ABSTRACT
The candidate vaccine HZ/su is being developed to prevent herpes-zoster disease (HZ). HZ occurrence is attributed to declines in varicella-zoster virus (VZV) specific T-cell immunity. HZ/su contains VZV antigen, gE, and Adjuvant System AS01B (liposome-based formulation of MPL and QS-21). In clinical trials, AS01B enhances CD4+ T-cell responses to gE. In clinical trials of other vaccines, Adjuvant Systems AS03 and AS04 also enhance antigen-specific CD4+ T-cell responses. Hence the purpose of this study was to evaluate gE formulated with AS01B, AS01E (50% less MPL and QS-21 than AS01B), AS03 or AS04 in C57BL6 mice primed with live-attenuated VZV. Four-weeks post-vaccination, the gE-specific CD4+ T-cell response to gE/AS01B was 5.4, 2.8 and 2.2-fold greater than those to gE/AS03, gE/AS04 and gE/AS03, respectively (p<0.001). Therefore in the VZV-primed mouse model, CD4+ T-cell responses to gE appeared most enhanced by AS01B, and adds further support for the use of AS01B in the HZ/su formulation.
KEYWORDS: herpes zoster Adjuvant System, MPL, QS-21, Varicella-zoster virus, vaccine
Herpes zoster (HZ) or shingles is a disease with symptoms including skin rash and postherpetic neuralgia, and is caused by the reactivation of dormant varicella zoster virus (VZV).1 The lifetime risk of having HZ has been estimated at around 30%, and the incidence of disease increases with age, with immunosuppressive treatments or with immunocompromised conditions.1-3 The occurrence of HZ has been attributed to a decline in T-cell mediated immunity to VZV.1,2,4,5 A live-attenuated vaccine is licensed (Zostavax®a, Merck),3,6,7 and its effectiveness against the incidence of disease has been estimated at around 50% in adults aged ≥60 years.6,8 Therefore there remains a need to increase vaccine effectiveness, especially in older adults.
In a recent Phase 3 placebo-controlled study of the candidate subunit vaccine HZ/su, the efficacy for the prevention of HZ was estimated at 97.2% in adults ≥50 years of age; and vaccine efficacy in the ≥70 years age group was similar to that in the 50–59 y and 60–69 y age groups.9 HZ/su includes a recombinant antigen based on the VZV glycoprotein E (gE). VZV gE is an abundant virion-envelope glycoprotein, essential for viral replication and cell-to-cell spreading;10,11 and gE-specific CD4+ T cells may play a role in preventing symptomatic VZV reactivation in healthy adults.5,12
HZ/su also contains the GSK-proprietary Adjuvant System AS01B, which enhances both gE-specific antibody and T-cell responses to antigen in mice and in clinical trials.13-17 AS01B contains liposomes and 2 immunostimulants, MPL (3-O-desacyl-4'-monophosphoryl lipid A) and QS-21d (Quillaja saponaria Molina, fraction 21).17 In mice, MPL and QS-21, in combination, synergistically induce gE-specific CD4+ T-cell responses to vaccination.13 AS01B contains 2-fold more MPL and QS-21 than AS01E, and induces higher gE-specific CD4+ T-cell responses to vaccination than AS01E in mice and in a clinical trial of human adults aged ≥50 years.13,15 AS01B also induces higher gE-specific CD4+ T-cell responses to vaccination in mice than an aluminum-salt adjuvant.13
In clinical trials, 2 other Adjuvant Systems, AS03 and AS04, have been shown to enhance antigen-specific CD4+ T-cell responses to influenza and human papillomavirus HPV vaccines, respectively, but have not been evaluated with the gE antigen.18,19 Therefore the objective of this study was to compare CD4+ T-cell responses to gE vaccines adjuvanted with AS01B or AS01E, with those to gE vaccines adjuvanted with AS03 or AS04, in mice primed with live-attenuated VZV. Antibody responses to vaccination were also evaluated.
Two independent experiments were performed in which C57Bl/6 mice (Harlan Horst, Netherlands) were primed with one sub-cutaneous dose of a live-attenuated varicella vaccine (full-human dose of Varilrix®bc; 104 pfu). Five weeks after priming on Days 0 and 28, mice were administered intramuscular (tibialis) injections of a gE vaccinec or saline (0.9% NaCl; control group). One gE-vaccine dose contained 5 µg gE and an Adjuvant System in 50 µl. The contents of the Adjuvant Systems AS01B, AS01E, AS03, or AS04 are defined by the quantities of the following components in a full-human dose: AS01B contains 50 µg MPL and 50 µg QS-21; AS01E contains 25 µg MPL and 25 µg QS-21; AS03 contains 11.86 mg α-tocopherol and squalene in an oil-in-water emulsion, and AS04 contains 50 μg MPL adsorbed on 500 μg aluminum salt. For the purpose of this article, gE/AS01B, gE/AS01E, gE/AS03, and gE/AS04 refer to the mouse vaccines in which the Adjuvant System contains one tenth of the respective quantities used in a full-human dose.
Antigen-specific CD4+ T cells expressing at least one of the 2 cytokines IFN-γ and IL-2, were detected in all vaccine groups at 30 d after dosing. In Experiment 1, the geometric mean frequency (GMF) of gE-specific CD4+ T cells was 6.2% in the gE/AS01B group; whereas it was 3.5% in the gE/AS04 group, 2.2% in the gE/AS01E group and 1.3% in the gE/AS03 group (Fig. 1A). In Experiment 2, the GMF of gE-specific CD4+ T cells was 9.1% in the gE/AS01B group; whereas it was 5.8% in the gE/AS01E group, 1.9% in the gE/AS04 group, and 1.5% in the gE/AS03 group (Fig. 1A). In Experiments 1 and 2, the frequencies of gE-specific CD4+ T cells in the NaCl group were either close to or below the cut-off for the assay (GMFs were 0.3% and 0.05%, respectively). The frequencies of gE-specific CD8+ T cells in any of the adjuvanted-vaccine groups were not significantly higher than the baseline frequencies observed in the NaCl group (not shown).
Figure 1.
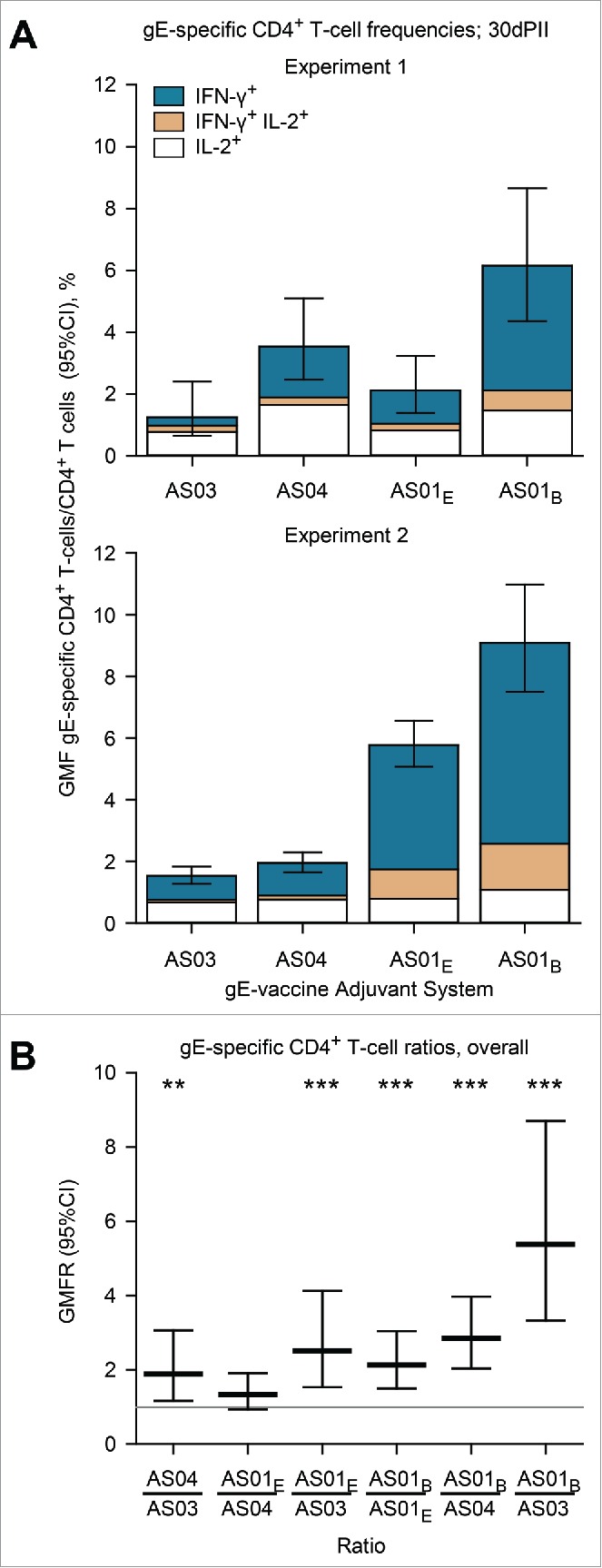
Geometric mean frequencies (GMFs) of (A) gE-specific CD4+ T cells and (B) ratios of GMFs from different adjuvanted-vaccine groups. Spleens (Experiment 1, N=8 and Experiment 2, N = 11; spleens pooled from 2 mice) were sampled at 30 d after the second vaccine dose (30dPII). The frequency of gE-specific CD4+ T cells was calculated as a percentage of cytokine-positive CD4+ T cells divided by all CD4+ T cells. Error bars represent 95% confidence intervals. In Experiments 1 and 2, the frequencies of antigen-specific CD4+ T cells in the NaCl group were either close to or below the cut-off for the assay (GMFs were 0.3% and 0.05%, respectively). In (B), horizontal gray reference line indicates a ratio = 1, and asterisks indicate significant differences from 1 (** p<0.01; *** p<0.001). Antigen-specific T cells were evaluated in splenocyte-restimulation cultures as described previously,13 but with some modifications. Briefly, splenocyte cultures (1106 cells per well of 96-well plate) were prepared from spleens of 2 mice and were incubated for 2 hours in the presence of gE peptides spanning the complete gE protein (6315-mer peptides, 11 amino-acid overlap) and then incubated ˜18 hours in the presence of brefeldin A. Subsequently, the cells were stained with fluorescent-monoclonal antibodies specific for CD4 and after permeabilization, for intracellular-cytokines IL-2 and IFN-γ. All antibodies were obtained from BD Biosciences, Belgium. Flow cytometry was performed using LSR II Facs (BD Biosciences, Belgium) and analyzed using FlowJo software (FlowJo, LLC, OR, USA). Statistical calculations were based on an analysis of variance with 2 factors (vaccine group, experiment) on log10 values using a heterogeneous variance model (i.e., identical variances were not assumed for the different levels of the factor). Estimates of the geometric-mean ratios between groups and their 95% confidence intervals (CI) were obtained using back-transformation of log10 values. Adjustments for multiple testing were performed using Tukey's method. All analyses were performed using SAS software (Version 9.2, SAS Institute Inc., NC, USA).
In both experiments, the differences between the vaccine groups were mostly associated with gE-specific CD4+ T cells that were IFN-γ positive (Fig. 1A). Moreover, the magnitude of IFN-γ production in IFN-γ+ CD4+ T cells relative to IFN-γ− CD4+ T cells appeared higher in the gE/AS01B group than in the other groups (measured by fluorescent-staining intensities; not shown). Overall, gE-specific CD4+T cells were 5.4, 2.8 and 2.2-fold more frequent in response to gE/AS01B than in response to gE/AS03 gE/AS04 and gE/AS01E (p<0.001), respectively; and were 2.5-fold more frequent in response to gE/AS01E than in response to gE/AS03 (p<0.001; Fig. 1B).
Antigen-specific antibodies were detected in all vaccine groups at 14 and 28 d after dosing but were not detected in the NaCl group (concentrations were below the cut-off of the assay; i.e. <500 EU/ml). For both experiments and in any given vaccine group, the magnitude of geometric mean concentrations (GMCs) of gE-specific antibodies appeared similar at 14 d compared with 28 d (Fig. 2A). Some significant differences were observed in the ratios of antibody concentrations between vaccine groups, although, no differences were more than 2-fold (Fig. 2B). In the gE/AS01B group at 14 and 28 days, GMCs were 1.6-fold (p<0.001) and 1.7-fold higher (p<0.001) than in the gE/AS03 group, respectively; 1.4-fold (p<0.001) and 1.7-fold (p<0.001) higher than in the gE/AS04 group, respectively; and 1.5-fold (p<0.001) and 1.4-fold (p<0.05) higher than in the gE/AS01E group, respectively (p<0.001).
Figure 2.
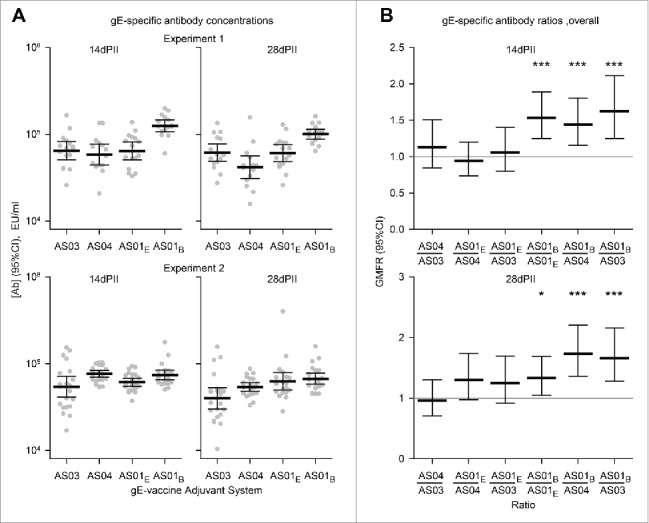
Geometric mean concentrations (GMCs) of (A) gE-specific antibodies and (B) ratios of GMCs (GMFRs) from different adjuvanted-vaccine groups. Sera (Experiment 1, N = 16 1 and Experiment 2, N = 22) were sampled at 14 and 28 d after the second vaccine dose (14dPII and 28dPII, respectively). Error bars represent 95% confidence intervals. Antigen-specific antibodies were not detected in the NaCl group (concentrations were below the cut-off of the assay; i.e.<500 EU/ml). In (B), horizontal gray reference lines indicate a ratio = 1, and asterisks indicate significant differences from 1 (*p<0.05; *** p<0.001). Antigen-specific antibody concentrations (in EU/ml and defined by internal standards) were measured by ELISA as previously described.13 Statistical calculations were performed as described in Figure 1.
Overall, the AS01B-based vaccine formulation induced the highest frequency of gE-specific CD4+ T cells compared with the AS03 and AS04 vaccine formulations, primarily reflecting differences in the frequencies of those T cells that were IFN-γ positive. The AS01E formulation also induced a higher frequency of gE-specific CD4+ T cells than AS03. The potential that these comparative differences are relevant in humans is suggested from the observation that gE-specific CD4+ T cell responses were higher to the AS01B formulation than to the AS01E formulation in VZV-primed mice (consistent with a previous study) as well as in the clinical setting.13,15
Although VZV antibodies are not considered essential to confer protection against HZ,2 the AS01B-based vaccine formulation induced marginally higher gE-specific antibody concentrations compared with the other formulations (and our unpublished observations suggest that these antibody concentrations correlate with VZV-neutralizing activity). Hence, the differences between AS01B-based vaccine formulation and the AS03- and AS04-based vaccine formulations were primarily reflected in differences in CD4+ T cell frequencies and in line with nonclinical and clinical experience of other vaccines.17
In humans, VZV-specific cell-mediated immunity appears to play an essential role in protection against both the occurrence and morbidity of HZ, although a clearly defined correlate of protection against HZ remains to be identified.1,2,4,5 CD4+ T cells expressing IFN-γ appear to predominate in the responses to VZV antigens in general or to gE alone,5,20 thus supporting the monitoring of CD4+ T cells in HZ-vaccine evaluations.13 Hence the present study and a previous preclinical study add further support for the use of AS01B rather than AS03, AS04, AS01E or aluminum salt in the candidate HZ vaccine formulation.
Abbreviations
- CI,
confidence interval;
- gE,
glycoprotein E;
- HZ,
herpes zoster;
- GMC,
geometric mean concentration;
- GMF,
geometric mean frequency;
- MPL,
3-O-desacyl-4'-monophosphoryl lipid A;
- QS-21,
Quillaja saponaria Molina, fraction 21;
- VZV,
varicella zoster virus
Abbreviations
- CI
confidence interval
- gE
glycoprotein E
- HZ
herpes zoster
- GMC
geometric mean concentration
- GMF
geometric mean frequency
- MPL
3-O-desacyl-4'-monophosphoryl lipid A
- QS-21
Quillaja saponaria Molina, fraction 21
- VZV
varicella zoster virus
Disclosure of potential conflicts of interest
All authors were involved in the conception and design of the studies. ND, MB, MF acquired the data. All authors analyzed and interpreted the results. All authors were involved in drafting the manuscript or revising it critically for important intellectual content. All authors had full access to the data and approved the manuscript before it was submitted by the corresponding author.
All authors have declared the following interests: all authors are employees of the GSK group of companies. MB and ND own GSK stocks.
Acknowlegements
Animal husbandry and experiments were ethically reviewed and carried out in accordance with European Directive 2010/63/EU and GlaxoSmithKline Biologicals SA policy. We thank Sandra Giannini for scientific advice and Frédéric Renaud for performing the statistical analyses (both GSK Vaccines, Belgium). Matthew Morgan (MG Science Communications, Belgium) provided scientific writing services, and Ulrike Krause (GSK Vaccines, Belgium) provided editorial advice and coordinated the manuscript's development.
Funding
This work was sponsored by GlaxoSmithKline Biologicals SA who also took responsibility for all costs associated with the development and publishing of the manuscript, including scientific writing assistance.
References
- [1].O'Connor KM, Paauw DS. Herpes zoster. Med Clin North Am 2013; 97:503-22, ix; PMID:23809711; http://dx.doi.org/ 10.1016/j.mcna.2013.02.002 [DOI] [PubMed] [Google Scholar]
- [2].Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol 2010; 48 Suppl 1:S2-S7; PMID:20510263; http://dx.doi.org/ 10.1016/S1386-6532(10)70002-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1-30; PMID:18528318 [PubMed] [Google Scholar]
- [4].Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol 2010; 342:341-57; PMID:20473790 [DOI] [PubMed] [Google Scholar]
- [5].Malavige GN, Jones L, Black AP, Ogg GS. Varicella zoster virus glycoprotein E-specific CD4+ T cells show evidence of recent activation and effector differentiation, consistent with frequent exposure to replicative cycle antigens in healthy immune donors. Clin Exp Immunol 2008; 152:522-31; PMID:18363743; http://dx.doi.org/ 10.1111/j.1365-2249.2008.03633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE et al.. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271-84; PMID:15930418; http://dx.doi.org/ 10.1056/NEJMoa051016 [DOI] [PubMed] [Google Scholar]
- [7].Doan HQ, Ung B, Ramirez-Fort MK, Khan F, Tyring SK. Zostavax : a subcutaneous vaccine for the prevention of herpes zoster. Expert Opin Biol Ther 2013; 13:1467-77; PMID:23984934; http://dx.doi.org/ 10.1517/14712598.2013.830101 [DOI] [PubMed] [Google Scholar]
- [8].Gagliardi AM, Gomes Silva BN, Torloni MR, Soares BG. Vaccines for preventing herpes zoster in older adults. Cochrane Database Syst Rev 2012; 10:CD008858; PMID:23076951 [DOI] [PubMed] [Google Scholar]
- [9].Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barbera J et al.. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087-96; PMID:25916341; http://dx.doi.org/ 10.1056/NEJMoa1501184 [DOI] [PubMed] [Google Scholar]
- [10].Berarducci B, Rajamani J, Zerboni L, Che X, Sommer M, Arvin AM. Functions of the unique N-terminal region of glycoprotein E in the pathogenesis of varicella-zoster virus infection. Proc Natl Acad Sci U S A 2010; 107:282-7; PMID:19966293; http://dx.doi.org/ 10.1073/pnas.0912373107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Berarducci B, Ikoma M, Stamatis S, Sommer M, Grose C, Arvin AM. Essential functions of the unique N-terminal region of the varicella-zoster virus glycoprotein E ectodomain in viral replication and in the pathogenesis of skin infection. J Virol 2006; 80:9481-96; PMID:16973553; http://dx.doi.org/ 10.1128/JVI.00533-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fowler WJ, Garcia-Valcarcel M, Hill-Perkins MS, Murphy G, Harper DR, Jeffries DJ, Burns NR, Adams SE, Kingsman AJ, Layton GT. Identification of immunodominant regions and linear B cell epitopes of the gE envelope protein of varicella-zoster virus. Virology 1995; 214:531-40; PMID:8553555; http://dx.doi.org/ 10.1006/viro.1995.0064 [DOI] [PubMed] [Google Scholar]
- [13].Dendouga N, Fochesato M, Lockman L, Mossman S, Giannini SL. Cell-mediated immune responses to a varicella-zoster virus glycoprotein E vaccine using both a TLR agonist and QS21 in mice. Vaccine 2012; 30:3126-35; PMID:22326899; http://dx.doi.org/ 10.1016/j.vaccine.2012.01.088 [DOI] [PubMed] [Google Scholar]
- [14].Didierlaurent AM, Collignon C, Bourguignon P, Wouters S, Fierens K, Fochesato M, Dendouga N, Langlet C, Malissen B, Lambrecht BN et al.. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J Immunol 2014; 193:1920-30; PMID:25024381; http://dx.doi.org/ 10.4049/jimmunol.1400948 [DOI] [PubMed] [Google Scholar]
- [15].Chlibek R, Bayas JM, Collins H, de la Pinta ML, Ledent E, Mols JF, Heineman TC. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults ≥50 years of age. J Infect Dis 2013; 208:1953-61; PMID:23904292; http://dx.doi.org/ 10.1093/infdis/jit365 [DOI] [PubMed] [Google Scholar]
- [16].Chlibek R, Smetana J, Pauksens K, Rombo L, Van den Hoek JA, Richardus JH, Plassmann G, Schwarz TF, Ledent E, Heineman TC. Safety and immunogenicity of three different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults: a phase II, randomized, controlled study. Vaccine 2014; 32:1745-53; PMID:24508036; http://dx.doi.org/ 10.1016/j.vaccine.2014.01.019 [DOI] [PubMed] [Google Scholar]
- [17].Garçon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines 2011; 10:471-86; http://dx.doi.org/ 10.1586/erv.11.29 [DOI] [PubMed] [Google Scholar]
- [18].Couch RB, Bayas JM, Caso C, Mbawuike IN, Lopez CN, Claeys C, El IM, Herve C, Laupeze B, Oostvogels L et al.. Superior antigen-specific CD4+ T-cell response with AS03-adjuvantation of a trivalent influenza vaccine in a randomised trial of adults aged 65 and older. BMC Infect Dis 2014; 14:425; PMID:25078387; http://dx.doi.org/ 10.1186/1471-2334-14-425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Van Damme P, Leroux-Roels G, Simon P, Foidart JM, Donders G, Hoppenbrouwers K, Levin M, Tibaldi F, Poncelet S, Moris P et al.. Effects of varying antigens and adjuvant systems on the immunogenicity and safety of investigational tetravalent human oncogenic papillomavirus vaccines: results from two randomized trials. Vaccine 2014; 32:3694-705; PMID:24674663; http://dx.doi.org/ 10.1016/j.vaccine.2014.03.040 [DOI] [PubMed] [Google Scholar]
- [20].Vossen MT, Gent MR, Weel JF, de Jong MD, van Lier RA, Kuijpers TW. Development of virus-specific CD4+ T cells on reexposure to Varicella-Zoster virus. J Infect Dis 2004; 190:72-82; PMID:15195245; http://dx.doi.org/ 10.1086/421277 [DOI] [PubMed] [Google Scholar]


