kuang WJ, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 23.Ulrich D, Ulrich F, Unglaub F, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with different types of scars and keloids. J Plast Reconstr Aesthet Surg. 2010;63(6):1015–1021. doi: 10.1016/j.bjps.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Yuan-yuan Wu. The role of protein kinase C-αin recombinant human endostatin inhibiting angiogenesis on wound healing of rabbit ear. Lanzhou: Lanzhou University; 2013. [Google Scholar]
- 25.Shen R, Li TZ, Qi SH, Liang HZ, Xu YB, Xie JL, et al. A research of endothelial cell-targeted therapy for cure of hypertrophic scar. Zhonghua Zheng Xing Wai Ke Za Zhi. 2003;19(4):254–257. [PubMed] [Google Scholar]
- 26.Ramadori G, Knittel T, Saile B. Fibrosis and altered matrix synthesis. Digestion. 1998;59(4):372–375. doi: 10.1159/000007518. [DOI] [PubMed] [Google Scholar]
- 27.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 28.Hetzel M, Bachem M, Anders D, et al. Different effects of growth factors on proliferation and matrix production of normal and fibrotic human lung fibroblasts. Lung. 2005;183(4):225–237. doi: 10.1007/s00408-004-2534-z. [DOI] [PubMed] [Google Scholar]
- 29.Mirastschijski U, Schnabel R, Claes J, et al. Matrix metalloproteinase inhibition delays wound healing and blocks the latent transforming growth factor-beta1-promoted myofibroblast formation and function. Wound Repair Regen. 2010;18(2):223–234. doi: 10.1111/j.1524-475X.2010.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egea V, Zahler S, Rieth N, et al. “Tissue inhibitor of metalloproteinase-1 (TIMP-1) regulates mesenchymal stem cells through let-7f microRNA and Wnt/β-catenin signaling,”. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(6):E309–E316. doi: 10.1073/pnas.1115083109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umenishi F, Umeda M, Miyazaki K. Efficient purification of TIMP-2 from culture medium conditioned by human hepatoma cell line, and its inhibitory effects on metalloproteinases and in vitro tumor invasion. J Biochem. 1991;110(2):189–195. doi: 10.1093/oxfordjournals.jbchem.a123555. [DOI] [PubMed] [Google Scholar]
- 32.Ulrich D, Ulrich F, Unglaub F, Piatkowski A, Pallua N. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with different types of scars and keloids. J Plast Reconstr Aesthet Surg. 2010;63(6):1015–1021. doi: 10.1016/j.bjps.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 33.Aoki M, Miyake K, Ogawa R, Dohi T, Akaishi S, Hyakusoku H, et al. siRNA Knockdown of Tissue Inhibitor of Metalloproteinase-1 in Keloid Fibroblasts Leads to Degradation of Collagen Type I. J Invest Dermatol. 2014;134(3):818–826. doi: 10.1038/jid.2013.396. [DOI] [PubMed] [Google Scholar]