ABSTRACT
To overcome drawbacks of current injection vaccines, such as causing needle phobia, needing health professionals for inoculation, and generating dangerous sharps wastes, researchers have designed novel vaccines that are combined with various microneedle arrays (MAs), in particular, with the multifunctional particle-constructed MAs (MPMAs). MPMAs prove able to enhance vaccine stability through incorporating vaccine ingredients in the carrier, and can be painlessly inoculated by minimally trained workers or by self-administration, leaving behind no metal needle pollution while eliciting robust systemic and mucosal immunity to antigens, thanks to delivering vaccines to cutaneous or mucosal compartments enriched in professional antigen-presenting cells (APCs). Especially, MPMAs can be easily integrated with functional molecules fulfilling targeting vaccine delivery or controlling immune response toward a Th1 or Th2 pathway to generate desired immunity against pathogens. Herein, we introduce the latest research and development of various MPMAs which are a novel but promising vaccine adjuvant delivery system (VADS).
KEYWORDS: adjuvant, controlled temperature chain, C-type lectin receptor, intradermal immunization, Liposome, mucosal immunity, microneedle array, nanoparticles, toll-like receptor
Introduction
Nowadays it is widely recognized that vaccination is the most cost-effective and the best prophylactic strategy for the treatment of many diseases, such as pathogenic infections, inflammations, and even malignant tumors.1 Unfortunately, most of the now available vaccines are administered by intramuscular or subcutaneous injection and are, thus, inevitably associated with numerous unwanted disadvantages, such as causing needle phobia and low patient compliance, the risk of potential contamination by needles, a need for highly trained personnel for inoculation,2 and also sometimes triggering poor immunity, especially, in mucosa.3 To overcome the drawbacks associated with injections, researchers have formulated novel vaccines that can be inoculated through alternative routes, such as oral uptake, inhalation, intranasal and transdermal administration.4-6 Encouragingly, some of these novel vaccines that are vaccinated through intranasal or oral uptake have been approved for marketing,4,6 offering great benefits to vaccinees, especially, children and the ones who have needle phobia.
In the past 2 decades, microneedle arrays (MAs) have been developed to deliver various vaccines through cutaneous or mucosal routes and have attracted much attention of researchers and pharmaceutical developers due to numerous advantages.7,8 Microneedles on MAs are needle-like structures, including the hollow or solid ones, both of which are usually shorter than 1 mm and used to pierce the stratum corneum or stratified squamous epithelia to facilitate the delivery of agents into skin or mucosa. For vaccine delivery, microneedles should be long enough to pierce the cutaneous or mucosal superficial layers, but preferably short enough to avoid bleeding and pain-causing. As such, MA vaccines have big advantages over others in that they have sub-millimeter structures designed to pierce the skin or mucosa painlessly and can very efficiently deliver vaccines to the cutaneous or mucosal compartments enriched in antigen-presenting cells (APCs), such as Langerhans cells, dendritic cells and macrophages, providing the basis for robust immunostimulatory effects and a significant antigen-sparing potential.9-12 In particular, MA vaccines possess the potential to simplify immunization programs by eliminating the use of hypodermic metal needles, allowing self-administration of vaccine during pandemics and, thus, facilitating large-scale immunization in developing nations with a critical shortage of healthcare workers.13,14
To date, various types of MAs have been designed for vaccine delivery and can be roughly summarized as 2 categories: the dissolvable and non-dissolvable ones.7 Usually, the non-dissolvable MAs include the hollow metal microneedles just for injection of the enclosed contents and the solid ones that are made of stiff inorganic materials, such as metal, silicon, glass and ceramics, bearing, or not, the active ingredients on microneedle surfaces and, therefore, function mainly as a poking device, micro-injection or as a master mold for fabrication of dissolving MAs. The dissolvable MAs are usually fabricated with biocompatible materials that can be degraded or dissolve in body fluids, and are either formulated as a simple homogeneous matrix entrapping antigen/adjuvant or engineered into specific multifunctional particles carrying the vaccine ingredients forming the multifunctional particle-constructed MAs (MPMAs).7,15
Usually, MPMAs are fabricated with materials in the form of particulate carriers, such as liposomes, polymeric particles, virus-like particles (VLPs), and non-virulent viruses, some of which may be further modified with functional molecules other than vaccine primary ingredients (i.e., antigen and/or adjuvant), rendering MAs an effective vaccine adjuvant and delivery system (VADS).16 Notably, these MPMAs deliver vaccines in particulate carriers as they penetrate and dissolve into skin/mucosa and, as a result, often play multiple roles, such as an antigen depot, targeting delivery, controlled release, and even the immunoresponse pathway-governor, depending on the function of decorating molecules. These molecules include TLR (toll-like receptor) ligands acting as an adjuvant, C-type receptor ligands working as an APC-targeting guide, and the micro-environmental cue-sensitive materials triggering agent release.17 Thus, the MPMA vaccines formulated with different ingredients in a specific way can induce a Th1, Th2 or mixed Th1/Th2 immune response resulting in strong cellular/humoral immunity against the loaded antigens.7 Especially, when administration through oral mucosal routes, the MPMAs can efficiently elicit systemic as well we mucosal immunity not only at the vaccination site but also throughout the mucosal network, establishing a multiple immunodefense against invading pathogens.18
To date, though various types of MPMA vaccines under development are still far away from markets, several conventional vaccines based on microneedle delivery- are undergoing clinical trials,19-23 hinting the tantalizing prospects of MPMA products, which have numerous ideal features including the high stability, convenient and painless inoculation process, the possibility of self-administration, and easy post-use disposal leaving no needle pollutions. In this review, different types of MPMAs fabricated as an effective VADS are introduced to provide a reference to readers who are going to commit to the development of MA vaccines that are suitable for wide vaccination.
Preparation of MPMAs
The MPMAs are usually fabricated using the specific microneedle array inverse molds (MAIMs), which define the number, geometry and size of the microneedles. To make the MPMAs, the functional micro/nanoparticles are firstly prepared with or without vaccine ingredients and then filled into the MAIMs, and after removal of water and, sometimes, any other solvents, the solidified MPMAs are formed.7 Although this casting-drying process for making MPMAs is simple,15,24 the premises for accomplishing such a fabrication process are that the vaccines with particulate characters have been well formulated and the MAIMs are already at hand. Unfortunately, production of either of the 2 entities is not as easy as the MPMA fabrication procedure and requires a comprehensive design and often involves rather complicated techniques.18
While the MAIMs are mainly fabricated with polydimethylsiloxane (PDMS),7,15 other materials such as ceramics and even purple sands have also been employed by some researchers to make the reverse molds with the advantage of not only simplifying the process by saving several steps and some types of special precision instruments but also omitting the use of organic solvents.25,26 By comparison, preparation of the MAIMs with PDMS is rather complex and involves several toxic solvents and numerous energy-consuming steps.27-29 Usually, the MAIMs of PDMS are fabricated from the master molds, of which the MPMAs are the exact replicates. The master molds are usually made of different stiff materials, such as silicon, titanium, stainless-steel and glass, are often fabricated by the microelectromechanical systems (MEMS) technology, including deposition, photolithography, and reactive ion etching (RIE).7,30 The details of the MAIM fabrication process can be found in several previous papers,27,30 but, herein, are briefly introduced, taking as an example the preparation of the PDMS MAIMs with silicon master molds. Figure 1 shows the process flow chart (top and side view) for fabrication of the silicon master molds and PDMS MAIMs, while the steps are briefly summarized as follows: (1) Coating a protective layer (Fig. 1a and b); (2) Patterning and isotropic etching (Figs. 1c and d);27,30 (3) Anisotropic etching; (Fig. 1e, f and g); (4) Polymerization and molding (Figs. 1h, and i).16
Figure 1.
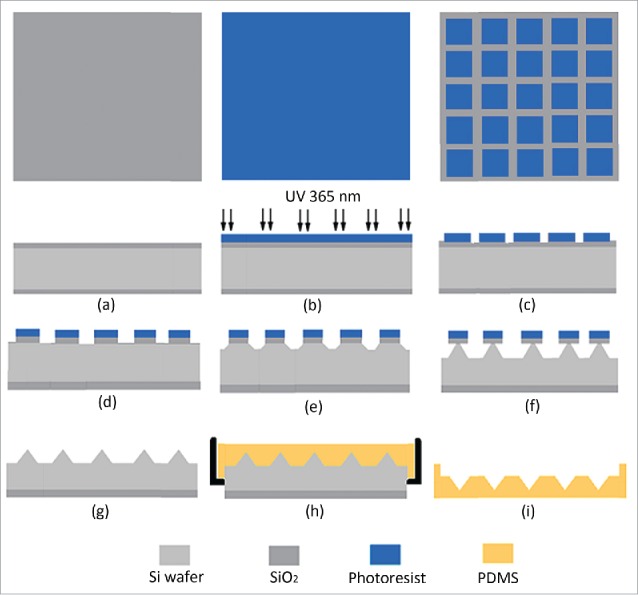
Schematic process for preparing the microneedle array reverse mold (MARM) with PDMS using photolithography and reactive ion etching (RIE) methods of the microelectromechanical systems (MEMS) technology.
The above steps are just a brief summarization of the fabrication process of the PDMS MAIMs, of which a prepared representative product is shown in Figure 2, with each of the main steps and operating conditions having been markedly simplified. However, this concise summary is a clear outline of the rather complexed process for preparing MAIMs and may provide a reference for pharmaceutical or biological researchers who do not have a strong MESM background but are facing a challenge to develop MAs for vaccine/drug delivery. For more professional details, readers are encouraged to refer to the related references.27,30
Figure 2.
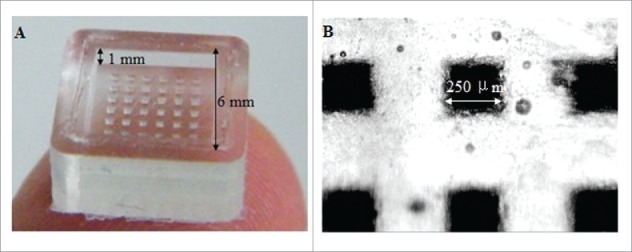
A photo of a microneedle array inverse mold (MAIM) made of PDMS taken in zoom lens with a digital camera (left) and the image of the microholes of a MARM observed under an optical microscope (right). Reprinted with permission from Reference 16.
Physical aspects and penetration of MPMAs
Size and geometry of MPMAs
The influences of microneedle geometry and size on agent delivery are theoretically identical for all solid microneedles and, therefore, are introduced here without distinguishing between MPMAs and other MAs.7,15 The microneedles on MAs are reported in literature to have various shapes including pyramidal, taper cone, cylindrical, rectangular, octagonal, and quadrangular columns with sharp tips,7,15 as shown in Figure 3. Obviously, the piercing features and the loading capacity, which usually counts on just the microneedle body but not the substrate of a MA, are the primary defining factors that have to be taken into consideration while deciding the optimum geometry of the microneedles to be prepared. Often, the microneedle length of different MAs ranges from 100 to 600 μm, while the width or diameter of the microneedle bottom plane extends from 100 to 300μm, to ensure efficient penetration, painless piercing, and also sufficient loading capacity for ingredients.7,15,18
Figure 3.
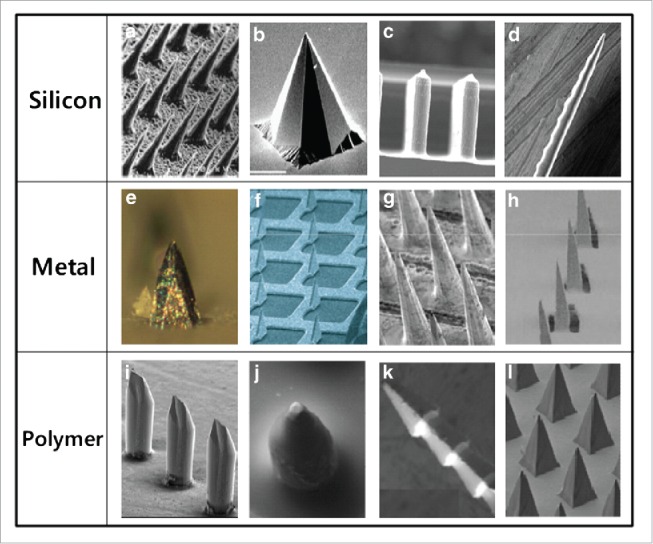
Different type of MAs made of silicon, metal and polymer with microneedles of different shapes. Reprinted with permission from Reference 7.
Factors influencing penetration
Up to now, there have been few reports about how the shape and geometry of the microneedles actually influence immunization efficacy, but there are a few reports providing evidence on their influences on the agent other than vaccine delivery. Based on physical mechanics and the physiological features of the inoculation site for the MPMAs, several factors are argued to influence the microneedle penetration into the skin or mucosa. Firstly, the elastic nature of the skin or mucosa can prevent microneedle penetration by unfolding around the needles during microneedle application. Secondly, the hardness and toughness of MAs limit the insertion forces not to exceed the tensile forces of the microneedles to avoid damaging the microstructures.15 Especially, this aspect should be taken into account when MAs with relatively long microneedles are designed for application to the tough skin rather than the much vulnerable mucosa. Thirdly, the sharpness of the microneedle tip is also an important factor for skin and mucosa penetration, since sharp microneedles will penetrate more efficiently than the blunt ones.31 Therefore, the microneedle geometry plays a crucial role in MA-based vaccine delivery since it defines the sharpness and strength of the microneedles and will finally influence insertion stress. Fourthly, the microneedle density of a MA (the number of microneedles per row or the number of microneedles related to the surface area of patch) will also have an influence on the penetration. Usually, MPMA microneedles with a low density can easily pierce the skin or mucosa because they can pull it tight between the needles. In contrast, very dense microneedles fixed on a MPMA are less efficient in piercing the vaccination site due to the ‘bed of nails’ effect they exhibit,15 as shown livingly by the action of an acrobat who lies on an array of sharp but dense nails without injury.
Recently, there has been an interest to investigate the influence of different variables related to the microneedle physical properties to reach optimum microneedle design and, hence, improve the transdermal drug delivery using MAs.32 Many relevant factors have been considered crucial to formulate an optimized microneedle array system, including the geometry and shape of the microneedles, microneedle density, microneedle length, and microneedle bottom and even tip radius.
Park et al. fabricated beveled- and chisel-tip and tapered-cone microneedles using biodegradable polymers, such as PLA and PLGA, measured needle mechanical properties, and assessed their ability to increase transport across the skin.29 Mechanical testing proved that the microneedle failure forces (the force at which needles broke during axial loading) increase with Young's modulus of the material and needle base diameter but decrease with needle length, while they are larger than the forces needed to insert microneedles into skin, allowing MAs to increase permeability of human cadaver skin to a low-molecular weight tracer calcein and a macromolecular protein bovine serum albumin (BSA) by up to 3 orders of magnitude.
To optimize the microneedle dimensions with a view to increase skin permeability, Al-Qallaf and Das using algorithm based on an in-house java program presented a framework, allowing one to choose dimensions according to one's need.33 Their optimization program suggests that the optimum patches have the number of microneedles per row (n) equal 20 and 13 for solid and hollow microneedles, respectively, while the surface area of patch and microneedle bottom radius equal 0.04 cm2 and 25 µm, respectively, in case of solid microneedles. The authors also showed that the aspect ratio (relates to the ratio of the center-to-center distance between 2 microneedles (pitch) to the microneedle bottom radius, R) should be greater than 2.0 so that an overlapping between any microneedles does not occur.
Bal et al. described the influence of microneedle geometry on agent transport through the formed conduits by LSCM (laser scanning confocal microscopy) visualization in human volunteers, who received 3 types of MAs with 300-μm length microneedles of different shapes (Fig. 4) with the model agent fluorescein which was applied either before or after piercing.32 They observed that the shape of the conduits formed by microneedle piercing was dependent on the type of MA used and the microneedles with the shape of oblique section-cut cylinder (Fig. 4A, named 300A for convenience) formed half-moon shaped conduits which were much larger and deeper than those visualized after treatment with the other 2 MAs, which had shapes of oblique section-cut square column (Fig. 4B, 300B) and regular cone (Fig. 4C, 300C), respectively, and left behind the more round-shaped conduits with similar size. Also, these piercing effects were observed at different times of 5, 10 and 15 min after MA administration. In addition, they found that the fluorescent dye preferably diffused through the lipid regions surrounding the corneocytes, outlining the cells. Though application of the MAs with 300-μm length microneedles was not perceived as painful, administration with the 300A microneedles, in contrast to the other 2 types, resulted in, occasionally, small blood spots which were regarded as the sign of side effects and the relatively large holes, whereby bacteria and other pathogens may, arguably, enter into skin.
Figure 4.
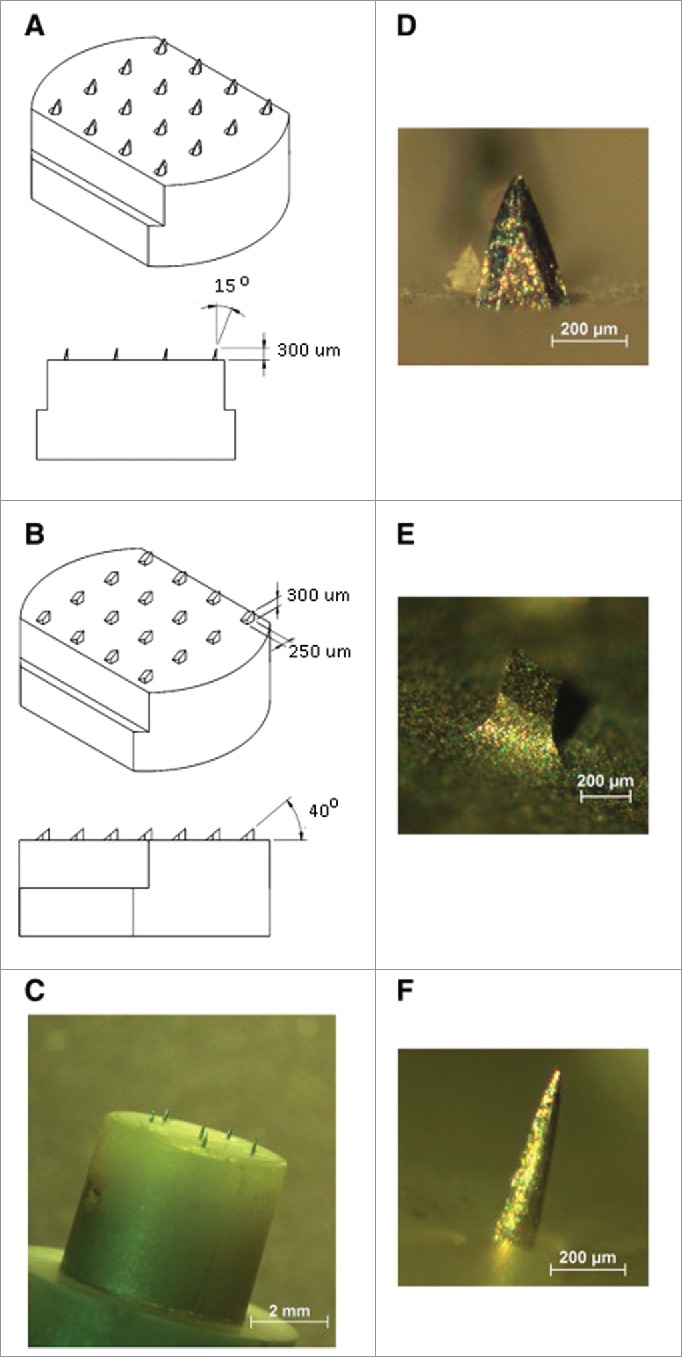
Images of the 3 different microneedle arrays used in this study. A: 300A microneedle array, assembled of 30 G needles. B: 300ED stainless steel microneedle array. C: Dermastamp consisting of 6 microneedles. In figure D, E and F higher magnification images of single microneedles are shown. Reprinted with permission from Reference 32.
Although microneedle geometry and size has an influence on the insertion ability of MAs, many issues, such as delivery efficiency, therapeutic or immunization efficacy and side effects, for different MAs with differently shaped microneedles remain yet unclear, despite the fact that several types of MAs with irregular shaped microneedles have been developed and investigated for agent delivery in animal models.7 Nevertheless, many novel MAs, especially MPMAs, have been successfully developed for drug and, increasingly, for vaccine delivery and proven highly effective in enhancing efficacy in preclinical investigations.
MPMAs for vaccine delivery
A MPMA constructed for vaccine delivery usually comprises 2 parts: the basement or pedestal and a number of microneedles fixed on the basement.15 While the basement is, in most cases, composed of inert materials as matrix, the microneedles contain the functional particles, antigens/adjuvants which may or not be incorporated into those particles, and the necessary excipients that are well-chosen for shaping, protecting, and increasing the adjuvanticity and efficacy of the vaccines. The vaccine antigens are often in the form of the antigenic proteins isolated from the surface expressions by pathogens or the whole live attenuated or inactivated pathogens, such as viruses and bacteria.34-36 The isolated antigenic proteins, used for constituting so called subunit vaccines, are often entrapped or integrated into the functional particles such as liposomes, PLGA particles, virus like particles (VLPs), which may be combined with biofunctional materials including adjuvants to increase the delivery and immunization efficiency of vaccines.35,37 The whole live attenuated or inactivated pathogens can rarely be entrapped in micro/nanoparticles due to their large individual size and therefore are usually directly incorporated in or coated on the surface of MA microneedles and, also, may be separately delivered under the assistance by poking with independent MAs. However, MA delivery of vaccines composed of the nonvirulent whole pathogens, which, though, are actually the micro/nano-sized particles, has, whether or not, been widely tested or validated in the clinic and is not yet included in this paper which focuses on only the artificial micro/nanoparticles.
Multifunctional liposomes constituted MPMAs
Liposomes were discovered by British haematologist Bangham and coworkers, in the early 1960s, and have since been widely explored for vaccine delivery due to their good biocompatibility, high loading capacity to a variety of agents and well-established production method.38-41 Also it is argued that when used as a vaccine carrier liposomes fulfil a dual function of delivery and adjuvanticity, although the latter is rather weak.42 Thus, to increase the adjuvanticity, antigen-loading capacity as well as delivery efficiency, conventional liposomes are often modified on surface or in membranes with functional materials, such as the ionizable lipidic ingredients, TLR ligands, and C-type receptor-binding molecules, engendering the multifunctional liposomes to act as a carrier that can efficiently capture the oppositely charged antigens and targetedly deliver the immunogenic agents to APCs.43,44
Currently, liposomal vaccines are also fabricated into MAs to enhance their transportation into superficial layers of skin or mucosa, wherein affluent APCs are patrolling in surveillance of the invading pathogens. Gao's group prepared the cationic CpG OND-liposomes entrapping antigens or hepatitis B virus DNA, which were subsequently fabricated into the microneedles (9 × 9 right square pyramid with a base width of 250 μm, and 550 μm in height) of MAs with PVP as a pedestal and matrix.45 When patched onto mice skin, this kind of MAs induced the production of high levels of anti-antigen IgG and IgG2a antibodies and directed a shift of the immunoresponse type from predominately Th2 type to a balance Th1/Th2 type. In particular, this kind of MPMAs loaded with hepatitis B virus DNAs could effectively deliver DNA vaccine into skin, inducing effective immune response toward the desired pathway establishing robust immunity against HBV in mouse model.46 By comparison, Bouwstra's group demonstrated that encapsulation of model antigen OVA into cationic DOTPA/CpG OND-liposomes had a beneficial effect on the quality of the antibody response in mice after intranodal or i.d. immunization via needle injection but, notably, impaird proper delivery of antigen and adjuvant to the lymph nodes when the formulations are administered intra-nasally or transcutaneously at the site pre-treated by metal MAs (4 × 4 microneedles with a length of 300 μm).47 These results suggest that, in contrast to liposomal vaccine-incorporated MAs, pre-treatment with independent MAs for micro-channel formation is not beneficial for vaccination with liposomal vaccines.
Irvine's group engineered a special kind of multifunctional liposomes, which were the negatively charged interbilayer-cross-linked multilamellar lipid vesicles (ICMVs) containing TLR4 ligand monophophoryl lipid A (MPLA) and proved to be a potent vaccine carrier.48 Using PLGA as the matrix and pedestal, the researchers fabricated MPMAs with individual microneedles coated with multilayer films via layer-by-layer assembly of the biodegradable cationic poly(β-amino ester) (PBAE) and negatively charged ICMVs (Fig. 5).49 Given to mice on skin, the MPMAs effectively transferred the multilayer films of PBAE/ICMV from microneedle surfaces into cutaneous tissues, where the ICMV loaded cargos remained over the course of 24 h, increasing local APC uptake of the delivered antigens and stimulating the immune systems in situ, which resulted in robust antigen-specific humoral immune responses with a balanced generation of Th1/Th2 types.
Figure 5.
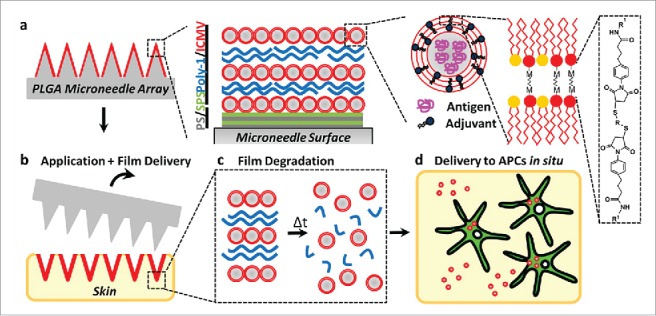
(a) Schematic illustration of (Poly-1/ICMV) multilayers deposited onto PLGA microneedle surfaces (Poly-1 = PBAE). ICMV lipid nanocapsules are prepared with interbilayer covalent cross-links between maleimide head groups (M) of adjacent phospholipid lamellae in the walls of multilamellar vesicles. (Poly-1/ICMV) PEMs were constructed on microneedles after (PS/SPS) base layer deposition. (b) Microneedles transfer (Poly-1/ICMV) coatings into the skin as cutaneous depots at microneedle insertion points. (c) Hydrolytic degradation of Poly-1 leads to PEM disintegration and ICMV release into the surrounding tissue. (d) ICMV delivery to skin-resident APCs provides coincident antigen exposure and immunostimulation, leading to initiation of adaptive immunity. Reprinted with permission from Reference 49.
Although most of the MPMA-based vaccines under development have been designed for percutaneous immunization, intramucosal administration provides an alternative inoculation route with numerous additional benefits, as reported by Wang's group in their papers on MPMA vaccines.16,50 Using emulsification-lyophilization procedure,39-41,51,52 the researchers successfully created multifunctional liposomes bearing decorations of C-type receptor ligand mannose-PEG-cholesterol conjugate and TLR4 ligand MPLA, forming the mannosylated/lipid A-liposomes (MLLs), which were confirmed to be an effective vaccine carrier for administration at oral cavity mucosa by inducing enhanced antigen-specific IgG, mucosal secreting IgA and chemookines such as IFN-γ and IL-4.44 Going further, they incorporated the MLLs into the microneedles of a new kind of MAs, which were called proMMAs and had an array of 6×6 pyramidal microneedles, each with a base width of 250 μm and a height of 650-μm, using PVP and sucrose as the matrix, base plates as well as the dehydration protectants for antigens (OVA or HBsAg) (Fig. 6).16 Thanks to lack of water and the presence of dehydration protectants, the proMMAs proved rather stable satisfying the handle condition of CTC,13 which is a very useful measure to guarantee the bioactivity of the vaccines transported and allocated in remote areas where the integrated cold chain is not often available.53 Patched onto mouse oral cavity mucosa other than skin, the proMMAs, which were tough enough to pierce porcine skin, dissolved rapidly recovering, driven by interstitial fluid rehydration force, in situ the initial multifunctional MLLs which subsequently functioned the roles of vaccines and stimulated robust systemic immune responses against the loaded antigens (OVA or HBsAg). Also, the researchers demonstrated that proMMAs could enormously save vaccine doses due to their direct delivery of MLLs across mucus barrier into oral mucosa, preventing the ingredient loss caused by oral fluids and swallowing. In contrast to the results by Irvine et al., who showed that transcutaneous administration of MA-based vaccines could still induce mucosal immune responses in mammals,54 Wang's group revealed that only through mucosal rather than cutaneous immunization could the effective mucosal immunity be established by proMMAs, as evidenced by their observation that high levels of antigen-specific IgA in the salivary, intestinal and vaginal secretions were only detected in the mucosally vaccinated mice.16 Also, the proMMAs induced mice to initiate a mixed Th1/Th2 immune response against the loaded antigens, such as OVA and HBsAg, due to the dual decoration of liposomes with a mannose derivative and adjuvant lipid A, which can respectively facilitate APC uptake of the pathogen-like MLLs through mannose receptor-mediated endocytosis and APC cross-presentation of the internalized particulated antigens with MHC-I to CD8+ T cells through activation of TLR-mediated signaling pathways.42,52,55,56 Thus, the proMMA-based vaccines have the capacity to induce both systemic and mucosal immune responses to the loaded antigens to set up strong cellular and humoral immunity against the related pathogens.
Figure 6.
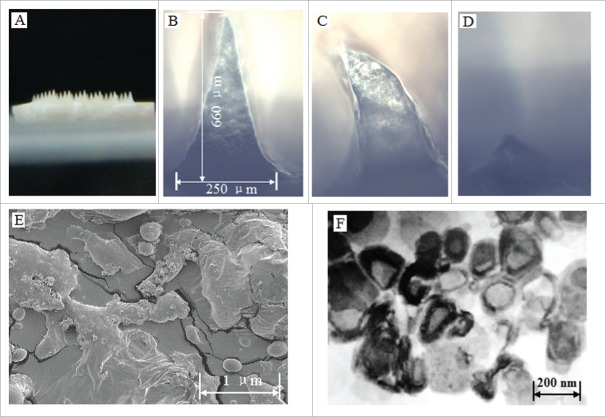
Characteristics of the prepared proMMA and MLLs. (A) Image of the prepared proHMA with 6 × 6 microneedles. (B) An optical microscopy image of a proHMA microneedle, which, upon rehydration, dissolved rapidly with changing its shape within 5 s (C) and almost disappeared in 1 min (D). (E) SEM of the powders of the proHMA microneedles. The within numerous nanospheres were proliposomes of the HBsAg-MLLs. (F) TEM of the HBsAg-MLLs prepared by procedure of emulsion-lyophilization. Reprinted with permission from Reference 16.
Clearly, different types of the multifunctional liposome-constituted MAs, including the proMMAs with oral cavity immunization, have created many novel vaccine platforms which, once well developed, will bring great benefits to humans, especially children, who need urgently the effective, convenient and safe vaccines to set up systemic as well as mucosal immunity as a first line of defense against numerous invasive pathogenic microorganisms, such as HBV, HIV, malaria, Dengue, and the high lethal Ebola viruses.57
PLGA particles constituted MPMAs
To adjust the pharmacodynamics and release kinetics of a drug as what is desired, numerous macromolecules have been synthesized for constructing pharmaceutical carriers. Among these, PLGA (poly(lactic-co-glycolic acid)) is a well-known biocompatible polymer which is rather safe and will hydrolyse into the original monomers of lactic acid and glycolic acid in the body.58 PLGA not only has been used in a number of Food and Drug Administration (FDA) approved therapeutic products,59 but also is engineered into micro/nanoparticles as a vaccine carrier.59-61 However, an effective carrier should have the ability to deliver vaccines to the professional APCs, such as dendritic cells (DCs) and macrophages, which are argued to be most efficient in antigen presentation for initiating immune responses but are usually lurking in plenty in superficial skin and mucosa.
To develop skin DC-targeting vaccines, Kissenpfennig's group prepared dissolving MAs with a 19 × 19 array of 600-μm height microneedles which were incorporated with OVA-encapsulated PLGA nanoparticles engineered with size of 357 nm, zeta-potential of −20 mV, and OVA entrapment efficiency of 35%.62 The researchers vaccinated mice by manually inserting the MPMAs onto the untreated dorsal side of both ears and showed that the skin-resident DCs first took up the antigen-PLGA nanoparticles and then delivered them to cutaneous draining lymph nodes where they subsequently induced significant expansion of antigen-specific T cells. Moreover, mice received this kind of MPMAs generated robust antigen-specific cellular immunity, which provided a complete protection against the challenges with either antigen-expressing B16 melanoma tumors or a murine model of para-influenza, through the activation of antigen-specific cytotoxic CD8+ T cells that resulted in efficient clearance of tumors and virus. Further investigation confirmed that following MPMA immunization, it was Langerhans cells (LCs) that constituted the major skin DC subset capable of cross-priming antigen-specific CD8+ T cells ex vivo.63 Although all DC subsets were equally efficient in priming CD4+ T cells, LCs were largely responsible for orchestrating the differentiation of CD4+ IFN-γ- and IL-17-producing effectors, as evidenced by the observation that depletion of LCs prior to immunization had a remarkable negative effect on CD8+ CTL responses in vivo, rendering the vaccinated animals the reduced protective anti-tumor and viral immunity. Notably, the cross-priming bias was lost following i.d. immunization with the soluble antigen-laden MAs, suggesting that processing and cross-presentation of nano-particulate antigen is favored by LCs highlighting the importance of LCs in skin immunization. Thus, selectively targeting the skin resident DCs with nano-particulate immunogens in dissolvable polymeric MAs potentially provides a promising technological platform for improved vaccination strategies.
Also, Irvine and his group manufactured PLGA microparticle MAs entrapping protein antigens and adjuvant Cy3-poly(I:C). The PLGA microparticles had a size of about 1580 nm in diameter and zeta potential of −26 mV and were fabricated to form the MPMAs which had conical microneedles of 700-μm in height and 250-μm in diameter at the base with water-soluble poly(acrylic acid) (PAA) as a supporting pedestal.64 The MA microneedles proved thermostable and able to perforate the stratum corneum and implant the antigen/adjuvant-loaded PLGA microparticles in the epidermal compartment following patch removal due to rapid separation of microneedles from PAA pedestal (Fig. 7). Notably, the formed depot of PLGA microparticles remained in the skin for weeks after patching and maintained the release of encapsulated cargos for sustained delivery, resulting in potent humoral and cellular immunity matching or exceeding that by traditional needle injection vaccination. These results suggest that PLGA microparticle-constituted MPMAs are an effective platform for straightforward and robust transcutaneous vaccine deliverythrough engendering the controlled release depot of vaccines.
Figure 7.
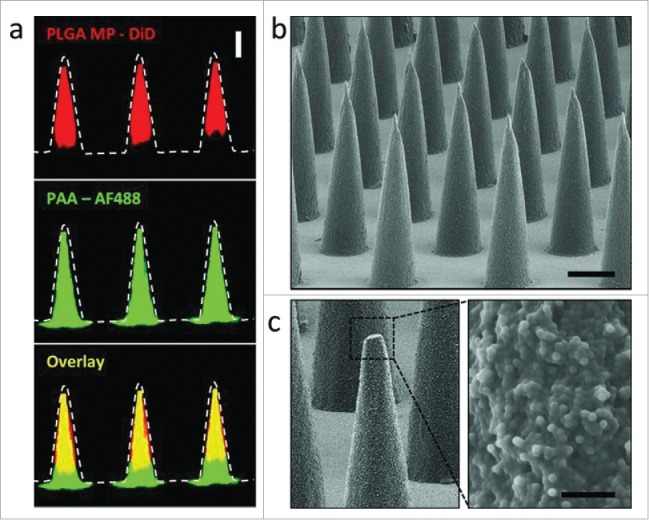
(a) Confocal microscopy images of PLGA-PAA composite microneedles fabricated to encapsulate DiD-loaded PLGA microparticles (MP) (right, scale bar 200 μm). SEM images of (b) resulting microparticle-encapsulating microneedle array (scale bar 200 μm) and (c) high magnification image of the composite needle interior of a fractured microneedle (scale bar 10 μm). Reprinted with permission from Reference 64.
Chitosan nanoparticles delivered with MAs
Chitosan is a linear glucosamine polysaccharide with mucoadhesive property and has a positive charge under acidic conditions, allowing it to be used widely for drug delivery.65 Recently, Bouwstra's group made a study to gain insight into the transcutaneous delivery and immunogenicity of N-trimethyl chitosan (TMC) nanoparticles entrapping diphtheria toxoid (DT) as an adjuvant, which were applied transcutaneously with an independent metal MA.66 Mice were vaccinated with DT-loaded TMC nanoparticles, a solution of TMC and DT (TMC/DT) or DT alone, which were applied onto the skin before or after microneedle treatment with 2 different 300-µm-long microneedle arrays and were also injected i.d.ly (ID). They found that independent of the MA used,the sequence of microneedle treatment and vaccine application, transcutaneous immunisation with the TMC/DT mixture solution elicited 8-fold higher IgG titres compared to the DT-loaded TMC nanoparticles or DT solution, while the toxin-neutralising antibody titres from this group were similar to those elicited by subcutaneous DT-alum. Confocal microscopy studies revealed that transport of the TMC nanoparticles across the microneedle conduits was limited compared to a TMC solution. Also, in another study this group demonstrated that after transcutaneous administration with metal MAs, TMC–OVA conjugates were more immunogenic than either TMC/OVA nanoparticles or the TMC + OVA mixtures, likely because they penetrate the skin more easily than nanoparticles and, consequently, were better delivered to DCs, while they showed higher uptake by DCs than TMC + OVA mixtures.67 Thus, it was argued that TMC had indeed an adjuvant function in transcutaneous immunization with microneedles, but only if applied in a solution, denying the necessity of engineering TMC into nanoparticles as vaccine in carriers when using independent MA for delivery. This suggests that the design and development of MPMA vaccines is much more complexed than the speculation just based on the existent data of specific MAs and, thus, requires sufficient precautions, considerations and explorations.
Polyelectrolyte multilayer constituted MPMAs
The polyelectrolyte multilayer (PEM) is an ultra-thin film which is formed by the alternate adsorption of positively and negatively charged polyelectrolytes on a substratum.68 The electrostatic attraction between multiple oppositely charged polyelectrolytes provides relatively firm interactions for immobilization of biomolecules within the PEMs, engendering a kind of useful carriers for loading various vaccine agents including antigenic peptides, DNAs or RNAs.69
However, the planar multilayer patches can rarely facilitate entry of released cargos from the PEM films into the epidermis due to their specific configuration. DeMuth and coworkers engineered DNA vaccine-entrapping PEMs which were further fabricated into MAs with 9 × 9 conic microneedles of 250 µm in diameter at base and 650 µm in height and a matrix/basement of poly(L-lactide) (PLA), forming this so called “multilayer tattoo” DNA vaccines.17 Using melt-molding protocol the authors produced the PLA-based MPMAs, which were then coated with a release layer of photo- and pH-sensitive PNMP, and then a PEM film of 20 bilayers of protamine-sulfate (PS) and poly(4-styrene-sulfonate) (SPS) to provide a uniform charge density through the use of layer-by-layer (LbL) self-assembly, and finally, by iterative adsorption, with an overlying PEM film composed of immune-stimulatory poly (I:C) and/or vaccine DNA (Cy5-labeled pDNA encoding luciferase, Cy5-pLUC) and a biodegradable poly(β-amino-ester) (PBAE), forming the MA coated with PEM films as “multilayer tattoo” DNA vaccines. Further investigation demonstrated that the MAs with pH-sensitive release layer allowed PEMs to transfer into the skin after brief microneedle application and promoted local transfection by increasing the retention of DNA and adjuvants in the skin of C57Bl/6 mice from days to weeks, with kinetics determined by the film composition. In addition, the “multilayer tattoo” DNA vaccines in the MPMAs induced immune responses against a model HIV antigen comparable with electroporation in mice; increased memory T-cell generation and produced 140-fold higher gene expression in non-human primate skin compared with i.d. DNA injection. These results suggest that this type of MPMAs composed of PEM films overlying a pH-sensitive release layer is a promising carrier for improving DNA vaccination against HIV viruses, which have claimed millions of human lives since their discovery due to lack of an effective vaccine for prophylaxis of their infection.
Virus-like Particle Constituted MPMAs
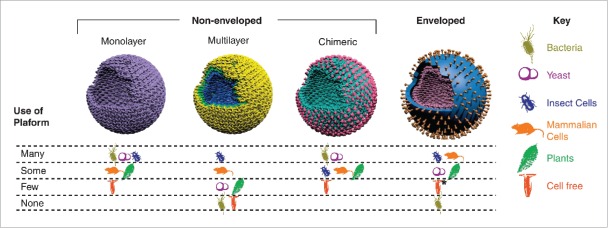
Virus-like particles (VLPs) are nanoparticles formed by multi-subunit protein complexes through self-assembly into the 3D structures that mimic conformation of native viruses.70 VLPs are non-infectious and unable to replicate due to lack of viral genetic material and, therefore, are considered excellent candidates for vaccine carriers, because the repetitive arrays on their surface resembling pathogen features can be recognized by the immune system to induce strong humoral and cellular responses even in the absence of frequently used adjuvants. To date, more than 110 types of VLPs from 35 different pathogen families have been constructed and evaluated in different fields, and Figure 8 summarizes different platforms available used to produce different VLP configurations.71 Notably, several VLP-based vaccines have already entered markets, including the HBV, HPV, HEV, and RTS,S vaccines,72 among which the HPV VLP vaccines have been tested with MPMA delivery in animal models.
Figure 8.
Different VLP platforms developed to produce VLP vaccines with different configurations. Reprinted with permission from Reference 71.
Influenza VLP vaccine constituted MPMAs
For i.d. delivery of influenza vaccines, Quan et al. using Spodoptera frugiperda Sf9 as factory cells manufactured influenza VLPs, which contained H1 HA and M1 proteins derived from H1N1 A/PR/8/34 virus and were coated onto steel MA 5-microneedle surfaces stabilized with disaccharide trehalose.73 In vivo experiments showed that mice received a single dose of MA influenza VLPs (0.35 μg of total VLP protein) in the skin produced high levels of IgG2a, HA inhibition titers, neutralizing antibodiesby increased antibody-secreting plasma cells, and acquired remarkable 100% protection immunity against the lethal influenza virus; while mice with intramuscular influenza VLPs (0.35 μg of total VLP protein) obtained inferior immunity with only partial protection (≤40%) from the lethal influenza virus. Using similar strategies, Song et al. proved that H5N1 avian influenza VLP-constituted MAs induced long-lasting B- and T-cell responses with improved protection in mice.74,75 Also, Pearton et al. found that influenza VLP-based MAs could initiate a stimulatory response in Langerhans cells (LCs) through deposition of the vaccine in human skin.12 And further exploration of the complex molecular and cellular host responses to influenza VLP MAs revealed up-regulation of a host of genes responsible for key immunomodulatory processes and host viral response following microneedle injection of VLPs.76
More recently, a broadly cross-protective influenza A intradermal MA vaccine was developed based on VLPs anchored with the M2 protein which is influenza virus extracellular domain and remains nearly invariant among different strains and is, thus, a promising candidate antigen for developing universal influenza vaccines.77 To further broaden the immunological protective scope, the researchers genetically engineered a new construct with a tandem repeat of M2e sequences (M2e5x) derived from human, swine, and avian origin influenza A viruses to better cover a broader range of influenza viruses. Then, M2e5x sequences were expressed on a VLP platform and manipulated into a membrane-anchored form of M2e5x VLPs, which were coated onto steel MA microneedles (700 μm in length and 200 μm in width) with carboxymethyl cellulose and trehalose as a stabilizer (Fig. 9). The MA M2e5x VLPs maintained stability for 8 weeks at room temperature and, when patched to the skin of mice, induced strong humoral and mucosal M2e antibody responses and conferred cross-protection against challenges with heterosubtypic H1N1, H3N2, and H5N1 influenza viruses through additional robust cellular immunity, as indicated by high levels of IgG2a isotype antibodies and IFN-γ. The potential immunological and logistic advantages of M2e5x VLP MA vaccines may offer a promising approach to develop easy-to-administer universal influenza vaccines.
Figure 9.
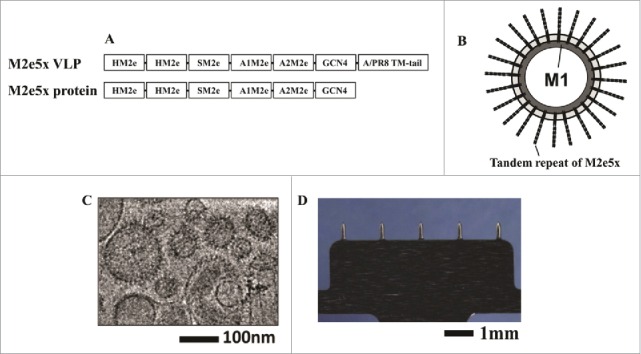
MAs and M2e5x VLPs or M2e5x proteins for vaccination. (A) Structure of M2e5x VLP or M2e5x proteins. HM2e: human M2e, SM2e: swine M2e (2009 pandemic flu), A1M2e: major avian M2e, A2M2e: minor avian M2e. (B) Schematic diagram of influenza M2e5x VLPs containing tandem repeat of heterologous M2e and matrix (M1) proteins. (C) Cryo-TEM (transmission electron microscopy) image of influenza M2e5x VLPs. (D) Microneedle array coated with M2e5x VLPs. Reprinted with permission from Reference 77.
Human papilloma VLP vaccine constituted MPMAs
Currently, the 2 successfully licensed human papilloma virus (HPV) vaccines are both VLP-based intramuscular injection products, bearing inconvenient immunization disadvantages, such as causing pain, needing professional personnel and producing metal needle wastes. Notably, because the HPV capsids confer tropism for basal epithelium, HPV VLPs represent attractive carriers for vaccination targeted to the skin using microneedles, providing the potential to abandon intramuscular injection for HPV vaccination.78 To develop convenient HPV vaccines as well as to fully exploit skin-targeting features of HPV VLPs, Kines et al. encapsidated DNA expressing the M/M2 or F-protein antigen of respiratory syncytial virus (RSV) in HPV16 pseudovirions (PsV) engendering the HPV16-M1/M2-PsV and HPV16-F-PsV, respectively, which were coated onto steel MA microneedle surfaces with carboxymethyl cellulose and trehalose as a stabilizer.78 Immunization of mice with HPV16-M/M2 MAs induced M/M2-specific T-cell responses which were detected post RSV challenge, while the HPV16 PsV-F immunized mice were fully protected from challenge with HPV16 PsV and had reduced RSV viral load in lung and nose upon intranasal RSV challenge. The results suggest that MA delivery of lyophilized HPV PsV as VLPs may provide a practical, thermostable combined vaccine approach against different pathogens.
Perspectives
Benefits of MPMA vaccines
Up to now, a variety types of MPMAs have been developed for vaccine delivery. Unfortunately, there are no MPMA vaccines that have been approved for use or even for clinical trials for prophylaxis of the existing infectious pathogens. The MPMAs contain the particles that can be easily decorated with different molecules to fulfil targeting delivery or governing immune response toward a Th1, Th2 or mixed Th1/Th2 pathway to generate the desired immunity against pathogens. In contrast to non-dissolvable MAs, the dissolvable MPMAs can deliver vaccines without sharp wastes left behind after vaccination and enhance vaccine stability through incorporating vaccine ingredients within micro/nanoparticles or microneedle anhydrous matrix.50 The dissolvable MPMA vaccines are also helpful for enhancing vaccination coverage rates,18 because their high thermostability makes the products applicable to the CTC for dispatch in areas lack of an integrated cold chain,13 their convenient vaccination needs no professionals and expands the inoculation coverage in poor districts,13,16 and also their high efficacy reduces the dose and dosing times for a vaccine that is in shortage, especially, during a pandemic period.35
Vaccination routes
Although most of the current MA vaccines are designed for administration via mammalian skin, oral cavity and vaginal mucosa provides indeed a practical option of sites through which the MPMAs can be vaccinated. Mucosal vaccination of MPMAs may bring in numerous benefits, such as good compliance, convenience, vaccination by self-administration or by minimally trained personnel, production of zero pollution thanks to uptake of the MPMA pedestal or other rests into gastrointestinal tract of recipients, and, especially, the induction of systemic as well as mucosal immunity, which is usually elicited mainly via mucosal route as the first line of immunodefenses and, thus, very crucial to protecting the body from attack by infectious pathogens.16 In addition, mammalian oral mucosa is usually much easier to pierce by MPMAs than the skin,9,15,79 and this markedly reduces the hardness requirement for microneedles and, therefore, greatly expands the scope of materials and procedures that can be used for producing MPMA vaccines.50 By comparison, i.d. MPMA vaccination may leave behind on the skin the ingredient residues, which upon hydration may be rather adhesive and are very likely to stain the clothes by contamination; in contrast, mucosal MPMA vaccination does not cause this kind of troubles. Therefore, in the authors' opinion, future efforts on the development of MPMA vaccines might well be put on designing the products suitable for mucosal vaccination.80
Hurdles to the development of MPMA vaccines
The dissolvable MPMAs made of micro/nanoparticles, polymers and saccharides are relatively brittle and frail presenting a big challenge to packaging, transportation and vaccination. For efficacious vaccine delivery, the MPMAs must be fabricated with appropriate ingredients to ensure their sufficient hardness for skin penetration and resistance to friction encountered before or during vaccination.81 It is reported that the MPMAs are tough enough to pierce mammalian stratum corneum when they are constructed with an optimized combination of biocompatible polymers, such as PVP and PLGA, and/or sugars, such as sucrose and trehalose, and the highly active adhesives, such as CMC-Na and starch. However, in practice, human individual variation in race, gender and even age of recipients may require much more high levels of hardness for MPMAs than the standards used in models with a limited sample size.82
In addition, preparation of MAs, especially the dissolvable MPMAs, is rather complex and usually involves numerous fine steps using a variety of precision instruments,18,27 with the bottle neck lying in the large scale production of MAIMs as preliminary reserves using MEMS technology, such as photoresistence deposition, lithography pattern and RIE.18 Recent progress and advances in related fields make it possible to massively produce the MAIMs, but, at the cost of production costs, which also arises from the exquisite package required to ensure the fragile microneedles remain intact before vaccination.83 Although MA delivery can reduce the cost of vaccination and the increased thermostability may lower dispatch spending by omitting the expensive cold chain, the production costs of MPMA vaccines can hardly be expected at levels offsetting the overall expenditures associated with final clinical use of the products. Nevertheless, it is the key attributes of MPMA vaccines, such as usability, wear time, and proper disposal that control the product acceptability in the marketplace, which, in turn, influences the inputs of social and industrial resources.84
Hopes and prospects of MPMA vaccines
Currently, there are no MPMA vaccines that have been approved for use or even for clinical trials for prophylaxis of the existing infectious pathogens. However, numerous prominent advantages of this novel VADS attract more and more academic and company researchers to devote their efforts to the development of vaccines based on MPMAs. Encouragingly, several clinical trials on other types of both dissolvable and non-dissolvable MA vaccines have already been carried out by researchers and physicians. And the safety and effectiveness of these MA-based vaccines have been primarily confirmed by the updated clinical trials.20,21,85 Also, a recent interview showed high support for administration of MA vaccines by key opinion leaders in the United States, who suggested that the priority considerations in the ongoing development of MA vaccines include confirming efficacy and ensuring safety for self-administration, ease of use, short wear times, and an easily accessible application site.84 All these suggest that MA vaccines have attracted attentions not only from the academic and industrial communities but also from the governmental administrations, as also indicated by the fact that MAs combine with several conventional vaccines, such as influenza, polio and measles, have been approved for clinical trials under collaboration between researchers, manufacturers and the administrative authorities.86-88 In summary, it is reasonable to argue that the wide collaborative and incessant efforts by manufacturers, governments and scientists from different fields, such as pharmacy, immunology, bioengineering and MEMS, may eventually push certain MA, even MPMA, vaccines into clinical use for prophylaxis of infectious diseases. If that is realized, an era of ‘vaccination without pain’ does come.
Abbreviations
- APC
antigen-presenting cells
- CDC
Centers for Disease Control and Prevention
- CMC
carboxymethyl cellulose
- CpG ODN
CpG oligodeoxynucleotides
- CTC
controlled temperature chain
- FITC
fluorescein-5-isothiocyanate
- HA
hemagglutinin
- HBsAg
hepatitis B virus surface antigen
- ICMV
interbilayer-cross-linked multilamellar lipid vesicle
- MAIM
microneedle array inverse mold
- MEMS
microelectromechanical system
- MHC
major histocompatibility complex
- MPLA
MPLA, monophosphoryl lipid A
- MPMA
multifunctional particle-constituted microneedle array
- OVA
OVA, ovalbumin
- PAA
polyacrylic acid
- PBAE
poly(β-amino ester)
- PDMS
polydimethylsiloxane
- PEG
polyethylene glycol
- PEM
polyelectrolyte multilayer
- PLA
polylactic acid
- PLGA
polylactic-co-glycolic acid
- PVP
polyvinyl pyrrolidone
- RIE
isotropic reactive ion etching
- TLR
toll-like receptor
- VADS
vaccine adjuvant-delivery system
Acknowledgments
The authors acknowledge that this work was financially supported by the Program of Visiting Research and Study Abroad for the University Middle-aged and Young Key Talents (Grant number gxfxZD2016045) and the University Natural Science Research Project (Grant number KJ2016SD28), both sponsored by the Department of Education of Anhui Province; and also by Program of Domestic Visiting Scholars for the University Young Key Teachers by the Ministry of Education of China, which was performed in University of Science and Technology of China.
Author Contributions
X. Wang, N. Wang wrote the primary manuscript. N. Li, Y. Zhen collected the data. T. Wang edited the final edition.
References
- [1].Ozawa S, Mirelman A, Stack ML, Walker DG, Levine OS. Cost-effectiveness and economic benefits of vaccines in low- and middle-income countries: a systematic review. Vaccine 2012; 31:96-108; PMID:23142307; http://dx.doi.org/ 10.1016/j.vaccine.2012.10.103 [DOI] [PubMed] [Google Scholar]
- [2].Levine MM. “IDEAL” vaccines for resource poor settings. Vaccine 2011; 29 Suppl 4:D116-25; PMID:22486974; http://dx.doi.org/ 10.1016/j.vaccine.2011.11.090 [DOI] [PubMed] [Google Scholar]
- [3].Chen D, Zehrung D. Desirable attributes of vaccines for deployment in low-resource settings. J Pharm Sci 2012; 102:29-33; PMID:23136115; http://dx.doi.org/ 10.1002/jps.23352 [DOI] [PubMed] [Google Scholar]
- [4].Childress BC, Montney JD, Albro EA. Making evidence-based selections of influenza vaccines. Hum Vaccin Immunother 2014; 10:2729-32; PMID:25483499; http://dx.doi.org/ 10.4161/hv.29340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Domingues CM, de Fatima Pereira S, Cunha Marreiros AC, Menezes N, Flannery B. Introduction of sequential inactivated polio vaccine-oral polio vaccine schedule for routine infant immunization in Brazil's National Immunization Program. J Infect Dis 2014; 210 Suppl 1:S143-51; PMID:25316829; http://dx.doi.org/ 10.1093/infdis/jit588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Low N, Bavdekar A, Jeyaseelan L, Hirve S, Ramanathan K, Andrews NJ, Shaikh N, Jadi RS, Rajagopal A, Brown KE, et al.. A randomized, controlled trial of an aerosolized vaccine against measles. N Engl J Med 2015; 372:1519-29; PMID:25875257; http://dx.doi.org/ 10.1056/NEJMoa1407417 [DOI] [PubMed] [Google Scholar]
- [7].Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev 2012; 64:1547-68; PMID:22575858; http://dx.doi.org/ 10.1016/j.addr.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Henry S, McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles: a novel approach to transdermal drug delivery. Journal of pharmaceutical sciences 1998; 87:922-5; PMID:9687334; http://dx.doi.org/ 10.1021/js980042+ [DOI] [PubMed] [Google Scholar]
- [9].Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol 2009; 333:369-93; PMID:19768415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pattani A, McKay PF, Garland MJ, Curran RM, Migalska K, Cassidy CM, Malcolm RK, Shattock RJ, McCarthy HO, Donnelly RF. Microneedle mediated intradermal delivery of adjuvanted recombinant HIV-1 CN54gp140 effectively primes mucosal boost inoculations. J Control Release 2012; 162:529-37; PMID:22960496; http://dx.doi.org/ 10.1016/j.jconrel.2012.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine 2014; 32:1856-62; PMID:24530146; http://dx.doi.org/ 10.1016/j.vaccine.2014.01.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pearton M, Kang SM, Song JM, Kim YC, Quan FS, Anstey A, Ivory M, Prausnitz MR, Compans RW, Birchall JC. Influenza virus-like particles coated onto microneedles can elicit stimulatory effects on Langerhans cells in human skin. Vaccine 2010; 28:6104-13; PMID:20685601; http://dx.doi.org/ 10.1016/j.vaccine.2010.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zipursky S, Djingarey MH, Lodjo JC, Olodo L, Tiendrebeogo S, Ronveaux O. Benefits of using vaccines out of the cold chain: Delivering Meningitis A vaccine in a controlled temperature chain during the mass immunization campaign in Benin. Vaccine 2014; 32:1431-5; PMID:24559895; http://dx.doi.org/ 10.1016/j.vaccine.2014.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Al-Zahrani S, Zaric M, McCrudden C, Scott C, Kissenpfennig A, Donnelly RF. Microneedle-mediated vaccine delivery: harnessing cutaneous immunobiology to improve efficacy. Expert Opin Drug Deliv 2012; 9:541-50; PMID:22475249; http://dx.doi.org/ 10.1517/17425247.2012.676038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release 2012; 161:645-55; PMID:22342643; http://dx.doi.org/ 10.1016/j.jconrel.2012.01.042 [DOI] [PubMed] [Google Scholar]
- [16].Wang T, Zhen YY, Ma XY, Wei BA, Li SQ, Wang NN. Mannosylated and lipid A-incorporating cationic liposomes constituting microneedle arrays as an effective oral mucosal HBV vaccine applicable in the controlled temperature chain. Colloid Surface B 2015; 126:520-30; http://dx.doi.org/ 10.1016/j.colsurfb.2015.01.005 [DOI] [PubMed] [Google Scholar]
- [17].DeMuth PC, Min Y, Huang B, Kramer JA, Miller AD, Barouch DH, Hammond PT, Irvine DJ. Polymer multilayer tattooing for enhanced DNA vaccination. Nature materials 2013; 12:367-76; PMID:23353628; http://dx.doi.org/ 10.1038/nmat3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang T, Wang N. Biocompatible Mater Constructed Microneedle Arrays as a Novel Vaccine Adjuvant-Delivery System for Cutaneous and Mucosal Vaccination. Curr Pharm Design 2015; 21:5245-55; http://dx.doi.org/ 10.2174/1381612821666150923100147 [DOI] [PubMed] [Google Scholar]
- [19].Haq MI, Smith E, John DN, Kalavala M, Edwards C, Anstey A, Morrissey A, Birchall JC. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices 2009; 11:35-47; PMID:18663579; http://dx.doi.org/ 10.1007/s10544-008-9208-1 [DOI] [PubMed] [Google Scholar]
- [20].Levin Y, Kochba E, Kenney R. Clinical evaluation of a novel microneedle device for intradermal delivery of an influenza vaccine: are all delivery methods the same? Vaccine 2014; 32:4249-52; PMID:24930715; http://dx.doi.org/ 10.1016/j.vaccine.2014.03.024 [DOI] [PubMed] [Google Scholar]
- [21].Hirobe S, Azukizawa H, Hanafusa T, Matsuo K, Quan YS, Kamiyama F, Katayama I, Okada N, Nakagawa S. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials 2015; 57:50-8; PMID:25913250; http://dx.doi.org/ 10.1016/j.biomaterials.2015.04.007 [DOI] [PubMed] [Google Scholar]
- [22].Bal SM, Caussin J, Pavel S, Bouwstra JA. In vivo assessment of safety of microneedle arrays in human skin. Eur J Pharm Sci 2008; 35:193-202; PMID:18657610; http://dx.doi.org/ 10.1016/j.ejps.2008.06.016 [DOI] [PubMed] [Google Scholar]
- [23].Anand A, Zaman K, Estivariz CF, Yunus M, Gary HE, Weldon WC, Bari TI, Steven Oberste M, Wassilak SG, Luby SP, et al.. Early priming with inactivated poliovirus vaccine (IPV) and intradermal fractional dose IPV administered by a microneedle device: A randomized controlled trial. Vaccine 2015; 33:6816-22; PMID:26476367; http://dx.doi.org/ 10.1016/j.vaccine.2015.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wendorf JR, Ghartey-Tagoe EB, Williams SC, Enioutina E, Singh P, Cleary GW. Transdermal Delivery of Macromolecules Using Solid-State Biodegradable Microstructures. Pharm Res-Dordr 2011; 28:22-30; http://dx.doi.org/ 10.1007/s11095-010-0174-y [DOI] [PubMed] [Google Scholar]
- [25].Yang S, Feng Y, Zhang L, Chen N, Yuan W, Jin T. A scalable fabrication process of polymer microneedles. International journal of nanomedicine 2012; 7:1415-22; PMID:22457598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bystrova S, Luttge R. Micromolding for ceramic microneedle arrays. Microelectron Eng 2011; 88:1681-4; http://dx.doi.org/ 10.1016/j.mee.2010.12.067 [DOI] [Google Scholar]
- [27].Wilke N, Mulcahy A, Ye SR, Morrissey A. Process optimization and characterization of silicon microneedles fabricated by wet etch technology. Microelectron J 2005; 36:650-6; http://dx.doi.org/ 10.1016/j.mejo.2005.04.044 [DOI] [Google Scholar]
- [28].Donnelly RF, Majithiya R, Singh TRR, Morrow DIJ, Garland MJ, Demir YK, Migalska K, Ryan E, Gillen D, Scott CJ, et al.. Design, Optimization and Characterisation of Polymeric Microneedle Arrays Prepared by a Novel Laser-Based Micromoulding Technique. Pharmaceutical research 2011; 28:41-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. Journal of Controlled Release 2005; 104:51-66; PMID:15866334; http://dx.doi.org/ 10.1016/j.jconrel.2005.02.002 [DOI] [PubMed] [Google Scholar]
- [30].Qin D, Xia YN, Whitesides GM. Soft lithography for micro- and nanoscale patterning. Nat Protoc 2010; 5:491-502; PMID:20203666; http://dx.doi.org/ 10.1038/nprot.2009.234 [DOI] [PubMed] [Google Scholar]
- [31].Watanabe T, Hagino K, Sato T. Evaluation of the effect of polymeric microneedle arrays of varying geometries in combination with a high-velocity applicator on skin permeability and irritation. Biomed Microdevices 2014; 16:591-7; PMID:24733417; http://dx.doi.org/ 10.1007/s10544-014-9861-5 [DOI] [PubMed] [Google Scholar]
- [32].Bal SM, Kruithof AC, Zwier R, Dietz E, Bouwstra JA, Lademann J, Meinke MC. Influence of microneedle shape on the transport of a fluorescent dye into human skin in vivo. Journal of Controlled Release 2010; 147:218-24; PMID:20650292; http://dx.doi.org/ 10.1016/j.jconrel.2010.07.104 [DOI] [PubMed] [Google Scholar]
- [33].Ai-Qallaf B, Das DB. Optimization of square microneedle arrays for increasing drug permeability in skin. Chem Eng Sci 2008; 63:2523-35; http://dx.doi.org/ 10.1016/j.ces.2008.02.007 [DOI] [Google Scholar]
- [34].Edens C, Collins ML, Goodson JL, Rota PA, Prausnitz MR. A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine 2015; 33:4712-4718. http://dx.doi.org/ 10.1016/j.vaccine.2015.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang T, Zhen Y, Ma X, Wei B, Li S, Wang N. Mannosylated and lipid A-incorporating cationic liposomes constituting microneedle arrays as an effective oral mucosal HBV vaccine applicable in the controlled temperature chain. Colloids Surf B Biointerfaces 2015; 126:520-30; PMID:25612819; http://dx.doi.org/ 10.1016/j.colsurfb.2015.01.005 [DOI] [PubMed] [Google Scholar]
- [36].Zhang J, Pritchard E, Hu X, Valentin T, Panilaitis B, Omenetto FG, Kaplan DL. Stabilization of vaccines and antibiotics in silk and eliminating the cold chain. Proceedings of the National Academy of Sciences of the United States of America 2012; 109:11981-6; PMID:22778443; http://dx.doi.org/ 10.1073/pnas.1206210109 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [37].Wang T, Zhen Y, Ma X, Wei B, Wang N. Phospholipid bilayer-coated aluminum nanoparticles as an effective vaccine adjuvant-delivery system. ACS applied materials & interfaces 2015; 7:6391-6; PMID:25780860; http://dx.doi.org/ 10.1021/acsami.5b00348 [DOI] [PubMed] [Google Scholar]
- [38].Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. Journal of molecular biology 1965; 13:238-52; PMID:5859039; http://dx.doi.org/ 10.1016/S0022-2836(65)80093-6 [DOI] [PubMed] [Google Scholar]
- [39].Wang T, Wang N, Sun W, Li T. Preparation of submicron liposomes exhibiting efficient entrapment of drugs by freeze-drying water-in-oil emulsions. Chem Phys Lipids 2011; 164:151-7; PMID:21185814; http://dx.doi.org/ 10.1016/j.chemphyslip.2010.12.005 [DOI] [PubMed] [Google Scholar]
- [40].Wang T, Deng Y, Geng Y, Gao Z, Zou J, Wang Z. Preparation of submicron unilamellar liposomes by freeze-drying double emulsions. Biochim Biophys Acta 2006; 1758:222-31; PMID:16563340; http://dx.doi.org/ 10.1016/j.bbamem.2006.01.023 [DOI] [PubMed] [Google Scholar]
- [41].Wang T, Wang N, Jin X, Zhang K, Li T. A novel procedure for preparation of submicron liposomes-lyophilization of oil-in-water emulsions. J Liposome Res 2009; 19:231-40; PMID:19263267; http://dx.doi.org/ 10.1080/08982100902788390 [DOI] [PubMed] [Google Scholar]
- [42].Alving CR, Rao M, Steers NJ, Matyas GR, Mayorov AV. Liposomes containing lipid A: an effective, safe, generic adjuvant system for synthetic vaccines. Expert Rev Vaccines 2012; 11:733-44; PMID:22873129; http://dx.doi.org/ 10.1586/erv.12.35 [DOI] [PubMed] [Google Scholar]
- [43].Shariat S, Badiee A, Jaafari MR, Mortazavi SA. Optimization of a Method to Prepare Liposomes Containing HER2/Neu-Derived Peptide as a Vaccine Delivery System for Breast Cancer. Iran J Pharm Res 2014; 13:15-25; PMID:24711825. [PMC free article] [PubMed] [Google Scholar]
- [44].Wang N, Wang T, Zhang ML, Chen RN, Niu RW, Deng YH. Mannose derivative and lipid A dually decorated cationic liposomes as an effective cold chain free oral mucosal vaccine adjuvant-delivery system. Eur J Pharm Biopharm 2014; 88:194-206; PMID:24769065; http://dx.doi.org/ 10.1016/j.ejpb.2014.04.007 [DOI] [PubMed] [Google Scholar]
- [45].Guo L, Chen J, Qiu Y, Zhang S, Xu B, Gao Y. Enhanced transcutaneous immunization via dissolving microneedle array loaded with liposome encapsulated antigen and adjuvant. Int J Pharm 2013; 447:22-30; PMID:23410987; http://dx.doi.org/ 10.1016/j.ijpharm.2013.02.006 [DOI] [PubMed] [Google Scholar]
- [46].Qiu Y, Guo L, Zhang S, Xu B, Gao Y, Hu Y, Hou J, Bai B, Shen H, Mao P. DNA-based vaccination against hepatitis B virus using dissolving microneedle arrays adjuvanted by cationic liposomes and CpG ODN. Drug delivery 2016. (in press); http://dx.doi.org/ 10.3109/10717544.2014.992497 [DOI] [PubMed] [Google Scholar]
- [47].Slutter B, Bal SM, Ding Z, Jiskoot W, Bouwstra JA. Adjuvant effect of cationic liposomes and CpG depends on administration route. J Control Release 2011; 154:123-30; PMID:21315779; http://dx.doi.org/ 10.1016/j.jconrel.2011.02.007 [DOI] [PubMed] [Google Scholar]
- [48].Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, Sohail M, Luo S, Um SH, Khant H, et al.. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nature materials 2011; 10:243-51; PMID:21336265; http://dx.doi.org/ 10.1038/nmat2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].DeMuth PC, Moon JJ, Suh H, Hammond PT, Irvine DJ. Releasable layer-by-layer assembly of stabilized lipid nanocapsules on microneedles for enhanced transcutaneous vaccine delivery. ACS Nano 2012; 6:8041-51; PMID:22920601; http://dx.doi.org/ 10.1021/nn302639r [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhen YY, Wang N, Gao ZB, Ma XY, Wei BA, Deng YH, Wang T. Multifunctional liposomes constituting microneedles induced robust systemic and mucosal immunoresponses against the loaded antigens via oral mucosal vaccination. Vaccine 2015; 33:4330-40; PMID:25858854; http://dx.doi.org/ 10.1016/j.vaccine.2015.03.081 [DOI] [PubMed] [Google Scholar]
- [51].Wang T, Wang N, Song H, Xi X, Wang J, Hao A, Li T. Preparation of an anhydrous reverse micelle delivery system to enhance oral bioavailability and anti-diabetic efficacy of berberine. Eur J Pharm Sci 2011; 44:127-35; PMID:21742030; http://dx.doi.org/ 10.1016/j.ejps.2011.06.015 [DOI] [PubMed] [Google Scholar]
- [52].Wang N, Wang T, Zhang M, Chen R, Deng Y. Using procedure of emulsification-lyophilization to form lipid A-incorporating cochleates as an effective oral mucosal vaccine adjuvant-delivery system (VADS). Int J Pharm 2014; 468:39-49; PMID:24704308; http://dx.doi.org/ 10.1016/j.ijpharm.2014.04.002 [DOI] [PubMed] [Google Scholar]
- [53].Desai SN, Kamat D. Closing the global immunization gap: delivery of lifesaving vaccines through innovation and technology. Pediatrics in review / American Academy of Pediatrics 2014; 35:e32-40; PMID:24986933; http://dx.doi.org/ 10.1542/pir.35-7-e32 [DOI] [PubMed] [Google Scholar]
- [54].DeMuth PC, Li AV, Abbink P, Liu J, Li H, Stanley KA, Smith KM, Lavine CL, Seaman MS, Kramer JA, et al.. Vaccine delivery with microneedle skin patches in nonhuman primates. Nature biotechnology 2013; 31:1082-5; PMID:24316643; http://dx.doi.org/ 10.1038/nbt.2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 2007; 316:1628-32; PMID:17569868; http://dx.doi.org/ 10.1126/science.1138963 [DOI] [PubMed] [Google Scholar]
- [56].Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci 2008; 65:3231-40; PMID:18668203; http://dx.doi.org/ 10.1007/s00018-008-8228-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ohimain EI. Recent advances in the development of vaccines for Ebola virus disease. Virus Res 2016; 211:174-85; PMID:26596227; http://dx.doi.org/ 10.1016/j.virusres.2015.10.021 [DOI] [PubMed] [Google Scholar]
- [58].Allison SD. Effect of structural relaxation on the preparation and drug release behavior of poly(lactic-co-glycolic)acid microparticle drug delivery systems. Journal of pharmaceutical sciences 2008; 97:2022-35; PMID:17828755; http://dx.doi.org/ 10.1002/jps.21124 [DOI] [PubMed] [Google Scholar]
- [59].Ungaro F, d'Angelo I, Miro A, La Rotonda MI, Quaglia F. Engineered PLGA nano- and micro-carriers for pulmonary delivery: challenges and promises. The Journal of pharmacy and pharmacology 2012; 64:1217-35; PMID:22881435; http://dx.doi.org/ 10.1111/j.2042-7158.2012.01486.x [DOI] [PubMed] [Google Scholar]
- [60].Joshi VB, Geary SM, Salem AK. Biodegradable particles as vaccine antigen delivery systems for stimulating cellular immune responses. Human vaccines & immunotherapeutics 2013; 9:2584-90; PMID:23978910; http://dx.doi.org/ 10.4161/hv.26136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Silva AL, Rosalia RA, Varypataki E, Sibuea S, Ossendorp F, Jiskoot W. Poly-(lactic-co-glycolic-acid)-based particulate vaccines: particle uptake by dendritic cells is a key parameter for immune activation. Vaccine 2015; 33:847-54; PMID:25576216; http://dx.doi.org/ 10.1016/j.vaccine.2014.12.059 [DOI] [PubMed] [Google Scholar]
- [62].Zaric M, Lyubomska O, Touzelet O, Poux C, Al-Zahrani S, Fay F, Wallace L, Terhorst D, Malissen B, Henri S, et al.. Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly-D,L-lactide-co-glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS nano 2013; 7:2042-55; PMID:23373658; http://dx.doi.org/ 10.1021/nn304235j [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zaric M, Lyubomska O, Poux C, Hanna ML, McCrudden MT, Malissen B, Ingram RJ, Power UF, Scott CJ, Donnelly RF, et al.. Dissolving microneedle delivery of nanoparticle-encapsulated antigen elicits efficient cross-priming and Th1 immune responses by murine Langerhans cells. J Invest Dermatol 2015; 135:425-34; PMID:25243789; http://dx.doi.org/ 10.1038/jid.2014.415 [DOI] [PubMed] [Google Scholar]
- [64].Demuth PC, Garcia-Beltran WF, Ai-Ling ML, Hammond PT, Irvine DJ. Composite dissolving microneedles for coordinated control of antigen and adjuvant delivery kinetics in transcutaneous vaccination. Advanced functional materials 2013; 23:161-72; PMID:23503923; http://dx.doi.org/ 10.1002/adfm.201201512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Smith A, Perelman M, Hinchcliffe M. Chitosan: a promising safe and immune-enhancing adjuvant for intranasal vaccines. Human vaccines & immunotherapeutics 2014; 10:797-807; PMID:24346613; http://dx.doi.org/ 10.4161/hv.27449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bal SM, Ding Z, Kersten GF, Jiskoot W, Bouwstra JA. Microneedle-based transcutaneous immunisation in mice with N-trimethyl chitosan adjuvanted diphtheria toxoid formulations. Pharm Res 2010; 27:1837-47; PMID:20559701; http://dx.doi.org/ 10.1007/s11095-010-0182-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Bal SM, Slutter B, Jiskoot W, Bouwstra JA. Small is beautiful: N-trimethyl chitosan-ovalbumin conjugates for microneedle-based transcutaneous immunisation. Vaccine 2011; 29:4025-32; PMID:21443959; http://dx.doi.org/ 10.1016/j.vaccine.2011.03.039 [DOI] [PubMed] [Google Scholar]
- [68].Leguen E, Chassepot A, Decher G, Schaaf P, Voegel JC, Jessel N. Bioactive coatings based on polyelectrolyte multilayer architectures functionalized by embedded proteins, peptides or drugs. Biomolecular engineering 2007; 24:33-41; PMID:16860599; http://dx.doi.org/ 10.1016/j.bioeng.2006.05.023 [DOI] [PubMed] [Google Scholar]
- [69].Kim BS, Park SW, Hammond PT. Hydrogen-bonding layer-by-layer assembled biodegradable polymeric micelles as drug delivery vehicles from surfaces. Acs Nano 2008; 2:386-92; PMID:19206641; http://dx.doi.org/ 10.1021/nn700408z [DOI] [PubMed] [Google Scholar]
- [70].Zeltins A. Construction and characterization of virus-like particles: a review. Mol Biotechnol 2013; 53:92-107; PMID:23001867; http://dx.doi.org/ 10.1007/s12033-012-9598-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Rodriguez-Limas WA, Sekar K, Tyo KEJ. Virus-like particles: the future of microbial factories and cell-free systems as platforms for vaccine development. Curr Opin Biotech 2013; 24:1089-93; PMID:23481378; http://dx.doi.org/ 10.1016/j.copbio.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hoffman SL, Vekemans J, Richie TL, Duffy PE. The march toward malaria vaccines. Vaccine 2015; 33 Suppl 4:D13-23; PMID:26324116; http://dx.doi.org/ 10.1016/j.vaccine.2015.07.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Quan FS, Kim YC, Vunnava A, Yoo DG, Song JM, Prausnitz MR, Compans RW, Kang SM. Intradermal Vaccination with Influenza Virus-Like Particles by Using Microneedles Induces Protection Superior to That with Intramuscular Immunization. Journal of virology 2010; 84:7760-9; PMID:20484519; http://dx.doi.org/ 10.1128/JVI.01849-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Song JM, Kim YC, Lipatov AS, Pearton M, Davis CT, Yoo DG, Park KM, Chen LM, Quan FS, Birchall JC, et al.. Microneedle delivery of H5N1 influenza virus-like particles to the skin induces long-lasting B- and T-cell responses in mice. Clin Vaccine Immunol 2010; 17:1381-9; PMID:20631330; http://dx.doi.org/ 10.1128/CVI.00100-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Song JM, Kim YC, Barlow PG, Hossain MJ, Park KM, Donis RO, Prausnitz MR, Compans RW, Kang SM. Improved protection against avian influenza H5N1 virus by a single vaccination with virus-like particles in skin using microneedles. Antivir Res 2010; 88:244-7; PMID:20851715; http://dx.doi.org/ 10.1016/j.antiviral.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Pearton M, Pirri D, Kang SM, Compans RW, Birchall JC. Host responses in human skin after conventional intradermal injection or microneedle administration of virus-like-particle influenza vaccine. Advanced healthcare materials 2013; 2:1401-10; PMID:23564440; http://dx.doi.org/ 10.1002/adhm.201300006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kim MC, Lee JW, Choi HJ, Lee YN, Hwang HS, Lee J, Kim C, Lee JS, Montemagno C, Prausnitz MR, et al.. Microneedle patch delivery to the skin of virus-like particles containing heterologous M2e extracellular domains of influenza virus induces broad heterosubtypic cross-protection. J Control Release 2015; 210:208-16; PMID:26003039; http://dx.doi.org/ 10.1016/j.jconrel.2015.05.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kines RC, Zarnitsyn V, Johnson TR, Pang YYS, Corbett KS, Nicewonger JD, Gangopadhyay A, Chen M, Liu J, Prausnitz MR, et al.. Vaccination with human papillomavirus pseudovirus-encapsidated plasmids targeted to skin using microneedles. PLos One 2015; 10: e0120797-0120814. PMID:25785935; http://dx.doi.org/ 10.1371/10.1371/journal.pone.0120797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Carey JB, Pearson FE, Vrdoljak A, McGrath MG, Crean AM, Walsh PT, Doody T, O'Mahony C, Hill AV, Moore AC. Microneedle array design determines the induction of protective memory CD8+ T cell responses induced by a recombinant live malaria vaccine in mice. PLoS One 2013; 6:e22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol 2006; 6:148-58; PMID:16491139; http://dx.doi.org/ 10.1038/nri1777 [DOI] [PubMed] [Google Scholar]
- [81].Lutton RE, Moore J, Larraneta E, Ligett S, Woolfson AD, Donnelly RF. Microneedle characterisation: the need for universal acceptance criteria and GMP specifications when moving towards commercialisation. Drug Deliv Transl Res 2015; 5:313-31; PMID:26022578; http://dx.doi.org/ 10.1007/s13346-015-0237-z [DOI] [PubMed] [Google Scholar]
- [82].Suh H, Shin J, Kim YC. Microneedle patches for vaccine delivery. Clin Exp Vaccine Res 2014; 3:42-9; PMID:24427762; http://dx.doi.org/ 10.7774/cevr.2014.3.1.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kolli CS. Microneedles: bench to bedside. Ther Deliv 2015; 6:1081-8; PMID:26419290; http://dx.doi.org/ 10.4155/tde.15.67 [DOI] [PubMed] [Google Scholar]
- [84].Jacoby E, Jarrahian C, Hull HF, Zehrung D. Opportunities and challenges in delivering influenza vaccine by microneedle patch. Vaccine 2015; 33:4699-704; PMID:25842218; http://dx.doi.org/ 10.1016/j.vaccine.2015.03.062 [DOI] [PubMed] [Google Scholar]
- [85].Arya J, Prausnitz MR. Microneedle patches for vaccination in developing countries. J Control Release 2016. (in press); PMID:26603347; http://dx.doi.org/ 10.1016/j.jconrel.2015.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Korschun H. Clinical study at Emory tests microneedle skin patches as alternative to flu shot Researchers are enrolling volunteers in a clinical study at the Hope Clinic of the Emory Vaccine Center. Emory University: Emory University Woodruff Health Sciences Center, August 28, 2015. [Google Scholar]
- [87].Toon J. Polio vaccination with microneedle patches receives funding for patch development, clinical trial Clinical Trial Will Evaluate Polio Vaccination Using Microneedle Patch. Georgia Institute of Technology, Atlanta, Georgia: 30332-0181 USA: Georgia Institute of Technology News Center, February 24, 2015. [Google Scholar]
- [88].Meek G. Microneedle Patch for Measles Vaccination Could Be a Game Changer Promises to Increase Reach of Immunization Coverage Globally: CDC Newsroom Releases, April 27, 2015. [Google Scholar]