Abstract
The term “lymphoma” describes a heterogeneous group of disorders involving monoclonal proliferation of malignant lymphocytes. As a group, lymphomas are among the most common tumors of dogs. Yet our enumeration and understanding of the many subtypes of lymphoma have been relatively slow, perhaps in part because for many years lymphoma was treated as a singular entity rather than a group of distinct diseases. The recognition that the full spectrum of lymphoid malignancies seen in humans also occurs in dogs, and that these tumors retain not only morphologic similarities and biological behavior but also synonymous driver molecular abnormalities, sets an ideal stage for dual-purpose research that can accelerate progress for these diseases in both species. Specifically, dogs represent exceptional models for defining causality, understanding progression, and developing new treatments for lymphoma in comparatively brief windows of time. Unique advantages of canine models include (1) spontaneous disease occurring without an isogenic background or genetic engineering; (2) chronology of disease adapted to lifespan, (3) shared environment and societal status that allows dogs to be treated as “patients,” while at the same time being able to ethically explore translational innovations that are not possible in human subjects; and (4) organization of dogs into breeds with relatively homogeneous genetic backgrounds and distinct predisposition for lymphomas. Here, we will review recent studies describing intrinsic and extrinsic factors that contribute to the pathogenesis of canine and human lymphomas, as well as newly developed tools that will enhance the fidelity of these models to improve diagnosis and develop new treatments.
2. Introduction
Non-Hodgkin lymphomas (NHL) are a heterogeneous group of relatively common human lymphoid malignancies that are increasing in incidence; the age-adjusted incidence of NHL has almost doubled in the past 30 years, with an estimated 70,130 new cases and 18,940 deaths in the UNITED STATES 2012 (Howlader et al., 2012). In Western countries, more than 80% of NHLs are mature B-cell tumors (Anon, 1997). The addition of Rituximab (chimeric anti-CD20) to multi-agent cytotoxic chemotherapy has significantly improved outcomes for patients with these B-cell malignancies (Traullé and Coiffier, 2005); however, causes for most subtypes of NHL remain unknown, therapeutic gains fail to extend life for all patients, and cures are elusive.
Lymphomas are among the most common types of tumors in dogs. There are remarkable similarities between the clinical features of canine lymphomas and human NHL, making the former attractive models for studying disease progression and therapy (reviewed in (Marconato et al., 2012)). Here, we will focus our attention on the molecular pathogenetic features of canine lymphomas, underscoring the concept that canine lymphomas also represent a heterogeneous group of diseases, and thus, each subtype is likely to best inform its unique disease homolog among human NHLs.
3. The Natural History of Canine Lymphomas
The data used to estimate the incidence of lymphomas in dogs in the United States date back to the 1960’s. In the most widely cited study, Dorn reported that lymphomas accounted for ~6% of all malignancies in dogs in Alameda and Contra Costa Counties, CA (Dorn et al., 1968), and for ~90% of all hematopoietic cancers (Dorn et al., 1967). The incidence was estimated at 24 cases per 100,000 dog at risk (Dorn et al., 1967), which is slightly higher than the contemporary incidence rate reported for humans by the SEER program (19.6 per 100,000) (Howlader et al., 2012). More recent studies have estimated the incidence of canine lymphoma at 20 – 107 cases per 100,000, supporting the conclusion that the incidence of this group of diseases is higher in dogs than in humans (Dobson et al., 2002; Edwards et al., 2003; Merlo et al., 2008; Teske, 1994).
Dorn’s work (Dorn et al., 1967), a large comprehensive survey of the Veterinary Medical Database in the United States and Canada by Priester and McKay in the late 1970’s (Priester and McKay, 1980), and reports from hospital records and insurance databases in the United Kingdom and the Netherlands (Edwards et al., 2003; Teske, 1994) showed that certain dog breeds had statistically significant increased risk to develop lymphoma as compared to the average risk of all dogs. This suggests that heritable risk factors for the disease were introduced with the derivation of specific breeds. Other evidence supporting the existence of strongly embedded heritable factors for this disease in dogs could be inferred by familial clustering observed in Bullmastiff, Rottweiler, and Scottish Terrier lines (Onions, 1984; Teske et al., 1994). It is possible, however, that the demographics, incidence, and dynamics of the canine disease may have changed between then and now (Merlo et al., 2008; Ritt, 2010).
The introduction of multi-agent chemotherapy protocols as the standard of care for canine lymphoma in the 1980’s and 1990’s made this disease treatable, if not curable. Without treatment, dogs diagnosed with high-grade lymphoma generally survive less than 6 weeks, but survival increases progressively with chemotherapy: about 50% of dogs with high-grade lymphoma treated with multi-agent chemotherapy will stay in remission 7 – 10 months and will survive 10 – 14 months (Garrett et al., 2002; Keller et al., 1993; MacDonald et al., 2005). Breed type also might influence disease progression and response to therapy; for example, in one study, German Shepherd Dogs showed worse survival compared to other hospital populations (Garrett et al., 2002).
4. Pathological Classification of Canine Lymphoma According to the World Health Organization
The classification of human NHL into distinct subtypes has evolved over the past five decades to achieve a worldwide consensus in the latest World Health Organization (WHO) classification (Swerdlow et al., 2008). This classification utilizes morphology, topography, immunophenotype, and clinical progression to define approximately 30 distinct subtypes of human NHL; however, it is not static and continues to be refined. Work that attempted to develop a similar classification of canine lymphomas (Valli, 2002) was initially met with more resistance, but a modified WHO classification for canine lymphomas was recently published (Valli et al., 2011) and is slowly gaining acceptance.
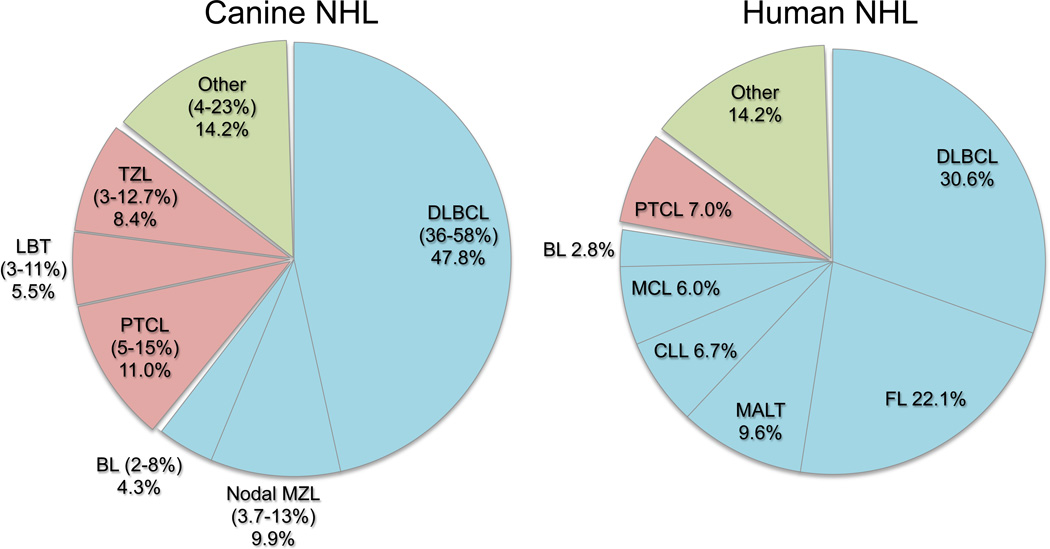
A careful parallel assessment of the distribution of disease between both species is instructive to illustrate the strengths of the canine model (Figure 1). Specifically, diffuse large B-cell lymphoma (DLBCL) is the most commonly seen subtype in both species, but among the other five common subtypes that occur in dogs (Frantz et al., 2012; Ponce et al., 2010; Thomas et al., 2011; Valli et al., 2011), marginal zone lymphoma (MZL), peripheral T-cell lymphoma not otherwise specified (PTCL), nodal T-zone lymphoma (TZL), and lymphoblastic T-cell lymphoma (LBT) are quite rare in humans (Anon, 1997), providing resources that can help us to better understand these diseases at the cellular level.
Figure 1.
Relative distribution of common subtypes of canine lymphoma (Frantz et al., 2012; Ponce et al., 2010; Thomas et al., 2011; Valli et al., 2011) and human NHL (Anon, 1997). DLBCL; diffuse large B-cell lymphoma, MZL; marginal zone lymphoma, BL; Burkitt lymphoma, PTCL; peripheral T cell lymphoma not otherwise specified, TZL; nodal T-zone lymphoma, LBT; lymphoblastic T-cell lymphoma, FL; follicular lymphoma, MALT; mucosa associated lymphoid tissue lymphoma (extranodal MZL), CLL; chronic lymphocytic leukemia, MCL; mantle cell lymphoma.
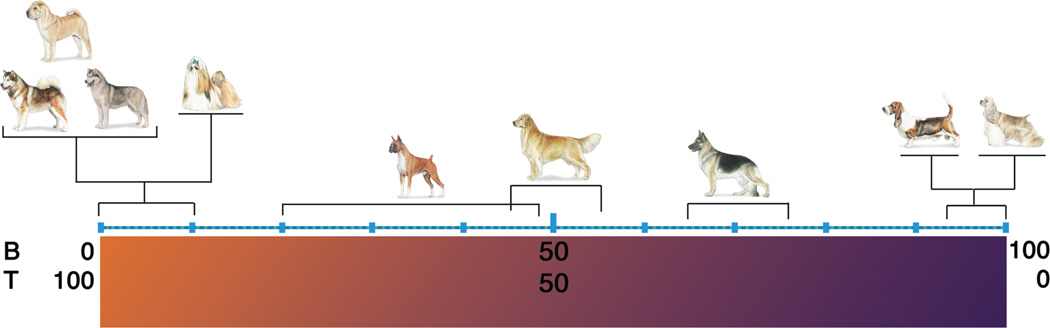
It is worth noting that there appears to be a peculiar predilection for some dog breeds to develop certain subtypes of lymphoma. Overall, T-cell lymphomas account for about 35–40% of all lymphoma diagnoses in dogs (Modiano et al., 2005). However, this prevalence might be disproportionately influenced by heritable traits. Some modern Spitz (Northern) and Asian breeds develop T-cell lymphomas almost exclusively, whereas some modern European breeds develop B-cell lymphomas almost exclusively (Figure 2). Golden Retrievers and Boxers, two breeds with a high incidence of lymphoma (Dorn et al., 1967; Glickman et al., 1999; Priester and McKay, 1980), also show statistically different distributions of B-cell lymphomas and T-cell lymphomas when compared with the average for all dogs (Modiano et al., 2005). Somewhat predictably, the distribution of B-cell lymphomas and T-cell lymphomas in mixed breed dogs is comparable to that seen when all purebred dogs are considered as a single group (Modiano et al., 2005). This suggests that distinct factors that modulate lymphoma risk have been enriched during the process of breed derivation, providing unique opportunities to identify these traits through contemporary genome- wide studies (Tonomura et al., 2012). Furthermore, each breed is likely to reflect a small segment of outbred human (and mixed breed canine) populations; thus, the reduced genetic heterogeneity seen in dog breeds will help to lower the background noise of genome-wide approaches to identify the frequency of homologous risk factors (mutations) that are etiologically significant in both species.
Figure 2.
Breed-specific distribution of B-cell and T-cell lymphomas in dogs. The prevalence of B-cell-derived and T-cell derived tumors in all dogs (considered as a single group) are ~65% and ~35%, respectively (Modiano et al., 2005). The incidence of B-cell tumors and T-cell tumors in most dogs and mixed breed dogs falls within these ranges (shown by German Shepherd Dog in this figure). In contrast, a prevalence of excess B-cell tumors and T-cell tumors has been shown in certain breeds. Reproduced with permission from (Modiano et al., 2006).
5. Comparative Molecular Pathogenesis of Human NHL and Canine Lymphomas
5a. Shared Molecular Aberrations
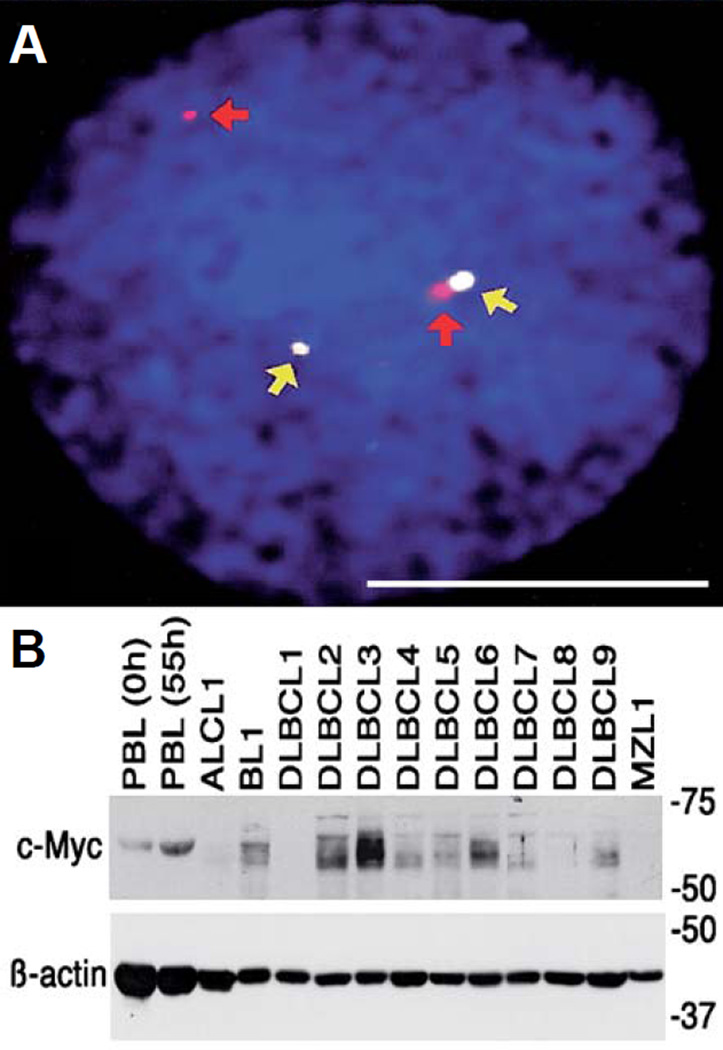
Previous work has shown that shared pathognomonic molecular aberrations occur in some human and canine hematological malignancies. For example, Burkitt lymphoma (BL) in humans is characterized by a balanced translocation, whereby a region of human chromosome 8 (HSA 8q24) containing the MYC oncogene is juxtaposed on the region of chromosome 14 that contains the immunoglobulin heavy-chain (IgH) enhancer (HSA 14q32). This leads to constitutive MYC expression in B-cells, contributing to malignant transformation. In the sporadic disease, this translocation seems to occur stochastically. In the endemic form of BL, the translocation may be driven by instability associated with proliferation induced by Epstein-Barr virus (EBV) infection, and it potentiates EBV-associated lymphomagenesis (Ferry, 2006). In the dog, a corresponding MYC-IgH translocation was characterized where MYC from canine chromosome (CFA) 13 was juxtaposed with the IGH locus on CFA 8 (Figure 3) in sporadic BL (Breen and Modiano, 2008).
Figure 3.
Conserved cytogenetic rearrangement in canine Burkitt lymphoma. (A) Interphase tumor cell from a dog with BL showing heterozygous co-localization of a canine BAC clone containing the MYC gene (white spots indicated by yellow arrows) and a BAC clone that maps to the same cytogenetic band as the IgH locus (red spots, identified by red arrows). Scale bar = 5 µm. (B) Myc expression in non-stimulated normal canine peripheral blood lymphocytes (PBL) (0 h), mitogen-stimulated PBL (55 h), or lymph node cells from dogs with anaplastic large cell lymphoma (ALCL), BL, DLBCL (1–9) or nodal MZL. Beta-actin was used as a loading control. Reproduced with permission from (Breen and Modiano, 2008).
5b. Viral Etiologies in Lymphoma
Various types of endemic (transmissible) lymphoma have been described in humans. As noted above, endemic Burkitt lymphoma is associated with EBV infection, although a superimposed infection with the malarial parasite Plasmodium falciparum appears to be necessary for the complete manifestation of the malignant phenotype in this disease (Ferry, 2006). Along with EBV, the Human T-lymphotropic viruses (HTLV) HTLV-I and HTLV-II also are associated with endemic forms of T-cell lymphoma (Müller et al., 2004). There are few reports of canine lymphotropic retroviruses associated with leukemia and lymphoma (Safran et al., 1992; Tomley et al., 1983). Recently, a possible gamma herpes virus-like agent also was observed in five cases of canine B-cell lymphoma, (Huang et al., 2012). However, the significance of viral agents in the pathogenesis of canine lymphoma remains to be determined, as Koch’s postulates have not been fulfilled through experimental transmission and there is no epidemiological evidence to suggest transmissibility in canine lymphomas.
5c. Comparative Cytogenetics – Heritable Traits and Gene Dosage
Human NHLs show relatively high levels of molecular diversity, consistent with their morphological diversity. Recurrent DNA copy number aberrations (CNA) exceed the frequency expected from normal copy number variability (Alvarez and Akey, 2012), but even within each WHO subtype, there is sufficient heterogeneity such that CNAs that are fundamentally associated with disease pathogenesis have remained largely elusive (Thomas et al., 2011).
CNAs are also relatively common in canine lymphomas, albeit the genomic imbalance in dogs is reduced compared to that seen in human NHL (Thomas et al., 2011). Breen et al have proposed that the confounding effects of genomic heterogeneity in domestic dogs are limited as a consequence of the development of distinct and genetically isolated breeds, and thus detection of genetic factors that are intimately associated with tumor pathogenesis may be more penetrant, even if less frequent (Thomas et al., 2011). Several evolutionarily conserved CNAs were identified in canine lymphoma and human NHL using an innovative “genomic recoding” model, which organizes the genomes of different species according to syntenic blocks. The frequency of canine T-cell lymphomas may be especially useful in defining recurrent, pathogenetically significant CNAs. What is more, the observed breed bias may be especially informative in defining the possible role of heritable traits in genomic instability, as reflected by aneuploidy, and in tumor-specific alterations in gene dosage.
5d. Gene Expression Profiling
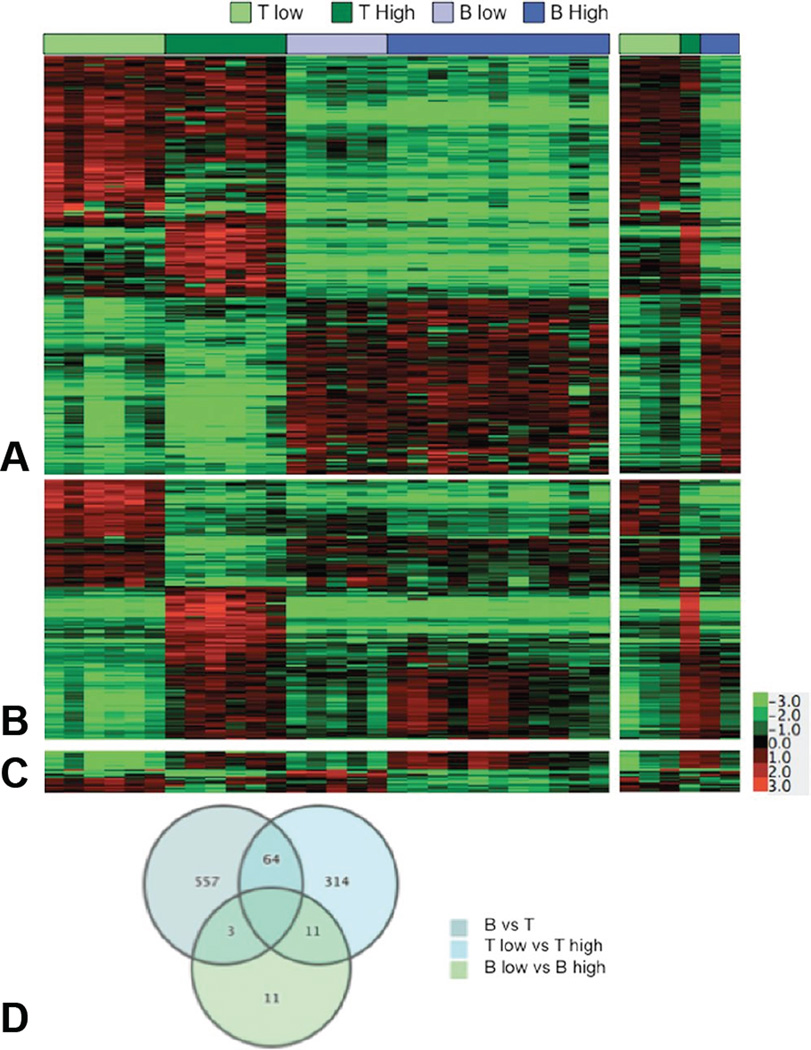
Gene expression profiling is another genome-wide method that has been applied to categorize NHLs based on their molecular features. This approach has been used most extensively in DLBCL, where a molecular classification is widely accepted (Alizadeh et al., 2000; Lossos et al., 2004; Rosenwald et al., 2002; Shipp et al., 2002). Recently, we reported that canine DLBCL, BL, MZL, LBT, PTCL, and TZL can be organized into three distinct molecular subgroups consisting of high-grade T-cell lymphomas (LBT, PTCL), low-grade T-cell lymphomas (TZL), and B-cell lymphomas (DLBCL, BL, and MZL) (Figure 4) (Frantz et al., 2012). Our data also suggest that DLBCL and nodal MZL may exist as a continuum of the same disease, with MZL representing a possibly distinct subgroup in both dogs and humans (Frantz et al., 2012). In the case of canine lymphomas, this molecular classification was prognostic and could be done using relatively simple quantitative real-time RT-PCR assays, which should make them translatable to more conventional diagnostic platforms (Frantz et al., 2012).
Figure 4.
Statistically significant genes define molecular subtypes of canine lymphoma. Genes differentially expressed with >3-fold average change and P values <0.001 were identified for the comparison of groups composed of (A) B-cell and T-cell lymphomas (n = 624), (B) high-grade and low-grade T-cell lymphomas (n = 389), and (C) high-grade and low-grade B-cell lymphomas (n = 25) using t test statistics. The second panel of (A–C) is an independent “validation” set (6 samples, right inset) of the results obtained in the initial set (29 samples, left panel). (D) Venn diagram showing the number of unique and overlapping genes for each 2-group test. Reproduced with permission from (Frantz et al., 2012).
Our data did not support the organization of canine DLBCL into molecular groups that resemble human activated B-cell (ABC) DLBCL and germinal center-like B-cell (GCB) DLBCL. This could reflect the heterogeneity that is intrinsic to DLBCL as the largest single group of lymphoma in both species, or it could suggest that canine DLBCL represents only one subtype (a possibility that would be challenging to confirm in studies with small sample size and in the absence of the respective comparison group). As far as we know, there is no report that satisfies the criteria for subtyping ABC and GCB DLBCL in dogs. At present, we would caution investigators to avoid using canine lymphoma to model specifically ABC or GCB DLBCL subtypes.
6. Model Systems to Study the Biology of Canine Lymphoma
6a. In vitro culture of primary B-cell lymphomas
In general, hematologic malignancies have proven substantially less tractable for in vitro manipulation than their solid tumor counterparts. Unlike normal lymphoid cells that can survive for days to weeks in culture and can be manipulated to divide synchronously, primary lymphoma cells rarely survive >24–48 hours in culture, with or without exogenous mitogens. Canine B-cell lymphomas are particularly unstable ex vivo, and indeed only one canine B-cell lymphoma cell line exists that has consistently retained its in vivo phenotype with high fidelity after repeated passage (Rütgen et al., 2010; Rütgen et al., 2012).
Nevertheless, the utility of human lymphoma cell lines that are available to the scientific community is evident in the countless citations by investigators who have used them as resources to define signaling pathways in lymphocyte activation and lymphocyte development and, to some extent, in therapeutic development. Even so, these cell lines represent only a sliver of the diversity seen both within and among tumors. Therefore, the development of reliable methods to culture primary lymphomas in vitro and in vivo would provide a useful resource for preclinical studies. To this end, we adapted the “CD40 system,” which was described as an effective method to maintain human B-cells in vitro (Andersen et al., 2000; Planken et al., 1996; Visser et al., 2000), to grow primary canine B-cell lymphoma cells in short-term and extended culture (Ito et al., 2012). We showed that CD40 signals can be delivered by feeder cells expressing CD40 ligand (CD40L, a member of the tumor necrosis factor family) (Mason et al., 2008) or in a cell-free culture system using soluble, trimeric CD40L. Canine B-cell lymphomas grown under these conditions retained their original phenotype, clonality, and known karyotypic abnormalities even after extended periods of expansion. The cell-free system was especially useful to assess targeted reagents against canine and human B-cell malignancies, highlighting its potential applications for preclinical development.
The observation that CD40 signaling supports growth of primary malignant canine B-cell lymphoma cells in culture is consistent with the notion that this pathway plays an important role in the oncogenic maintenance of B-cell malignancies. It is well established that the interaction of CD40L with CD40 promotes activation of nuclear factor kappa B (NFκB) transcription factors, which in turn promote survival of human and canine B-cell lymphoma cells (Challa et al., 2002; Davis et al., 2001; Gaurnier-Hausser et al., 2011; Pham et al., 2002), thus providing a series of rational targets for therapeutic development.
6b. Xenotransplantation models of primary B-cell lymphomas
In vivo experiments have been essential to understand the effects of potential therapies in complex systems that include the supporting tumor microenvironment. Xenotransplantation of human lymphoma cell lines into receptive, immunodeficient mice has been a powerful tool for preclinical development, and similar models are reported for two canine lymphoma cell lines (Kisseberth et al., 2007; Rütgen et al., 2012). However, the use of cell lines for in vivo xenotransplantation is subject to the same limitations regarding incomplete representation of tumor diversity, and most attempts to transplant primary B-cell lymphomas into mice have been unrewarding. In what is probably the most comprehensive study to address this, Mori and colleagues showed that only 10 of 50 human NHL tumors (and specifically 8 of 30 derived from B-cell NHL) injected into mice with severe combined immunodeficiency engrafted successfully based on the retention of phenotypic and genotypic properties of the primary tumors (Itoh et al., 1993). The other 13 engrafted tumors were found to be newly developed clones composed of EBV-transformed B-cells (Itoh et al., 1993). Two important limitations are apparent from this study; (1) only a limited number of human NHLs were able to engraft successfully in immunodeficient mice, and (2) the high prevalence of EBV infection in human populations will remain a persistent challenge to establish primary human lymphoma xenograft models.
As described above, EBV infection is unlikely to present a problem in development of xenotransplantation of canine lymphomas. So, we chose a different mouse model to enhance the rate of engraftment, using NOD/SCID/IL-2Rgnull (NSG) mice as recipients. These mice show improved engraftment of human hematopoietic stem cells over other mouse strain (Shultz et al., 2005), and in one study, they were used successfully to engraft a primary human follicular lymphoma and a DLBCL (Chao et al., 2010). We also added a conditioning step to improve engraftment. In our initial experiments, we noted that residual T-cells present in canine B-cell lymphomas caused severe graft-versus-host disease. But increasing the stringency of depletion was sufficient to overcome this problem and produced reliable, serially transplantable canine B-cell lymphoma xenografts (Figure 5) (Ito et al., 2011). In our experience, canine T-cell lymphomas also engraft with low frequency, but the conditioned NSG system may be sufficiently robust to overcome this challenge. Altogether, models using xenotransplantation of primary tumors will offer better models for assessing preclinical efficacy, especially when used as part of comparative approaches where primary tumor cells from humans and dogs are analyzed side by side.
Figure 5.
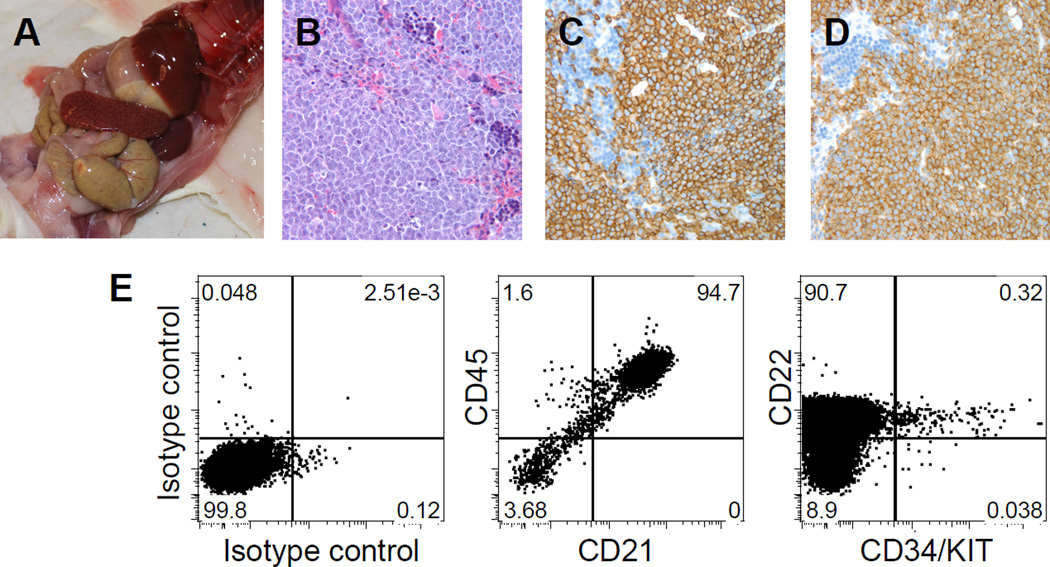
Tumor engraftment of primary canine B-cell lymphoma in NSG mice. (A) Photomicrograph showing splenomegaly in an NSG recipient at autopsy. (B) Photomicrograph of diffuse infiltration of tumor cells in spleen. (C and D) Immunostaining of the donor (dog) cells for expression of canine CD20 (C) and CD79a (D). (E) Spleen cells of a secondary NSG recipient are virtually all canine B-cells, expressing CD21, CD22, and CD45, analyzed by multi-parameter flow cytometry. A small population of hematopoietic progenitor antigens CD34 and KIT positive cells is found within the CD22+ B-cell tumor population. Reproduced with permission from (Ito et al., 2011).
7. Tumor-Initiating Cells in Lymphoma
The importance of cancer stem cells or tumor-initiating cells (TICs) in the pathogenesis of cancer is becoming increasingly well recognized (Nguyen et al., 2012). However, there are only a few reports supporting the existence of TICs in human lymphoma cell lines or in transgenic lymphoma mouse models (Lee et al., 2012; Vega et al., 2009; Wang et al., 2011). Our group identified a putative lymphoma-initiating cell (Ly-IC) population in primary canine B-cell lymphomas, which was characterized by co-expression of hematopoietic progenitor antigens CD34, c-Kit, and CD133, the lymphoid lineage marker CD22, and the common leukocyte antigen CD45 (Figure 6A) (Ito et al., 2011). When compared with the bulk of the tumor cells (BTCs), the putative Ly-IC population (enriched for CD34 expression) showed significantly lower expression of 44 genes across the genome that mapped to “Cell Cycle” and to “Membrane and Raft proteins” pathways using Ingenuity Pathway Analysis (Figure 6B). This suggests that Ly-ICs may exhibit the characteristic “slow proliferation” that is seen in normal bone marrow-derived hematopoietic stem cells. Importantly, the putative Ly-IC population persisted in the xenotransplantation setting (Figure 5), suggesting it is relevant to the biology of this disease in vivo (Ito et al., 2011).
Figure 6.
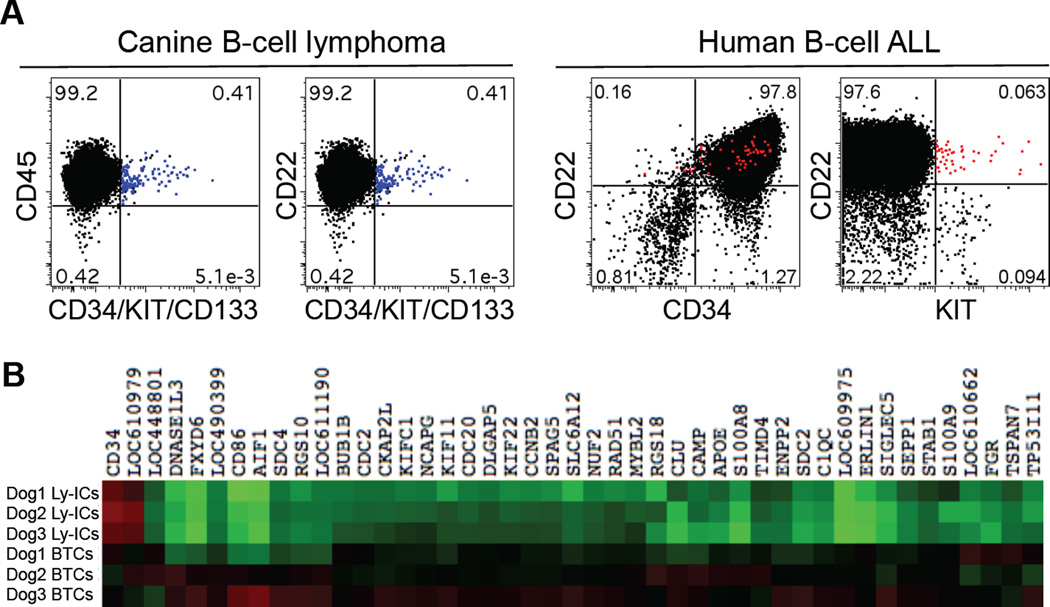
Putative TICs in primary canine B-cell lymphomas and human B-cell ALLs. (A) Existence of a putative Ly-IC population (CD22+CD34/KIT/CD133+; blue dots) in primary canine B-cell lymphoma samples (n = 24) and a putative TIC population (CD22+KIT+; red dots) in primary human B-cell ALL samples (n = 2) were shown using multi-parameter flow cytometry. Reproduced with permission from (Ito et al., 2011). (B) Heat map showing 44 differentially expressed transcripts between enriched Ly-ICs and BTCs (greater than 2-fold change by a two group T-test, p <0.05). Heat map colors represent median-centered fold change expression in log-space. Up-regulated genes are shown in red and down-regulated genes are shown in green.
The existence of TICs in other lymphoid malignancies is similarly controversial (Bernt and Armstrong, 2009). Several groups have reported putative TICs in acute lymphoblastic leukemia (ALL) based on co-expression of CD34, CD133, and CD19 (Castor et al., 2005; Cox et al., 2009; Cox et al., 2004; Hotfilder et al., 2002; le Viseur et al., 2008). We identified an Ly-IC-like population in human B-cell ALL that co-expressed KIT, CD19, and CD22 (Figure 6A), which may be equivalent to the canine B-cell Ly-ICs we described above. Additional work is needed to establish the significance of TICs in the pathogenesis of lymphoid malignancies of humans and dogs. However, if the hierarchical model applies to these tumors, it will be imperative to develop strategies that can target the TIC compartment in order to improve the outcome of patients with these tumors.
8. Canine Lymphoma in Pre-clinical Development
One of the major advantages of working with canine lymphomas is our capacity to utilize these tumors as spontaneous models of NHL for pre-clinical development. The capability of achieving rapid accrual and the potential benefit afforded by the shorter chronology of disease have been described in detail elsewhere (Khanna et al., 2006; Marconato et al., 2012; Paoloni and Khanna, 2008). As noted above, however, the capacity of these models to inform the process of pre-clinical development will require rigorous proof of anatomic, morphologic, phenotypic, and molecular homology to ensure that the canine disease is comparable to the subtype of NHL that is being modeled.
In their recent review of this topic ((Marconato et al., 2012), Comazzi and colleagues summarized recent drug trials that demonstrated safety and efficacy for treatment of canine lymphoma, including GS-9219 (a prodrug of the nucleotide analogue 9-(2-phosphonylmethoxyethyl) guanine), ABT526 (a modified thrombospondin-I peptide), and a NEMO-binding domain peptide that acts as an inhibitor of the NFκB pathway. Other contemporary trials include documentation of Btk target modulation by the small molecule inhibitor PCI-32765 (Honigberg et al., 2010) and tolerability of a pro-caspase-3 activating compound (Peterson et al., 2010).
Data from several multi-institutional efforts to develop new reagents and protocols also are reaching maturity. One double-blinded, placebo-controlled study examined safety and efficacy of neoadjuvant PSC-833 (Novartis, Inc.), a selective inhibitor of the ATP binding cassette B1 transporter (ABCB1, a.k.a., p-glycoprotein/multidrug resistance protein-1), in dogs with therapy-naïve DLBCL. This study was designed to test the hypothesis that modulation of ABCB1 activity in Ly-ICs by PSC-833 would sensitize these to doxorubicin-induced cytotoxicity and thus lengthen remission intervals. Interim data analyses showed a trend towards delayed progression in the PSC-833 group compared to placebo, and significantly delayed progression associated with stabilization or reduction in the percent of Ly-ICs in lymph nodes at the end of the neoadjuvant therapy period (Ito et al., 2013). Another study examined the therapeutic potential of KPT-335 (Karyopharm Therapeutics, Inc.), an orally available selective inhibitor of nuclear export (SINE) that binds exportin 1 (a.k.a., CRM1). SINE compounds inhibit export of tumor suppressors and other proteins, which in turn leads to selective death of tumor cells in vitro. This study identified a maximum tolerated dose for KPT-335, with disease stabilization and tumor reduction when used as a single agent, in dogs with relapsed DLBCL (Schacam et al., 2012).
9. Future directions
There has been remarkable progress in the development of new therapies that can improve or replace traditional chemotherapy protocols for NHL. Perhaps the best example is rituximab, which revolutionized treatment for most B-cell malignancies (Traullé and Coiffier, 2005); however, there is a wide array of additional promising reagents in the FDA pipeline, including Ibrutinib (PCI-32765) (Wiestner, 2013), Bortezomib (a proteasome/NFκB inhibitor) (Mato et al., 2012), Brentuximab vedotin (a CD30-specific antibody-drug conjugate) (Deng et al., 2013), and others. We are particularly enthusiastic about a new multi-institutional effort using CD47 blockade (Chao et al., 2010) and a new anti-canine CD20 antibody to model development of this combinatorial approach for DLBCL and nodal MZL.
In summary, we advocate setting a high bar where spontaneous canine lymphomas are used judiciously to model homologous subtypes of human NHL. Careful attention to anatomic, morphologic, molecular, and clinical features of these diseases can accelerate discovery and improve the efficiency of translation, ultimately benefiting dogs and humans, and addressing unmet medical needs.
Acknowledgments
The authors would like to thank Ms. Mitzi Lewellen and Ms. Ashley Graef for editorial assistance. We also wish to acknowledge the assistance of Dr. Aaron Sarver (University of Minnesota) for microarray data analysis. This work was supported in part by MAF First Award Grant D12CA-302 (DI), Morris Animal Foundation D13CA-033 (JFM), Masonic Cancer Center Internal Grants Program - Hematologic Malignancy Innovations Award (JFM), Skippy Frank Fund for Life Sciences and Translational Research (DI and JFM), the Starlight Fund, The Land of PureGold Foundation, the WillPower Fund, and other philanthropic donations to the University of Minnesota Animal Cancer Care and Research Program. AMF was supported by the DVM/PhD combined degree program of the College of Veterinary Medicine, University of Minnesota and by a pre-doctoral fellowship from Morris Animal Foundation (D09CA-405).
References
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- Alvarez CE, Akey JM. Copy number variation in the domestic dog. Mammalian Genome. 2012;23:144–163. doi: 10.1007/s00335-011-9369-8. [DOI] [PubMed] [Google Scholar]
- Andersen NS, Larsen JK, Christiansen J, Pedersen LB, Christophersen NS, Geisler CH, Jurlander J. Soluble CD40 ligand induces selective proliferation of lymphoma cells in primary mantle cell lymphoma cell cultures. Blood. 2000;96:2219–2225. [PubMed] [Google Scholar]
- Anon A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The non-Hodgkin's lymphoma classification project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- Bernt KM, Armstrong SA. Leukemia stem cells and human acute lymphoblastic leukemia. Semin Hematol. 2009;46:33–38. doi: 10.1053/j.seminhematol.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen M, Modiano JF. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans – man and his best friend share more than companionship. Chromosome Res. 2008;16:145–154. doi: 10.1007/s10577-007-1212-4. [DOI] [PubMed] [Google Scholar]
- Castor A, Nilsson L, Astrand-Grundström I, Buitenhuis M, Ramirez C, Anderson K, Strömbeck B, Garwicz S, Békássy AN, Schmiegelow K, Lausen B, Hokland P, Lehmann S, Juliusson G, Johansson B, Jacobsen SEW. Distinct patterns of hematopoietic stem cell involvement in acute lymphoblastic leukemia. Nat Med. 2005;11:630–637. doi: 10.1038/nm1253. [DOI] [PubMed] [Google Scholar]
- Challa A, Eliopoulos AG, Holder MJ, Burguete AS, Pound JD, Chamba A, Grafton G, Armitage RJ, Gregory CD, Martinez-Valdez H, Young L, Gordon J. Population depletion activates autonomous CD154-dependent survival in biopsylike Burkitt lymphoma cells. Blood. 2002;99:3411–3418. doi: 10.1182/blood.v99.9.3411. [DOI] [PubMed] [Google Scholar]
- Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, Jan M, Cha AC, Chan CK, Tan BT, Park CY, Zhao F, Kohrt HE, Malumbres R, Briones J, Gascoyne RD, Lossos IS, Levy R, Weissman IL, Majeti R. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CV, Diamanti P, Evely RS, Kearns PR, Blair A. Expression of CD133 on leukemia-initiating cells in childhood ALL. Blood. 2009;113:3287–3296. doi: 10.1182/blood-2008-04-154187. [DOI] [PubMed] [Google Scholar]
- Cox CV, Evely RS, Oakhill A, Pamphilon DH, Goulden NJ, Blair A. Characterization of acute lymphoblastic leukemia progenitor cells. Blood. 2004;104:2919–2925. doi: 10.1182/blood-2004-03-0901. [DOI] [PubMed] [Google Scholar]
- Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194:1861–1874. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Pan B, O'Connor OA. Brentuximab vedotin. Clin Cancer Res. 2013;19:22–27. doi: 10.1158/1078-0432.CCR-12-0290. [DOI] [PubMed] [Google Scholar]
- Dobson JM, Samuel S, Milstein H, Rogers K, Wood JL. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J Small Anim Pract. 2002;43:240–246. doi: 10.1111/j.1748-5827.2002.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Dorn CR, Taylor DO, Hibbard HH. Epizootiologic characteristics of canine and feline leukemia and lymphoma. Am J Vet Res. 1967;28:993–1001. [PubMed] [Google Scholar]
- Dorn CR, Taylor DO, Schneider R, Hibbard HH, Klauber MR. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J Natl Cancer Inst. 1968;40:307–318. [PubMed] [Google Scholar]
- Edwards DS, Henley WE, Harding EF, Dobson JM, Wood JL. Breed incidence of lymphoma in a UK population of insured dogs. Vet Comp Oncol. 2003;1:200–206. doi: 10.1111/j.1476-5810.2003.00025.x. [DOI] [PubMed] [Google Scholar]
- Ferry JA. Burkitt's lymphoma: clinicopathologic features and differential diagnosis. Oncologist. 2006;11:375–383. doi: 10.1634/theoncologist.11-4-375. [DOI] [PubMed] [Google Scholar]
- Frantz AM, Sarver AL, Ito D, Phang TL, Karimpour-Fard A, Scott MC, Valli VEO, Lindblad-Toh K, Burgess KE, Husbands BD, Henson MS, Borgatti A, Kisseberth WC, Hunter LE, Breen M, O'Brien TD, Modiano JF. Molecular Profiling Reveals Prognostically Significant Subtypes of Canine Lymphoma. Vet Pathol. 2012 doi: 10.1177/0300985812465325. (published online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett LD, Thamm DH, Chun R, Dudley R, Vail DM. Evaluation of a 6-month chemotherapy protocol with no maintenance therapy for dogs with lymphoma. J Vet Intern Med. 2002;16:704–709. doi: 10.1892/0891-6640(2002)016<0704:eoacpw>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Gaurnier-Hausser A, Patel R, Baldwin AS, May MJ, Mason NJ. NEMO-binding domain peptide inhibits constitutive NF-κB activity and reduces tumor burden in a canine model of relapsed, refractory diffuse large B-cell lymphoma. Clin Cancer Res. 2011;17:4661–4671. doi: 10.1158/1078-0432.CCR-10-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman L, Glickman N, Thorpe R. The Golden Retriever Club of America National Health Survey 1998–1999. The Golden Retriever Club of America National Health Survey. 1999 Available online at http://www.grca.org/health/reports.html. [Google Scholar]
- Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, Buggy JJ. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotfilder M, Röttgers S, Rosemann A, Jürgens H, Harbott J, Vormoor J. Immature CD34+CD19− progenitor/stem cells in TEL/AML1-positive acute lymphoblastic leukemia are genetically and functionally normal. Blood. 2002;100:640–646. doi: 10.1182/blood.v100.2.640. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA. Bethesda, MD: National Cancer Institute; 2012. Apr, SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site. [Google Scholar]
- Huang S-H, Kozak PJ, Kim J, Habineza-Ndikuyeze G, Meade C, Gaurnier-Hausser A, Patel R, Robertson E, Mason NJ. Evidence of an oncogenic gammaherpesvirus in domestic dogs. Virology. 2012;427:107–117. doi: 10.1016/j.virol.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Endicott MM, Jubala CM, Helm KM, Burnett RC, Husbands BD, Borgatti A, Henson MS, Burgess KE, Bell JS, Kisseberth WC, Valli VE, Cutter GR, Avery AC, Hahn KA, O'Brien TD, Modiano JF. A tumor-related lymphoid progenitor population supports hierarchical tumor organization in canine B-cell lymphoma. J Vet Intern Med. 2011;25:890–896. doi: 10.1111/j.1939-1676.2011.0756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Frantz AM, Williams C, Thomas R, Burnett RC, Avery AC, Breen M, Mason NJ, O'Brien TD, Modiano JF. CD40 ligand is necessary and sufficient to support primary diffuse large B-cell lymphoma cells in culture: a tool for in vitropreclinical studies with primary B-cell malignancies. Leuk Lymphoma. 2012;53:1390–1398. doi: 10.3109/10428194.2011.654337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Modiano JF, Childress MO, Mason NJ, Leary JF, O'Brien TD, Henson MS, Borgatti A, Krick E, Stuebner K, Winter A, Stewart JC, Lahrman SA, Meyers JL, Ruetz S. Targeting lymphoid progenitor cells in canine B-cell lymphoma using neoadjuvant ABCB1 transporter inhibition and doxorubicin chemotherapy; Proc 2013 ACVIM Forum; Seattle, WA. 2013. [Google Scholar]
- Itoh T, Shiota M, Takanashi M, Hojo I, Satoh H, Matsuzawa A, Moriyama T, Watanabe T, Hirai K, Mori S. Engraftment of human non-Hodgkin lymphomas in mice with severe combined immunodeficiency. Cancer. 1993;72:2686–2694. doi: 10.1002/1097-0142(19931101)72:9<2686::aid-cncr2820720927>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Keller ET, MacEwen EG, Rosenthal RC, Helfand SC, Fox LE. Evaluation of prognostic factors and sequential combination chemotherapy with doxorubicin for canine lymphoma. J Vet Intern Med. 1993;7:289–295. doi: 10.1111/j.1939-1676.1993.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Khanna C, Lindblad-Toh K, Vail D, London C, Bergman P, Barber L, Breen M, Kitchell B, McNeil E, Modiano JF, Niemi S, Comstock KE, Ostrander E, Westmoreland S, Withrow S. The dog as a cancer model. Nat Biotechnol. 2006;24:1065–1066. doi: 10.1038/nbt0906-1065b. [DOI] [PubMed] [Google Scholar]
- Kisseberth WC, Nadella MVP, Breen M, Thomas R, Duke SE, Murahari S, Kosarek CE, Vernau W, Avery AC, Burkhard MJ, Rosol TJ. A novel canine lymphoma cell line: a translational and comparative model for lymphoma research. Leuk Res. 2007;31:1709–1720. doi: 10.1016/j.leukres.2007.04.003. [DOI] [PubMed] [Google Scholar]
- le Viseur C, Hotfilder M, Bomken S, Wilson K, Röttgers S, Schrauder A, Rosemann A, Irving J, Stam RW, Shultz LD, Harbott J, Jürgens H, Schrappe M, Pieters R, Vormoor J. In childhood acute lymphoblastic leukemia, blasts at different stages of immunophenotypic maturation have stem cell properties. Cancer Cell. 2008;14:47–58. doi: 10.1016/j.ccr.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Das B, Lin TL, Grimes C, Zhang X, Lavezzi T, Huang L, Cole J, Yau L, Li L. A rare fraction of drug-resistant follicular lymphoma cancer stem cells interacts with follicular dendritic cells to maintain tumourigenic potential. Br J Haematol. 2012;158:79–90. doi: 10.1111/j.1365-2141.2012.09123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossos IS, Czerwinski DK, Alizadeh AA, Wechser MA, Tibshirani R, Botstein D, Levy R. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med. 2004;350:1828–1837. doi: 10.1056/NEJMoa032520. [DOI] [PubMed] [Google Scholar]
- MacDonald VS, Thamm DH, Kurzman ID, Turek MM, Vail DM. Does L-asparaginase influence efficacy or toxicity when added to a standard CHOP protocol for dogs with lymphoma? J Vet Intern Med. 2005;19:732–736. doi: 10.1892/0891-6640(2005)19[732:dlieot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Marconato L, Gelain ME, Comazzi S. The dog as a possible animal model for human non-Hodgkin lymphoma: a review. Hematol Oncol. 2012 doi: 10.1002/hon.2017. [DOI] [PubMed] [Google Scholar]
- Mason NJ, Coughlin CM, Overley B, Cohen JN, Mitchell EL, Colligon TA, Clifford CA, Zurbriggen A, Sorenmo KU, Vonderheide RH. RNA-loaded CD40-activated B cells stimulate antigen-specific T-cell responses in dogs with spontaneous lymphoma. Gene Ther. 2008;15:955–965. doi: 10.1038/gt.2008.22. [DOI] [PubMed] [Google Scholar]
- Mato AR, Feldman T, Goy A. Proteasome inhibition and combination therapy for non-Hodgkin's lymphoma: from bench to bedside. Oncologist. 2012;17:694–707. doi: 10.1634/theoncologist.2011-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo DF, Rossi L, Pellegrino C, Ceppi M, Cardellino U, Capurro C, Ratto A, Sambucco PL, Sestito V, Tanara G, Bocchini V. Cancer incidence in pet dogs: findings of the Animal Tumor Registry of Genoa, Italy. J Vet Intern Med. 2008;22:976–984. doi: 10.1111/j.1939-1676.2008.0133.x. [DOI] [PubMed] [Google Scholar]
- Modiano JF, Breen M, Avery AC, London CA. Breed-specific Canine Lymphoproliferative Diseases. In: Ostrander EA, Giger U, Lindblad-Toh K, editors. The Dog and its Genome. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2006. pp. 439–450. [Google Scholar]
- Modiano JF, Breen M, Burnett RC, Parker HG, Inusah S, Thomas R, Avery PR, Lindblad-Toh K, Ostrander EA, Cutter GC, Avery AC. Distinct B-Cell and T-Cell Lymphoproliferative disease prevalence among dog breeds indicates heritable risk. Cancer Res. 2005;65:5654–5661. doi: 10.1158/0008-5472.CAN-04-4613. [DOI] [PubMed] [Google Scholar]
- Müller AMS, Ihorst G, Mertelsmann R, Engelhardt M. Epidemiology of non-Hodgkin's lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol. 2004;84:1–12. doi: 10.1007/s00277-004-0939-7. [DOI] [PubMed] [Google Scholar]
- Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- Onions DE. A prospective survey of familial canine lymphosarcoma. J Natl Cancer Inst. 1984;72:909–912. [PubMed] [Google Scholar]
- Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–156. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- Peterson QP, Hsu DC, Novotny CJ, West DC, Kim D, Schmit JM, Dirikolu L, Hergenrother PJ, Fan TM. Discovery and canine preclinical assessment of a nontoxic procaspase-3-activating compound. Cancer Res. 2010;70:7232–7241. doi: 10.1158/0008-5472.CAN-10-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham LV, Tamayo AT, Yoshimura LC, Lo P, Terry N, Reid PS, Ford RJ. A CD40 Signalosome anchored in lipid rafts leads to constitutive activation of NF-kappaB and autonomous cell growth in B cell lymphomas. Immunity. 2002;16:37–50. doi: 10.1016/s1074-7613(01)00258-8. [DOI] [PubMed] [Google Scholar]
- Planken EV, Dijkstra NH, Willemze R, Kluin-Nelemans JC. Proliferation of B cell malignancies in all stages of differentiation upon stimulation in the 'CD40 system'. Leukemia. 1996;10:488–493. [PubMed] [Google Scholar]
- Ponce F, Marchal T, Magnol JP, Turinelli V, Ledieu D, Bonnefont C, Pastor M, Delignette ML, Fournel-Fleury C. A morphological study of 608 cases of canine malignant lymphoma in France with a focus on comparative similarities between canine and human lymphoma morphology. Vet Pathol. 2010;47:414–433. doi: 10.1177/0300985810363902. [DOI] [PubMed] [Google Scholar]
- Priester WA, McKay FW. The occurrence of tumors in domestic animals. Natl Cancer Inst Monogr. 1980:1–210. [PubMed] [Google Scholar]
- Ritt MG. Epidemiology of Hematopoietic Neoplasia. In: Weiss DJ, Wardrop JK, editors. Schalm's Veterinary Hematology. 6th. Ames, IA: Wiley-Blackwell; 2010. pp. 427–432. [Google Scholar]
- Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland EB, Giltnane JM, Hurt EM, Zhao H, Averett L, Yang L, Wilson WH, Jaffe ES, Simon R, Klausner RD, Powell J, Duffey PL, Longo DL, Greiner TC, Weisenburger DD, Sanger WG, Dave BJ, Lynch JC, Vose J, Armitage JO, Montserrat E, López-Guillermo A, Grogan TM, Miller TP, LeBlanc M, Ott G, Kvaloy S, Delabie J, Holte H, Krajci P, Stokke T, Staudt LM Project, L.L.M.P. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- Rütgen BC, Hammer SE, Gerner W, Christian M, de Arespacochaga AG, Willmann M, Kleiter M, Schwendenwein I, Saalmüller A. Establishment and characterization of a novel canine B-cell line derived from a spontaneously occurring diffuse large cell lymphoma. Leuk Res. 2010;34:932–938. doi: 10.1016/j.leukres.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Rütgen BC, Willenbrock S, Reimann-Berg N, Walter I, Fuchs-Baumgartinger A, Wagner S, Kovacic B, Essler SE, Schwendenwein I, Nolte I, Saalmüller A, Escobar HM. Authentication of Primordial Characteristics of the CLBL-1 Cell Line Prove the Integrity of a Canine B-Cell Lymphoma in a Murine In Vivo Model. PLoS One. 2012;7:e40078. doi: 10.1371/journal.pone.0040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safran N, Perk K, Eyal O, Dahlberg JE. Isolation and preliminary characterisation of a novel retrovirus isolated from a leukaemic dog. Res Vet Sci. 1992;52:250–255. doi: 10.1016/0034-5288(92)90018-w. [DOI] [PubMed] [Google Scholar]
- Schacam S, Barnard S, Kisseberth W, Ito D, Jensen K, Borgatti A, Henson M, Wilson H, McCauley D, Modiano JF, Kauffman M, London C. Results of a Phase I Dose Escalation Study of the Novel, Oral CRM1 Selective Inhibitor of Nuclear Export (SINE) KPT-335 in Dogs with Spontaneous Non-Hodgkin’s Lymphomas (NHL). 54th Annual Meeting of the American Society for Hematology; Atlanta, GA. 2012. [Google Scholar]
- Shipp MA, Ross KN, Tamayo P, Weng AP, Kutok JL, Aguiar RCT, Gaasenbeek M, Angelo M, Reich M, Pinkus GS, Ray TS, Koval MA, Last KW, Norton A, Lister TA, Mesirov J, Neuberg DS, Lander ES, Aster JC, Golub TR. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med. 2002;8:68–74. doi: 10.1038/nm0102-68. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic. Stem Cells. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th. Lyon: International Agency for Research on Cancer; 2008. [Google Scholar]
- Teske E. Canine malignant lymphoma: a review and comparison with human non-Hodgkin's lymphoma. Vet Q. 1994;16:209–219. doi: 10.1080/01652176.1994.9694451. [DOI] [PubMed] [Google Scholar]
- Teske E, de Vos JP, Egberink HF, Vos JH. Clustering in canine malignant lymphoma. Vet Q. 1994;16:134–136. doi: 10.1080/01652176.1994.9694435. [DOI] [PubMed] [Google Scholar]
- Thomas R, Seiser EL, Motsinger-Reif A, Borst L, Valli VE, Kelley K, Suter SE, Argyle D, Burgess K, Bell J, Lindblad-Toh K, Modiano JF, Breen M. Refining tumor-associated aneuploidy through ‘genomic recoding’ of recurrent DNA copy number aberrations in 150 canine non-Hodgkin lymphomas. Leuk Lymphoma. 2011;52:1321–1335. doi: 10.3109/10428194.2011.559802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomley FM, Armstrong SJ, Mahy BW, Owen LN. Reverse transcriptase activity and particles of retroviral density in cultured canine lymphosarcoma supernatants. Br J Cancer. 1983;47:277–284. doi: 10.1038/bjc.1983.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonomura N, Thomas R, Megquier K, Perloski M, Swofford R, Davis B, Barber L, Burgess K, Azuma C, Modiano JF, Breen M, Lindblad-Toh K. The 6th International Conference on Advances in Canine and Feline Genomics and Inherited Disease. Sweden: Visby; 2012. GWAS identifies risk loci for two hematologic malignancies in golden retriever. [Google Scholar]
- Traullé C, Coiffier BB. Evolving role of rituximab in the treatment of patients with non-Hodgkin's lymphoma. Future Oncol. 2005;1:297–306. doi: 10.1517/14796694.1.3.297. [DOI] [PubMed] [Google Scholar]
- Valli VE. Histological classification of hematopoietic tumors of domestic animals. Washington, D.C.: AFIP Monograph; 2002. [Google Scholar]
- Valli VE, Myint MS, Barthel A, Bienzle D, Caswell J, Colbatzky F, Durham A, Ehrhart EJ, Johnson Y, Jones C, Kiupel M, Labelle P, Lester S, Miller M, Moore P, Moroff S, Roccabianca P, Ramos-Vara J, Ross A, Scase T, Tvedten H, Vernau W. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet Pathol. 2011;48:198–211. doi: 10.1177/0300985810379428. [DOI] [PubMed] [Google Scholar]
- Vega F, Davuluri Y, Cho-Vega JH, Singh RR, Ma S, Wang R-Y, Multani AS, Drakos I, Pham L, Lee Y-CL, Shen L, Ambrus J, Medeiros LJ, Ford RJ. Side population of a murine mantle cell lymphoma model contains tumor-initiating cells responsible for lymphoma maintenance and dissemination. J Cell Mol Med. 2009;14:1532–1545. doi: 10.1111/j.1582-4934.2009.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser HP, Tewis M, Willemze R, Kluin-Nelemans JC. Mantle cell lymphoma proliferates upon IL-10 in the CD40 system. Leukemia. 2000;14:1483–1489. doi: 10.1038/sj.leu.2401829. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu Y, Malek SN, Zheng P, Liu Y. Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem Cell. 2011;8:399–411. doi: 10.1016/j.stem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiestner A. Targeting B-cell receptor signaling for anticancer therapy: the Bruton's tyrosine kinase inhibitor Ibrutinib induces impressive responses in B-cell malignancies. J Clin Oncol. 2013;31:128–130. doi: 10.1200/JCO.2012.44.4281. [DOI] [PubMed] [Google Scholar]