ABSTRACT
The European Medicines Agency has approved a multicomponent serogroup B meningococcal vaccine (Bexsero®) for use in individuals of 2 months of age and older. A cost-effectiveness analysis (CEA) from the societal and Italian National Health Service perspectives was performed in order to evaluate the impact of vaccinating Italian infants less than 1 y of age with Bexsero®, as opposed to non-vaccination. The analysis was carried out by means of Excel Version 2011 and the TreeAge Pro® software Version 2012. Two basal scenarios that differed in terms of disease incidence (official and estimated data to correct for underreporting) were considered. In the basal scenarios, we considered a primary vaccination cycle with 4 doses (at 2, 4, 6 and 12 months of age) and 1 booster dose at the age of 11 y, the societal perspective and no cost for death. Sensitivity analyses were carried out in which crucial variables were changed over probable ranges. In Italy, on the basis of official data on disease incidence, vaccination with Bexsero® could prevent 82.97 cases and 5.61 deaths in each birth cohort, while these figures proved to be three times higher on considering the estimated incidence. The results of the CEA showed that the Incremental Cost Effectiveness Ratio (ICER) per QALY was €109,762 in the basal scenario if official data on disease incidence are considered and €26,599 if estimated data are considered. The tornado diagram indicated that the most influential factor on ICER was the incidence of disease. The probability of sequelae, the cost of the vaccine and vaccine effectiveness also had an impact. Our results suggest that vaccinating infants in Italy with Bexsero® has the ability to significantly reduce meningococcal disease and, if the probable underestimation of disease incidence is considered, routine vaccination is advisable.
KEYWORDS: Bexsero®, Cost-Effectiveness Analysis (CEA), health economics, meningococcus, Neisseria meningitidis B, vaccination
Introduction
Invasive disease caused by Neisseria meningitidis (Nm) is a serious public health problem and has a heavy economic impact.1,2 The incidence of invasive disease is highly variable according to geographical area.3 In Europe, since the introduction of massive meningococcal serogroup C vaccination, serogroup B has become the main causative agent of meningococcal disease, and is associated with high rates of mortality and disability; children under 1 y of age are mainly affected.4,5 In Italy, about 60% of typed cases of meningococcal invasive disease are now caused by Neisseria meningitidis B (NmB).6-8
In the past, many attempts to produce an effective vaccine against NmB were made in various parts of the world. However, owing to the great similarity between NmB capsular polysaccharide and human neural components,9 preparation of an effective vaccine has proved very difficult.4,5 Consequently, research has focused on sub-capsular components, particularly on the antigens of the outer membrane vesicles (OMVs). Vaccines based on OMVs have been developed and have proved to be efficacious in Norway,10 Cuba,11 Brazil,12 Chile,13 and New Zealand.14 These experiences have shown that, because OMV vaccines are strictly strain-specific, they are useful in epidemics sustained by the same strain as that contained in the vaccine; this is logical, given the great variability of the outer membrane proteins.15
A new multicomponent vaccine (Bexsero®), produced by means of reverse vaccinology, has now gained marketing authorisation in Europe,16 Canada,17 Australia18 and the US.19 We therefore felt that it would be useful to carry out a cost-effectiveness analysis (CEA) on the possible use of Bexsero® in the Italian epidemiological scenario.
Results
Introducing vaccination with Bexsero® in Italian infants could prevent 82.97 cases and 5.61 deaths in each birth cohort, considering official data on disease incidence (0.23 per 100,000 subjects),8 and 248.91 cases and 16.83 deaths considering estimated data on disease incidence (0.69 per 100,000).20
Table 1 shows the results of CEA. As can be seen, the Incremental Cost Effectiveness Ratios (ICERs) per QALY were €109,762 for basal scenario 1 and €26,599 for basal scenario 5. The ICERs were €120,990 and €37,827 from the National Health Service (NHS) perspective, considering the official and estimated data on disease incidence, respectively.
Table 1.
Results of CEA analysis broken down by different scenarios.
| Scenario | Perspective | Cost of death | Number of vaccine doses | Disease incidence | ICER per QALY (€) |
|---|---|---|---|---|---|
| 1* | Social | 0 | 5 | official data | 109,762 |
| 2 | Social | SHC | 5 | official data | 109,191 |
| 3 | Social | WTP | 5 | official data | 104,657 |
| 4 | NHS | 0 | 5 | official data | 120,990 |
| 5* | Social | 0 | 5 | estimated data | 26,599 |
| 6 | Social | SHC | 5 | estimated data | 26,029 |
| 7 | Social | WTP | 5 | estimated data | 21,494 |
| 8 | NHS | 0 | 5 | estimated data | 37,827 |
With regard to the 8 scenarios developed, the 4 scenarios that considered estimated data on disease incidence proved cost-effective at a threshold value of €50,000 (Table 1).
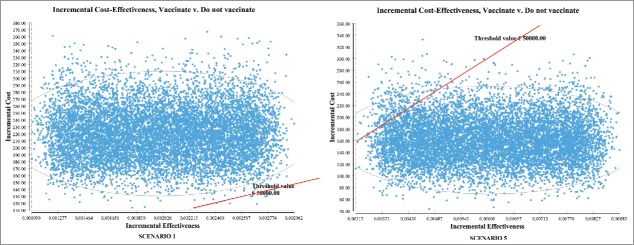
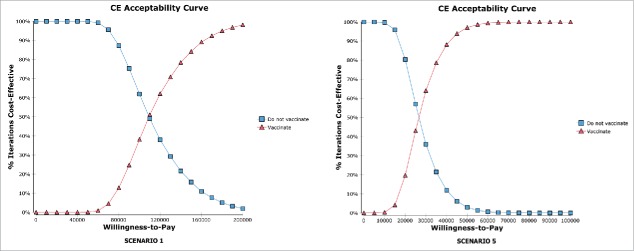
The Incremental Cost-Effectiveness (ICE) ellipse scatterplots (Fig. 1) showed that, at a threshold value of €50,000 per QALY, the introduction of vaccination had 0.04% of probability of being cost-effective in scenario 1 and 97.08% of probability of being cost-effective in scenario 5. These findings were confirmed by the cost-effectiveness acceptability curves (Fig. 2).
Figure 1.
Incremental Cost-Effectiveness (ICE) ellipse scatterplots showing the distribution of values of incremental costs and incremental effectiveness resulting from the Monte Carlo simulation. Points below the threshold value indicate cost-effectiveness.
Figure 2.
Cost-effectiveness acceptability curves (“vaccinate” and “do not vaccinate”). The figures show the probability of being cost-effective on varying the threshold value.
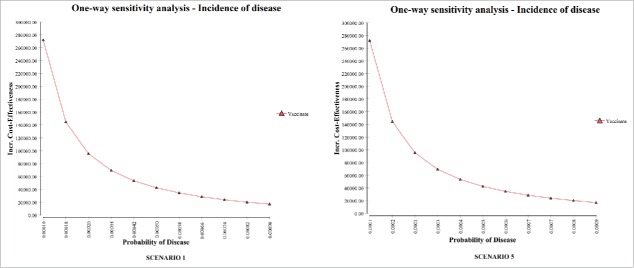
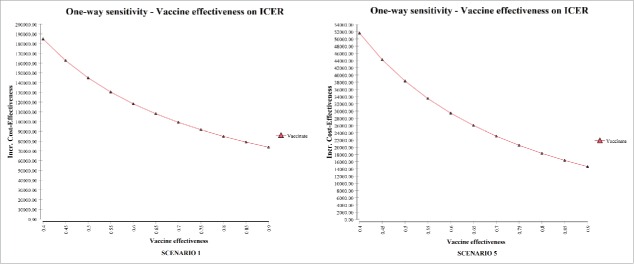
One-way sensitivity analyses were developed on considering the influence of disease incidence on the ICER for the vaccination strategy (Fig. 3). Further one-way sensitivity analyses were developed on considering the influence of vaccine effectiveness on the ICER for the vaccination strategy (Fig. 4).
Figure 3.
Impact of average annual incidence (per 100,000) of serogroup B invasive disease on the ICER (one-way sensitivity analysis). The probability of disease is per person.
Figure 4.
Impact of vaccine effectiveness on the ICER (one-way sensitivity analysis).
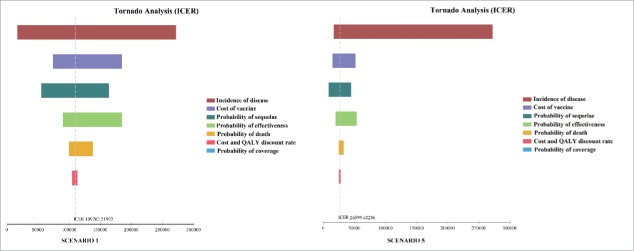
Figure 5 illustrates the results yielded by Tornado analyses on considering the ICER. The tornado diagrams indicate that the most influential factor is disease incidence. The probability of sequelae, the cost of the vaccine and vaccine effectiveness also have an impact.
Figure 5.
Tornado diagrams evaluating the influence of each parameter of the model on ICER.
Discussion
When a new vaccine, such as Bexsero®, becomes available, decision-makers must decide whether to introduce it into the National Immunization Program (NIP) or to wait until more evidence has been obtained (cost–effectiveness, etc.) or until conditions change (price, financial resources, supply, program strength, etc.). World Health Organization (WHO) guidelines on this issue21 suggest considering: public health priorities (disease burden; other inventions; efficacy, quality and safety of the vaccine; and economic and financial issues), the available of the vaccine on the market, and the availability of supply. In this regard, cost-effectiveness studies are helpful to decision-makers.
Our study showed that vaccinating infants in Italy with Bexsero® has the ability to significantly reduce meningococcal disease, and that the vaccine program could be cost-effective if the possible underestimation of disease incidence is considered. It is important to highlight that the mathematical and economic models applied to the transmission and prevention of infectious diseases necessarily adopt a reductionist approach.22 In the case of meningococcal disease, this is particularly true with regard to the possible underestimation of disease incidence and the evaluation of possible sequelae and their costs. Regarding the underestimation of disease incidence, although the Italian system of surveillance of invasive bacterial diseases is well established and well structured, it does have limitations. For instance, in most cases when the patient is taken to hospital, antibiotic therapy has already been started. Moreover, as the Regional Centers do not always promptly dispatch isolated strains to the National Institute of Health (ISS) for typing, a certain proportion of isolated strains cannot be typed. Finally, many fulminant cases are not recognizable as such according to the evidence requested by the WHO.23 Indeed, 2 recent Italian studies demonstrated that in Italy the real incidence of bacterial invasive diseases is greatly underestimated.20,24 Azzari et al.20 reported that culture has so far been the most frequently used technique for meningococcal surveillance in Italy. However, bacterial culture leads to considerable underestimation of the number of cases. In that study, the authors compared the culture method with the molecular method and found that the sensitivity of culture was less than one third of that of the molecular method. Furthermore, culture displayed lower sensitivity than the molecular method when patients had been treated with antibiotics.20 Therefore, on the basis of the results of the study by Azzari et al.20 and in order to limit the possible underestimation of disease incidence, we implemented option 2 considering an annual estimated disease incidence of 0.69 per 100,000 (scenarios 5, 6, 7 and 8). This issue is very important as disease incidence is the major factor influencing ICER, as highlighted by our results and also by previous economic evaluations.25-27
Another key issue that affects ICER is the estimation of sequelae. Indeed, many of the studies in the medical literature have not considered all relevant sequelae. Moreover, as it is not rare for survivors to suffer multiple sequelae, it is very difficult to evaluate their frequency and combinations. Therefore, we tried to limit this factor of underestimation (probability of long-term sequelae) as far as possible. We believe that this approach is one of the strengths of our study. Indeed, cost-effectiveness studies often neglect the issue of underestimation and tend to be very conservative.25-29 Our study indicated that the parameter “probability of sequelae” was one of the parameters impacting ICER.
An HTA analysis30 performed in Italy estimated that introducing Bexsero® into the infant immunization program would be cost-effective from the social perspective under specific assumptions, in particular on considering discount rates lower than 3% (ICER = €44.872 considering discount rate 1.5% for outcomes and 3% for costs; ICER = €26,806 considering discount rate 1.5% both for outcomes and costs). Furthermore, a recent Italian cost-effectiveness analysis found that routine infant immunization with Bexsero® would not be cost-effective with an ICER of > 350,000 €/QALY.25 In this latter study, Tirani et al.25 considered a 3-dose vaccine immunization schedule at 2, 3 and 4 months followed by 1 catch-up dose between 12 and 23 months, a cost per vaccine dose of €67, vaccine efficacy of 75% and 3-y duration of protection; however, their analysis only considered the direct costs associated with meningococcal invasive disease (NHS perspective). Notably, it is difficult to compare the results of these study25,30 with our findings, owing to the different values of the parameters used.
So far, some economic analyses of the introduction of vaccination against NmB have been published in developed countries.26-29 Pouwels et al. concluded that: at the current low level of disease incidence, the introduction of routine infant vaccination (4-dose schedule) is unlikely to be cost-effective in the Netherlands28; Tu et al. also reported the same conclusions with regard to the Canadian setting.26 Christensen et al. claimed that the new MenB vaccine could substantially reduce the disease in England and be cost-effective if competitively priced, particularly if the vaccine can prevent carriage as well as disease.27,29
It is important to consider that some economic studies on vaccinations are performed only from the perspective of the third-party payer (usually the NHS).25,26 However, meningococcal disease generates very high indirect costs, such as, for instance, the loss of productivity of patients and their parents, and the need for special education for the subjects affected by severe complications (for example mental retardation, cognitive problems, hearing loss and severe speech or communication problems, etc.). We therefore considered the societal perspective as the basal scenario, precisely in order to provide a complete picture of the general costs of the disease. Indeed, WHO recommends to evaluating the widest perspective.31
The cost and effectiveness of the vaccine and the duration of protection may also play an important role in influencing ICER. With regard to vaccine effectiveness, we considered the results of clinical trials on vaccine efficacy and the results of the study by Vogel et al.32 on predicted strain coverage. Their study assessed the predicted strain coverage by using the Meningococcal Antigen Typing System (MATS) method, which a very recent study considered to be a conservative predictor of strain coverage by Bexsero® in infants. Indeed, the authors demonstrated that, although MATS and hSBA yielded significantly associated results, hSBA more often also revealed protection against strains which did not prove positive on MATS.33 Strain coverage could therefore be higher.
With regard to the duration of protection, complete information is not yet available, as the vaccine is recent; the duration of protection can therefore only be hypothesized. We assumed a 10-y duration of full vaccine protection26 and subsequent waning of protection, whereby vaccination would only directly prevent one-quarter of cases over the lifetime of a vaccinated birth cohort.34 It must be noted that the majority of cases occur in the first years of life,4,5,20 followed by a secondary lower peak in adolescents and young adults.35 Given the epidemiological trend of meningococcal disease, an adolescent booster dose may be useful in order to prevent the cases that occur during this period of life. This strategy has also been advocated by other researchers.34 Our model therefore considered a booster dose at 11 y of age.
Like all pharmaco-economic analyses, the present study has limitations because the models are a simplification of the real world setting. Firstly, as only limited data are available on some parameters, we had to make certain assumptions, particularly with regard to the duration of protection conferred by the vaccine. For what concerns sequelae-related costs, we had to use data from studies conducted outside Italy, as no Italian study reports the data needed in order to carry out a pharmaco-economic analysis. We assumed that the life expectancy of subjects affected by disease who survive with sequelae was the same as that of unaffected subjects (except for the sequela “renal failure”). This is not completely true, though the differences seem to be small.29,36 The whole cohort (531,372 individuals) experienced a naturally occurring, age-related decline in quality of life.
We implemented a static model and did not consider herd immunity. However, it is probable that Bexsero® would have an impact on carriage; this issue was investigated in a very recent study.37 Nevertheless, herd protection is more likely to occur if a booster dose is administered in adolescence, as carriage is particularly high in teenagers.28,38,39
In conclusion, our results suggest that vaccinating infants in Italy with Bexsero® has the ability to significantly reduce meningococcal disease and, if the probable underestimation of disease incidence is considered, routine vaccination is advisable.
Surveillance after vaccine implementation will be crucial in order to evaluate some parameters, as the true effectiveness of the vaccine and the duration of protection are not yet fully known. Furthermore, potential benefits due to cross-protection against non-B serogroups40-42 and the vaccine ability to induce herd protection will need to be assessed.
Materials and methods
Design
A static cohort-simulation model was developed. In order to evaluate the vaccination strategy, a decisional-tree model was created by means of TreeAge Pro® software (Microsoft Inc., Redmond, WA; 1988–2012 TreeAge Software, Inc.). Figure 6 shows a simplified version of the decisional tree; the complete decisional tree is reported in Electronic Supplementary Material A1. Our decisional tree defined two strategies: vaccinating or not vaccinating infants less than 1 y of age. A cohort of 531,372 individuals was used, corresponding to the number of children less than 1 y of age who were resident in Italy at the 2012 census.43
Figure 6.
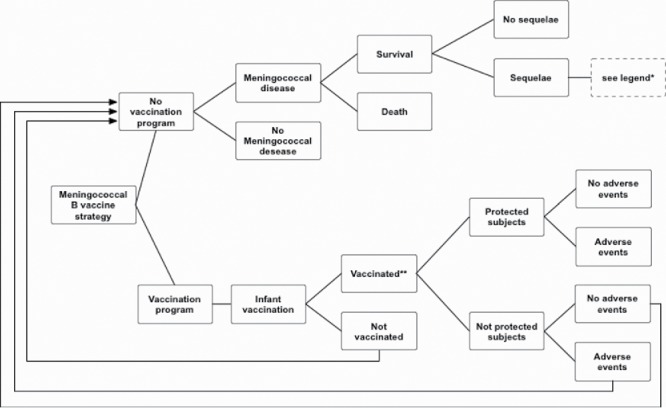
Simplified decisional tree: meningococcal serogroup B vaccination in infants.*Legend: amputation with substantial disability, anxiety, arthritis, depression, motor deficits, blindness, epilepsy or seizure, severe neurological disability, mental retardation (cognitive problems), hearing loss requiring cochlear implantation, moderate/severe bilateral hearing loss, moderate unilateral hearing loss, skin necrosis, scars, severe speech or communication problems, renal failure, chronic migraine.**Protection was assumed to begin after the second dose of the vaccine.
The cost-effectiveness study was modeled on routine immunization with 4 doses (at 2, 4, 6 and 12 months of age) of Bexsero® (primary cycle) and 1 booster dose at the age of 11 y (Table 2).
Table 2.
Scenarios evaluated.
| Scenario | Perspective | Cost of death | Number of vaccine doses [Primary cycle (2, 4, 6 and 12 months of age) and booster dose at 11 y] | Disease incidence |
|---|---|---|---|---|
| 1* | Social | 0 | 5 | official data |
| 2 | Social | SHC | 5 | official data |
| 3 | Social | WTP | 5 | official data |
| 4 | NHS | 0 | 5 | official data |
| 5* | Social | 0 | 5 | Estimated data |
| 6 | Social | SHC | 5 | Estimated data |
| 7 | Social | WTP | 5 | Estimated data |
| 8 | NHS | 0 | 5 | Estimated data |
Basal scenarios.
The “Vaccination” branch of the tree (Fig. 6) was divided into two branches, according to whether or not parents chose to have their children vaccinated (compliance node). Compliance influences the coverage rate. We considered a coverage rate of 90% as the base case. In the sensitivity analysis, a range of 50–100% was considered.
Vaccinated children may or may not be protected, depending on the predicted effectiveness of the vaccine (see sub-section “Vaccine efficacy, predicted effectiveness and duration of protection”). Unprotected infants were considered to be susceptible; their probability of acquiring meningococcal disease was therefore deemed to be the same as that of unvaccinated subjects (“Not Vaccinated” branch).
We assumed that protection would wane over time (see sub-section “Vaccine efficacy, predicted effectiveness and duration of protection”). Protection began after 2 doses of the vaccine.27,44
Unvaccinated infants (“Not Vaccinated” branch) were regarded as having the possibility to live their whole lives without contracting the disease. If, however, they contracted the disease, 2 outcomes were possible: death or survival. Survivors were then divided into 2 different categories: without or with sequelae. We assumed that any subjects who had had the disease would no longer be susceptible (as repeat invasive disease is rare and is associated with individuals with immune deficiencies and anatomical defects27).
We considered the acute-phase and lifetime consequences of invasive disease caused by NmB (direct and indirect costs). For each case, the burden of meningococcal disease was assessed on the basis of age-adjusted life expectancy on disease acquisition. We considered an average life expectancy of 82.03 y as reported by the Italian National Statistics Institute (ISTAT).43
We calculated the costs of the vaccination strategy, including the costs due to the treatment of adverse events caused by vaccination. On the basis of the difference between the costs of both strategies (vaccination and no vaccination), the potential benefits of the new vaccine were evaluated. The study was conducted from the perspectives of both the third-party payer [National Health Service (NHS)] and society. In the societal perspective, two options were considered: no cost for death and cost for death.
All costs were adjusted to the value of € in 2013,45 and were discounted as indicated by Italian guidelines (discount rate of 3% both for costs and utilities).46,47 In the sensitivity analysis, a range of 0 – 8% was considered.
Our model considered 2 options on the basis of 2 different probabilities of disease, as reported in the sub-section “disease incidence.”7,20
Eight scenarios were evaluated (Table 2), the basal scenarios being scenarios 1 and 5. Scenario 1 considered the following parameters: social perspective, no cost of death, primary vaccination cycle with 4 doses (2, 4, 6 and 12 months of age) and booster dose at the age of 11 y and official data on disease incidence. Scenario 5 considered the following parameters: social perspective, no cost of death, primary vaccination cycle with 4 doses (2, 4, 6 and 12 months of age) and booster dose at the age of 11 y and estimated data on disease incidence.
Disease incidence
Disease-incidence estimates were based on the special Invasive Diseases Surveillance System (MIB) of the Italian Ministry of Health.48 In Italy, it is mandatory to notify the MIB of laboratory-confirmed cases of invasive disease caused by Nm. Cases are reported to the Local Health Unit (LHU), which communicates the data to the Regional and National Authorities. Concurrently, all hospitals should report any confirmed or suspected cases of bacterial invasive disease to the LHU. Cases are described on individual case-report forms, which include information on the clinical status, microbiological results and vaccination status.49
All labs that isolate a strain of Nm are requested to send the isolate to the National Reference labs, based at the National Institute of Health (ISS), for confirmation, serotyping and molecular typing. In the reports of the ISS, cases are subdivided into 7 age-classes: 0, 1–4, 5–9, 10–14, 15–24, 25–64, and >64 y and by Nm serogroup.7,8
Our model considered 2 options on the basis of 2 different probabilities of disease: option 1 considered the average number of confirmed NmB cases that occurred annually in Italy from 2007 to 2012 (133 cases/y) (official data)8; option 2 considered a number of cases (399 cases/y) 3 times higher than in option 1, on the basis of 2 recent Italian studies which demonstrated that the real incidence of bacterial invasive disease in Italy is greatly underestimated (estimated data).20,24
In option 1, we assumed an annual disease incidence of 0.23 per 100,000 subjects.8 In option 2, we assumed an annual disease incidence of 0.69 per 100,000. In the sensitivity analysis, a range of 0.1–0.7 per 100,000 was considered.
The annual distribution of cases broken down by age is reported in Supplementary Material (Electronic Supplementary Material A2).
Disease consequences
Death, survival without sequelae, and survival with long-term sequelae were the 3 outcomes considered in our model.
The case-fatality rate, broken down by age, was applied according to the 2006 EU-IBIS report.50 In our model, we assumed a global probability of death of 6.73%. In the sensitivity analysis, a range of 0–10% was considered.
While it is fairly easy to find papers on the sequelae from invasive bacterial disease,51 it is difficult to obtain accurate measurements of the long-term consequences of serogroup B disease. We assumed that 40.2% of survivors would have at least 1 sequela, as reported by a recent study.52 In the sensitivity analysis, a variation of 20% was considered. The single sequelae28,53-57 considered in our study and their frequency, are reported in Table 3. Additionally, a personal communication by Magnus Gottfredsson allowed us to evaluate the incidence of some sequelae in the cohort study performed by this author.55 Although meningococcal disease can cause multiple sequelae, in our study, as in other health economics studies,27 we assumed that each survivor would have only 1 sequela. Other sequelae, such as brain abscess, cranial nerve palsy, obstructive hydrocephalus, ataxia, chronic organ damage, etc, as defined by Woods58 and by Brandtzaeg,59 were not considered.
Table 3.
Probability of sequelae.
| Sequelae | Base case | Distribution |
|---|---|---|
| Amputation with substantial disabilitya | 0.01 | Uniform (0.008; 0.012) |
| Anxietyb | 0.068 | Uniform (0.0544; 0.0816) |
| Arthritisb | 0.025 | Uniform (0.02; 0.03) |
| Depressionb | 0.05 | Uniform (0.04; 0.06) |
| Motor deficitsc | 0.019 | Uniform (0.0152; 0.0228) |
| Blindnessa | 0.004 | Uniform (0.0032; 0.0048) |
| Epilepsy or Seizurea | 0.02 | Uniform (0.016; 0.024) |
| Severe Neurological disabilityd | 0.021 | Uniform (0.0168; 0.0252) |
| Mental retardation (cognitive problems)b,e | 0.254 | Uniform (0.196; 0.312) |
| Hearing loss requiring cochlear implantationa | 0.02 | Uniform (0.016–0.024) |
| Moderate/severe bilateral hearing lossa | 0.05 | Uniform (0.04; 0.06) |
| Moderate unilateral hearing lossa | 0.05 | Uniform (0.04; 0.06) |
| Skin necrosisb | 0.015 | Uniform (0.012; 0.018) |
| Scarsf | 0.03 | Uniform (0.024; 0.036) |
| Severe speech or communication problemsa | 0.037 | Uniform (0.0296; 0.0444) |
| Renal failureb | 0.019 | Uniform (0.0152; 0.0228) |
| Chronic migraineb | 0.10 | Uniform (0.08; 0.12) |
According to [56];
According to [55];
According to [28];
According to [53];
According to [57];
According to [54].
Quality of life
Our model also considered the impairment of the quality of life (QoL) of survivors with long-term sequelae. This assessment was necessary in order to evaluate the permanent consequences for health status. Furthermore, because few data are available on the QoL of survivors of meningococcal disease,60 we sometimes used QoL evaluations for sequelae or pathologies similar to those caused by meningitis; for instance, for chronic migraine, the results reported by Xu et al. were used.61
Table 4 shows the health utilities broken down by sequelae.53,60-69
Table 4.
Health utilities of single sequelae.
| Sequelae | Base case | Distribution |
|---|---|---|
| Amputation with substantial disabilitya | 0.613 | Uniform (0.490; 0.7356) |
| Anxietyb | 0.687 | Uniform (0.5496; 0.8244) |
| Arthritisc | 0.690 | Uniform (0.552; 0.828) |
| Depressionb | 0.729 | Uniform (0.583; 0.875) |
| Motor deficitsd | 0.830 | Uniform (0.664; 0.996) |
| Blindnesse | 0.260 | Uniform (0.208; 0.312) |
| Epilepsy or Seizuref | 0.830 | Uniform (0.664; 0.996) |
| Severe Neurological disabilitya | 0.060 | Uniform (0.048; 0.072) |
| Mental retardation (cognitive problems mild/moderate)g | 0.541 | Uniform (0.4328; 0.6492) |
| Hearing loss requiring cochlear implantationf | 0.810 | Uniform (0.648; 0.972) |
| Moderate/severe bilateral hearing lossf | 0.910 | Uniform (0.728; 1) |
| Moderate unilateral hearing lossf | 0.910 | Uniform (0.728; 1) |
| Skin necrosish | 0.900 | Uniform (0.720; 1) |
| Scarsa | 1.000 | Uniform (0.8; 1) |
| Severe speech or communication problemsi | 0.390 | Uniform (0.312; 0.468) |
| Renal failurel | 0.820 | Uniform (0.656; 0.984) |
| Chronic migrainem | 0.814 | Uniform (0.6512; 0.9768) |
According to [53];
According to [62];
According to [63];
According to [64];
According to [65];
According to [60];
According to [66];
According to [67];
According to [68];
According to [69];
According to [61].
Vaccine efficacy, predicted effectiveness and duration of protection
In clinical trials conducted with rMenB+OMV (Bexsero®), vaccine efficacy has been studied by means of the serum bactericidal antibody assay by human complement (hSBA).44,70-72
In studies involving infants, adolescents and young adults, the 4CMenB vaccine has been shown to induce robust bactericidal antibodies against strains expressing the vaccine antigens.73 Therefore, on the basis of the results of clinical trials,74,75 and considering another economic evaluation,29 we assumed that vaccinated subjects would have 95% protection against disease. Furthermore, a study estimating the strain coverage of Bexsero® predicted strain coverage in Italy to be 87%.32 Therefore, we used this estimate in our model. In the sensitivity analysis, a variation of 20% was considered both for vaccine efficacy and for predicted strain coverage.
Data on the duration of protection conferred by Bexsero® are incomplete. Considering the basic assumptions of immunology,76 the results obtained in clinical trials of Bexsero®77 and an economic evaluation conducted in Canada,26 we assumed 10-y duration of full vaccine protection and subsequent waning of protection, whereby vaccination would only directly prevent one-quarter of cases over the lifetime of a vaccinated birth cohort.34 Furthermore, clinical trials have demonstrated the ability of Bexsero® to induce immunological memory.35,74,75,77 If immunological memory is induced by the vaccine, the natural MenB infection could acts as booster and help to maintain long-term protection.
Given the epidemiological trend of meningococcal disease, an adolescent booster dose may be useful in order to prevent the cases that occur during this period of life. Our model therefore considered a booster dose at 11 y of age.
Costs associated with the disease
The study was conducted from the perspectives of both the Italian NHS and society. All costs were measured in € at January 2013 values, with previous years being adjusted to January 2013 levels.45 To determine the total cost of meningococcal disease, we considered the following categories of costs: costs related to the acute phase of disease (direct and indirect costs)8,53,78,79 (Table 5), costs related to meningococcal sequelae (direct and indirect costs)30,53,80-93 (Table 6) and the social costs of death94 (Table 7).
Table 5.
Acute phase of disease: costs (no discount rate) were measured in € at January 2013 values and were referred to 1 case.
| Parameter | Base case | Distribution |
|---|---|---|
| Medical care: cost of hospitalization per casea | 7,900 | Gamma (25; 316) |
| Public Health Responseb | 3,223 | Gamma (25; 128) |
| Lost productivity of parent or relativesc | 870 | Gamma (25; 35) |
| Lost productivity of patientc | 1,426 | Gamma (25; 57) |
According to [78];
Assumed based upon [53];
According to [8,79].
Table 6.
Meningococcal sequelae. Costs (no discount rate) were measured in € at January 2013 values.
| Parameters |
Base Case |
Distribution |
|---|---|---|
| Annual direct costs (1 case) | ||
| Amputation with substantial disabilitya | 7,339 | Gamma (25; 293.6) |
| Anxietyb | 1,146 | Gamma (25; 45.8) |
| Arthritis(1-y cost)c | 1,184 | Gamma (25; 47.4) |
| Depressiond | 3,192 | Gamma (25; 127.7) |
| Motor deficitse | 7,682 | Gamma (25; 307.3) |
| Blindnessf | 4,076 | Gamma (25; 163.0) |
| Epilepsy or seizureg | 2,272 | Gamma (25; 90.9) |
| Severe neurological disabilityh | 94,880 | Gamma (25; 3795.0) |
| Mental retardation (cognitive problems)e | 7,507 | Gamma (25; 300.3) |
| Hearing loss requiring cochlear implantationa | 6,327 | Gamma (25; 253.1) |
| Moderate/severe bilateral/unilateral hearing lossa | 3,163 | Gamma (25; 126.5) |
| Skin necrosisa | 1,066 | Gamma (25; 42.6) |
| Scarsh | 533 | Gamma (25; 21.3) |
| Severe speech or communication problemsi | 9,796 | Gamma (25; 391.8) |
| Renal failurel | 56,126 | Gamma (25; 2245.0) |
| Chronic migrainem |
892 |
Gamma (25; 35.7) |
| |
Annual indirect costs (1 case) |
|
| Special case education*h | 14,566 | Gamma (25; 582.2) |
| Lost productivity of parent^n | 24,500 | Gamma (25; 980.0) |
| Lost productivity of patient°o | 24,500 | Gamma (25; 980.0) |
According to [53];
According to [80];
According to [81];
According to [82];
According to [83];
According to [84];
According to [85,86];
According to [87];
According to [30];
According to [88];
According to [89];
According to [90,91,92];
According to [90,92,93].
Applied to: motor deficits, blindness, epilepsy or seizure, mental retardation (cognitive problems), hearing loss and severe speech or communication problems.
Applied to: mental retardation (cognitive problems), severe neurological disability, severe speech or communication problems, epilepsy, blindness, motor deficit, severe amputations and hearing loss.
Applied to: severe amputations, anxiety, depression, motor deficit, blindness, epilepsy or seizure, severe neurological disability, mental retardation (cognitive problems), Hearing loss requiring cochlear implantation, Moderate/severe bilateral/unilateral hearing loss, renal failure, severe speech or communication problems.
Table 7.
The social costs (no discount rate) of death were measured in € at January 2013 values.
| Age (years) | Willingness to pay (WTP)a | Distribution | Human Standard Capital (HSC)a | Distribution |
|---|---|---|---|---|
| <1 | 1,513,985 | Gamma (25; 60559.4) | 81,434 | Gamma (25; 3257.1) |
| 1–4 | 1,594,678 | Gamma (25; 63787.2) | 101,143 | Gamma (25; 4045.7) |
| 5–9 | 1,743,967 | Gamma (25; 69758.5) | 148,817 | Gamma (25; 5941.9) |
| 10–14 | 1,924,832 | Gamma (25; 76993.1) | 228,391 | Gamma (25; 9135.6) |
| 15–24 | 2,122,126 | Gamma (25; 84887.8) | 368,015 | Gamma (25; 14720.6) |
| 25–64 | 1,260,459 | Gamma (25; 50418.1) | 336,674 | Gamma (25; 13466.9) |
| >64 | 96,178 | Gamma (25; 3847.2) | 40,377 | Gamma (25; 1615.1) |
According to [94].
Costs related to the acute phase of disease
The costs of the acute phase of disease were applied to all cases and are shown in Table 5.
The direct costs related to the acute phase of meningococcal disease were those of medical care, i.e. hospitalization and public health response. In evaluating the costs of hospitalization, we used the cost of the Diagnosis Related Group (DRG) associated to meningococcal disease.78 We calculated the cost of the public health response to a case of meningococcal disease by considering the average number of contacts that required chemoprophylaxis treatment, the average cost of a course of chemoprophylaxis treatment, and the average working time devoted by public health departments to a single reported case of meningococcal disease.53
The indirect costs were those associated with lost productivity of the patient and lost productivity of the parents or relatives. With regard to costs of lost productivity of the patient, we only considered the cases which occurred during working age (18–64 y). We considered an average cost of €16.2 per working hour79 for 8 hours/day (€129.6) and an average length of stay in hospital of 11 d (€1,426).8 The costs of lost productivity of parents or relatives were applied to all cases.8,79
Costs related to meningococcal sequelae
Survivors with sequelae constituted a subset of nonfatal cases; these incurred sequela-specific direct medical costs. For some complications, in addition to direct medical costs, other indirect costs (special education, lost productivity of parents and lost productivity of patients) were also considered. The annual direct and indirect costs of meningococcal sequelae are shown in Table 6.
The direct costs of the sequela “amputation with substantial disability” included both the costs of acute treatment (e.g. cost of the amputation procedure) and lifetime medical costs (e.g., maintenance of prosthetics, rehabilitation etc).53 The direct costs of the sequela “hearing loss requiring cochlear implantation” included not only the costs of the cochlear implant, but also that of its implantation and lifetime maintenance.53 With regard to severe neurological disability, our estimate of the direct costs also considered the costs of long-term institutional care,87 while for the sequela “arthritis,” we only considered the medical costs for 1 y, as this complication is usually resolved by short-term therapy.81,95 Finally, our estimate of the costs of the sequela “renal failure” took into account the permanent organ damage that can lead to kidney transplantation or dialysis, assuming a life expectancy of 5 y.88
With regard to indirect costs, we considered the costs of special education87 for the following sequelae: motor deficits, blindness, epilepsy or seizure, mental retardation (cognitive problems), hearing loss and severe speech or communication problems. These costs were calculated by determining the age-specific additional costs per child per year in comparison with regular education, and were applied to subjects younger than 17 y of age, as education is compulsory up to that age in Italy. Moreover, some severe sequelae require a parent to give up work in order to assist her child. We therefore evaluated the additional cost of lost productivity of a parent for the following severe complications: mental retardation (cognitive problems), severe neurological disability, severe speech or communication problems, epilepsy, blindness, motor deficit, severe amputations and hearing loss. This additional cost was considered up to the age of 17 y. In our calculations, we supposed that it would be the mother who gave up her job. The calculation of lost productivity of the mother considered the following parameters: the mean age at which women in Italy have their first child, the mean number of potential working years lost by these women, the percentage of women employed and their per capita income.90-92
To evaluate the lost productivity of patients, we estimated the residual earning capacity of patients affected by each sequela; this calculation was based on the disability percentages defined by Italian law93 and considered the year of disease onset, income per capita, residual working years and unemployment rate in Italy.90,92 We evaluated the additional cost of lost productivity of a patient for the following severe complications: severe amputations, anxiety, depression, motor deficit, blindness, epilepsy or seizure, severe neurological disability, mental retardation (cognitive problems), hearing loss requiring cochlear implantation, moderate/severe bilateral/unilateral hearing loss, renal failure, severe speech or communication problems.
Social cost of death (indirect cost)
To evaluate the social cost of death, 2 approaches are usually used: the “willingness to pay” and the “human standard capital” methods. In our model, we calculated the social cost of death by means of each method separately.94 The cost was computed on considering the age of death. These values are reported in Table 7.
Costs associated with vaccination
The costs associated with vaccination are reported in Table 8.78,96-98
Table 8.
Costs associated with vaccination (€).
| Direct costs | ||||
|---|---|---|---|---|
| Range | ||||
| Parameter |
Base Case |
Min |
Max |
Distribution |
| Cost of the primary cycle of vaccination (4 doses)a | 200.00 | 100.00 | 300.00 | Fixed |
| Cost of vaccine administration per doseb | 5.80 | – | – | Fixed |
| Cost of hospitalization for 1 anaphylactic reactionc | 1,175 | – | – | Fixed |
| Cost of 1 mild or moderate adverse eventd | 3.40 | – | – | Fixed |
According to [96];
According to [97];
According to [78];
According to [98].
Costs of vaccine
We considered a cost of €200,00 for the primary cycle of vaccination (4 doses) from the NHS and societal perspectives.96 The cost of the booster dose at 11 y was set at €36.12 (discount rate of 3% applied). Private sector prices were not considered. In the sensitivity analysis, a range of costs for the primary cycle of vaccination of €100–300 was considered.
A cost of €5.80 was attributed to the administration of each dose of vaccine.97
Costs of vaccine-associated adverse events
Our assessment of the costs determined by mild and moderate adverse events after vaccine administration was based on the study by Gossger et al.99 These authors estimated a total frequency of local and systemic adverse events of 30%, 26%, 28% and 28% after the first, second, third and fourth doses, respectively.99 We considered a frequency of local and systemic adverse events of 28% for the booster dose. As fever is the most frequent adverse event, our evaluation considered the cost of the most widely used antipyretic in Italy (1 box of paracetamol)98 as the cost of 1 mild or moderate adverse event. In the case of moderate adverse events, we did not consider the cost of consultation of the pediatrician or general practitioner, as house calls and outpatient visits are free of charge in Italy. Regarding severe adverse events, we considered the frequency (1 case per 719,790 doses) and costs of anaphylactic reactions on the basis of evaluations by Christensen et al. and AGENAS.27,78
Economic analysis
In order to evaluate the effect of introducing the meningococcal B vaccine into the Italian immunization program, we conducted a cost–effectiveness analysis (CEA). The analysis was carried out by means of Excel Version 2011 and the TreeAge Pro® software Version 2012 (Build 12.2.3.0).
The analysis is expressed in terms of Incremental Cost Effectiveness Ratio (ICER), where the denominator is the health gain in quality-adjusted life years (QALYs) and the numerator is the difference between the costs of the vaccination strategy and those of a no-vaccination strategy.
Sensitivity analysis
One-way sensitivity analyses were performed in order to evaluate how the uncertainty of disease incidence and vaccine effectiveness can influence the ICER.
Furthermore, to obtain the best set of parameters for the Monte Carlo simulation, a multivariate sensitivity analysis was performed. In this analysis, we varied: disease incidence, the probability of sequelae, the probability of death, vaccine effectiveness, the probability of coverage, the cost of the vaccine, and the cost and QALY discount rate.
The sensitivity analysis was performed for the 2 basal scenarios (Table 2, scenarios 1 and 5).
Monte Carlo simulation
We developed a second-order Monte Carlo simulation, which is a 2-dimensional simulation used to propagate variability and uncertainty separately. This procedure consists of multiple realizations of model parameters and iterations of input variables. The outcome is a collection of cumulative distribution functions that simultaneously display the uncertainty and variability in the results.100 This simulation was developed by using 10,000 samples.
We then performed a CEA in which incremental costs and health outcomes were computed and plotted on an X-Y scatter plot. Furthermore, we drew cost-effectiveness acceptability curves, whereby “threshold value” was plotted against the proportion of runs (samples) that resulted in incremental cost-effectiveness ratios below this threshold value.101
The Monte Carlo simulation was performed for the 2 basal scenarios (Table 2, scenarios 1 and 5).
Tables 4, 5, 6, 7 and 8 report the range of values used in the sensitivity analysis and Monte Carlo simulation.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Dr Bernard Patrick for his linguistic review of the manuscript.
Funding
The study was financed by the Italian Ministry of University and Research (MIUR, project PRIN 2009; Grant number: 2009ZPM4×4).
Author Contributions
RG designed, coordinated and supervised the research. PL, ET, AT developed the economic model and carried out the economic analysis. RG, DP, DA, CdW, WR, GI, PB collected and analyzed epidemiological data. RG, DP, DA, CL collected and analyzed economic parameters. RG, DP, DA, PB, WR, GI carried out the epidemiological data-quality control. AT, PL, CL carried out the economic data-quality control. RG, DA, DP wrote the manuscript. All authors revised the manuscript and contributed to improving the paper; all authors read and approved the final manuscript.
References
- [1].Anonychuk A, Woo G, Vyse A, Demarteau N, Tricco AC. The cost and public health burden of invasive meningococcal disease outbreaks: a systematic review. Pharmacoeconomics 2013; 31(7):563-76; PMID:23673904; http://dx.doi.org/ 10.1007/s40273-013-0057-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Davis KL, Misurski D, Miller J, Karve S. Cost impact of complications in meningococcal disease: evidence from a United States managed care population. Hum Vaccin 2011; 7(4):458-65; PMID:21795848; http://dx.doi.org/ 10.4161/hv.7.4.14434 [DOI] [PubMed] [Google Scholar]
- [3].Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009; 27 Suppl 2:B51-63; PMID:19477562; http://dx.doi.org/ 10.1016/j.vaccine.2009.04.063 [DOI] [PubMed] [Google Scholar]
- [4].Gasparini R, Panatto D. Meningococcal glycoconjugate vaccines. Hum Vaccin 2011; 7:170-82; PMID:21178398; http://dx.doi.org/ 10.4161/hv.7.2.13717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Panatto D, Amicizia D, Lai PL, Gasparini R. Neisseria meningitidis B vaccines. Expert Rev Vaccines 2011; 10:1337-51; PMID:21919622; http://dx.doi.org/ 10.1586/erv.11.103 [DOI] [PubMed] [Google Scholar]
- [6].European Centre for Disease Prevention and Control: Surveillance of invasive bacterial diseases in Europe 2008/2009 http://www.ecdc.europa.eu. Accessed 2013 [Google Scholar]
- [7].Istituto Superiore di Sanità (ISS): Dati di sorveglianza delle malattie batteriche invasive aggiornati al 10/04/2013 http://www.simi.iss.it/dati.htm. Accessed May2014 [Google Scholar]
- [8].ISS-CNESPS: Dati e evidenze disponibili per l'introduzione della vaccinazione anti-meningococco B nei nuovi nati e negli adolescenti http://www.epicentro.iss.it/temi/vaccinazioni/pdf/Istruttoria%20MENINGOCOCCO%20B.pdf. Accessed September2015 [Google Scholar]
- [9].Finne J, Leinonen M, Mäkelä P. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet 1983; 2:355-57; PMID:6135869; http://dx.doi.org/ 10.1016/S0140-6736(83)90340-9 [DOI] [PubMed] [Google Scholar]
- [10].Bjune G, Høiby EA, Grønnesby JK, Arnesen O, Fredriksen JH, Halstensen A, Holten E, Lindbak AK, Nøkleby H, Rosenqvist E, et al.. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 1991; 338:1093-96; PMID:1682541; http://dx.doi.org/ 10.1016/0140-6736(91)91961-S [DOI] [PubMed] [Google Scholar]
- [11].Sierra GV, Campa HC, Varcacel NM, Garcia IL, Izquierdo PL, Sotolongo PF, Casanueva GV, Rico CO, Rodriguez CR, Terry MH. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann 1991; 14:195-207; PMID:1812432 [PubMed] [Google Scholar]
- [12].de Moraes JC, Perkins BA, Camargo MC, Hidalgo NT, Barbosa HA, Sacchi CT, et al.. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet 1992; 340:1074-78; PMID:1357461; http://dx.doi.org/ 10.1016/0140-6736(92)93086-3 [DOI] [PubMed] [Google Scholar]
- [13].Boslego J, Garcia J, Cruz C, Zollinger W, Brandt B, Ruiz S, Martinez M, Arthur J, Underwood P, Silva W. Efficacy, safety, and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Vaccine 1995; 13:821-29; PMID:7483804; http://dx.doi.org/ 10.1016/0264-410X(94)00037-N [DOI] [PubMed] [Google Scholar]
- [14].Oster P, Lennon D, O'Hallahan J, Mulholland K, Reid S, Martin D. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine 2005; 23(17–18):2191-96; PMID:15755593; http://dx.doi.org/ 10.1016/j.vaccine.2005.01.063 [DOI] [PubMed] [Google Scholar]
- [15].Panatto D, Amicizia D, Lai PL, Cristina ML, Domnich A, Gasparini R. New versus old meningococcal group B vaccines: how the new ones may benefit infants & toddlers. Indian J Med Res 2013; 138(6):835-46; PMID:24521624 [PMC free article] [PubMed] [Google Scholar]
- [16].European Medicines Agency (EMA): Bexsero http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002333/human_med_001614.jsp&mid=WC0b01ac058001d124. Accessed June2014 [Google Scholar]
- [17].Health Canada: Bexsero http://www.hc-sc.gc.ca/dhp-mps/prodpharma/sbd-smd/drug-med/sbd_smd_2014_bexsero_147275-eng.php. Accessed December2014 [Google Scholar]
- [18].Australian Government. Department of Health, Therapeutic Goods Administration ARTG ID 190718. http://search-au.funnelback.com/s/search.html?collection=tga-artg&profile=record&meta_i=190718. Accessed December2014 [Google Scholar]
- [19].FDA US. Food and Drug Administration FDA approves a second vaccine to prevent serogroup B meningococcal disease. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm431370.htm#%2EVMK_tQ3TMfs%2Elinkedin. Accessed January2015 [Google Scholar]
- [20].Azzari C, Canessa C, Filippi F, Moriondo M, Indolfi G, Nieddu F, Martini M, de Martino M, Castiglia P, Baldo V, et al.. Distribution of invasive meningococcal B disease in Italian pediatric population: implications for vaccination timing. Vaccine 2014; 32:1187-91; PMID:24120548; http://dx.doi.org/ 10.1016/j.vaccine.2013.09.055 [DOI] [PubMed] [Google Scholar]
- [21].WHO: Principles and considerations for adding a vaccine to a national immunization programme From decision to implementation and monitoring. http://www.who.int/immunization/programmes_systems/policies_strategies/vaccine_intro_resources/nvi_guidelines/en/Accessed October 2014 [Google Scholar]
- [22].Anderson RM, Nokes DJ: Mathematical models of transmission and control. In Holland WW, Detels R, Knox G. Oxford Textbook of Public Health, 2nd Edition. Oxford University Press, New York, 1991. Pp 225-52. [Google Scholar]
- [23].WHO Laboratory methods for the diagnosis of meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. http://www.who.int/csr/resources/publications/meningitis/WHO_CDS_CSR_EDC_99_7_EN/en/. Accessed August2014 [Google Scholar]
- [24].Baldovin T, Furlan P, Lazzari R, Cocchio S, Baldo V. Flussi informativi delle meningiti e delle malattie batteriche invasive presenti nella Regione Veneto. http://www.vaccinarsi.org/assets/uploads/news/report_situazione_meningiti_veneto/report_%202007_2012_ssm-simi-mib.pdf. Accessed September2014 [Google Scholar]
- [25].Tirani M, Meregaglia M, Melegaro A. Health and economic outcomes of introducing the new MenB vaccine (Bexsero) into the Italian routine infant immunisation programme. Plos One 2015; 10(4):e0123383; PMID:25874805; http://dx.doi.org/ 10.1371/journal.pone.0123383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tu HA, Deeks SL, Morris SK, Strifler L, Crowcroft N, Jamieson FB, Kwong JC, Coyte PC, Krahn M, Sander B. Economic evaluation of meningococcal serogroup B childhood vaccination in Ontario, Canada. Vaccine 2014; 32(42):5436-46; PMID:25131732; http://dx.doi.org/ 10.1016/j.vaccine.2014.07.096 [DOI] [PubMed] [Google Scholar]
- [27].Christensen H, Hickman M, Edmunds WJ, Trotter CL. Introducing vaccination against serogroup B meningococcal disease: an economic and mathematical modelling study of potential impact. Vaccine 2013; 31:2638-46; PMID:23566946; http://dx.doi.org/ 10.1016/j.vaccine.2013.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pouwels KB, Hak E, van der Ende A, Christensen H, van den Dobbelsteen GP, Postma MJ. Cost-effectiveness of vaccination against meningococcal B among Dutch infants: Crucial impact of changes in incidence. Hum Vaccin Immunother 2013; 9:1129-38; PMID:23406816; http://dx.doi.org/ 10.4161/hv.23888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Christensen H, Trotter CL, Hickman M, Edmuds WJ. Re-evaluanting cost effectiveness of universal meningitis vaccination (Bexsero) in England: modeling study. BMJ 2014; 349:g5725; PMID:25301037; http://dx.doi.org/ 10.1136/bmj.g5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].di Pietro ML, Capri S, Kheiraoui F, de Waure C, Quaranta G, Poscia A, Specchia ML, Veneziano MA, Di Nardo F, Cadeddu C, et al.. Health Technology Assessment della vaccinazione contro Meningococco B. QIJPH 2013; 2:1-113. [Google Scholar]
- [31].WHO – Guide to cost-effectiveness analysis http://www.who.int/choice/publications/p_2003_generalised_cea.pdf. Accessed on September2015. [Google Scholar]
- [32].Vogel U, Taha MK, Vazquez JA, Findlow J, Claus H, Stefanelli S, Caugant DA, Kriz P, Abad R, Bambini S, et al.. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis 2013; 13:416-25; PMID:23414709; http://dx.doi.org/ 10.1016/S1473-3099(13)70006-9 [DOI] [PubMed] [Google Scholar]
- [33].Frosi G, Biolchi A, Lo Sapio M, Rigat F, Gilchrist S, Lucidarme J, Findlow J, Borrow R, Pizza M, Giuliani MM, et al.. Bacterial antibody against a representative epidemiocological meningococcal serogroup B panel confirms that MATS underestimate 4CMenB vaccine strain coverage. Vaccine 2013; 31:4968-74; PMID:23954380; http://dx.doi.org/ 10.1016/j.vaccine.2013.08.006 [DOI] [PubMed] [Google Scholar]
- [34].McQuaid F, Snape MD. Will booster doses be required for serogroup B meningococcal vaccine? Expert Rev Vaccines 2014; 13(3):313-5; PMID:24451002; http://dx.doi.org/ 10.1586/14760584.2014.878654 [DOI] [PubMed] [Google Scholar]
- [35].Gasparini R, Amicizia D, Domnich A, Lai PL, Panatto D. Neisseria meningitidis B vaccines: recent advances and possible immunization policies. Expert Rev Vaccines 2014; 13:345-64; PMID:24476428; http://dx.doi.org/ 10.1586/14760584.2014.880341 [DOI] [PubMed] [Google Scholar]
- [36].Roed C, Omland LH, Engsig FN, Skinhoj P, Obel N. Long-term mortality in patients diagnosed with meningococcal disease: a Danish nationwide cohort study. PloS One 2012; 5:e9662; http://dx.doi.org/ 10.1371/journal.pone.0009662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Read RC, Baxter D, Chadwick DR, Faust SN, Finn A, Gordon SB, Heath PT, Lewis DJ, Pollard AJ, Turner DP, et al.. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet 2014; 384:2123-31; PMID:25145775; http://dx.doi.org/ 10.1016/S0140-6736(14)60842-4 [DOI] [PubMed] [Google Scholar]
- [38].Trotter CL, Maiden M. Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert Rev Vaccines 2009; 8(7):851-61; PMID:19538112; http://dx.doi.org/ 10.1586/erv.09.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gasparini R, Comanducci M, Amicizia D, Ansaldi F, Canepa P, Orsi A, Icardi G, Rizzitelli E, De Angelis G, Bambini S, et al.. Molecular and serological diversity of Neisseria meningitidis carrier strains isolated from Italian students aged 14 to 22 years. J Clin Microbiol 2014; 52:1901-10; PMID:24648565; http://dx.doi.org/ 10.1128/JCM.03584-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Muzzi A, Mora M, Pizza M, Rappuoli R, Donati C. Conservation of meningococcal antigens in the genus Neisseria. M Bio 2013; 4:e00163-13; PMID:23760461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Vogel U, Claus H. Vaccine development against Neisseria meningitidis. Microb Biotechnol 2011; 4(1):20-31; PMID:21255369; http://dx.doi.org/ 10.1111/j.1751-7915.2010.00178.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ladhani SN, Giuliani MM, Biolchi A, Pizza M, Beebeejaun K, Lucidarme J, Findlow J, Ramsay ME, Borrow R. Effectiveness of Meningococcal B Vaccine against Endemic Hypervirulent Neisseria meningitidis W Strain, England. Emerg Infect Dis 2016; 22(2):309-11; PMID:26811872; http://dx.doi.org/ 10.3201/eid2202.150369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Istituto Nazionale di Statistica (ISTAT): Demografia in cifre http://demo.istat.it/ Accessed July2014 [Google Scholar]
- [44].Snape M, Dawson T, Oster P, Evans A, John TM, Ohene-Kena B, et al.. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr Infect Dis 2010; 29:e71–9; http://dx.doi.org/ 10.1097/INF.0b013e3181faa6be [DOI] [PubMed] [Google Scholar]
- [45].Rivaluta: Rivalutazioni e documentazione su prezzi, costi e retribuzioni contrattuali. http://rivaluta.istat.it. Accessed June2014 [Google Scholar]
- [46].AIES. Proposta di line guida per la valutazione economica degli interventi sanitari in Italia PharmacoEconomics – Italian Research Articles 2009; 11(2):83-93. [Google Scholar]
- [47].Capri S, Ceci A, Terranova L, Merlo F, Mantovani L. Guidelines for economic evaluations in Italy: Recommendations from the Italian Group of Pharmacoeconomic Studies. Drug Information J 2001; 35:189-201. [Google Scholar]
- [48].Circolare del Ministero della Sanità , 400.2/15/5709 del 29 dicembre 1993: Sorveglianza delle meningiti batteriche. http://:www.epicentro.it. Accessed June2014 [Google Scholar]
- [49].SIMI. Malattie batteriche invasive: Protocollo per la sorveglianza nazionale delle malattie invasive da meningococco, pneumococco ed emofilo in Italia. 12/03/2007. http://www.simi.iss.it/meningite_batterica.htm. Accessed July2014 [Google Scholar]
- [50].EUIBIS Invasive Neisseria meningitidis in Europe 2006 Commission of the European communities ECDC Agreement ECD.312. http://www.ecdc.it Accessed July2014 [Google Scholar]
- [51].Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10(5):317-28; PMID:20417414; http://dx.doi.org/ 10.1016/S1473-3099(10)70048-7 [DOI] [PubMed] [Google Scholar]
- [52].Wang B, Haji Ali Afzali H, Marshall H. The inpatient costs and hospital service use associated with invasive meningococcal disease in South Australian children. Vaccine 2014; 32(37):4791-8; PMID:24998605; http://dx.doi.org/ 10.1016/j.vaccine.2014.05.069 [DOI] [PubMed] [Google Scholar]
- [53].Shepard CW, Ortega-Sanchez IR, Scott RD 2nd, Rosenstein NE; ABCs Team . Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics 2005; 115(5):1220-32; PMID:15867028; http://dx.doi.org/ 10.1542/peds.2004-2514 [DOI] [PubMed] [Google Scholar]
- [54].Healy CM, Butler KM, Smith E, Hensey OP, Bate T, Moloney AC, MacMahon P, Cosgrove J, Cafferkey MT. Influence of serogroup on the presentation, course, and outcome of invasive meningococcal disease in children in the Republic of Ireland, 1995-2000. Clin Infect Dis 2002; 34(10):1323-30; PMID:11981727; http://dx.doi.org/ 10.1086/340050 [DOI] [PubMed] [Google Scholar]
- [55].Gottfredsson M, Reynisson IK, Ingvarsson RF, Kristjansdottir H, Nardini MV, Sigurdsson JF, Schneerson R, Robbins JB, Miller MA. Comparative Long-term adverse effects elicited by invasive group B and C meningococcal infections. CID 2011; 53:e117; http://dx.doi.org/ 10.1093/cid/cir500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Viner RM, Booy R, Johnson H, Edmunds WJ, Hudson L, Bedford H, Kaczmarski E, Rajput K, Ramsay M, Christie D. Outcomes of invasive meningococcal serogroup B disease in children and adolescents (MOSAIC): a case-control study. Lancet Neurol 2012; 11:774-83; PMID:22863608; http://dx.doi.org/ 10.1016/S1474-4422(12)70180-1 [DOI] [PubMed] [Google Scholar]
- [57].van de Beek D. The burden of serogroup B meningococcal disease. Lancet Neurol 2012; 11:743-5; PMID:22863607; http://dx.doi.org/ 10.1016/S1474-4422(12)70184-9 [DOI] [PubMed] [Google Scholar]
- [58].Woods CR. Neisseria meningitidis (Meningococcus) In: Behrman RE, Kliegman RM, Jenson HB, eds. Nelson Textbook of Pediatrics, 17th edition. Philadelphia, PA: Saunders; 2004. pp 896-9. [Google Scholar]
- [59].Brandtzaeg P. Pathogenesis and Pathophysiology of Invasive Meningococcal Disease In: Frosch M, Maiden MCJ, eds. Handbook of Meningococcal Disease: Infection Biology, Vaccination, Clinical Management. Weinheim, Germany: Wiley-VCH; 2006. Pp 427-80. [Google Scholar]
- [60].Oostenbrink R, A Moll HA, Essink-Bot ML. The EQ-5D and the Health Utilities Index for permanent sequelae after meningitis: a head-to-head comparison. J Clin Epidemiol 2002; 55:791-99; PMID:12384194; http://dx.doi.org/ 10.1016/S0895-4356(02)00448-1 [DOI] [PubMed] [Google Scholar]
- [61].Xu R, Insinga RP, Golden W, Hu XH. EuroQol (EQ-5D) health utility scores for patients with migraine. Qual Life Res 2011; 20:601-08; PMID:21063786; http://dx.doi.org/ 10.1007/s11136-010-9783-5 [DOI] [PubMed] [Google Scholar]
- [62].Saarni SI, Suvisaari J, Sintonen H, Pirkola S, Koskinen S, Aromaa A, Lönnqvist J. Impact of psychiatric disorders on health-related quality of life: general population survey. Br J Psychiatry 2007; 190:326-32; PMID:17401039; http://dx.doi.org/ 10.1192/bjp.bp.106.025106 [DOI] [PubMed] [Google Scholar]
- [63].Caban-Martinez AJ, Lee DJ, Fleming LE, Tancredi DJ, Arheart KL, LeBlanc WG, McCollister KE, Christ SL, Louie GH, Muennig PA. Arthritis, occupational class, and the aging US workforce. Am J Public Health. 2011; 101:1729-34; PMID:21778483; http://dx.doi.org/ 10.2105/AJPH.2011.300173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Stouthard MEA, Essink-Bot ML, Bonsel GJ, Barendregt JJ, Kramers PGN, van der Water HPA, Gunning-Schepers LJ, van der Maas PJ (1997) Disability weights for disease in The Netherlands. Amsterdan Medical Centre, Institute for Social Health Care, Amsterdam. http://www.moh.govt.nz/notebook/nbbooks.nsf/0/2D7B9CE4FAF56E1ACC256C41000FF92C/$file/Disability%20Weights.pdf. Accessed September2014s [Google Scholar]
- [65].Brown MM, Brown GC, Sharma S, Kistler J, Brown H. Utility values associated with blindness in an adult population. Br J Ophthalmol 2001; 85(3):327-31; PMID:11222340; http://dx.doi.org/ 10.1136/bjo.85.3.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Donev D, Zalatel-Kragelj, Bjegovic V, Burazeri G. Measuring the burden of disease: Disability Adjusted Life Year (DALY). http://www.mf.uni-lj.si/dokumenti/6b695fc9385e3e2ab8fb41ec7d34660d.pdf Accessed 30July2014 [Google Scholar]
- [67].Thein HH, Gomes T, Krahn MD, Wodchis WP. Health status utilities and the impact of pressure ulcers in long-term care residents in Ontario. Qual Life Res 2010; 19(1):81-9; PMID:20033300; http://dx.doi.org/ 10.1007/s11136-009-9563-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yfantopoulos J. Quality of life and QALYs in themeasurement of health. Arch Hellenic Med 2001; 18(2):114-30. [Google Scholar]
- [69].Wyld M, Morton RL, Hayen A, Howard K, Webster AC. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. Plos Med 2012; 9(9):e1001307; PMID:22984353; http://dx.doi.org/ 10.1371/journal.pmed.1001307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, et al.. Multicenter, open-label, randomized Phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis 2010; 51(5):1127-37; PMID:20954968; http://dx.doi.org/ 10.1086/656741 [DOI] [PubMed] [Google Scholar]
- [71].Giuliani MM, Adu-Bobie J, Comanducci M, Aric∫ B, Savino S, Santini L, Brunelli B, Bambini S, Biolchi A, Capecchi B, et al.. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A 2006; 103:10834-39; PMID:16825336; http://dx.doi.org/ 10.1073/pnas.0603940103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lucidarme J, Comanducci M, Findlow J, Gray SJ, Kaczmarski EB, Guiver M, Kugelberg E, Vallely PJ, Oster P, Pizza M, et al.. Characterization of fHbp, NHBA (gna2132), nadA, porA, sequence type (ST), and genomic presence of IS1301 in group B meningococcal ST269 clonal complex isolates from England and Wales. J Clin Microbiol 2009; 47:3577-85; PMID:19759227; http://dx.doi.org/ 10.1128/JCM.00936-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Nolan T, O'Ryan M, Wassil J, Abitbol V, Dull P. Vaccination with a multicomponent meningococcal B vaccine in prevention of disease in adolescents and young adults. Vaccine 2015; 33(36):4437-45; PMID:26187261; http://dx.doi.org/ 10.1016/j.vaccine.2015.06.011 [DOI] [PubMed] [Google Scholar]
- [74].Vesikari T, Esposito S, Prymula R, Ypma E, Kohl I, Toneatto D, Dull P, Kimura A, EU Meningococcal B Infant Vaccine Study group . Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet 2013; 381(9869):825-35; PMID:23324563; http://dx.doi.org/ 10.1016/S0140-6736(12)61961-8 [DOI] [PubMed] [Google Scholar]
- [75].Santolaya ME, O'Ryan ML, Valenzuela MT, Prado V, Vergara R, Muñoz A, Toneatto D, Graña G, Wang H, Clemens R, Dull PM, V72P10 Meningococcal B Adolescent Vaccine Study group . Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. Lancet 2012; 379(9816):617; PMID:22260988; http://dx.doi.org/ 10.1016/S0140-6736(11)61713-3 [DOI] [PubMed] [Google Scholar]
- [76].Atkinson W, Wolfe S, Hamborsky J. Epidemiology and prevention of vaccine-preventable diseases. Public Health Foundation 2011. [Google Scholar]
- [77].McQuaid F, Snape MD, John TM, Kelly S, Robinson H, Houlden J, Voysey M, Toneatto D, Kitte C, Dull PM, et al.. Persistence of bactericidal antibodies to 5 years of age after immunization with serogroup B meningococcal vaccines at 6, 8, 12 and 40 months of age. Pediatr Infect Dis J 2014; 33:760-66; PMID:24722351; http://dx.doi.org/ 10.1097/INF.0000000000000327 [DOI] [PubMed] [Google Scholar]
- [78].AGENAS. Agenzia Nazionale per i Servizi Sanitari Regionali: Ricoveri ospedalieri I sistemi tariffari regionali vigenti nell'anno 2009. Gennaio 2010. http://www.quotidianosanita.it/allegati/allegato5477154.pdf Accessed July2014 [Google Scholar]
- [79].ISTAT: retribuzioni http://www.istat.it/it/archivio/retribuzioni. Accessed July2014 [Google Scholar]
- [80].Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E, Dodel R, Ekman M, Faravelli C, Fratiglioni L, et al.. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 2011; 21:718-79; PMID:21924589; http://dx.doi.org/ 10.1016/j.euroneuro.2011.08.008 [DOI] [PubMed] [Google Scholar]
- [81].Oxford Economic: The economic costs of arthritis for the UK economy Final Report March 2010. http://www.oxfordeconomics.com/publication/download/222531. Accessed July2014 [Google Scholar]
- [82].Deambrosis P, Terrazzani G, Giusti P, Pullia G. Il costo del paziente depresso: l'esperienza della Ulss 9 di Treviso. Pharmaco Economics Italian Res 2007; 9(1):1-8; http://dx.doi.org/ 10.1007/BF03320565 [DOI] [Google Scholar]
- [83].Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jönsson B. CDBE2010 study group; European Brain Council. The economic cost of brain disorders in Europe. Eur J Neurol 2012; 19(1):155-62; PMID:22175760; http://dx.doi.org/ 10.1111/j.1468-1331.2011.03590.x [DOI] [PubMed] [Google Scholar]
- [84].Frick KD, Walt JG, Chiang TH, Doyle JJ, Stern LS, Katz LM, Dolgitser M, Hendlish SK. Direct costs of blindness experienced by patients enrolled in managed care. Ophthalmology 2008; 115:11-7; PMID:17475331; http://dx.doi.org/ 10.1016/j.ophtha.2007.02.007 [DOI] [PubMed] [Google Scholar]
- [85].Guerrini R, Battini R, Ferrari AR, Veggiotti P, Besana D, Gobbi G, Pezzani M, Berta E, Tetto A, Beghi E, et al.. The costs of childhood epilepsy in Italy: comparative findings from three health care settings. Epilepsia 2001; 42:641-46; PMID:11380572; http://dx.doi.org/ 10.1046/j.1528-1157.2001.27300.x [DOI] [PubMed] [Google Scholar]
- [86].Beghi E, Garattini L, Ricci E, Cornago D, Parazzini F. EPICOS Group. Direct cost of medical management of epilepsy among adults in Italy: a prospective cost-of-illness study (EPICOS). Epilepsia 2004; 45:171-8; PMID:14738425; http://dx.doi.org/ 10.1111/j.0013-9580.2004.14103.x [DOI] [PubMed] [Google Scholar]
- [87].Hepkema H, Pouwels KB, van der Ende A, Westra TA, Postma MJ. Meningococcal serogroup A, C, W-135 and Y conjugated vaccine: a cost-effectiveness analysis in the Netherlands. PLOS One 2013; 8:e65036; PMID:23741448; http://dx.doi.org/ 10.1371/journal.pone.0065036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Gopal I, Bhonagiri S, Ronco C, Bellomo R. Out of hospital outcome and quality of life in survivors of combined acute multiple organ and renal failure treated with continuous venovenous hemofiltration/hemodiafiltration. Intensive Care Med 1997; 23:766-72; PMID:9290991; http://dx.doi.org/ 10.1007/s001340050407 [DOI] [PubMed] [Google Scholar]
- [89].Bloudek LM, Stokes M, Buse DC, Wilcox TK, Lipton RB, Goadsby PJ, Varon SF, Blumenfeld AM, Katsarava Z, Pascual J, et al.. Cost of healthcare for patients with migraine in five European countries: results from the International Burden of Migraine Study (IBMS). J Headache Pain 2012; 13:361-78; PMID:22644214; http://dx.doi.org/ 10.1007/s10194-012-0460-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].ISTAT: PIL Pro CaPite Italia. http://noi-italia.istat.itindex.php?id7&user_100ind_pi1[id_pagina]=91. Accessed July2014 [Google Scholar]
- [91].ISTAT: Noi Italia 2013 – fecondità http://www.istat.it/it/archivio/fecondità. Accessed July2014 [Google Scholar]
- [92].ISTAT: Noi Italia 2013 – mercato del lavoro http://www.istat.it/it/archivio/mercatodellavoro. Accessed July2014 [Google Scholar]
- [93].Decreto Ministeriale - Ministero della Sanità - 5 febbraio 1992 “Approvazione della nuova tabella indicativa delle percentuali d'invalidità per le minorazioni e malattie invalidanti.” Gazz. Uff. 26 febbraio 1992, n. 47, S.O. http://www.gazzettaufficiale.it Accessed July2014 [Google Scholar]
- [94].Landefeld JS, Seskin EP. The economic value of life: linking theory to practice. Am J Public Health 1982; 72:555-66; PMID:6803602; http://dx.doi.org/ 10.2105/AJPH.72.6.555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Cabellos C, Nolla JM, Verdaguer R, Pelegrin I, Ribera A, Ariza J, Viladrich PF. Arthritis related to systemic meningococcal disease: 34 years' experience. Eur J Clin Microbiol Infect Dis 2012; 31:2661-66; PMID:22476361; http://dx.doi.org/ 10.1007/s10096-012-1610-1 [DOI] [PubMed] [Google Scholar]
- [96].Bollettino Ufficiale della Regione Basilicata N5 - 24-2-2014: Parte I http://:www.basilicatanet.it. Accessed July2014 [Google Scholar]
- [97].Mennini FS, Giorgi Rossi P, Palazzo F, Largeron N. Health and economic impact associated with a quadrivalent HPV vaccine in Italy. Gynecol Oncol 2009; 112:370-6; PMID:19041125; http://dx.doi.org/ 10.1016/j.ygyno.2008.09.031 [DOI] [PubMed] [Google Scholar]
- [98].Tutto sui farmaci Available: www.torrinomedica.it. Accessed August2014 [Google Scholar]
- [99].Gossger N, Snape MD, Yu LM, Finn A, Bona G, Esposito S, Principi N, Diez-Domingo J, Sokal E, Becker B, et al.. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA 2012; 307:573-82; PMID:22318278; http://dx.doi.org/ 10.1001/jama.2012.85 [DOI] [PubMed] [Google Scholar]
- [100].Wu FC, Tsang YP. Second-order Monte Carlo uncertainty/variability analysis using correlated model parameters: applications to salmonid embryo survival risk assessment. Ecol Modell 2004; 177(3–4):393-414; http://dx.doi.org/ 10.1016/j.ecolmodel.2004.02.016 [DOI] [Google Scholar]
- [101].Fenwick E, O'Brien BJ, Briggs A. Cost-effectiveness acceptability curves-facts, fallacies and frequently asked questions. Health Econ 2004; 13(5):405-15; PMID:15127421; http://dx.doi.org/ 10.1002/hec.903 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.