Abstract
Background
This article examines the concepts of low glycemic indices (GIs) and glycemic load (GL) foods as key drivers in the dietary management of type 2 diabetes as well as their shortcomings. The controversies arising from the analysis of glycemic index (GI) and GL of foods such as their reproducibility as well as their relevance to the dietary management of type 2 diabetes are also discussed.
Methods
Search was conducted in relevant electronic databases such as: Pubmed, Google Scholar, HINARI, the Cochrane library, Popline, LILACS, CINAHL, EMBASE, etc to identify the current status of knowledge regarding the controversies surrounding management of diabetes with low GI and GL foods.
Conclusion
This article suggests that in view of discrepancies that surround the results of GI versus GL of foods, any assay on the GI and GL of a food with the aim of recommending the food for the dietary management of type 2 diabetes, could be balanced with glycated hemoglobin assays before they are adopted as useful antidiabetic foods.
Keywords: Glycemic index, carbohydrates, type 2 diabetes, nutrition, foods
Introduction
The incidence of type 2 diabetes which accounts for more than 90 to 95% of all cases of diabetes mellitus with its attendant economic stress on the health care system has been rising.
A number of factors that have been attributed to this include: increase in sedentary lifestyle, obesity, lack of physical activity, consumption of an energy-rich diet, longer life span, smoking, and more1 but the role of dietary carbohydrate has been controversial. Little relation has been found between total carbohydrate intake and the risk of type 2 diabetes2.
Previously, it was widely held that blood glucose response to different diets is determined mainly by the amount of carbohydrates they contain. This consequently resulted in traditional diabetes diet plans in which the amount of foods allowed were based on their carbohydrate contents. However, the concept of glycemic index (GI) which classifies the blood glucose-raising potential of carbohydrate foods relative to glucose or white bread3 has shown that foods with similar carbohydrate contents did not usually have the same impact on blood glucose levels4–9.
Nowadays, GI has been transformed by its popularizers from a potentially useful tool in planning diets for diabetic patients to a key player for the prevention and management of diabetes, prevention of dyslipidemia, cardiovascular diseases and even certain types of cancers2. The question then is whether such a transformation especially for the prevention and management of diabetes (which is the focus of this review), is justified and whether it is wise to set as a public health policy for an entire population, the avoidance of certain foods because of their high glycemic indices (GIs). Although several studies5–9 have suggested the application of the concept of low GIs and GL foods in the prevention and management of type 2 diabetes, the American Diabetes Association is still very much hesitant in the adoption of this concept as a useful tool for management of type 2 diabetes due to the challenges associated with this concept.
Therefore, in this review, an attempt was made to enumerate the prospects and challenges associated with this concept in the management of type 2 diabetes as well as to proffer solutions to these challenges which is lacking in previous reports on this concept.
To answer this question, the following issues, as discussed in this review, need to be considered.
Methods
Several relevant electronic databases such as: Pubmed, Medline, Google Scholar, HINARI, Agora, the Cochrane library, EMBASE, etc were searched up to 2014 to identify the current status of knowledge regarding the controversies surrounding management of diabetes with low GI and GL foods.
Results
The results of the findings obtained from these data bases are reported in this review.
Terminologies used for digestibility of carbohydrates
Glycemic Index (GI)
GI is defined as the area under the glucose response curve after consumption of 50 g carbohydrate from a test food divided by the area under the curve after consumption of 50 g carbohydrate from a control food (either white bread or glucose)10–11. It is a classification of the blood glucose-raising potential of carbohydrate foods relative to glucose or white bread3. Generally, there are three categories of foods based on their GI values: The high-GI foods (> 70), intermediate-GI foods (>55 – < 70) and low-GI foods (< 55)7.
Glycemic Response
This refers to the effect the food will have on blood glucose after consumption.
Glycemic Load (GL)
The glycemic response to an ingested food was found to depend not only on the GI but also on the total amount of carbohydrates ingested, and this led to the concept of GL.
GL accounts for how much of carbohydrate is in the food and how each gram of carbohydrate in the food raises blood glucose levels. GL is classified as: low (< 10), intermediate (11–19) and high (> 20). GL is a metric used as a basis for weight loss, or diabetes control12.
Mathematically, GL = GI × available carbohydrate (g) /100
Where available carbohydrate = total carbohydrate - dietary fiber.
One unit of GL approximates the glycemic effect of 1 g of glucose. Typical diets contain from 60–180 GL units per day. Dietary glycemic overload could eventually result in increased risk of diabetes and obesity12.
The GL of a food is dependent on 2 factors: the GI of the food and the serving size and as such, increases or decreases in GL can be achieved by varying either or both terms. Therefore, a low GL food can be achieved by either decreasing the GI of the food or by eliminating most of the carbohydrates from the diet11.
Factors affecting GIs and GLs of foods
Carbohydrate contents of foods
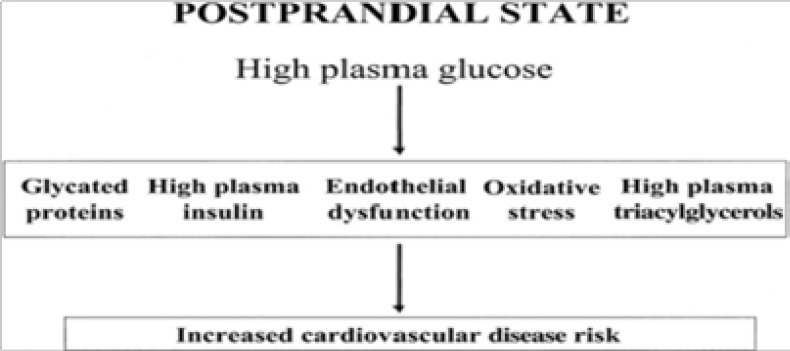
Dietary carbohydrates could increase blood glucose levels especially in the postprandial period. Therefore, for people with diabetes, either those with type 1 or those who have more severe forms of type 2, a carbohydrate-rich diet could be detrimental to glycemic control resulting in microvascular and macrovascular complications13. In parallel with plasma glucose elevation, plasma insulin and triacylglycerol concentrations have also been reported13 to increase with a high-carbohydrate diet, along with other cardiovascular disease risk factors as shown in Figure 1.
Fig 1.
Relationship between postprandial hyperglycemia and cardiovascular disease risk. Source:13
However, not all carbohydrate-rich foods result in hyperglycemia when consumed. Differences in postprandial blood glucose responses to various carbohydrate-containing foods have also been demonstrated in both healthy and diabetic subjects, even when consumed in portions containing identical amounts of carbohydrate13,14. This indicates that there could be differences in individual components of carbohydrates that are responsible for the reported variations to postprandial blood glucose responses after consumption of the various carbohydrate containing foods by both healthy and diabetic individuals.
These variations in glycemic responses to carbohydrate foods and which also tend to affect the GL of foods, were reported to arise from different components of carbohydrates present in foods and their properties such as: Starch composition/properties (digestible, indigestible, amylose/amylopectin ratio, gelatinization, retrogradation), dietary fiber contents15–16, sugars as well as other factors such as: insulin response, protein contents, processing techniques, variety, particle size, fat, acidity, storage and time of harvest. However, for the purpose of this review, the discussion is concentrated on starch composition/properties, dietary fiber, insulin response, protein contents, processing, variety, particle size, fat and acidity.
Starch composition/properties
Starch contributes about 70–80% of the total carbohydrates in normal diets7,16. For nutritional purposes, starches are classified on the basis of their rate and extent of digestion into 3 categories, namely: rapidly digestible starch (RDS), slowly digestible (SDS), and resistant starch (RS)17,18.
RDS is the starch fraction that is rapidly digested and absorbed in the duodenum and proximal regions of the small intestine leading to a rapid elevation of blood glucose and usually a subsequent episode of hypoglycaemia17. RDS consists mainly of amorphous and dispersed starch and it is found in high amounts in starchy foods cooked by moist heat, such as bread and potatoes. It is measured chemically as the starch that is converted to the constituent glucose molecules within 20 min of enzyme digestion17.These rapid and large increases in blood glucose levels can further lead to cell, tissue and organ damage19.
SDS is the starch fraction that is digested slowly but completely in the small intestine to provide sustained glucose release with a low initial glycemia and subsequently a slow and prolonged release of glucose, leading to prolonged energy availability19. This category consists of physically inaccessible amorphous and raw starch with types A and type C crystalline structures, such as cereals and type B starch, either in granule form or retrograded form in cooked foods.
It is measured chemically as the starch that is converted to glucose after a further 100 min of enzyme digestion17. Examples include: most raw cereals and others19. The potential health benefits of SDS include stable glucose metabolism, diabetes management, etc.
RS is not digested in the upper gastrointestinal tract but is fermented by the gut microflora, producing short chain fatty acids that provide additional energy to the body. Hence its definition by Sagilata and colleagues as the fraction of dietary starch that escapes digestion in the small intestine17. RS is measured chemically as the difference between total starch (TS) (obtained from homogenized and chemically treated sample) and the sum of RDS and SDS, generated from non-homogenized food samples by enzyme digestion.
Mathematically, RS = TS − (RDS + SDS)17. It is not all resistant starches that are the same.
There are 4 classes of resistant starches and they include:
Type 1 which refers to a starch that resists digestion because it is bound within the fibrous cell walls that make it physically inaccessible. Examples include: partly milled grains and seeds as well as legumes. Type 1 is heat stable in most normal cooking operations and enables its use as an ingredient in a wide variety of conventional foods.
Type 2 which refers to a starch that is in a certain granular form and resistant to enzyme digestion. This type is found in some starchy foods, including raw potatoes and green (unripe) bananas.
Type 3 which represent the most RS fraction. It is formed when certain starchy foods, including potatoes and rice, are cooked and cooled. The cooling converts some of the gelatinized digestible starches into resistant starches through the process of retrogradation17. A lot of starchy foods cooked by moist heat can therefore contain some fractions of Type 3 as a result of retrogradation. Type 3 is entirely resistant to digestion by pancreatic amylases.
Type 4 which is man-made and it refers to the RS where new chemical bonds other than α-(1, 4) or α-(1, 6) are formed. Modified starches obtained by various chemical processes fall under this category.
Studies in humans show that RS can have powerful health benefits such as improved insulin sensitivity in type 2 diabetic patients and reduction of blood glucose levels20. Other health benefits that have been reported to be associated with consumption of foods rich in RS include: prevention of colon cancer, substrate for the growth of probiotics, reduction of gall stone formation, hypocholesterolemic effects, inhibition of fat accumulation, and increase in absorption of minerals17,21.
Various physical factors like stirring, water-starch ratio, cooking and cooling regimes affect resistant starch formation22. RS formation is also influenced by amyloseamylopectin ratio23.
Amylose-amylopectin ratio
Amylose
Amylose is a helical polymer made of α-D-glucose units, bound to each other through α(1→4) glycosidic bonds. This polysaccharide is one of the two components of starch, making up approximately 20–30% of the structure.
Amylopectin
Amylopectin is a soluble polysaccharide and highly branched polymer of glucose found in plants, making up approximately 70% of starch. Amylopectin is highly branched, being formed of 2,000 to 200,000 glucose units.
Evidence shows that amylose slows digestion and insulin response time, providing a lower glycaemic index24. Studies by Miller et al25 showed that rice with higher amylose content (Doongara, 28% amylose) gave a significantly lower glycemic index (GI) and insulin index than the normal amylose versus amylopectin rice varieties (Calrose and Pelde, 20% amylose).
Studies by Miller et al25 showed that rice with higher amylose content gave a significantly lower GI than normal amylose versus amylopectin rice varieties.
Furthermore, amylopectin/amylose ratio and amylose complexes with lipids affect the rate of starch hydrolysis. The extent of digestibility of starches generally decreases as amylose content increases26 although amylose content alone is not a sole predictor of digestibility27,28. Similarly, amylose complexed with lipid is more resistant to attack by hydrolytic enzymes than is free carbohydrate29.
Gelatinization
When starch is heated to a temperature of about 50°C, in the presence of water, the amylose in the granule swells; the crystalline structure of the amylopectin disintegrates and the granule ruptures. The polysaccharide chains take up a random configuration, causing swelling of the starch and thickening of the surrounding matrix. The process, known as gelatinization, makes the starch easily digestible17. The greater the gelatinization, the more viscous the starch would be and the higher will be its GI and this could also affect the GL.
Gelatinized starch samples are far more susceptible to degradation by α-amylase than are native starch granules7,26–29.
Retrogradation
After starch is gelatinized, when it gets cold (low temperature), the gelatinized starch gradually begins to undergo rearrangement of its amylose and amylopectin macromolecules which leads to increase in crystalline nature of starch molecules. This process is known as retrogradation. Retrogradation becomes more intense as time passes and temperatures go down. The higher the amylose content of starch, the greater the effectiveness of the retrogradation process and the more resistant to digestion the starch will be due to stronger hydrogen bonding leading to lower GI of the starch15 and this could also lower the GL of starch.
Through the process of retrogradation, gelatinized or solubilised starch can be transformed from an unstructured into a more ordered or crystalline state. This physical change causes heat processed starchy foods to harden or become stale as they spontaneously approach a meta stable state of lower free energy. This has been reported to decrease the GI value, due to an increased resistance to amylase30. Consequently, the duration of the first stage of retrogradation depends on the amylose content of starch. High molecular weight amylose promotes retrogradation more than lower molecular weight polymers7.
Dietary fiber
The American Association of Cereal Chemists31, defined dietary fiber as carbohydrate polymers with more than a three degree polymerization which are neither digested nor absorbed in the small intestine.
Recently, The British Nutrition Foundation32 defined dietary fiber as a group of substances in plant foods that cannot be completely broken down by human digestive enzymes. These include: waxes, lignin and polysaccharides such as cellulose and pectin.
According to the classification of dietary fibre by the American Association of Cereal Chemists as described by James and Mark33, dietary fiber is divided into 3 constituents: non starch polysaccharides (NSP) and oligosaccharides (cellulose, hemicellulose, inulin, pectin, gums, mucilages, polufructoses, arabinogalactans and others), analogous carbohydrates (indigestible dextrins, polydextrose, resistant starches, resistant potato dextrins, resistant maltodextrins, methyl cellulose and others) and lignin substances associated with NSP and lignin complex (waxes, phytate, cutin, saponin, tannins, and suberins).
Slavin et al34 showed that viscous soluble fibre plays an important role in controlling postprandial glycemic and insulin responses because of its effect on gastric emptying and macronutrient absorption from the gut34. Surprisingly, some prospective studies found that insoluble and not soluble fibre was inversely related to the incidence of type 2 diabetes mellitus35,36.
Several studies conducted during the last decade regarding the effect of dietary fibre on insulin sensitivity provided controversial results37–42. In a randomized cross-over study, Pereira et al38 used a euglycemic-hyperinsulinemic clamp test to measure insulin sensitivity in eleven obese hyperinsulinaemic participants. Their study showed that consumption of a whole grain diet led to a postprandial improvement in insulin sensitivity when compared to a refined grain diet38.
Similarly, Weickert et al39 used the same method to measure insulin sensitivity in overweight and obese women and found that this increased after 3 days of a diet containing bread enriched with insoluble fibre compared to another diet containing white bread. Giacco et al40 carried out a 6 months randomized parallel study comparing a diet containing 50 g/d of soluble fibre with a diet containing only 15 g/d of fibre. They found an improvement in the daily blood glucose profile and glycated hemoglobin (HbA1C) levels and a marked reduction in the number of hypoglycemic events. Chandalia et al41 also demonstrated that high fibre diets contributed to better metabolic control in thirteen type 2 diabetic patients.
In a cross-over study in which patients were randomized to a diet containing moderate amount of fibre (8 g of soluble fibre and 16 g insoluble fibre) or to a diet containing high amount of fibre (25 g of soluble fibre and 25 g insoluble fibre), plasma glucose concentrations were significantly lower for the high fibre diet than for the lowfibre diet37.
In contrast, Jenkins et al42 used a cross-over design to study the effects of a diet high in cereal fibre in type 2 diabetic patients and found no improvement in conventional markers of glycemic control after 3 months of intervention.
In clinical studies using fibre supplements, it appears that only the viscous variety of soluble fibre plays a significant role in reducing postprandial glycemia. This is in contrast to the findings from different authors where insoluble fibre, but not soluble fibre, from natural food sources was inversely related to the risk of diabetes35–36,43.
Currently, the American Diabetes Association recommends that diabetic patients consume 14 g/1000 kcal/day of fibre because a high amount of fibre is necessary to improve glycemic control37,44.
However, while the short term beneficial effects of fibre on the glycemic profile may have been documented; there have not been enough trials to prove categorically that soluble fibre supplements would be an effective tool for ameliorating glycemic control in the long term. Although prospective studies have shown that fibre in the diet could protect an individual from diabetes, clinical trials are still needed to corroborate these reports37.
Sugars
GI is affected by the composition of sugar in a food. For example, sucrose which is made up of glucose and fructose, has a lower GI than glucose because half of the sucrose molecule is made up of fructose, a type of sugar that elicits low blood sugar response45. In addition, while the GI of sucrose is 68, the GI of glucose is 10045. This variation in GI as a result of composition of sugar could also affect the GL.
Other factors
Insulin response
Another factor that has been found to have considerable effect on the blood glucose level of an individual following consumption of a carbohydrate rich diet is insulin response. Insulin is the primary hormone the body uses to maintain blood glucose levels within a healthy range. Therefore, in a well fed state or when foods especially with considerable amounts of carbohydrates (depending on their composition) are eaten, elevated levels of intracellular glucose in the hepatocyte allows glucokinase to phosphorylate the excess glucose to glucose-6-phosphate. This reaction stimulates insulin response or activation which makes for conversion of the excess glucose to glycogen. The conversion of glucose-6-phosphate to glycogen (glycogenesis) is favoured by inactivation of glycogen phosphorylase and activation of glycogen synthase. Elevated insulin level results in overall increased glycogen synthesis or glycogenesis and inadequate secretion of insulin (poor response) could result in improper metabolism of carbohydrates, proteins and fats, leading to hyperglycemia.
Miller et al25 in their study noted a negative relationship between the GI and insulin response of some foods, a typical example being Calrose brown rice with a GI of 83 but with a contrasting insulin index (response) of 51. Although it is known that insulin responses to carbohydrate foods is potentiated by addition of fats to the food, the reports of Miller et al25 indicated that the fat contents of the rice was negligible. Therefore, insulin response should be a factor of consideration when determining the optimal carbohydrate foods for diabetic patients.
Protein content
Protein-rich foods increase insulin secretion leading to lowering of postprandial blood glucose concentrations. Thus, the natural protein contents of some foods might be the reason why their starches are not easily hydrolyzed which confers them with lower GIs. A typical example is the case of pasta in cereals and gluten that slow the action of pancreatic amylases, thereby leading to lower GIs.
Processing techniques
It has been established that different food processing techniques affect the digestibility of starch which has some implications on the GIs of these foods. Processing techniques may affect both the gelatinization and retrogradation processes, influencing resistant starch formation. For example, roasted and fried foods were reported to have higher GIs than boiled foods22. In another study, steam cooking was reported to aid the production of RS17 and starches isolated from several steam-heated legumes were reported to be rich in indigestible RS (19% to 31%, dry matter basis), which was not observed in raw beans46. Again, Bahado-Singh et al15 reported that processing of sweet potatoes by boiling elicited lower GI values when compared to frying, baking, and roasting of sweet potatoes. The study of Deepa et al22 showed that the beneficial effects of dietary fibre in hindering the actions of hydrolytic enzymes are nullified when whole grains are ground as they are hydrolyzed at the same rate as polished grain flour.
A typical case of the effect of processing a food on its effect on blood glucose levels is seen in the study of Alegbejo et al47 which reported that boiled cocoyam (C. esculenta) has a high GI and so may not be good for diabetics, a finding that contradicted the traditional use of C. esculenta (either boiled or other processed forms) in Nigerian ethnomedicine in the management of diabetes whereas the study of Eleazu et al48 reported the hypoglycemic action of oven dried C. esculenta in experimental diabetic rats and which findings corroborated the traditional use of C. esculenta (either boiled or other processed forms) in Nigerian ethnomedicine in the management of diabetes whereas the study of Eleazu et al48 reported the hypoglycemic action of oven dried C. esculenta in experimental diabetic rats and which findings corroborated the traditional use of C. esculenta in Nigerian ethnomedicine in the management of diabetes but contradicted the reports of Alegbejo and colleagues.
These reports point to the fact that methods of processing of food samples usually affect their GIs. Finally, processing of foods using high temperatures could induce gelatinization, thereby permanently disrupting the amylose-amylopectin structure of the starch complex, making it more readily accessible by digestive enzymes.
Variety
Variety is one key factor that affects the GIs of foods. For example, there are discrepancies in the reported GI of rice and one of the factors that have been implicated as playing contributory roles to these discrepancies is the difference in their varieties22. Another example is the case of boiled dasheen (Colocassia esculenta) that was reported to have a GI of 77, whereas boiled eddoes (Colocassia esculenta) were reported to have a GI of 6649. Even with the same variety, glycemic indices may vary probably due to differences in accessions within the same variety. For example, the GI of white potatoes tends to range from moderate to very high even with the same variety4.
Particle size
When starchy foods are ground, their particles become much finer which makes for ease of their hydrolysis by digestive enzymes, thereby increasing their GIs10,16,50–52. For example, there are discrepancies in the reported GI of rice due to particle size22,25. Digestibility of starch is affected by the size of the granule and surface area to starch ratio for action of hydrolytic enzymes16.
Fat
Fat increases the time it takes for food to leave the stomach and enter the intestine. By slowing the rate at which dietary carbohydrates are digested in the intestine, fatcontaining foods may affect the rise in blood sugar and yield a lower GI than similar foods without fat. For example, the GI of potato chips is 57, French fries is 75 and baked potato is 8553.
Acidity
Acid in food slows down stomach emptying, thereby slowing the rate at which dietary carbohydrates are digested. Thus increasing the acidity in a meal can lower its GI and blood glucose45. These factors as enumerated above therefore tend to affect the validity and reproducibility of GIs and GLs of foods.
Therefore, in calculating the GIs of foods, all these parameters have to be considered otherwise, such data reported may not be a true representation of the GI of the food material being investigated.
Prospects of the concept of GIs and GL in the management of type 2 diabetes mellitus
Diet has been accepted as one of the key factors associated with a number of diseases one of which is diabetes. Diet constitutes a crucial aspect of the overall prevention/management of diabetes, which may involve diet alone, diet with oral hypoglycemic drugs, or diet with insulin54. The concept of GIs and GL have been reported to be useful tools in the management of diabetes54. This health inference derives from blood glucose concentration and the insulin response of diabetics, relating directly to the rate of digestion of carbohydrates42,55–56.
One of the major achievements of the concept of GIs and GL is that the classification of foods based on their GIs and loads has dispelled the repeatedly suggested dietary notion that carbohydrate-rich foods have deleterious health effects and as such, consumption should be limited56. This has been corroborated by several evidence-based studies that have demonstrated that not all carbohydrates are equal15,25. Furthermore, variations in the physiochemical properties of complex carbohydrates have been shown to elicit dissimilar physiological effects when consumed57. Long-term consumption of high-GI foods was proposed to increase insulin demand, promote insulin resistance, impair pancreatic β-cell function, and eventually lead to type 2 diabetes53,58–59. Studies with large numbers of people with diabetes indicated that those who maintained their blood glucose levels under tight control avoided the complications arising from this disease60. Other studies also associated reductions in the incidence and prevalence of heart disease, diabetes, and some forms of cancer with long-term consumption of low GI foods12,51 61–62. A potential mechanism of reduction of diabetes following consumption of foods with high GIs was postulated to be stimulation of increased insulin synthesis63.
Limitations of the concept of GI and GL
The immediate effects of carbohydrates on an individual's blood glucose concentration are of interest not only for nutritional guidance but the glucose concentration has various health implications as well42.
Although several studies have focused on GI and GL to determine the rates of digestion and absorption of different carbohydrate sources58 and though they are widely used, commercially and for research purposes to measure the rates at which dietary carbohydrates are hydrolysed in the digestive system42 and absorbed into the bloodstream with the aim of managing or preventing type 2 diabetes, the validity of GI and GL as therapeutic guides for these purposes is still controversial.
Skeptics have over time, questioned the fundamental properties of these functional measures for these purposes, including their reproducibility64–66.
A major reason for these controversies is the fact that the rate of digestion of carbohydrates varies with health status, race, and gender and as such, the human subjects used to test varieties of food for their GI and GL in in vivo assays must be carefully chosen.
Another argument that has been put up is that the effect of high GI diet is affected by the degree of insulin resistance. Some studies even found greater insulin sensitivity with high GI diets45.
Other studies of dietary GI and GL in relation to insulin resistance and the risk of type 2 diabetes also showed inconsistent results. For example, the insulin resistance atherosclerosis study that evaluated the effect of a higher versus a lower GI or GL diet on insulin sensitivity in a large epidemiologic setting showed no correlation between either GI or GL and insulin sensitivity67.
In the Framingham offspring cohort study68, both dietary GI and GL were positively related to insulin resistance whereas in the Zutphen elderly study69, the insulin resistance atherosclerosis study67, the health, aging, and body composition study70, no associations were found.
In the health professionals follow-up study58, the nurses' health study II71, and the Melbourne collaborative cohort study72, dietary GI but not GL was positively associated with risk of type 2 diabetes while reports by Salmeron et al58 indicated a positive correlation between high GI and risk of type II diabetes.
Although most international diabetes organizations advocate the use of GI in the prevention and management of type 2 diabetes73, the American Diabetes Association (ADA) does not fully endorse the use of the GI because it considers current evidence insufficient to support a relation between dietary GI or GL and the development of diabetes74,75.
Another problem that has limited the effective use of GI values in therapeutic guide for diabetic patients is that the methods for defining GI are not standardized, with values having large inter- and intra-individual variability56,76–77. For instance, the GI values reported by different authors11,73,78 for potato and rice varied widely.
The accuracy of in vivo GI measurements is also influenced by other factors such as: method of calculating IAUC, methods used in blood glucose measurement, defining the amount of tested food which contains 50 g of hyperglycemic (i.e. absorbable, digestible) carbohydrates, variability within the same individual and amongst the subjects included in GI determination, day to day glycemia variability, and time of the day when the test is carried out56. Another reason for varied differences in GI values of similar foods reported by different investigators could be due to differences in starch structure or digestibility, variation in methodology, or to the effects of random variation10.
Due to the complexities and costs of GI and GL evaluations in humans, in vitro measurements of starch digestion in foods was proposed52 to predict the GI of foods.
Although good correlation was found between this model and the GIs of foods determined in vivo, a range of intrinsic and extrinsic factors that alter the rate of gastrointestinal motility, digestion and absorption also influence the GI and these cannot always be predicted using the in vitro model12. This major limitation for in vitro assay of GI thus makes the in vivo model to be the only model that could be used to establish the GI of a food.
Going by the discrepancies in the results of GI and GL of foods determined in vivo as well as other shortcomings associated with them as outlined in this review, it is plausible to suggest that cthe concept of low GIs and GL foods may not be the panacea for type 2 diabetes.
Role of glycated hemoglobin in glycemic control
Glycated hemoglobin is a glycoprotein formed as a result of non-enzymatic interaction between glucose and the amino groups of valine and lysine residues in hemoglobin. Formation of HbA1c is irreversible and the level in the red blood cell depends on the blood glucose concentration. The amount of HbA1c in the blood is dependent on mean glucose levels present during the 1–2 months preceding measurement, as HbA1c accumulates in red blood cells during their lifespan79. Thus, the level of HbA1c is an indicator of glycemic levels on a longterm basis79.
Since the standardization of HbA1c assays, it has been recommended as a useful tool in the diagnosis and follow up of diabetes80.
In view of the variations and discrepancies that surround the results of GIs versus GL of foods, it is suggested that any assay of the GI and GL of a food with the aim of recommending the food for the dietary management of type 2 diabetes, be balanced with HbA1c assays before they are adopted.
Conclusion
It is suggested that one should be careful in interpreting the results of determination of GI of foods, especially when such results are used as the sole basis for therapeutic recommendations in type 2 diabetes management. Such studies could be confirmed using HbA1c assays.
Disclosure statement
The author declares no conflicts of interest
References
- 1.Al-Jiffri O, Abd El-Kader S. Psychological wellbeing and biochemical modulation in response to weight loss in obese type 2 diabetes patients. Afr Health Sci. 2015;15:503–511. doi: 10.4314/ahs.v15i2.25. doi: http://dx.doi.org/10.4314/ahs.v15i2.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omoregie ES, Osagie AU. Glycemic indices and glycemic load of some Nigerian Foods. Pakistan J Nutr. 2008;7:710–716. [Google Scholar]
- 3.Wolever TMS, Katzman-Relle L, Jenkins AL, Vuksan V, Josse RG, Jenkins DJA. Glycaemic index of 102 complex carbohydrate foods in patients with diabetes. Nutr Res. 1994;4:651–669. doi: 10.1016/S0271-5317(05)80201-5. [DOI] [Google Scholar]
- 4.Michael M. Annemasse. France: 2004. The GI pioneer; pp. 343–345. [Google Scholar]
- 5.Pamela CC, Richard AH. Lippincott's Illustrated Reviews: Biochemistry. 2nd Edition. 1994. pp. 123–124. [Google Scholar]
- 6.Hoebler C, Devaux MF, Karinthi A, Belleville C, Barry JL. Particle size of solid food after human mastication and in vitro simulation of oral breakdown. Int J Food Sci Nutri. 2000;51:353–366. doi: 10.1080/096374800426948. [DOI] [PubMed] [Google Scholar]
- 7.Dona AC, Guilhem P, Robert GG, Philip WK. Digestion of starch: In vivo and in vitro kinetic models used to characterize oligosaccharide or glucose release. Carbohydrate Polym. 2010;80:599–617. doi: 10.1016/j.carbpol.2010.01.002. [DOI] [Google Scholar]
- 8.Kati H, Riitta T, Isabel B, Jenna P, et al. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci. 2010:1365–1402. doi: 10.3390/ijms11041365.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon YI, Vattem DA, Shetty K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac J Clin Nutr. 2006;15:107e18. [PubMed] [Google Scholar]
- 10.Wolever TMS, Jenkins DJA, Jenkins A, Josse RG. The glycemic index: Methodology and Clinical implications. Am J Clin Nutr. 1991;54:846–854. doi: 10.1093/ajcn/54.5.846. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins AL. The glycemic index: Looking back 25 years. Cereal Foods World. 2007;52:50–53. doi: 10.1094/cfw-52-1-0050. [DOI] [Google Scholar]
- 12.Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: A 1-y randomized controlled trial. Am J Clin Nutr. 2007;85:1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- 13.Gabriele R, Angela AR, Rosalba G. Role of glycemic index and glycemic load in the healthy state, in prediabetes, and in diabetes. Am J Clin Nutr. 2008;87:269S–274S. doi: 10.1093/ajcn/87.1.269S. [DOI] [PubMed] [Google Scholar]
- 14.Liljeberg H, Granfeldt Y, Bjorck I. Metabolic responses to starch in bread containing intact kernels versus milled flour. Eur J Clin Nutr. 1992;46:561–565. [PubMed] [Google Scholar]
- 15.Bahado-Singh PS, Riley CK, Wheatley AO, Lowe HIC. Relationship between processing method and the glycemic indices of ten Sweet Potato (Ipomoea batatas) cultivars commonly consumed in Jamaica. J Nutr and Metab. 2011;2011 doi: 10.1155/2011/584832. Article ID 584832: 6 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urooj A, Puttraj S. Digestibility index and factors affecting rate of starch digestion in vitro in conventional food preparation. Starch/Staerke. 1999;51:430–435. doi: 10.1002/(SICI)1521-3803(19990101)43:1<42::AID-FOOD42>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 17.Sajilata MG, Singhal RS, Kulkarni PR. Resistant starch - A review. Comp Rev in Food Sci and Food Saf. 2006;5:1–17. doi: 10.1111/j.1541-4337.2006.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 18.Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;46:S33–S50. [PubMed] [Google Scholar]
- 19.Erik EJGA, Itziar A, Arne AJ, Alfredo M, et al. Starches, Sugars and Obesity. Nutrients. 2011;3:341–369. doi: 10.3390/nu3030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nurgent AP. Health properties of resistant starch. Nutrition Bull. 2005;30:27–54. [Google Scholar]
- 21.Hyun-Jung C, Dong-Hoon S, Seung-Taik L. In vitro starch digestibility and estimated glycemic index of chemically modified corn starches. Food Research International. 2008;41:579–585. doi: 10.1016/j.foodres.2008.04.006. [DOI] [Google Scholar]
- 22.Deepa G, Singh V, Naidu KA. A comparative study on starch digestibility, glycemic index and resistant starch of pigmented ('Njavara’ and ‘Jyothi') and a non-pigmented ('IR 64') rice varieties. J Food Sci Technol. 2010;47:644–649. doi: 10.1007/s13197-010-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frei M, Siddhuraju P, Becker K. Studies on the in vitro starch digestibility and the glycemic index of six different indigenous rice cultivars from the Philippines. Food Chem. 2003;83:395–402. [Google Scholar]
- 24.Higgins JA. Resistant Starch: Metabolic Effects and Potential Health Benefits. Journal of AOAC International. 2004;87:761–768. [PubMed] [Google Scholar]
- 25.Miller JB, Pang E, Bramall L. Rice: A high or low glycemic index food?”. Am J Clin Nutr. 1992;56:1034–1036. doi: 10.1093/ajcn/56.6.1034. [DOI] [PubMed] [Google Scholar]
- 26.Vesterinen E, Myllärinen P, Forssell P, Söderling E, Autio K. Structural properties in relation to oral enzymatic digestibility of starch gels based on pure starch components and high amylose content. Food Hydrocoll. 2002;16:161–167. [Google Scholar]
- 27.Htoon A, Shrestha AK, Flanagan BM, Lopez-Rubio A, Bird AR, Gilbert EP, et al. Effects of processing high amylose maize starches under controlled conditions on structural organization and amylase digestibility. Carbohydrate Polym. 2009;75:236–245. doi: 10.1016/j.carbpol.2008.06.016. [DOI] [Google Scholar]
- 28.Lopez-Rubio A, Flanagan BM, Shrestha AK, Gidley MJ, Gilbert EP. Molecular rearrangement of starch during in vitro digestion: Toward a better understanding of enzyme resistant starch formation in processed starches. Biomacromol. 2008;9:1951–1958. doi: 10.1021/bm800213h. [DOI] [PubMed] [Google Scholar]
- 29.Nebesny E, Rosicka J, Tkaczyk M. Influence of conditions of maize starch enzymatic hydrolysis on physicochemical properties of glucose syrups. Starch/Staerke. 2004;56:132–137. [Google Scholar]
- 30.Chung HJ, Lim HS, Lim ST. Effect of partial gelatinization and retrogradation on the enzymatic digestion of waxy rice starch. J Cereal Sci. 2006;43:353–359. doi: 10.1016/j.jcs.2005.12.001. [DOI] [Google Scholar]
- 31.AAAC, author. AACC adopts oat bran definition. [15th Novemberr 2010]. Available online: http://www.aaccnet.org/news/pdfs/OatBran.pdf.
- 32.The British Nutrition Foundation, author. [26/07/2015]. http://www.nutrition.org.uk/nutritionscience/nutrients/dietary-fibre.html.
- 33.James ML, Mark DH. Effects of Dietary Fiber and Its Components on metabolic health. Nutrients. 2010;2:1266–1289. doi: 10.3390/nu2121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slavin JL, Martini MC, Jacobs DR, Marquart L. Plausible mechanisms for the protectiveness of whole grains. Am J Clin Nutr. 1999;70:459S–463S. doi: 10.1093/ajcn/70.3.459s. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan S, Rosenberg L, Singer M, Hu FB, Djoussé L, Cupples LA, Palmer JR. Glycemic index, glycemic load, and cereal fibre intake and risk of T2DM in US black women. Arch Intern Med. 2007;167:2304–2309. doi: 10.1001/archinte.167.21.2304. [DOI] [PubMed] [Google Scholar]
- 36.Montonen J, Knekt P, Järvinen R, Aromaa A, Reunanen A. Whole-grain and fibre intake and the incidence of type 2 diabetes. Am J Clin Nutr. 2003;77:622–629. doi: 10.1093/ajcn/77.3.622. [DOI] [PubMed] [Google Scholar]
- 37.Babio N, Balanza R, Basulto J, Bullo M, Salas-Salvado J. Dietary fibre: influence on body weight, glycemic control and plasma cholesterol profile. Nutr Hosp. 2010;25:327–340. [PubMed] [Google Scholar]
- 38.Pereira MA, Jacobs DR, Jr, Pins JJ, Raatz SK, Gross MD, Slavin JL, Seaquist ER. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr. 2002;75:848–855. doi: 10.1093/ajcn/75.5.848. [DOI] [PubMed] [Google Scholar]
- 39.Weickert MO, Möhlig M, Schöfl C, Arafat AM, Otto B, Viehoff H, Koebnick C, Kohl A, Spranger J, Pfeiffer AF. Cereal fiber improves whole-body insulin sensitivity in overweight and obese women. Diabetes Care. 2006;29:775–780. doi: 10.2337/diacare.29.04.06.dc05-2374. [DOI] [PubMed] [Google Scholar]
- 40.Giacco R, Parillo M, Rivellese AA, Lasorella G, Giacco A, D'Episcopo L, Riccardi G. Long-term dietary treatment with increased amounts of fiber-rich low-glycemic index natural foods improves blood glucose control and reduces the number of hypoglycemic events in type 1 diabetic patients. Diabetes Care. 2000;23:1461–1466. doi: 10.2337/diacare.23.10.1461. [DOI] [PubMed] [Google Scholar]
- 41.Chandalia M, Garg A, Lutjohann D, Von Bergmann K, Grundy SM, Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med. 2000;342:1392–1398. doi: 10.1056/NEJM200005113421903. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins DJ, Kendall CW, Augustin LS, Martini MC, Axelsen M, Faulkner D, et al. Effect of wheat bran on glycemic control and risk factors for cardiovascular disease in type 2 diabetes. Diabetes Care. 2002;25:1522–1528. doi: 10.2337/diacare.25.9.1522. [DOI] [PubMed] [Google Scholar]
- 43.Meyer KA, Kushi LH, Jacobs DR, Jr, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr. 2000;71:921–930. doi: 10.1093/ajcn/71.4.921. [DOI] [PubMed] [Google Scholar]
- 44.American Diabetes Association. Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31:S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 45.Pi SFX. Glycemic index and disease. Am J Clin Nutr. 2002;76:290S–298S. doi: 10.1093/ajcn/76.1.264S. [DOI] [PubMed] [Google Scholar]
- 46.Tovar J, Melito C. Steam-cooking and dry heating produce resistant starch in legumes. J Agric Food Chem. 1996;44:2642–2645. [Google Scholar]
- 47.Alegbejo JA, Ameh DA, Ogala WN, Ibrahim S. Glycemic index of boiled cocoyam and stew. Sahel Med J. 2008;11:80–83. [Google Scholar]
- 48.Eleazu CO, Okafor PN, Ifeoma I. Biochemical basis of the use of cocoyam (Colocassia esculenta L.) in the dietary management of diabetes and its complications in streptozotocin induced diabetes in rats. Asian Pac J Trop Dis. 2014;4:S705–S711. doi: 10.1016/S2222-1808(14)60711-8. [DOI] [Google Scholar]
- 49.Dan RD, Renee LCI, Surujpa T, Thomas MSW. Glycaemic index of selected staples commonly eaten in the Caribbean and the effects of boiling v. crushing. British J Nutr. 2004;91:971–977. doi: 10.1079/bjn20041125. [DOI] [PubMed] [Google Scholar]
- 50.Bjorck I, Granfeldt Y, Liljeberg H, Tovar J, Asp NG. Food properties affecting the digestion and absorption of Carbohydrates. Am J Clin Nutr. 1994;59:6995–7055. doi: 10.1093/ajcn/59.3.699S. [DOI] [PubMed] [Google Scholar]
- 51.Roberts SB. High-glycemic index foods, hunger, and obesity: Is there a connection? Nutr Rev. 2000;58:163–169. doi: 10.1111/j.1753-4887.2000.tb01855.x. [DOI] [PubMed] [Google Scholar]
- 52.Goni I, Garcia-Alonso A, Saura-Calixto F. A starch hydrolysis procedure to determine glycemic index. Nutr Res. 1997;17:427–437. [Google Scholar]
- 53.Brand-Miller JC. Glycemic load and chronic disease. Nutr Rev. 2003;61:S49–S55. doi: 10.1301/nr.2003.may.S49-S55. [DOI] [PubMed] [Google Scholar]
- 54.Wolever TMS, Mehling C, Chiasson JL, Josse RG, Leiter LA, Maheux P, et al. Low glycaemic index diet and disposition index in type 2 diabetes (the Canadian trial of Carbohydrates in Diabetes): A randomized controlled trial. Diabetologia. 2008;51:1607–1615. doi: 10.1007/s00125-008-1093-x. [DOI] [PubMed] [Google Scholar]
- 55.Brand-Miller JC, Holt SHA, Pawlak DB, McMillan J. Glycemic index and obesity. Am J Clin Nutr. 2002;76:281S–285S. doi: 10.1093/ajcn/76/1.281S. [DOI] [PubMed] [Google Scholar]
- 56.Rudolf C, Josef Ba, Martina Ř, Jana Z, Blanka D, Ludmila C, et al. Determination of the glycaemic index of selected foods (white bread and cereal bars) in healthy persons. Biomed Papers. 2004;148:17–25. doi: 10.5507/bp.2004.003. [DOI] [PubMed] [Google Scholar]
- 57.Riley CK, Bahado-Singh PS, Wheatley AO, Ahmad MH, Asemota HN. “Relationship between the physicochemical properties of starches and the glycemic indices of some Jamaican yams (Dioscorea spp.),”. Molecular Nutrition and Food Res. 2008;52:1372–1376. doi: 10.1002/mnfr.200700461. [DOI] [PubMed] [Google Scholar]
- 58.Salmeron J, Ascherio A, Rimm EB. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997a;20:545–550. doi: 10.2337/diacare.20.4.545. [DOI] [PubMed] [Google Scholar]
- 59.Wheatley AO, Asemota HN, Morrison EY. Glycemic index: not all carbohydrates are created equal. West Indian Med J. 2002;51(Suppl. 60) [Google Scholar]
- 60.Gilbertson HR, Jennie C, Brand-Miller JC, Thorburn AW, Evans S, Chondros P, et al. The effect of flexible low glycemic index dietary advice versus measured carbohydrate exchange diets on glycemic control in children with type 1 diabetes. Diabetes Care. 2001;24:1137–1143. doi: 10.2337/diacare.24.7.1137. [DOI] [PubMed] [Google Scholar]
- 61.Wolever TMS, Mehling C. High-carbohydrate-lowglycaemic index dietary advice improves glucose disposition index in subjects with impaired glucose tolerance. British J Nutr. 2002;87:477–487. doi: 10.1079/BJNBJN2002568. [DOI] [PubMed] [Google Scholar]
- 62.Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997b;277:472–477. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- 63.Wolever TMS, Bolognesi C. Source and amount of carbohydrate affect postprandial glucose and insulin on normal subjects. J Nutr. 1996;126:2798–2806. doi: 10.1093/jn/126.11.2798. [DOI] [PubMed] [Google Scholar]
- 64.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, et al. Glycemic index, glycemic load, and chronic disease risk-a meta-analysis of observational studies. Am J Clin Nutr. 2008;87:627–637. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- 65.DeVries JW. Glycemic index: The analytical perspective. Cereal Foods World. 2007;52:45–49. doi: 10.1094/CFW-52-2-0045. [DOI] [Google Scholar]
- 66.Livesey G, Taylor R, Hulshof T, Howlett J. Glycemic response and health- a systematic review and meta-analysis: Relations between dietary glycemic properties and health outcomes. American Journal of Clin Nutr. 2008;87:258S–268S. doi: 10.1093/ajcn/87.1.258S. [DOI] [PubMed] [Google Scholar]
- 67.Liese AD, Schulz M, Fang F, et al. Dietary glycemic index and glycemic load, carbohydrate and fiber intake, and measures of insulin sensitivity, secretion, and adiposity in the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2005;28:2832–2838. doi: 10.2337/diacare.28.12.2832. [DOI] [PubMed] [Google Scholar]
- 68.McKeown NM, Meigs JB, Liu S, Saltzman E, Wilson PW, Jacques PF. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care. 2004;27:538–546. doi: 10.2337/diacare.27.2.538. [DOI] [PubMed] [Google Scholar]
- 69.VanDam RM, Visscher AW, Feskens EJ, Verhoef P, Kromhout D. Dietary glycemic index in relation to metabolic risk factors and incidence of coronary heart disease: the Zutphen Elderly Study. Eur J Clin Nutr. 2000;54:726–731. doi: 10.1038/sj.ejcn.1601086. [DOI] [PubMed] [Google Scholar]
- 70.Sahyoun NR, Anderson AL, Tylavsky AF, Lee SJ, Sellmeyer DE, Harris TB. Dietary glycemic index and glycemic load and the risk of type 2 diabetes in older adults. Am J Clin Nutr. 2008;87:126–131. doi: 10.1093/ajcn/87.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr. 2004;80:348–356. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- 72.Hodge AM, English DR, O'Dea K, Giles GG. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care. 2004;27:2701–2706. doi: 10.2337/diacare.27.11.2701. [DOI] [PubMed] [Google Scholar]
- 73.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 74.Sheard NF, Clark NG, Brand-Miller JC, et al. Dietary carbohydrate (amount and type) in the prevention and management of diabetes: a statement by the American Diabetes Association. Diabetes Care. 2004;27:2266–2271. doi: 10.2337/diacare.27.9.2266. [DOI] [PubMed] [Google Scholar]
- 75.Bantle JP, Wylie-Rosett J, Albright AL, et al. Nutrition recommendations and interventions for diabetes- 2006: a position statement of the American Diabetes Association. Diabetes Care. 2006;29:2140–2157. doi: 10.2337/dc06-9914. [DOI] [PubMed] [Google Scholar]
- 76.Rasmussen O, Gregersen S, Hermansen K. The predictive capability of the glycemic response to spaghetti in non-insulin dependent diabetic subjects. J Intern Med. 1990;228:97–101. doi: 10.1111/j.1365-2796.1990.tb00201.x. [DOI] [PubMed] [Google Scholar]
- 77.Klein O, Nosek L, Plate J, Landvogt A, Heise T. Determination of the glycaemic index in diet products: how to overcome the limitations of the classical approach; 18th Congress of the International Diabetes Federation 2013; 24–29 August; Paris, France. [Google Scholar]
- 78.Soh NL, Brand-Miller J. The glycaemic index of potatoes: the effect of variety, cooking method and maturity. Eur J Clin Nutr. 1999;53:249–254. doi: 10.1038/sj.ejcn.1600713. [DOI] [PubMed] [Google Scholar]
- 79.Terhi K, Antti S, Tiina V, Teija V, Katarina L. Measuring postmortem glycated hemoglobin - A comparison of three methods. Legal Medicine. 2013;15:72–78. doi: 10.1016/j.legalmed.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 80.American Diabetes Association, author. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]