ABSTRACT
Introduction: Pneumococcal infection is a leading cause of illness and death in HIV-infected adults. Current United States guidelines for HIV-infected adults recommend a single dose of the 13-valent pneumococcal conjugate vaccine (PCV-13) at any CD4 count and at least 1 y after receipt of the 23-valent pneumococcal polysaccharide vaccine (PPV). PPV is known to lead to hyporesponsiveness to subsequent pneumococcal vaccines for at least 1 y Whether PCV-13 would be more immunogenic if administered later after PPV receipt or at higher CD4 counts has not been tested.
Methods: We prospectively collected serum from 96 HIV-infected adults before and after PCV-13 receipt, and measured antibody concentrations against 4 pneumococcal serotypes (3, 6A, 7F, and 19A) via indirect ELISA according to the WHO protocol. Post-booster antibody concentrations and fold-rise in antibody concentrations were compared according to time from PPV receipt and baseline CD4 count using univariate and multivariate analyses.
Results: PPV receipt >3 versus 1–3 y prior did not significantly change post-vaccination antibody concentrations, but was associated with slightly higher fold-rise in antibody concentration for the 3 tested serotypes included in PPV, though this only reached significance for serotype 7F. CD4 count was significantly associated with post-vaccination antibody concentrations for 3 of 4 serotypes, but not for fold-rise in antibody concentration for any serotype.
Conclusion: Waiting longer than 1 y after PPV receipt to administer PCV-13 may slightly improve the antibody response to serotypes included in both vaccines. While higher CD4 count at PCV-13 administration results in higher post-vaccination antibody concentrations, this is likely because higher CD4 count is also associated with higher pre-vaccination antibody concentrations.
KEYWORDS: adults, CD4 Count, HIV, Prevnar, pneumococcus, Pneumovax, vaccine
Introduction
Pneumococcal disease is a leading cause of illness and death among adults infected with the Human Immunodeficiency Virus (HIV). Although invasive pneumococcal disease (IPD) rates have dropped in the era of effective antiretroviral therapy, HIV-infected adults still have a 35-fold greater risk of IPD than the general population.1 The 7-valent pneumococcal conjugate vaccine (PCV-7) has been shown to reduce recurrent IPD in HIV-infected adults, even in subjects with CD4 counts <200 cells/mm3.2 Consequently, in 2012 the Unites States Advisory Committee on Immunization Practices (ACIP) recommended that HIV-infected adults with any CD4 count (no lower limit defined) receive a single dose of the newer 13-valent pneumococcal conjugate vaccine (PCV-13) if at least 1 y has passed since receipt of the 23-valent pneumococcal polysaccharide vaccine (PPV).3,4 If possible, PCV-13 is recommended prior to the 2–3 recommended doses of PPV, with the first dose of PPV at least 8 weeks after PCV-13.4 The addition of PCV-13 will enhance IPD protection for this vulnerable group, and possibly also enhance protection against community-acquired pneumonia due to vaccine-type pneumococcal strains, as demonstrated for elderly adults in the recent large CAPITA trial.5 However, the timing recommendations are based on limited data.
Although the recommendation to give PCV-13 before PPV is sound, the recommendation to wait only 1 y after PPV receipt to give PCV-13 is based on limited data. Polysaccharide vaccines are known to cause hyporesponsiveness to subsequent vaccine doses, an effect that is likely time-limited.6 Two studies suggested that hyporesponsiveness may no longer be present if >3 y elapse between administration of PPV and PCV.7,8 An additional study demonstrated that hyporesponsiveness was still present if only 1 y had elapsed between the dosing of PPV and PCV.6 However, whether hyporesponsiveness to PCV-13 would still be present 1–3 y after PPV receipt is unknown.
Secondly, studies of earlier PCV containing 4–7 serotypes showed that HIV-infected subjects with higher CD4 counts had increased vaccine responses.7,9–11 Thus, administering a single dose of PCV-13 at low CD4 counts may not provide optimal protection. Conjugate vaccine were developed because they can activate CD4 cells and consequently elicit a T-cell dependent B cell response resulting in memory B cells. Consequently, giving PCV-13 after the CD4 count increases on antiretroviral therapy might elicit a better immune response.
To help fill these knowledge gaps, we measured the antibody response in HIV-infected adults who were receiving PCV-13 according to the ACIP guidelines, and analyzed the effect of time interval since PPV receipt and CD4 count.
Results
Of the 105 subjects enrolled in Group 1 (serum taken before and 1 month after PCV-13), 4 subjects were excluded because of additional prior PCV-13 doses, and 5 subjects failed to return for the second visit, leaving 96 subjects for the analysis. Of the 50 subjects enrolled in Group 2 (serum taken 1 y after PCV-13), 1 was excluded because of additional prior PCV-13 doses.
The demographics of the subjects are shown in Table 1. Of the 42 subjects in Group 1 who received PPV 1–3 y prior, 2 had received 3 lifetime doses, 11 had received 2 lifetime doses, and 29 had received 1 lifetime dose of PPV. Of the 54 subjects who received PPV >3 y prior, 5 had received 2 lifetime doses, 28 had received 1 lifetime dose, and 21 had no record of receiving PPV. All subjects who had received multiple PPV doses received them at intervals of at least 5 y.
Table 1.
Demographics of study subjects.
| Group 1 Total | Group 1 PPV 1–3 yrs prior | Group 1 PPV >3 yrs prior | Group 1 CD4 <500 cells/mm3 | Group 1 CD4 >/=500 cells/mm3 | Group 2 | |
|---|---|---|---|---|---|---|
| n | 96 | 42 | 54 | 37 | 59 | 49 |
| Age in years | ||||||
| mean ± SD | 44 ± 11˜ | 46 ± 11 | 42 ± 11 | 45 ± 10 | 43 ± 11 | 50 ± 11˜ |
| range | 20–65 | 22–65 | 20–62 | 26–63 | 20–65 | 21–71 |
| Gender: no (%) male | 66 (69%) | 26 (62%) | 40 (74%) | 30 (81%)ˆ | 36 (61%)ˆ | 38 (78%) |
| Race | ||||||
| no (%) black | 76 (79%)˜ | 33 (79%) | 43 (80%) | 28 (76%) | 48 (81%) | 27 (55%)˜ |
| no (%) white | 19 (20%)˜ | 9 (21%) | 10 (19%) | 1 (3%) | 0 (0%) | 22 (45%)˜ |
| no (%) other | 1 (1%)˜ | 0 (0%) | 1 (2%) | 8 (22%) | 11 (19%) | 0 (0%)˜ |
| Body weight (lbs): mean ± SD | 188 ± 47 | 188 ± 48 | 189 ± 47 | 188 ± 46 | 188 ± 48 | 189±52 |
| No (%) prescribed a statin | 23 (24%) | 11 (26%) | 12 (22%) | 9 (24%) | 14 (24%) | 20 (41%) |
| No (%) with hx pneumonia | 38 (40%) | 15 (36%) | 23 (43%) | 22 (59%)ˆ | 16 (27%)ˆ | 23 (47%) |
| No (%) with diabetes | 11 (11%) | 5 (12%) | 6 (11%) | 2 (5%) | 9 (15%) | 10 (20%) |
| No (%) ever homeless | 26 (27%) | 12 (29%) | 14 (26%) | 10 (27%) | 16 (27%) | 8 (16%) |
| No (%) currently smoking | 37 (39%) | 15 (36%) | 22 (41%) | 16 (43%) | 21 (36%) | 22 (45%) |
| No (%) with hepatitis C | 11 (11%) | 6 (14%) | 5 (9%) | 4 (11%) | 7 (12%) | 12 (25%) |
| No (%) with AIDS diagnosis | 57 (59%) | 23 (55%) | 34 (63%) | 28 (76%)ˆ | 29 (49%)ˆ | 29 (59%) |
| No (%) on cART | 100 (100%) | 42 (100%) | 54 (100%) | 37 (100%) | 54 (100%) | 48 (98%) |
| CD4 count | ||||||
| mean + SD (cells/mm3) | 626 + 342 | 657 + 331 | 602 + 352 | — | — | 642 + 309 |
| no (%) <200 cells/mm3 | 11 (11%) | 4 (10%)* | 7 (13%)* | — | — | 3 (6%) |
| no (%) 200–499 cells/mm3 | 26 (27%) | 8 (19%)* | 18 (33%)* | — | — | 16 (33%) |
| no (%) 500–799 cells/mm3 | 34 (35%) | 18 (43%)* | 16 (30%)* | — | — | 15 (31%) |
| no (%) >800 cells/mm3 | 25 (26%) | 12 (29%)* | 13 (24%)* | — | — | 15 (31%) |
| Time from PPV | ||||||
| no (%) 1–3 y | 42 (44%) | — | — | 12 (32%) | 30 (51%) | 27 (55%) |
| no (%) >3 y | 54 (56%) | — | — | 25 (68%) | 29 (49%) | 22 (45%) |
SD = standard deviation, no = number, cART = combination antiretroviral therapy. Group 1 had serum taken before and 1 month after and Group 2 had serum taken 1 year after PCV-13 receipt. Comparisons were done by t-test for continuous variables, Fisher's exact test for binary variables, and Chi square test for categorical variables with >2 categories. Bold indicates variables with significant differences: ∼ p=0.003 for age and 0.001 for racial distribution between groups 1 and 2; *p=0.03 for CD4 count distribution between groups divided by PPV receipt; ^p=0.04 for gender, 0.002 for pneumonia history, and 0.01 for AIDS diagnosis between groups divided by CD4 count.
The antibody geometric mean concentrations (GMCs) at baseline (Group 1), 1 month post-vaccination (Group 1), and 1 y post-vaccination (Group 2) are shown in Fig. 1. Of note, there were demographic differences between Group 1 and Group 2 (Table 1), making any GMC comparisons between the 1 y time point and the other time points difficult. The remainder of the analysis is restricted to Group 1 alone.
Figure 1.
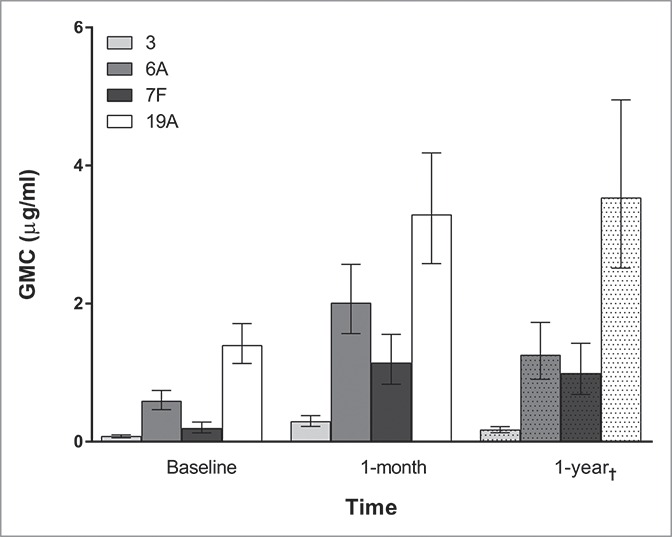
Antibody geometric mean concentrations (GMCs) for each serotype grouped by time since PCV-13 receipt. Bars represent 95% confidence intervals. †One year GMCs are from a different group of subjects (Group 2) than the baseline and one month GMCs (Group 1).
Time from PPV receipt (>3 y vs. 1–3 y) was not significantly associated with 1 month post-vaccination antibody concentration for any serotype on either univariate or multivariate analysis (Fig. 2, Table 2). Fold-rise in antibody concentration was higher for the subjects who had the >3 y versus 1–3 y interval between PPV and PCV-13 receipt for the 3 tested serotypes included in PPV (3, 7F, and 19A). However, this only reached significance for serotype 7F on univariate analysis (geometric mean of 8.0vs. 4.0 fold-rise for >3 versus 1–3 y, p = 0.04), and did not remain significant on multivariate analysis.
Figure 2.
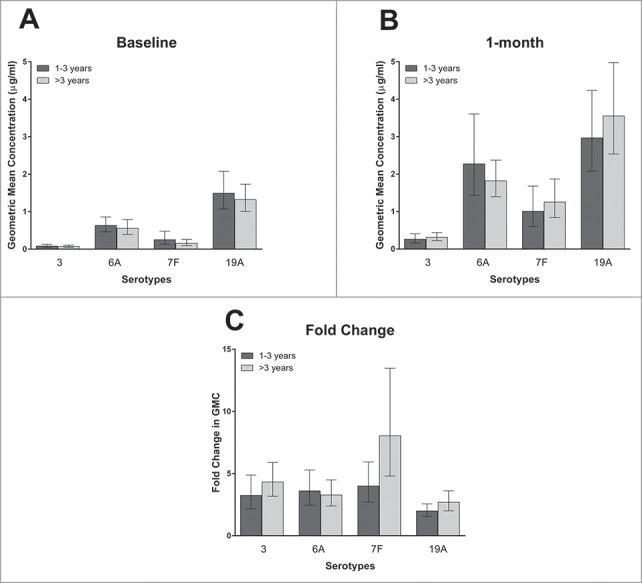
The geometric mean of the antibody concentrations at baseline (2A) and 1 month post –vaccination (2B), and the geometric mean of the fold-change in antibody concentrations (2C), according to time since PPV receipt by serotype. Bars represent 95% confidence intervals. There were 42 and 54 subjects in the 1–3 and >3 y since PPV receipt groups respectively. Of note, serotype 6A is included in PCV-13 but not PPV, but serotypes 3, 7F, and 19A are included in both PCV-13 and PPV.
Table 1.
Post-PCV-13 antibody concentration and fold-rise in antibody concentration by time since PPV receipt and CD4 count.
| By Years Since Pneumovax Receipt |
By CD4 Count at Vaccination |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1-3 years (n=42) | >3 years (n=54) | unadjusted p value | adjusted p value | <500 cells/mm3 (n=37) | >500 cells/mm3 (n=59) | unadjusted p value | adjusted p value | |
| Serotype 3 | ||||||||
| Post Booster GMC (95% CI) | 0.26 (0.17-0.41) | 0.31 (0.22-0.44) | NS | NS | 0.16 (0.11-0.25) | 0.41 (0.30-0.57) | 0.0008 | 0.01 |
| Fold-rise geometric mean (95% CI) | 3.24 (2.16-4.87) | 4.32 (3.18-5.89) | NS | NS | 3.17 (2.14-4.70) | 4.28 (3.11-5.90) | NS | NS |
| Serotype 6A | ||||||||
| Post Booster GMC (95% CI) | 2.27 (1.43-3.61) | 1.82 (1.40-2.37) | NS | NS | 1.74 (1.19-2.55) | 2.19 (1.58-3.05) | NS | NS |
| Fold-rise geometric mean (95% CI) | 3.61 (2.46-5.29) | 3.28 (2.40-4.49) | NS | NS | 3.88 (2.48-6.07) | 3.16 (2.39-4.17) | NS | NS |
| Serotype 7F | ||||||||
| Post Booster GMC (95% CI) | 1.00 (0.60-1.68) | 1.25 (0.84-1.87) | NS | NS | 0.81 (0.48-1.37) | 1.41 (0.96-2.08) | NS (0.09) | NS |
| Fold-rise geometric mean (95% CI) | 4.01 (2.70-5.94) | 8.04 (4.8-13.47) | 0.043 | NS (0.06) | 6.22 (3.96-9.76) | 5.75 (3.54-9.34) | NS | NS |
| Serotype 19A | ||||||||
| Post Booster GMC (95% CI) | 2.97 (2.08-4.24) | 3.55 (2.54-4.98) | NS | NS | 2.34 (1.54-3.56) | 4.06 (3.04-5.43) | 0.03 | NS (0.07) |
| Fold-rise geometric mean (95% CI) | 1.99 (1.55-2.56) | 2.69 (2.00-3.61) | NS | NS (0.09) | 2.00 (1.49-2.68) | 2.62 (2.00-3.42) | NS | NS |
GMC = geometric mean concentration. CI = confidence interval. NS = nonsignificant. Adjusted p values were from multivariate regression models including the following covariates: age, gender, race, statin use, AIDS diagnosis, history of pneumonia, diabetes, history of homelessness, current smoking status, and hepatitis C coinfection. Of note, serotypes 3, 7F, and 19A are included in both PCV-13 and PPV, but 6A is only in PCV-13.
CD4 count as a continuous variable was significantly associated with 1 month post-vaccination antibody concentrations for 3 of 4 serotypes on univariate analysis (p = 0.007, 0.1, 0.03, and 0.005 for serotypes 3, 6A, 7F, and 19A respectively), and for all serotypes on multivariate analysis (p = 0.04, 0.02, 0.04, and 0.003 for serotypes 3, 6A, 7F, and 19A respectively). Of note, the multivariate regression model did not include baseline antibody concentrations, and baseline antibody concentrations were significantly associated with post-vaccination antibody concentrations (p < 0.0001 for all serotypes). Fold-rise in antibody concentration, which does take baseline antibody concentrations into account, was not significantly associated with CD4 count for any serotype on univariate or multivariate analysis. The relationships of CD4 count to antibody concentrations before and 1 month after PCV-13 receipt, and to fold-rise in antibody concentration, are shown in Fig. 3. The relationships of CD4 count as a categorical variable (< or > 500 cells/mm3) to 1 month antibody concentrations and fold-rise in antibody concentration are shown in Table 2.
Figure 3.
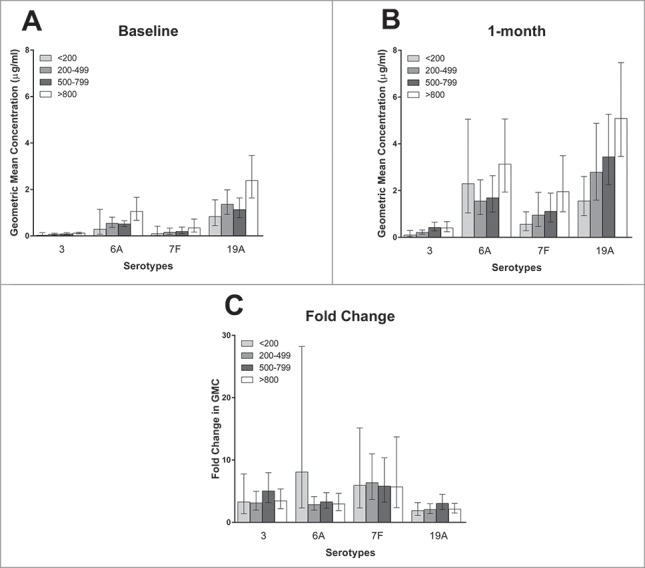
The geometric mean of the antibody concentrations at baseline (3A) and one month post –vaccination (3B), and the geometric mean of the fold-change in antibody concentrations (3C), according to CD4 count by serotype. Bars represent 95% confidence intervals. There were 11, 26, 34, and 25 subjects in the<200, 200–499, 500–799, and >800 CD4 cells/mm3 groups respectively.
Covariates with significant associations with 1 month post-booster antibody concentrations on multivariate analysis included age and smoking status (serotypes 3, 7F, and 19A). Covariates with significant associations with fold-rise in antibody concentrations on multivariate analysis included age and race (serotype 19A only).
Discussion
Our observational study measured antibody response to PCV-13 in adults with well-controlled HIV infection following ACIP recommendations. Subjects responded to vaccination, with significant increases in GMC 1 month post-vaccination for all serotypes tested. The fold rises in antibody concentration for serotypes 3, 7F, and 19A (the 3 tested serotypes included in both PPV and PCV-13), were slightly higher in subjects who had received PPV >3vs. 1–3 y prior to their PCV-13 vaccination, though this only reached significance for serotype 7F. This suggests that PPV-induced hyporesponsiveness may still be present 1–3 y after PPV receipt, although not to a high degree.
A number of studies have shown that PPV does not induce memory cells and can even decrease the response to subsequent pneumococcal vaccination.12 It has been theorized that the hyporesponsiveness following receipt of polysaccharide vaccines like PPV results from polysaccharide antigens persisting in the body for prolonged periods of time, leading to downregulation of B cells and binding of neutralizing polysaccharide-specific antibodies in the serum. This may also explain why in the only clinical trial testing the efficacy of PPV in HIV-infected adults, there were significantly higher rates of all-cause pneumonia in the vaccine versus placebo group, particularly in the first 6 months after PPV receipt.13 The hyporesponsiveness appears to be time-limited. In an American study, adult subjects who had recovered from pneumococcal pneumonia had a substantially lower antibody response to PCV-7 if they had received PPV 6 months earlier vs. not having recently received PPV.6 Further, subjects who had received PPV within the past year had distinctly lower antibody responses to PCV-7 than those vaccinated >5 y before, and those vaccinated 1–5 y before had an intermediate response. However, another study among 204 HIV-infected adults in the United States showed that prior PPV receipt 3–5 versus 5–8 y prior did not significantly affect antibody response to revaccination with either PPV or PCV-7, suggesting that hyporesponsiveness might wane by 3 y.8 In support of this, a Ugandan study demonstrated that HIV-infected adults vaccinated with PCV-7 who had received PPV 3.5–6.5 y prior had similar immune responses to those who had never received a prior PPV dose.7 In addition, in a recent PCV-13 immunogenicity trial in 329 HIV-infected adults who had received PPV a mean of 3.7 y prior (range not reported), time from PPV receipt did not correlate with vaccine response.14 Our results extend on these findings, demonstrating that subjects who received PPV 1–3vs. >3 y prior to PCV-13 had a trend toward a lower antibody response, but that this only reached significance for 1 serotype on univariate analysis. The results of this study on PCV-13, which are similar to prior studies of PCV-7, are likely applicable to PPV and PCV in general, including PCV-10, the other pneumococcal conjugate vaccine supported by Gavi (the global vaccine alliance) for use in developing countries.15
Antibody concentrations 1 month post-vaccination were significantly associated with CD4 count. However, this seems to be related to the fact that baseline antibody concentrations were also significantly associated with CD4 count, as fold-rise in antibody concentration was not significantly associated with CD4 count. Elevated baseline concentrations could have been due to prior natural pneumococcal exposure, prior PPV receipt (serotypes 3, 7F, or 19A), or cross-reaction with serotype 6B from prior PPV receipt (serotype 6A).16 Other studies on earlier pneumococcal conjugate vaccines have also shown that subjects with higher CD4 counts have higher baseline antibody concentrations, and that baseline antibody concentrations strongly correlate with post-vaccination antibody concentrations.17 This is presumably from a stronger immune response to prior pneumococcal exposure in subjects with higher CD4 counts. This would make sense in that CD4 cells are critical to the creation of germinal centers and the development of long-lasting memory B cells, either from pneumococcal natural infection or PCV, that can be boosted though subsequent pneumococcal antigen exposure.18
Serologic correlates of protection for pneumococcus in adults are not clearly defined. In studies of children receiving their primary PCV series, serotype-specific IgG concentrations of 0.35 ug/ml have been used as the threshold for immunity against IPD17 However, adults tend to have higher baseline antibody concentrations due to prior pneumococcal exposure and nasal colonization, so the threshold for immunity in adults is less clear. Furthermore, higher antibody concentrations may be needed to protect against non-invasive pneumococcal disease than against IPD, and IgG levels sufficient for protection seem to differ between different serotypes.19 Despite these uncertainties, common sense would dictate that higher post-vaccination antibody concentrations should correlate with improved protection. For other vaccines like hepatitis B vaccine, it has been shown that the antibody concentration 1 month after completing the initial vaccination series directly correlates with duration of immunity in HIV-infected adults.20 In HIV-infected children, memory response to pneumococcal vaccination was shown to directly correlate with antibody concentration 8 weeks after initial vaccination.21 Consequently, as HIV-infected subjects vaccinated at higher CD4 counts had higher post-vaccination antibody concentrations, one would expect them to have higher duration of protection after PCV-13 vaccination, regardless of the etiology.
The ACIP likely recommended a single dose of PCV-13 at any CD4 count because patients with lower CD4 counts are at increased risk of IPD. The only efficacy trial of pneumococcal conjugate vaccines in HIV-infected adults showed that 2 doses of PCV-7 reduced IPD due to vaccine serotypes by 86% in subjects with CD4 counts <200 cells/mm3.2 Furthermore, 75% of the IPD cases due to vaccine serotypes in this trial occurred in subjects with CD4 counts <200 cells/mm3. However, efficacy dropped from 85% the first year after vaccination to 25% thereafter, suggesting that duration of protection might be suboptimal. In contrast, in the recent large CAPITA trial, in which 84,496 healthy elderly adults (>65 y old) were randomized to PCV-13 versus placebo, the vaccine efficacy of 75% against IPD and 46% against community-acquired pneumonia due to vaccine-type strains persisted throughout the 4 y of follow-up.5 However, immunocompromised individuals (including HIV-infected individuals) were excluded from the trial, and those who became immunocompromised after enrollment demonstrated decreased vaccine efficacy. This further suggests that duration of protection in HIV-infected subjects vaccinated at low CD4 counts could be suboptimal.
To balance the need to provide protection for HIV-infected adults with low CD4 counts and the need to provide long-lasting protection, giving an additional dose of PCV-13 at a higher CD4 count could be considered. Although 2 recent clinical trials which evaluated the immunogenicity of multiple doses of PCV-13 in HIV-infected adults found limited benefit after a second and third dose, both trials limited enrollment to subjects with CD4 counts >200 cells/mm3.14,22 Further studies evaluating the immunologic response to a second dose of PCV-13, given after the CD4 count increases over 200 cells/mm3, in HIV-infected adults originally vaccinated at low CD4 counts, are needed.
Our study has several limitations. Our primary outcomes are only a surrogate for protection against pneumococcal disease. The mean CD4 count of our subjects was high, and only 11 subjects had a CD4 count <200 cells/mm3, which may have made it more difficult to detect differences in antibody response based on CD4 count. We only analyzed the serologic response to 4 of the 13 serotypes in PCV-13. Finally, it would have been ideal to follow the same cohort of subjects longer than 1 month post-vaccination. However, to our knowledge, our study is the first to evaluate the association between CD4 count and PCV-13 response in HIV-infected adults, to assess the response to PCV-13 in subjects with CD4 counts <200 cells/mm3, or to report antibody concentrations 1 y after PCV-13 receipt in HIV-infected adults.
Conclusions
In conclusion, we present data on the antibody response to PCV-13 administered to HIV-infected adults according to ACIP guidelines. Our data suggests that waiting longer than 1 y after PPV receipt to administer PCV-13 may slightly improve the antibody response to serotypes included in both vaccines. We show that post-vaccination antibody concentrations significantly correlate with CD4 count, but that this is due in large part to higher baseline antibody concentrations in subjects with higher CD4 counts. Future studies are needed to determine if a second PCV-13 dose would be beneficial after the CD4 count increases in subjects originally vaccinated at low CD4 counts.
Methods
Subjects
This was a prospective, observational study conducted at the primary HIV clinic (C3ID) at Eastern Virginia Medical School (EVMS) in Norfolk, VA. The EVMS Institutional Review Board approved the study. All subjects underwent informed consent.
Between August 2013 and February 2014, we enrolled 105 subjects whose provider had ordered PCV-13 (trade name Prevnar-13, manufactured by Wyeth Pharmaceuticals Inc.), and collected blood, questionnaire data, and information from the medical record such as dates of prior receipt of PPV (trade name Pneumovax 23, manufactured by Merck & Co., Inc.) and laboratory values including current CD4 count, before and 4–6 weeks after PCV-13 receipt (Group 1). Inclusion criteria included documented HIV infection, age >18 y, and HIV viral load <400 copies/ml on the most recent test. We limited enrollment to subjects with well-controlled HIV infection to exclude HIV viremia as a confounding factor, because subjects compliant with HIV medications were felt to be more likely to show up for the second study visit, and because subjects with uncontrolled HIV viremia have more immediate threats to their health than suboptimal vaccine timing. Due to budgetary constraints, we did not follow our subjects past 4–6 weeks. However, to assess antibody concentrations 1 y after PCV-13 receipt, we simultaneously enrolled and collected blood, questionnaire data, and information from the medical record from 50 additional subjects who fit the same inclusion criteria and who were retrospectively identified as having received PCV-13 11–13 months prior through review of clinic records (Group 2). Of note, none of the subjects in Group 2 had received PPV after PCV-13 receipt.
Laboratory analysis
On the day of collection, blood samples were centrifuged, and the serum stored at −80°C until laboratory analysis. Serum was tested via Indirect ELISA for IgG antibody levels against serotypes 3, 6A, 7F, and 19A according to the WHO protocol,23 using United States reference pneumococcal antiserum 007sp. Our analysis was restricted to 4 serotypes for logistic reasons, but we chose the 3 most prevalent serotypes in the United States since 2008 that are included in PCV-13 (3, 7F, and 19A), as well as the only serotype included in PCV-13 but not PPV (6A).24
Statistical analysis
We calculated that we would need at least 81 subjects to provide samples before and 1 month after PCV-13 receipt to detect a 1.5-fold difference in increase in geometric mean antibody concentrations (GMCs) between subjects with PPV receipt >3 y vs. 1–3 y prior, and subjects with CD4 counts of >500 vs. <500 cells/mm3, with 80% power and α of 0.05. We over-enrolled by 30% (105 subjects) to account for loss-to-follow up.
Descriptive statistics were reported. The 2 variables of interest were time from PPV receipt (>3 y vs. 1–3 y) and CD4 count. Of note, PPV receipt >3 y prior included both subjects who had record of last PPV receipt >3 y prior and subjects who had no record of ever receiving PPV, as other studies indicate that PPV receipt >3 y prior does not diminish PCV response in comparison to subjects who never received PPV.7,8 The primary outcomes were 1 month post-booster antibody concentrations and fold-rise in antibody concentrations for each of the 4 serotypes, and were logarithmically transformed prior to analysis. We chose continuous outcomes because serologic correlates of protection for pneumococcus in adults are not well-defined, prior studies have used various definitions of serologic response, and therefore assigning a specific categorical definition seemed arbitrary. Of note, the 2 clinical trials of PCV-13 in HIV-infected adults also used continuous outcomes.14,22 To analyze the association between variables and primary outcomes, we used linear regression for continuous variables, and t-test for binary variables. Multivariate regression models were constructed to investigate the association between the variables of interest and the primary outcomes. The covariates included in the models were determined a priori, and included age, gender, race, statin use, AIDS diagnosis, history of pneumonia, diabetes, history of homelessness, current smoking status, and hepatitis C coinfection. Two-sided statistical tests were conducted at an α level of 0.05. Statistical analysis was performed using SAS version 9.3 (SAS institute, Cary, North Carolina).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank the providers, staff, and especially the patients at the EVMS C3ID clinic.
Part of this work was presented at the 25th European Congress of Clinical Microbiology and Infectious Diseases (Poster #P0935), Copenhagen, Denmark. April 25–28, 2015.
Funding
This work was supported by the Doris Duke Charitable Foundation: (Clinical Scientist Development Award 2012061 [principal investigator, S.Troy]). S.Troy and A. Rossheim also received salary support while working on this project from the US National Institutes of Health: (Career Development Award 5K23AI093678 [principal investigator, S. Troy.]).
References
- [1].Heffernan RT, Barrett NL, Gallagher KM, Hadler JL, Harrison LH, Reingold AL, Khoshnood K, Holford TR, Schuchat A. Declining incidence of invasive Streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy, 1995–2000. J Infect Dis 2005; 191:2038-45; PMID:15897989; http://dx.doi.org/ 10.1086/430356 [DOI] [PubMed] [Google Scholar]
- [2].French N, Gordon SB, Mwalukomo T, White SA, Mwafulirwa G, Longwe H, Mwaiponya M, Zijlstra EE, Molyneux ME, Gilks CF. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med 2010; 362:812-22; PMID:20200385; http://dx.doi.org/ 10.1056/NEJMoa0903029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Info.NIH A Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents. 2015 [Google Scholar]
- [4].(CDC) CfDCaP . Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816-9; PMID:23051612 [PubMed] [Google Scholar]
- [5].Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, et al.. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114-25; PMID:25785969; http://dx.doi.org/ 10.1056/NEJMoa1408544 [DOI] [PubMed] [Google Scholar]
- [6].Musher DM, Rueda AM, Nahm MH, Graviss EA, Rodriguez-Barradas MC. Initial and subsequent response to pneumococcal polysaccharide and protein-conjugate vaccines administered sequentially to adults who have recovered from pneumococcal pneumonia. J Infect Dis 2008; 198:1019-27; PMID:18710324; http://dx.doi.org/ 10.1086/591629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Miiro G, Kayhty H, Watera C, Tolmie H, Whitworth JA, Gilks CF, French N. Conjugate pneumococcal vaccine in HIV-infected Ugandans and the effect of past receipt of polysaccharide vaccine. J Infect Dis 2005; 192:1801-5; PMID:16235180; http://dx.doi.org/ 10.1086/497144 [DOI] [PubMed] [Google Scholar]
- [8].Crum-Cianflone NF, Huppler Hullsiek K, Roediger M, Ganesan A, Patel S, Landrum ML, Weintrob A, Agan BK, Medina S, Rahkola J, et al.. A randomized clinical trial comparing revaccination with pneumococcal conjugate vaccine to polysaccharide vaccine among HIV-infected adults. J Infect Dis 2010; 202:1114-25; PMID:20795819; http://dx.doi.org/ 10.1086/656147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ahmed F, Steinhoff MC, Rodriguez-Barradas MC, Hamilton RG, Musher DM, Nelson KE. Effect of human immunodeficiency virus type 1 infection on the antibody response to a glycoprotein conjugate pneumococcal vaccine: results from a randomized trial. J Infect Dis 1996; 173:83-90; PMID:8537687; http://dx.doi.org/ 10.1093/infdis/173.1.83 [DOI] [PubMed] [Google Scholar]
- [10].Chen M, Ssali F, Mulungi M, Awio P, Yoshimine H, Kuroki R, Furumoto A, Tanimura S, Kityo C, Nagatake T, et al.. Induction of opsonophagocytic killing activity with pneumococcal conjugate vaccine in human immunodeficiency virus-infected Ugandan adults. Vaccine 2008; 26:4962-8; PMID:18639599; http://dx.doi.org/ 10.1016/j.vaccine.2008.06.093 [DOI] [PubMed] [Google Scholar]
- [11].Kroon FP, van Dissel JT, Ravensbergen E, Nibbering PH, van Furth R. Enhanced antibody response to pneumococcal polysaccharide vaccine after prior immunization with conjugate pneumococcal vaccine in HIV-infected adults. Vaccine 2000; 19:886-94; PMID:11115712; http://dx.doi.org/ 10.1016/S0264-410X(00)00232-2 [DOI] [PubMed] [Google Scholar]
- [12].O'Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis 2007; 7:597-606; PMID:17714673; http://dx.doi.org/ 10.1016/S1473-3099(07)70210-4 [DOI] [PubMed] [Google Scholar]
- [13].French N, Nakiyingi J, Carpenter LM, Lugada E, Watera C, Moi K, Moore M, Antvelink D, Mulder D, Janoff EN, et al.. Twenty-three-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet 2000; 355:2106-11; PMID:10902624; http://dx.doi.org/ 10.1016/S0140-6736(00)02377-1 [DOI] [PubMed] [Google Scholar]
- [14].Glesby MJ, Watson W, Brinson C, Greenberg RN, Lalezari JP, Skiest D, Sundaraiyer V, Natuk R, Gurtman A, Scott DA, et al.. Immunogenicity and Safety of 13-Valent Pneumococcal Conjugate Vaccine in HIV-Infected Adults Previously Vaccinated With Pneumococcal Polysaccharide Vaccine. J Infect Dis 2014; 212(1):18-27 [DOI] [PubMed] [Google Scholar]
- [15].MSF. Pneumococcal Conjugate Vaccines (PCV) The Right Shot: Bringing down barriers to affordable and adapted vaccines. Second ed 2015. p. 72-7 [Google Scholar]
- [16].Väkeväinen M, Eklund C, Eskola J, Käyhty H. Cross-reactivity of antibodies to type 6B and 6A polysaccharides of Streptococcus pneumoniae, evoked by pneumococcal conjugate vaccines, in infants. J Infect Dis 2001; 184:789-93; http://dx.doi.org/ 10.1086/322984 [DOI] [PubMed] [Google Scholar]
- [17].Nunes MC, Madhi SA. Safety, immunogenicity and efficacy of pneumococcal conjugate vaccine in HIV-infected individuals. Hum Vaccin Immunother 2012; 8:161-73; PMID:22426374; http://dx.doi.org/ 10.4161/hv.18432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Siegrist C-A. Vaccine immunology In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th ed. China: Elsevier Saunders; 2013. p. 14-32 [Google Scholar]
- [19].Klugman K, Black S, Dagan R, Malley R, Whitney C. Pneumococcal conjugate vaccine and pneumococcal common protein vaccines In: Plotkin O, and Offit. , editor. Vaccines. China: Elsevier Saunders; 2013. p. 504-41 [Google Scholar]
- [20].Lopes VB, Hassing RJ, de Vries-Sluijs TE, El Barzouhi A, Hansen BE, Schutten M, de Man RA, van der Ende ME. Long-term response rates of successful hepatitis B vaccination in HIV-infected patients. Vaccine 2013; 31:1040-4; PMID:23273969; http://dx.doi.org/ 10.1016/j.vaccine.2012.12.047 [DOI] [PubMed] [Google Scholar]
- [21].Abzug MJ, Song LY, Levin MJ, Nachman SA, Borkowsky W, Pelton SI, International Maternal Pediatric Adolescent AIDS Clinical Trials Group P1024 and P1061s Protocol Teams . Antibody persistence and immunologic memory after sequential pneumococcal conjugate and polysaccharide vaccination in HIV-infected children on highly active antiretroviral therapy. Vaccine 2013; 31:4782-90; PMID:23954381; http://dx.doi.org/ 10.1016/j.vaccine.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bhorat AE, Madhi SA, Laudat F, Sundaraiyer V, Gurtman A, Jansen KU, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine in HIV-infected individuals naive to pneumococcal vaccination. AIDS. 2015; 29(11):1345-54; PMID:25888646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nahm MH, David Goldblatt. Training manual for Enzyme linked immunosorbent assay for the quantitation of Streptococcus pneumoniae serotype specific IgG (Pn PS ELISA). (007sp Version). World Health Organization Pneumococcal Serology Reference Laboratories: WHO; 2015. Available at http://www.vaccine.uab.edu/ELISAProtocol(007sp).pdf. [Google Scholar]
- [24].Richter SS, Heilmann KP, Dohrn CL, Riahi F, Diekema DJ, Doern GV. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999–2011(1.). Emerg Infect Dis 2013; 19:1074-83; PMID:23763847; http://dx.doi.org/ 10.3201/eid1907.121830 [DOI] [PMC free article] [PubMed] [Google Scholar]


