ABSTRACT
Objective To estimate the cost-effectiveness of hepatitis E vaccination among pregnant women in epidemic regions. Methods A decision tree model was constructed to evaluate the cost-effectiveness of 3 hepatitis E virus vaccination strategies from societal perspectives. The model parameters were estimated on the basis of published studies and experts' experience. Sensitivity analysis was used to evaluate the uncertainties of the model. Results Vaccination was more economically effective on the basis of the incremental cost-effectiveness ratio (ICER< 3 times China's per capital gross domestic product/quality-adjusted life years); moreover, screening and vaccination had higher QALYs and lower costs compared with universal vaccination. No parameters significantly impacted ICER in one-way sensitivity analysis, and probabilistic sensitivity analysis also showed screening and vaccination to be the dominant strategy. Conclusion Screening and vaccination is the most economical strategy for pregnant women in epidemic regions; however, further studies are necessary to confirm the efficacy and safety of the hepatitis E vaccines.
KEYWORDS: cost-effectiveness analysis, decision tree model, hepatitis E vaccines, pregnant women
Introduction
Hepatitis E is an enterically transmitted infectious disease that is an important public health concern in developing countries in Asia and Africa. It is generally characterized by an acute self-limited infection; however, 1%−4% of acute cases can develop to fulminant hepatic failure (FHF), which is a risk factor for death among patients infected with hepatitis E virus (HEV).1,2 The fecal–oral route of infection with HEV genotypes 1 and 2 is responsible for waterborne epidemics or outbreaks in regions without sufficient sanitation measures.3,4 Compared with the general population, the probability of death among pregnant women is estimate to 0.198 in 9 Global Burden of Diseases regions,5 and the adverse pregnancy outcomes include, but are not limited to abortion, stillbirth, and intra-uterine death.6,7 Moreover, several surveys have reported vertical transmission of hepatitis E,8-10 which may increase the costs of health-care services and decrease quality of life.
Conventional strategies, such as improvements in sanitation and treatment of drinking water in developing regions, may not be efficient tools to prevent epidemics or outbreaks of HEV infection, and hepatitis E vaccines are a powerful measure to halt transmission.11 To date, only 2 vaccine candidates have completed phase 2 trials in humans, among which a genotype 1 HEV recombinant vaccine12-14 has a reported efficacy of 95.5% (95% CI: 85.6%–98.6%) in a phase 2 trial after 3 doses administered to 2,000 healthy Nepalese adults; however, further development of this vaccine has not been reported, likely due to the lack of long-term efficacy or commercial benefits.15 Another candidate, the HEV239 vaccine, is the only vaccine that has completed a phase 3 trial in China 16,17 and has been approved for use in Chinese adults aged 16 y and older.18 The efficacy of 3 doses of HEV239 vaccine during the phrase 3 trial comprising 11,165 participants was 100% (95% CI: 72.1%–100%).17 A previous study reported that the protection offered by the HEV239 vaccine can last several years in healthy adults, and it is effective and safe for the general population living in China.19 Moreover, one study reported that its adverse effects were not significantly different between incidentally vaccinated pregnant women and vaccinated non-pregnant women, which may provide evidence for research on immunization strategies.20 Currently, only inactivated influenza and tetanus, diphtheria, and pertussis (Tdap) vaccines are approved for use in pregnant women during their second or third trimesters,21-23 and other vaccines available to pregnant women should be used with caution. Thus, our study assessed the economics of pregnant women who had vaccinated before conception.
Decision tree models have been used to simulate vaccination strategies in pregnant populations.21,24-26 Therefore, the aim of the present study was to use a decision tree model to evaluate the cost-effectiveness of hepatitis E vaccination among pregnant women living in epidemic regions in order to determine if hepatitis E vaccination should be considered a useful strategy.
Results
Incremental cost-effectiveness ratio (ICER) of HEV vaccination
Table 1 showed the ICER values of 3 scenarios assessed by the decision tree model. The results demonstrate that the strategy of vaccination was dominant on the baseline value of the parameters (ICER < 3 times per capital GDP/ QALYs). Compared with the lack of vaccination, pregnant women in the group of individuals who received universal vaccination could gain a total net effectiveness of 123.61 QALYs and a total net cost of $−7,616.40, while vaccination after screening could result in net QALYs of 124.40 and savings of $69,900.54; moreover, screening and vaccination might acquire a net effectiveness of 0.78 QALYs and savings of $77,516.94 in the cohort that received universal vaccination.
Table 1.
Costs and effectiveness outcomes using the model (10,000 pregnant women).
| Scenarios | Total cost ($) | Incremental cost ($) | Total QALYs | Incremental QALYs | ICER* | ICER** |
|---|---|---|---|---|---|---|
| Screening and vaccination | 1262425.72 | −69900.54 | 9991.91 | 124.40 | <0 | <0 |
| Universal vaccination | 1339942.66 | 7616.40 | 9991.12 | 123.61 | 61.61 | — |
| No vaccination | 1332326.26 | — | 9867.51 | — | — | — |
Note. QALYs: quality adjusted life years; ICER: incremental cost-effectiveness ratio.
no-vaccination strategy was used as the control;
universal vaccination strategy was used as the control.
Sensitivity analysis
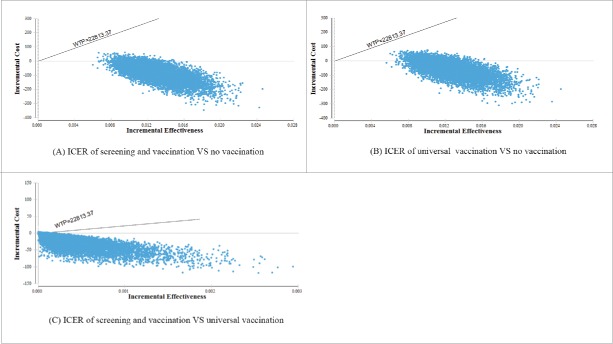
According to one-way sensitivity analysis, no variables had a significant effect on the change of the dominant strategy, thus, vaccination remained cost-effective. The results of probabilistic sensitivity analysis based on Monte Carlo simulations (Fig. 1) revealed that, compared with the lack of vaccination, points of the other 2 strategies were located below willingness-to-pay thresholds ($22,813.37/QALYs), indicating that vaccination was the dominant scenario in the cohort of 10,000 women living in epidemic regions. Compared with universal vaccination, most points of the screening and vaccination strategy were located in the fourth quadrant, indicating an incremental effectiveness and incremental cost above and below zero, respectively, supporting the conclusion that screening and vaccination was the most effective strategy for decreasing costs and increasing QALYs.
Figure 1.
Results of probabilistic sensitivity analysis The x-coordinate corresponds to the incremental QALYs gained, while the y-coordinate corresponds to the incremental cost saved ($); WTP=3 times China's per capital gross domestic product ($) /QALYsAbbreviations: ICER: incremental cost-effectiveness ratio; QALYs: quality-adjusted life years.
Discussion
HEV infection is responsible for 50% of acute viral hepatitis (AVH) cases in endemic regions.15,27 More than one-third of the world's population is infected with HEV.15 In developing countries, several outbreaks of hepatitis E have been reported,28-30 with distinct characteristics including high morbidity and mortality due to FHF in pregnant women.31 Measures to decrease exposure to HEV and enhance immunity via vaccination are considered appropriate preventive strategies.32 The phase 3 trial of the HEV239 vaccine is complete,17,18 and this vaccine is commercially available in China.
Our preliminary analysis of epidemic regions indicated that HE vaccines could be cost-effective in pregnant women. Moreover, the gained QALYs and saved costs using the screening and vaccination strategy were higher than those obtained using universal vaccination. Therefore, based on the reported efficacy and safety of HE vaccines, target vaccination of women who plan to conceive and who are living in epidemic regions is a viable strategy for policy makers.
The sensitivity analysis indicated that none of the parameters' ranges included in Table 2 had the ability to change the dominant strategy, but HEV antibody seroprevalence, vaccine efficacy, and vaccine price might be the most important factors for policy makers to consider when determining the advisability of vaccination, as the seroprevalence of HEV antibody among pregnant women in different countries or regions differs as a function of the gestational or pregnant age.33-35 In developing countries, the seroprevalence has been reported to be 84.3% among those living in rural villages in Egypt,36 33.67% in Northern India,37 14.17% in 5 cities in Gabon,38 10.24% in Yunnan in China,39 and 3.68% in Urmia in Iran.40 Vaccination should be performed after determining the prevalence of HEV antibodies in the population using national or regional field surveys. The long-term efficacy of the HEV239 vaccine in the general population did not decrease significantly compared with the 56-KD vaccine,13,19 but the efficacy of HE vaccines in pregnant women has not yet been confirmed. Moreover, although the cost range of HE vaccines did not significantly affect the dominant strategy in epidemic regions, more appropriate pricing of HE vaccines may influence policy makers' decisions regarding vaccination in areas with sporadic feature, which are the more common phenomenon worldwide.41
Table 2.
Model parameters.
| Parameters | Baseline | Range | Source |
|---|---|---|---|
| Probability | |||
| Incidence | 0.238 | 0.1727–0.3333 | 6,48-50 |
| Symptomatic infection | 0.198 | 0.167–0.229 | 5 |
| Seroprevalence of HEV antibody | 0.0883 | 0.005–0.67 | 63,64 |
| Probability of non-FHF | 0.55 | 0.19–0.75 | 51-53 |
| Probability of FHF | 0.45 | 0.25–−0.81 | 51-53 |
| Hospitalization rate | 0.328 | 0.273–0.391 | 17,19 |
| Death | |||
| Non-FHF | |||
| Pregnant woman | 0 | 0.00–0.025 | 51-53,57, 58 |
| Fetus# | 0.05 | 0–0.10 | 51-53,57, 58 |
| FHF | |||
| Pregnant woman | 0.48 | 0.13–0.79 | 54-56 |
| Fetus# | 0.21 | 0.14–0.38 | 54-56 |
| Efficacy of vaccine | 0.933 | 0.721–1 | 17,19 |
| Cost ($) | |||
| Vaccine (3 doses) | 121.33 | 16.31–146.78 | 59,65-67 |
| Administration (3 doses) | 3.73 | 1.47-18 | 59, 60,68 |
| Screening | 4.08 | 59 | |
| Outpatient medical cost | 134.24 | 74.98–219.29 | 59, 61 |
| Inpatient medical cost | |||
| Non-FHF | 2,169.10 | 510.46–6,771.39 | 59, 61 |
| FHF | 1,0581.68 | 1,025.25–2,7856.49 | 60 |
| Medical cost of death | |||
| Non-FHF | 5,000 | 2446.26–8,377.96 | 21, 24 |
| FHF | 13,600 | 12,000–24,932.46 | 22, 62 |
| Fetus | 2,956.68 | 2,446.26–5,000 | 21, 24 |
| Work days lost | |||
| Outpatient | 3.63 | 3–10 | 59, 60 |
| Inpatient | |||
| Non-FHF | 24 | 17.47–33.20 | 59, 69 |
| FHF | 30 | 17-40 | 54 |
| Death | 40 | 20–60 | 68 |
| QALYs | |||
| Healthy/asymptomatic | 1.00 | 60, 68 | |
| Outpatient | 0.83 | 24, 60 | |
| Non-FHF | 0.75 | 24, 60 | |
| Non-FHF (fetal death) | 0.41 | 60 | |
| FHF | 0.42 | 60, 70 | |
| FHF (fetal death) | 0.21 | 60 | |
| Death | 0 | 60, 68 | |
Note. HEV: hepatitis E virus; FHF: fulminant hepatic failure; non-FHF: non fulminant hepatic failure; QALYs: quality-adjusted life years.
probability of fetal death with maternal survival.
This study had some limitations. First, seasonality32 and gestational age42 which are both related to the morbidity and mortality of HEV infections, were not considered in this study; second, with regard to the cost-effectiveness of vaccination,43-45 although we attempted to improve the accuracy of the parameters, data concerning the QALYs of HEV-infected patients was lacking. Moreover, the HE vaccinees belonged to the general population in China, where genotype 4 HEV infection predominates. When rhesus monkeys were used as the infection model, the protection conferred by HEV vaccination against genotype 4 HEV was not significantly different from the protection against genotype 1.46 However, its efficiency and safety against acute infections or FHF due to genotype 1 or 2 HEV infection in the general or target population, such as pregnant women, requires further elucidation.15,47
In conclusion, the current model predicts that vaccination could save life-years and decrease total future costs during pregnancy. The costs of vaccination, efficacy of vaccines, and seroprevalence of HEV antibodies may serve as relevant information for policy makers developing more comprehensive evaluations of vaccination at national and international levels.
Materials and methods
Decision tree model
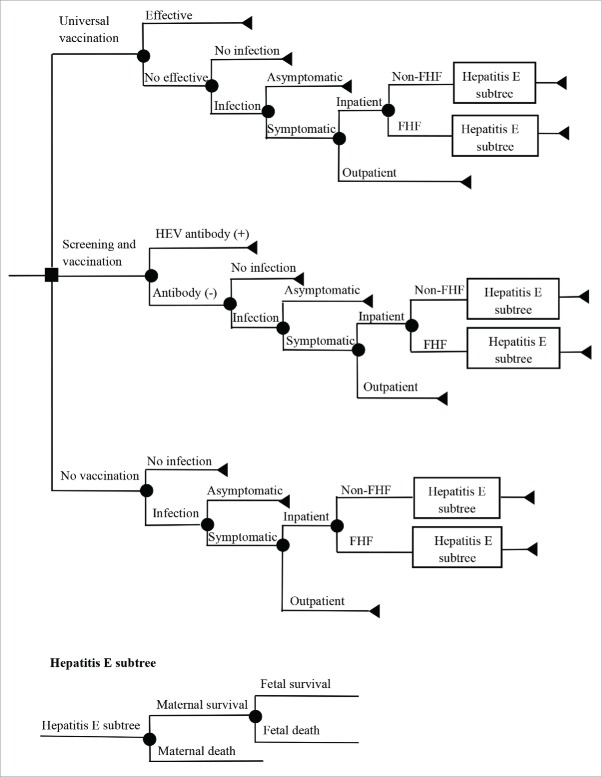
Based on the assumption of a single outbreak or epidemic per year, a decision tree (details shown in Fig. 2) was constructed to evaluate 3 vaccination scenarios: universal vaccination, screening and vaccination, and no vaccination. The screening and vaccination scenario predicted that women of childbearing age negative for HEV antibodies after screening would be vaccinated, and that all vaccinated members would receive 3-dose vaccines, with 100% of vaccine coverage. A cohort of 10,000 pregnant women who received these strategies before becoming pregnant was used to evaluate the economics during the outbreak or epidemic. In addition, the model was run from societal perspectives.
Figure 2.
Cost-effectiveness model Abbreviations: HEV: hepatitis E virus; FHF: fulminate hepatitis failure; Non-FHF: non-fulminate hepatitis failure.
In this study, each woman of childbearing age has the right to choose to be vaccinated, vaccinated while negative for HEV antibodies, or not vaccinated before becoming pregnant. The outbreak or epidemic would be occurred to this population who were during pregnant. Based on the reported efficacy and safety of the HEV239 vaccine in the general population and incidence of outbreaks or epidemics, each pregnant woman had a probability of having hepatitis E, and each patient diagnosed with acute hepatitis E might develop FHF, and all fulminant cases were assumed to require hospitalization. Other cases of pregnant women with acute infections had a probability of visiting the outpatient department, where only hospitalized individuals had a probability of death. Admitted patients had 2 kinds of outcome: survival or death, and surviving pregnant women could have live or dead fetuses. We assumed that fetuses that died during pregnancy in these women died due to HEV infection.
Several hypotheses were used in the model: first, cohort members received HE vaccine at 0, 1, and 6 months via intramuscular injection, according to the phrase 3 trial protocols;17 second, vaccines were administered to women who intended to become pregnant women, rather than to pregnant women, and adverse effects were ignored; third, all vaccinated members would be pregnant women within one year after immunization, and the probability of failure to conceive in this time was ignored; fourth, declining vaccine protection before or during pregnancy was ignored; 5, pregnant women with natural immunity would never be infected with HEV during this pregnancy.
Model inputs
The model parameters were identified based on searches of published data and experts' opinions (Table 2).
Probabilities
Morbidity was defined as the median reported incidence of pregnant women during outbreaks or epidemics.6,48-50 We assumed a symptomatic rate of 19.8% in HEV-infected cases, that 45% of symptomatic cases could develop to FHF,51-53 and that 48% of pregnant women diagnosed with FHF might die,54-56 a mortality rate much higher than that of non-fulminate cases.51, 52,57,58
Costs
The cost of HE vaccine was 248 RMB/per dose according to Innovax (Xiamen, China), and the cost of administration per dose was estimated at 7.63 RMB based on published articles. 59 The medical costs of infected pregnant women were also obtained from surveys and published articles,21, 24,59-62 and the medical costs of pregnant women who died due to AVH or FHF included the costs of fetal death; we assumed that fetuses died when their infected mothers died, and the costs of fetal death were only used in the calculation of surviving pregnant women whose developing fetus died (Table 2). The costs and QALYs of the fetuses themselves were ignored, but the influence of the status of the fetus on pregnant woman was considered in this study. The equation described by Rein et al.60 was used to calculate the costs of work loss (Productivity Losses = (per capital GDP/365.25)*( Duration of work loss)). In China, per capital GDP in 2014 was 46,629 RMB, and this study assumed that per capital GDP in 2015 was equal to GDP/capita in 2014 (http://data.stats.gov.cn/easyquery.htm?cn=C01). All costs were in 2015 US dollars (1 dollar =6.1318 RMB yuan in 2015).
QALYs
QALYs were used to quantify the effectiveness of vaccination and clinical outcomes associated with hepatitis E in pregnant women, but QALYs of HEV-infected cases were not assessed using field surveys in the world, and QALYs of another enterically-transmitted hepatitis (hepatitis A) might be an appropriate alternative to hepatitis E; moreover, pregnancy were considered in this study, QALYs of influenza which could be prevent by vaccines in pregnancy, might be another suitable choice to hepatitis E; furthermore, unlike costs, there was not appropriate method to convert QALYs of patients living in other countries to QALYs of Chinese person. Therefore, this parameter was calculated according to studies by Rein et al. and Richard et al.,24,60 which performed economic evaluations of HAV or influenza vaccination outside China.
Model analysis
All analyses were conducted using TreeAge Pro 2014 software. The ICER was used to compare 3 alternative strategies. One-way sensitivity analysis was applied to all parameters using the range values shown in Table 2, and probabilistic sensitivity analysis with 10,000 simulations was performed to assess the impact of parameter uncertainty on the ICER. The probability of symptomatic infection was assigned a β distribution as described by Rein et al.,5 and other parameters were assigned triangle distributions due to limited published data.
Abbreviations
- GDP
Gross domestic product
- FHF
Fulminant hepatic failure
- HEV
Hepatitis E virus
- HE
Hepatitis E
- AVH
Acute viral hepatitis
- ICER
Incremental cost-effectiveness ratio
- QALYs
Quality-adjusted life years.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was funded by the National Natural Fund (Code 81573258), the Jiangsu Provincial Social Development Fund (Code BE2013723, SBE2015710027).
Reference
- [1].Bru JP. Forgotten hepatitis: the hepatitis E. Rev Med Suisse 2012; 8:986-8; PMID:22662626 [PubMed] [Google Scholar]
- [2].Freshwater DA, Hepatitis E. the forgotten virus. J R Army Med Corps 2013; 159:167-8; PMID:24109137; http://dx.doi.org/ 10.1136/jramc-2013-000095 [DOI] [PubMed] [Google Scholar]
- [3].Aggarwal R. Clinical presentation of hepatitis. E. Virus Res 2011; 161:15-22; http://dx.doi.org/ 10.1016/j.virusres.2011.03.017 [DOI] [PubMed] [Google Scholar]
- [4].Perez-Gracia MT, Suay B, Mateos-Lindemann ML. Hepatitis E: an emerging disease. Infect Genet Evol 2014; 22:40-59; PMID:24434240; http://dx.doi.org/ 10.1016/j.meegid.2014.01.002 [DOI] [PubMed] [Google Scholar]
- [5].Rein DB, Wiersma S, Stevens GA. Modeling the global burden of hepatitis E virus infection and disease in 2005. Hepatology 2011; 54:1176A. [DOI] [PubMed] [Google Scholar]
- [6].Gou AL, Wu LY, Ma XZ, Zhou YC, Ai NW, Mi JT, et al.. An epidemiologic survey on a type E hepatitis (HE) outbreak in pregancy. Chin J Public Health 1989; 5:5-6. [Google Scholar]
- [7].Shinde N, Patil T, Deshpande A, Gulhane R, Patil M, Bansod Y. Clinical profile, maternal and fetal outcomes of acute hepatitis E in pregnancy. Ann Med Health Sci Res 2014; 4:S133-9; PMID:25184080; http://dx.doi.org/ 10.4103/2141-9248.138033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Khuroo MS, Kamili S, Jameel S. Vertical transmission of hepatitis E virus. Lancet 1995;345:1025-6; PMID:7723501; http://dx.doi.org/ 10.1016/S0140-6736(95)90761-0 [DOI] [PubMed] [Google Scholar]
- [9].Singh S, Mohanty A, Joshi YK, Deka D, Mohanty S, Panda SK. Mother-to-child transmission of hepatitis E virus infection. Indian J Pediatr 2003;70:37-9; PMID:12619951; http://dx.doi.org/ 10.1007/BF02722743 [DOI] [PubMed] [Google Scholar]
- [10].Khuroo MS, Kamili S, Khuroo MS. Clinical course and duration of viremia in vertically transmitted hepatitis E virus (HEV) infection in babies born to HEV-infected mothers. J Viral Hepat 2009;16:519-23; PMID:19228284; http://dx.doi.org/ 10.1111/j.1365-2893.2009.01101.x [DOI] [PubMed] [Google Scholar]
- [11].Teshale E, Ward JW. Making hepatitis E a vaccine-preventable disease. N Engl J Med 2015; 372:899-901; PMID:25738664; http://dx.doi.org/ 10.1056/NEJMp1415240 [DOI] [PubMed] [Google Scholar]
- [12].Safary A. Perspectives of vaccination against hepatitis E. Intervirology 2001; 44:162-6; PMID:11509877; http://dx.doi.org/ 10.1159/000050043 [DOI] [PubMed] [Google Scholar]
- [13].Shrestha MP, Scott RM, Joshi DM, Mammen MP, Thapa GB, Thapa N, Myint KS, Fourneau M, Kuschner RA, Shrestha SK, et al.. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med 2007; 356:895-903; PMID:17329696; http://dx.doi.org/ 10.1056/NEJMoa061847 [DOI] [PubMed] [Google Scholar]
- [14].Emerson SU, Purcell RH. Recombinant vaccines for hepatitis E. Trends Mol Med 2001; 7:462-6; PMID:11597521; http://dx.doi.org/ 10.1016/S1471-4914(01)02106-2 [DOI] [PubMed] [Google Scholar]
- [15].Hepatitis E vaccine: why wait? Lancet 2010; 376:845; PMID:20833284; http://dx.doi.org/ 10.1016/S0140-6736(10)61393-1 [DOI] [PubMed] [Google Scholar]
- [16].Xia NS, Zhang J, Li SW, Ge SX, Wu T, Zheng ZZ, et al.. Study Progress no Hepatitis E. J Xiamen Univ (Natural Science) 2011; 50:431-6. [Google Scholar]
- [17].Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, Yang CL, Jiang HM, Cai JP, et al.. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010; 376:895-902; PMID:20728932; http://dx.doi.org/ 10.1016/S0140-6736(10)61030-6 [DOI] [PubMed] [Google Scholar]
- [18].Li SW, Zhao Q, Wu T, Chen S, Zhang J, Xia NS. The development of a recombinant hepatitis E vaccine HEV 239. Hum Vaccin Immunother 2015; 11:908-14; PMID:25714510; http://dx.doi.org/ 10.1080/21645515.2015.1008870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang J, Zhang XF, Huang SJ, Wu T, Hu YM, Wang ZZ, Wang H, Jiang HM, Wang YJ, Yan Q, et al.. Long-term efficacy of a hepatitis E vaccine. N Engl J Med 2015; 372:914-22; PMID:25738667; http://dx.doi.org/ 10.1056/NEJMoa1406011 [DOI] [PubMed] [Google Scholar]
- [20].Wu T, Zhu FC, Huang SJ, Zhang XF, Wang ZZ, Zhang J, Xia NS. Safety of the hepatitis E vaccine for pregnant women: a preliminary analysis. Hepatology 2012; 55:2038; PMID:22161542; http://dx.doi.org/ 10.1002/hep.25522 [DOI] [PubMed] [Google Scholar]
- [21].Blommaert A, Bilcke J, Vandendijck Y, Hanquet G, Hens N, Beutels P. Cost-effectiveness of seasonal influenza vaccination in pregnant women, health care workers and persons with underlying illnesses in Belgium. Vaccine 2014; 32:6075-83; PMID:25239481; http://dx.doi.org/ 10.1016/j.vaccine.2014.08.085 [DOI] [PubMed] [Google Scholar]
- [22].Jit M, Cromer D, Baguelin M, Stowe J, Andrews N, Miller E. The cost-effectiveness of vaccinating pregnant women against seasonal influenza in England and Wales. Vaccine 2010; 29:115-22; PMID:21055501; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.078 [DOI] [PubMed] [Google Scholar]
- [23].Swamy GK, Garcia-Putnam R. Vaccine-Preventable Diseases in Pregnancy. Am J Perinatol 2013; 30:89-97; PMID:23271378 [DOI] [PubMed] [Google Scholar]
- [24].Beigi RH, Wiringa AE, Bailey RR, Assi TM, Lee BY. Economic value of seasonal and pandemic influenza vaccination during pregnancy. Clin Infect Dis 2009; 49:1784-92; PMID:19911967; http://dx.doi.org/ 10.1086/649013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kim SY, Russell LB, Park J, Verani JR, Madhi SA, Cutland CL, Schrag SJ, Sinha A. Cost-effectiveness of a potential group B streptococcal vaccine program for pregnant women in South Africa. Vaccine 2014; 32:1954-63; PMID:24530145; http://dx.doi.org/ 10.1016/j.vaccine.2014.01.062 [DOI] [PubMed] [Google Scholar]
- [26].Roberts S, Hollier LM, Sheffield J, Laibl V, Wendel GD. Cost-effectiveness of universal influenza vaccination in a pregnant population. Obstet Gynecol 2006; 107:1323-9; PMID:16738159; http://dx.doi.org/ 10.1097/01.AOG.0000210225.45986.99 [DOI] [PubMed] [Google Scholar]
- [27].Khuroo MS. Discovery of hepatitis E: the epidemic non-A, non-B hepatitis 30 years down the memory lane. Virus Res 2011; 161:3-14; PMID:21320558; http://dx.doi.org/ 10.1016/j.virusres.2011.02.007 [DOI] [PubMed] [Google Scholar]
- [28].Centers for Disease Control and Prevention. Investigation of hepatitis E outbreak among refugees-Upper Nile, South Sudan, 2012-2013. MMWR Morb Mortal Wkly Rep 2013;62:581-6; PMID:23884344 [PMC free article] [PubMed] [Google Scholar]
- [29].Ahmed JA, Moturi E, Spiegel P, Schilperoord M, Burton W, Kassim NH, Mohamed A, Ochieng M, Nderitu L, Navarro-Colorado C, et al.. Hepatitis E outbreak, Dadaab refugee camp, Kenya, 2012. Emerg Infect Dis 2013; 19:1010-2; PMID:23735820; http://dx.doi.org/ 10.3201/eid1906.130275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gurley ES, Hossain MJ, Paul RC, Sazzad HM, Islam MS, Parveen S, Faruque LI, Husain M, Ara K, Jahan Y, et al.. Outbreak of hepatitis E in urban bangladesh resulting in maternal and perinatal mortality. Clin Infect Dis 2014; 59:658-65; PMID:24855146; http://dx.doi.org/ 10.1093/cid/ciu383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shrestha SM. Hepatitis E in Nepal. Kathmandu Univ Med J 2006;4:530-44; PMID:18603971 [PubMed] [Google Scholar]
- [32].Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. Hepatitis E. Lancet 2012; 379:2477-88; PMID:22549046; http://dx.doi.org/ 10.1016/S0140-6736(11)61849-7 [DOI] [PubMed] [Google Scholar]
- [33].Adjei AA, Tettey Y, Aviyase JT, Adu-Gyamfi C, Obed S, Mingle JA, Ayeh-Kumi PF, Adiku TK. Hepatitis E virus infection is highly prevalent among pregnant women in Accra, Ghana. Virol J 2009;6:108; PMID:19619291; http://dx.doi.org/ 10.1186/1743-422X-6-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cevrioglu AS, Altindis M, Tanir HM, Aksoy F. Investigation of the incidence of hepatitis E virus among pregnant women in Turkey. J Obstet Gynaecol Res 2004;30:48-52; PMID:14718021; http://dx.doi.org/ 10.1111/j.1341-8076.2004.00155.x [DOI] [PubMed] [Google Scholar]
- [35].Alvarado-Esquivel C, Sanchez-Anguiano LF, Hernandez-Tinoco J. Hepatitis E virus exposure in pregnant women in rural Durango, Mexico. Ann Hepatol 2014; 13:510-7; PMID:25152983 [PubMed] [Google Scholar]
- [36].Stoszek SK, Abdel-Hamid M, Saleh DA, El Kafrawy S, Narooz S, Hawash Y, Shebl FM, El Daly M, Said A, Kassem E, et al.. High prevalence of hepatitis E antibodies in pregnant Egyptian women. Trans R Soc Trop Med Hyg 2006; 100:95-101; PMID:16257426; http://dx.doi.org/ 10.1016/j.trstmh.2004.12.005 [DOI] [PubMed] [Google Scholar]
- [37].Begum N, Devi SG, Husain SA, Kar P. Seroprevalence of subclinical HEV infection in pregnant women from north India: a hospital based study. Indian J Med Res 2009;130:709-13; PMID:20090131 [PubMed] [Google Scholar]
- [38].Caron M, Kazanji M. Hepatitis E virus is highly prevalent among pregnant women in Gabon, central Africa, with different patterns between rural and urban areas. Virol J 2008; 5:158; PMID:19102767; http://dx.doi.org/ 10.1186/1743-422X-5-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Huang F, Ma T, Li L, Zeng W, Jing S. Low seroprevalence of hepatitis E virus infection in pregnant women in Yunnan, China. Braz J Infect Dis 2013;17:716-7; PMID:24055390; http://dx.doi.org/ 10.1016/j.bjid.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rostamzadeh Khameneh Z, Sepehrvand N, Khalkhali HR. Seroprevalence of hepatitis E among pregnant women in urmia, iran. Hepat Mon 2013;13:e10931; PMID:24348644; http://dx.doi.org/ 10.5812/hepatmon.10931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nelson KE, Shih JW, Zhang J, Zhao Q, Xia N, Ticehurst JR, Labrique AB. Hepatitis E vaccine to prevent morbidity and mortality during epidemics. Open Forum Infect Dis 2014; 1:ofu098; PMID:25734166; http://dx.doi.org/ 10.1093/ofid/ofu098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Myers ER, Misurski DA, Swamy GK. Influence of timing of seasonal influenza vaccination on effectiveness and cost-effectiveness in pregnancy. Am J Obstet Gynecol 2011; 204:S128-40; PMID:21640230; http://dx.doi.org/ 10.1016/j.ajog.2011.04.009 [DOI] [PubMed] [Google Scholar]
- [43].Clements KM, Meier G, McGarry LJ, Pruttivarasin N, Misurski DA. Cost-effectiveness analysis of universal influenza vaccination with quadrivalent inactivated vaccine in the United States. Hum Vaccin Immunother 2014;10:1171-80; PMID:24609063; http://dx.doi.org/ 10.4161/hv.28221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jia Y, Li L, Cui F, Zhang D, Zhang G, Wang F, Gong X, Zheng H, Wu Z, Miao N, et al.. Cost-effectiveness analysis of a hepatitis B vaccination catch-up program among children in Shandong Province, China. Hum Vaccin Immunother 2014; 10:2983-91; PMID:25483678; http://dx.doi.org/ 10.4161/hv.29944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Boccalini S, Bechini A, Levi M, Tiscione E, Gasparini R, Bonanni P. Cost-effectiveness of new adult pneumococcal vaccination strategies in Italy. Hum Vaccin Immunother 2013; 9:699-706; PMID:23295824; http://dx.doi.org/ 10.4161/hv.23268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Li SW, Zhang J, Li YM, Ou SH, Huang GY, He ZQ, Ge SX, Xian YL, Pang SQ, Ng MH, et al.. A bacterially expressed particulate hepatitis E vaccine: antigenicity, immunogenicity and protectivity on primates. Vaccine 2005; 23:2893-901; PMID:15780738; http://dx.doi.org/ 10.1016/j.vaccine.2004.11.064 [DOI] [PubMed] [Google Scholar]
- [47].Kamili S. Toward the development of a hepatitis E vaccine. Virus Res 2011; 161:93-100; PMID:21620908; http://dx.doi.org/ 10.1016/j.virusres.2011.05.008 [DOI] [PubMed] [Google Scholar]
- [48].Cao XY, Ma XZ, Liu YZ. The epidemiology study of ENT-Erically transmitted non-A non-B hepatitis in the South area of Xinjiang, China. Chin J Public Health 1989; 8:193-9; PMID:NOT_FOUND [Google Scholar]
- [49].Xia XZ, Ni YZ, Ai EK, Sai LMJ, Chai P, et al.. An epidemiologic survey on a type E hepatitis (HE) outbreak. Chin J Epidemiol 1991; 12:257-60. [PubMed] [Google Scholar]
- [50].Rab MA BM, Mubarik MM, Asghar H, Sami Z, Siddiqi S, et al.. Water-borne hepatitis E virus epidemic in Islamabad, Pakistan: a common source outbreak traced to the malfunction of a modern water treatment plant. Tam J Trop Med Hyg 1997; 57:151-7. [DOI] [PubMed] [Google Scholar]
- [51].Jaiswal SP, Jain AK, Naik G, Soni N, Chitnis DS. Viral hepatitis during pregnancy. Int J Gynaecol Obstet 2001;72:103-8; PMID:11166742; http://dx.doi.org/ 10.1016/S0020-7292(00)00264-2 [DOI] [PubMed] [Google Scholar]
- [52].Zhang Y, Hai L, Yin M, Guli N, Ma M. Analysis of 500 cases in pregnant women with HNANB(E). J Xinjiang Med Univ 1990;13:54-8; PMID:NOT_FOUND [Google Scholar]
- [53].Jain AK JS, Jhamad S, Soni N, Sircar S, Jain M. To find out maternal and foetal outcome in pregnant women infected with acute viral hepatitis E(AVH-E) during third trimeter of pregnancy- a retrospective analysis. J Hepatol 2013;58:S500; PMID:NOT_FOUND; http://dx.doi.org/ 10.1016/S0168-8278(13)61234-9 [DOI] [Google Scholar]
- [54].Hamid SS, Jafri SM, Khan H, Shah H, Abbas Z, Fields H. Fulminant hepatic failure in pregnant women: acute fatty liver or acute viral hepatitis? J Hepatol 1996;25:20-7; PMID:8836897; http://dx.doi.org/ 10.1016/S0168-8278(96)80323-0 [DOI] [PubMed] [Google Scholar]
- [55].Banait VS, Sandur V, Parikh F, Murugesh M, Ranka P, Ramesh VS, Sasidharan M, Sattar A, Kamat S, Dalal A, et al.. Outcome of acute liver failure due to acute hepatitis E in pregnant women. Indian J Gastroenterol 2007; 26:6-10; PMID:17401226 [PubMed] [Google Scholar]
- [56].Bose PD, Das BC, Hazam RK, Kumar A, Medhi S, Kar P. Evidence of extrahepatic replication of hepatitis E virus in human placenta. J Gen Virol 2014; 95:1266-71; PMID:24622580; http://dx.doi.org/ 10.1099/vir.0.063602-0 [DOI] [PubMed] [Google Scholar]
- [57].Kumar A, Devi SG, Kar P, Agarwal S, Husain SA, Gupta RK, Sharma S. Association of cytokines in hepatitis E with pregnancy outcome. Cytokine 2014; 65:95-104; PMID:24416783; http://dx.doi.org/ 10.1016/j.cyto.2013.09.022 [DOI] [PubMed] [Google Scholar]
- [58].Patra S, Kumar A, Trivedi SS, Puri M, Sarin SK. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Intern Med 2007; 147:28-33; PMID:17606958; http://dx.doi.org/ 10.7326/0003-4819-147-1-200707030-00005 [DOI] [PubMed] [Google Scholar]
- [59].Xiu SX. Disease Burden of Hepatitis E and Health Economic Evaluation of Vaccine Interventions in Dongtai Area. Southeast Univ 2011. [Google Scholar]
- [60].Rein DB, Hicks KA, Wirth KE, Billah K, Finelli L, Fiore AE, Hoerger TJ, Bell BP, Armstrong GL. Cost-effectiveness of routine childhood vaccination for hepatitis A in the United States. Pediatrics 2007; 119:e12-21; PMID:17200237; http://dx.doi.org/ 10.1542/peds.2006-1573 [DOI] [PubMed] [Google Scholar]
- [61].Xiu SX, Zhang XF, Jiang HM, Yang CL, Wang ZZ, Huang SJ, et al.. Disease burden of hepatitis E in rural areas in east China. Chin J Zoonoses. 2011; 27:450-4. [Google Scholar]
- [62].Peasah SK, Azziz-Baumgartner E, Breese J, Meltzer MI, Widdowson MA. Influenza cost and cost-effectiveness studies globally-a review. Vaccine 2013; 31:5339-48; PMID:24055351; http://dx.doi.org/ 10.1016/j.vaccine.2013.09.013 [DOI] [PubMed] [Google Scholar]
- [63].http://www.who.int/csr/disease/hepatitis/whocdscsredc200112/en/index3.html. [Google Scholar]
- [64].Echevarria JM. Light and Darkness: Prevalence of Hepatitis E Virus Infection among the General Population. Scientifica 2014; 2014:481016; PMID:24672733; http://dx.doi.org/ 10.1155/2014/481016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Das A, Sinha M. A cost-effectiveness analysis of a candidate vaccine against hepatitis E virus using the institute of medicine model. Am J Gastroenterol 2001;96:S263; PMID:NOT_FOUNDAMBIGUOUS [Google Scholar]
- [66].Riedmann EM. Chinese biotech partnership brings first hepatitis E vaccine to the market. Hum Vaccin Immunother 2012;8:1743-4; PMID:AMBIGUOUS [PubMed] [Google Scholar]
- [67].Lee BY, Wateska AR, Bailey RR, Tai JH, Bacon KM, Smith KJ. Forecasting the economic value of an Enterovirus 71 (EV71) vaccine. Vaccine 2010; 28:7731-6; PMID:20923711; http://dx.doi.org/ 10.1016/j.vaccine.2010.09.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhuang GH, Pan XJ, Wang XL. A cost-effectiveness analysis of universal childhood hepatitis A vaccination in China. Vaccine 2008; 26:4608-16; PMID:18597903; http://dx.doi.org/ 10.1016/j.vaccine.2008.05.086 [DOI] [PubMed] [Google Scholar]
- [69].Berge JJ, Drennan DP, Jacobs RJ, Jakins A, Meyerhoff AS, Stubblefield W, Weinberg M. The cost of hepatitis A infections in American adolescents and adults in 1997. Hepatology 2000; 31:469-73; PMID:10655272; http://dx.doi.org/ 10.1002/hep.510310229 [DOI] [PubMed] [Google Scholar]
- [70].Karam V, Castaing D, Danet C, Delvart V, Gasquet I, Adam R, Azoulay D, Samuel D, Bismuth H. Longitudinal prospective evaluation of quality of life in adult patients before and one year after liver transplantation. Liver Transpl 2003;9:703-11; PMID:12827557; http://dx.doi.org/ 10.1053/jlts.2003.50148 [DOI] [PubMed] [Google Scholar]