ABSTRACT
PNEUMOVAX™ 23, a 23-valent polysaccharide pneumococcal vaccine (PPV23), covers 65% to 91% of the isolates recovered from adult cases of invasive pneumococcal disease. Several studies have demonstrated that pneumococcal serotypes 31, 11A, 35F, 17F, 3, 16F, 19F, 15B, and 10A are associated with higher case-fatality or meningitis rates than other pneumococcal serotypes. This study (U05-PnPS-403; EudraCT: 2008-003648-12) evaluated the immune response followings administration of PPV23 for 4 of these serotypes (10A, 11A, 15B, and 17F), that are included in PPV23 but not in licensed pneumococcal conjugate vaccines. Serotype-specific IgG geometric mean concentrations (GMCs) and geometric mean fold-rises (GMFRs) for these 4 serotypes were measured by a validated enzyme-linked immunosorbent assay (ELISA) in 104 subjects >50 y of age who were enrolled in a study evaluating the safety and immunogenicity of a single-dose of PPV23. At 1 month post-vaccination, GMCs for serotypes10A, 11A, 15B and 17F were 6.5, 4.3, 14.7, and 5.1 µg/mL, respectively. GMFRs from baseline were 9.0, 4.5, 8.4, and 11.5, respectively. The percentages of subjects achieving >2-fold increases in IgG GMCs between pre-vaccination and 1 month post-vaccination were 90%, 85%, 88% and 89%, respectively. In conclusion, PPV23 induces a robust immune response in adults to pneumococcal serotypes 10A, 11A, 15B, and 17F, which have been associated with elevated case-fatality or meningitis rates.
KEYWORDS: immunogenicity, pneumococcal polysaccharide vaccine, safety
Introduction
Pneumococcal infection is a major cause of pneumonia, bacteremia, and meningitis. Invasive pneumococcal disease (IPD) has been associated with increased risk of premature death in adults ≥65 y of age, especially in those with underlying chronic heart or lung disease, diabetes, cancer, or asplenia.1 Despite the availability of potent antibiotic therapies and intensive care, the case-fatality rate for patients hospitalized with IPD has remained at approximately 12% for many decades.2 More specifically, Streptococcus pneumoniae is a leading cause of community-acquired acute bacterial meningitis, accounting for approximately 50% of disease cases in adult patients. The overall mortality rate associated with bacterial meningitis was 21%, but was significantly higher among patients with pneumococcal meningitis (30%) than among those with meningococcal meningitis (7%).3
Pneumococcal serotypes differ substantially in their potential for colonization, invasiveness, and virulence. The majority of pneumococcal serotypes with highest density in the nasopharynx are less likely to be associated with invasiveness, although the biological factors required for increased virulence and invasiveness are not fully understood. In particular, a recent review cataloged the propensity for certain pneumococcal serotypes in adults to be associated with increased rates of serious clinical outcomes, including meningitis and elevated case-fatality rates, compared to other serotypes.4 That review highlighted multiple serotypes with elevated risk profiles that are represented in 23-valent pneumococcal polysaccharide vaccine (PPV23; Pneumovax™ 23, Merck & Co., Inc., Kenilworth, NJ), but not included in 13-valent pneumococcal conjugate vaccine (PCV-13; Prevnar 13™, Pfizer Inc., Philadelphia, PA).
Vaccination with pneumococcal polysaccharide vaccine is effective in reducing the burden of pneumococcal disease. Management of pneumococcal disease also requires appropriate management of patients, including the use of effective antibacterial treatment. PPV23 was first approved in 1983 and is currently licensed in >60 countries worldwide. To date, more than 220 million doses of PPV23 have been distributed worldwide. Multiple clinical trials and observational studies have shown that PPV23 displays an acceptable safety profile and is effective in the prevention of serious pneumococcal disease, with point estimates of efficacy ranging from 56% to 81% in immunocompetent persons.5-8 Additional studies have also demonstrated the effectiveness of PPV23 in adults who are at increased risk for pneumococcal disease.5,9-12
Although the concentration of anti-capsular antibody required to protect against pneumococcal infection caused by any specific capsular type has not been established in adults, a ≥2-fold increase in antibody level following vaccination was associated with efficacy in clinical trials of polyvalent pneumococcal polysaccharide vaccines.13,14 The present study was conducted to assess the antibody responses induced by PPV23 against 4 serotypes (10A, 11A, 15B, and 17F) that are associated with serious clinical outcomes and are only present in PPV23. These serotypes were among the 9 serotypes (i.e., 3, 10A, 11A, 15B, 16F, 17F, 19F, 31, 35F) found to be associated with highly increased mortality as compared to serotype 1, in a study of individuals 5 y of age and older.15 Two of these 7 serotypes (3 and 19F) are included in both licensed adult pneumococcal vaccines while 4 serotypes are unique to PPV23 (10A, 11A, 15B, and 17F); the remaining 3 serotypes (16F, 31, and 35F) are not included in either licensed pneumococcal vaccine. Several clinical trials have repeatedly found serotypes 10A, 11A, 15B, and 17F to have significantly elevated risk profiles for meningitis, case-fatality rates, or both.15-21 None of the serotypes associated with elevated risk for these serious clinical outcomes were unique to PCV13. We tested the antibody responses to PPV23 against these 4 serotypes using serum specimens from older adult subjects previously enrolled in a clinical trial sponsored by Sanofi Pasteur MSD. The original study had demonstrated that a newly revised manufacturing process of PPV23 (the process used for currently marketed product) is generally well tolerated and immunogenic.22
Results
Study population
Of the 111 subjects who received PPV23 manufactured using the new process in the original study, 104 (93.7%) were included in the present study since they had sufficient volume of serum for the measurement of serotype-specific antibodies to the 4 selected serotypes (10A, 11A, 15B, and 17F). Table 1 summarizes the demographic characteristics of these subjects at baseline. The mean age was 58.2 y (range, 50.1 to 71.9 y) and 57% were women; mean body mass index was 27.0 kg/m2 (range 19.7 to 38.9). In addition, 83% reported at least one medical condition or intercurrent disease at baseline and the most frequently reported conditions were depression (10%), back pain (9%), and headache (6%). The demographics of subjects included in this additional immunogenicity assessment were consistent with the subjects who received the revised manufacturing process vaccine in the original clinical trial.22
Table 1.
Baseline characteristics of study population (N = 104).
| Characteristics | |
|---|---|
| Age at vaccination (years) | |
| Mean (SD) | 58.2 (4.8) |
| Median | 57.8 |
| Minimum - Maximum | 50.1 – 71.9 |
| Gender | |
| Female n (%) | 59 (56.7%) |
| Male n (%) | 45 (43.3%) |
| Body Mass Index (kg/m2) | |
| Mean (SD) | 27.0 (4.0) |
| Median | 26.7 |
| Minimum - Maximum | 19.7 – 38.9 |
Immunogenicity
Baseline antibody concentrations varied among serotypes, ranging from 0.4 µg/mL for serotype 17F to 1.8 µg/mL for serotype 15B. PPV23 was highly immunogenic and was associated with at least 4-fold increase in serotype-specific antibodies to all 4 serotypes at 1 month post-vaccination. Between baseline and 1 month post-vaccination, serotype-specific IgG GMCs increased from 0.7 µg/mL to 6.5 µg/mL for serotype 10A, from 1.0 µg/mL to 4.3 µg/mL for serotype 11A, from 1.8 µg/mL to 14.7 µg/mL for serotype 15B, and from 0.4 µg/mL to 5.1 µg/mL for serotype 17F (Table 2). Serotype-specific GMFRs were 9.0 for serotype 10A, 4.5 for serotype 11A, 8.4 for serotype 15B and 11.5 for serotype 17F. Across the 4 serotypes tested, levels of serotype-specific IgG GMCs measured at baseline did not correlate with the magnitude of IgG GMCs measured following vaccination with PPV23. Although the highest increase from baseline (11.5-fold) was observed for serotype 17F, which also had the lowest IgG GMC at baseline (0.4 µg/mL), the serotype with the highest IgG GMC at baseline (1.8 µg/mL for serotype 15B) had comparable or higher GMFR (8.4-fold rise) than those observed for serotypes 10A or 11A (9.0-fold rise and 4.5-fold rise, respectively) which had lower baseline IgG GMCs, measured at 0.73 µg/mL and 1.0 µg/mL, respectively) (Table 2).
Table 2.
Antibody concentration (µg/mL) for pneumococcal serotypes 10A, 11A, 15B and 17F as measured by ELISA.
| Parameter |
Pneumococcal Serotype |
|||||||
|---|---|---|---|---|---|---|---|---|
| 10A |
11A |
15B |
17F |
|||||
| Baseline | Post-vaccination* | Baseline | Post-vaccination | Baseline | Post-vaccination | Baseline | Post-vaccination | |
| Geometric Mean Concentration (µg/mL) | 0.7 | 6.5 | 1.0 | 4.3 | 1.8 | 14.7 | 0.4 | 5.1 |
| 95% Confidence Interval (CI) | 0.6, 1.0 | 4.9, 8.7 | 0.8, 1.2 | 3.6, 5.1 | 1.4, 2.3 | 11.9, 18.3 | 0.3, 0.6 | 3.9, 6.5 |
| Median | 0.7 | 6.7 | 1.0 | 4.5 | 1.9 | 14.1 | 0.4 | 5.1 |
| Mean GMC Fold-rise | 9.0 | 4.5 | 8.4 | 11.5 | ||||
| 95% CI | 7.2, 11.2 | 3.8, 5.4 | 6.8, 10.3 | 8.9, 14.9 | ||||
| Median | 9.9 | 4.0 | 9.1 | 12.3 | ||||
| Proportion of Subjects with ≥2 -fold rise from baseline (Mean) | 90.4% | 84.6% | 88.5% | 89.4% | ||||
| 95% CI | 83.0, 95.3 | 76.2, 90.9 | 80.7, 93.9 | 81.9, 94.6 | ||||
Post-vaccination refers to 1 month (i.e., 21 to 35 d) following vaccination with PPV23
When compared to baseline levels, the percentages of subjects achieving at least 2-fold increase in serotype-specific IgG GMCs at 1 month post-vaccination with PPV23 were 90%, 85%, 88% and 89%, for serotypes 10A, 11A, 15B and 17F, respectively (Table 2). The proportions of subjects with antibody concentration before and after vaccination equal to or above the pre-specified cutoff values of 0.5, 1, 5, and 10 µg/mL for the 4 serotypes tested were also evaluated. For all 4 serotypes, the proportion of subjects meeting these pre-specified threshold values increased following vaccination with PPV23. More than 95% of study subjects had IgG GMC ≥0.5 µg/ml and at least 88.5% still had serotype-specific IgG GMC above 1 µg/ml for all 4 serotypes at 1 month post-vaccination. The proportion of subjects with serotype-specific IgG GMC ≥5 µg/ml and ≥10 µg/ml varied between serotypes, with lowest and highest proportions of subjects achieving these thresholds being observed for serotype 11A and serotype 15B, respectively.
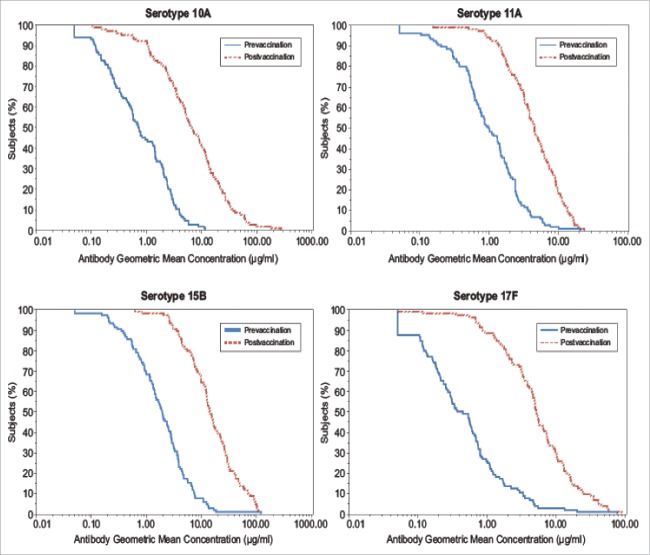
The overall changes in vaccine-induced immune responses from baseline are also depicted in the reverse cumulative distribution curves (RCDCs) for all 4 serotypes (Fig. 1). In comparison to baseline curves, post-vaccination curves were substantially shifted to the right for all 4 serotypes, indicating an increase in serotype-specific IgG GMCs from baseline.
Figure 1.
Serotype-specific IgG GMC Reverse Cumulative Distribution Curves at baseline and 30 d post-vaccination.
Discussion
Serotype-specific humoral antibodies are generally considered to be effective in preventing pneumococcal disease.13,23-29 The assays used to measure vaccine-induced immune responses following vaccination with pneumococcal vaccines in adults have evolved over time. In recent years, serotype-specific IgG antibodies have mostly been measured using the WHO-accepted ELISA and functional antibodies have been measured using the opsonophagocytic activity (OPA) assay. Serotype-specific IgG levels measured with the ELISA assay used in the present study have been shown to correlate with levels measured using OPA assays.30 Although previous studies have demonstrated that PPV23 can induce serotype-specific antibodies to all serotypes included in the vaccine, the present study links the immune responses to serotypes unique to PPV23 to the serious clinical outcomes associated with these pneumococcal serotypes, underscoring the need for continued vaccination of older adults with PPV23.
With the widespread use of PCVs in children and adults, significant decrease in overall incidence of pneumococcal disease has been observed, mostly disease caused by serotypes included in PCV13; however, disease caused by non-vaccine types has increased during the same time in both children and older adults. Increase in cases of pneumococcal disease caused by serotypes 10A, 11A, 15B, and 17F has been observed in some countries among children and adults following the introduction of PCV in children23,24 and these serotypes are among those associated with elevated risks of meningitis and lethality.15-21 The present study finds a robust response to all 4 serotypes in healthy adults 50 y and older following vaccination with PPV23 and underscores the need to continue vaccination of adults with PPV23 for the control of disease caused by serotypes not included in PCV13. In comparison to levels measured at baseline, IgG GMCs increased between 4.5-fold and 11.5-fold at 1 month post-vaccination. We also evaluated vaccine-induced immune response using a cutoff value (2–fold rise from pre-vaccination to post-vaccination) that was previously shown to be a surrogate for vaccine efficacy.13,14 In comparison to levels measured prior to vaccination, approximately 90% of study subjects in the present study achieved a ≥2-fold increase in serotype-specific IgG GMC and an IgG concentration of ≥1 µg/mL against pneumococcal serotypes 10A, 11A, 15B, and 17F at 1 month post-vaccination. Despite the use of different immunological assays to measure serotype-specific IgG, the results from our study are consistent with those previously described by Robbins et al., showing comparable magnitude in antibody fold-rise and comparable proportion of subjects with ≥2-fold rise in IgG GMCs from baseline for the selected 4 serotypes.13 Altogether, these findings further substantiate that PPV23 induces strong antibody responses against pneumococcal serotypes that are associated with serious clinical outcomes.
Several clinical trials have demonstrated that PPVs can prevent pneumococcal disease. The vaccine efficacy against invasive pneumococcal disease in immunocompetent adults is clearly established, but its clinical impact on pneumococcal pneumonia remains controversial.6,31 Early studies conducted in South African miners and in Papua New Guinea Highlanders demonstrated that pneumococcal polysaccharide vaccines were efficacious against pneumonia. The vaccine efficacy against type-specific pneumococcal pneumonia was 78.584%– in these populations;14,32-34 however, subsequent randomized trials in elderly or high-risk populations in the United States and other developed countries show variable results, possibly due to differences in study design, case definition of the clinical endpoint used in the trial, and characteristics of the population evaluated (outpatient, nursing residents, or high-risk individuals with co-morbidities). For example, effectiveness of PPV23 against all pneumococcal pneumonia was 48% in a population-based case control study among adults 50 y and older who had an episode of radiologically confirmed pneumococcal pneumonia (bacteremic and nonbacteremic cases) in Spain and 63.8% among nursing home residents (mean 84.7 y of age; range 55–105 y) who were enrolled in a randomized controlled trial in Japan.9,35 However, other studies failed to demonstrate any benefit of PPV23 in preventing pneumococcal pneumonia in middle-aged and elderly individuals.36,37 A recent meta-analysis of 18 randomized clinical trials found strong evidence of the efficacy of PPV against IPD (OR 0.26, 95% CI 0.14 to 0.45); although efficacy against all-cause pneumonia was demonstrated in low-income countries (OR 0.54, 95% CI 0.43 to 0.67), it was lower in high-income countries (OR 0.71, 95% CI 0.45 to 1.12).38 These results were consistent with those found in 3 previous meta-analyses evaluating the effectiveness of PPV against IPD and pneumonia.39-41
Most PPV clinical efficacy trials predated the ability to reliably identify nonbacteremic pneumococcal pneumonia from among all cases of pneumonia. Among studies that have recently evaluated the efficacy/effectiveness of PPV that relied on an urine antigen detection assay for the diagnosis of non-bacteremic pneumonia, Maruyama et al found that PPV23 was 63.8% efficacious (95% CI: 32, 81%) against pneumococcal pneumonia.9 Also, a study comparing adult subjects vaccinated with PPV23 within the previous 5 y with those who were never vaccinated found a vaccine efficacy of 48% against nonbacteremic pneumococcal pneumonia.42
Several epidemiologic studies performed in various countries around the world found that widespread use of PCVs among children has indirectly changed the distribution of pneumococcal serotypes associated with pneumococcal disease in adults.43-45 Prior to the licensure and widespread use of PCV7 for infant immunization against pneumococcal disease, the median difference in the proportions of adult IPD cases caused by the serotypes included in PPV23 compared to PCV13 was 16.3%. The median difference increased to 24.4% following the implementation of PCV7 in infant immunization schedule in the United States.43,45 Following the introduction of PCV13 in Canada, the difference in the proportion of pneumococcal isolates causing pneumococcal disease for serotypes included in PPV23 and PCV13 among Canadians ≥65 y of age increased from 24.8% in 2010 to 30.6% in 2012.45 The differentials can be expected to widen further as coverage rates for PCVs increase in countries that have already adopted the vaccine and as more countries worldwide are including PCVs in their infant immunization programs.46 More than the numerical difference in serotype coverage between PCV13 and PPV23, the intrinsic characteristics of these 11 serotypes unique to PPV23 as regards their potential for invasiveness, antibiotic resistance, and elevated risks for meningitis and/or case-fatality rate need to be closely monitored.4 More importantly, the findings described in this study underscore the need to increase the uptake of PPV23 in older adults for the prevention of pneumococcal diseases caused by these 4 serotypes that are unique to PPV23 and were found to be associated with high fatality rate.
Conclusion
PPV23 induces a robust IgG antibody response against pneumococcal serotypes 10A, 11A, 15B, and 17F, which are associated with elevated risk for mortality and meningitis in adults.
Methods
In the original study (U05-PnPS-403; EudraCT: 2008-003648-12), 220 healthy adults 50 y of age and older were enrolled in a clinical study comparing the safety and immunogenicity of 2 different formulations (manufactured using a new or former process) of PPV23. All eligible study subjects were immunocompetent, were naïve for any pneumococcal vaccine, and had no known allergy to any component of the study vaccines. All subjects were followed for safety for 14 d post-vaccination and serious AEs were monitored throughout the duration of the study. Blood samples were taken prior to vaccination and 28 d post-vaccination for the measurement of antibody concentrations to pneumococcal serotypes 3 and 8 by enzyme-linked immunosorbent assay (ELISA).22
In this present extension study, serum samples were only used from subjects in the original study who consented to future use of their leftover samples and who received PPV23 that was manufactured using the new manufacturing process. No safety measurements were assessed in the present study, as this evaluation was performed during the original study.22
Serum samples from the original study were stored at −20°C and those from study subjects eligible for the extension study were thawed approximately 7 y later, then aliquoted, anonymized, and sent to the testing laboratory (PPD Vaccines and Biologics, LLC; Wayne, Pennsylvania, USA). Serotype-specific IgG geometric mean concentrations (GMCs) to pneumococcal serotypes 10A, 11A, 15B, and 17F were measured in serum collected before and 21 to 35 d after vaccination using a previously described laboratory technique.47 Serotype-specific pneumococcal IgG antibodies were quantitated by a validated sandwich-type ELISA, using adsorption with pneumococcal cell wall polysaccharide (CPs) and non-vaccine heterologous capsular polysaccharides (types 25 and 72) to reduce cross-reacting antibody. The standard curve was prepared from the international anti-pneumococcal calibrator serum, 89SF (Center for Biologics Evaluation and Research, US Food & Drug Administration). The serotype-specific IgG GMC for each serum sample was calculated by comparing the optical density to that of the reference standard.
Anti-pneumococcal antibodies induced by PPV23 to serotypes 10A, 11A, 15B and 17F were assessed by computing the following measurements for each serotype: GMCs at the pre-vaccination and post-vaccination time points, the geometric mean fold rise (GMFR) as measured by the post-vaccination/pre-vaccination IgG GMC ratio, the proportion of subjects with at least 2-fold increase of antibody concentration from baseline to post-vaccination, and the proportion of subjects with pre-vaccination and post-vaccination IgG GMCs ≥0.5 µg/mL, 1 µg/mL, 5 µg/mL, and 10 µg/mL; as correlate of protection against pneumococcal disease in adults is not known, the proposed threshold values of IgG concentrations were used as points of reference and do not necessarily correspond to a seroprotective level.30 Reverse cumulative distribution (RCD) curves for the GMC values were also plotted.
Disclosure of potential conflicts of interest
All authors are employees or former employees of Merck & Co., Inc. and Sanofi-Pasteur MSD. Employees may hold stock and/or stock options in the company.
Author contributions
Ciprero, Manoff, Samson, Grabenstein, and Musey: study concept and design, analysis and interpretation of data, and preparation of manuscript.
Marchese, Sterling, Stek, Radley, Soubeyrand, Baudin, Richard: analysis and interpretation of data, and preparation of manuscript.
Sponsor's role
This study was funded by Sanofi Pasteur-MSD (sponsor). The study was designed, executed, and analyzed by the sponsor and Merck & Co., Inc. The sponsor and Merck & Co., Inc. formally reviewed a penultimate draft. All co-authors approved the final version of the manuscript.
Funding
Sanofi Pasteur-MSD Study Identification Number PMV01C, an extension of Sanofi Pasteur-MSD Study U05-PNPS-403 EudraCT Number: 2008-003648-12.
References
- [1].Jackson ML, Neuzil KM, Thompson WW, Shay DK, Yu O, Hanson CA, Jackson LA. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Inf Dis 2004; 39:1642-50; http://dx.doi.org/ 10.1086/425615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ludwig E, Bonanni P, Rohde G, Sayiner A, Torres A. The remaining challenges of pneumococcal disease in adults. Eur Respir Rev 2012; 21:57-65; PMID:22379175; http://dx.doi.org/ 10.1183/09059180.00008911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 2004; 351:1849-59; PMID:15509818; http://dx.doi.org/ 10.1056/NEJMoa040845 [DOI] [PubMed] [Google Scholar]
- [4].Grabenstein JD, Musey LK. Differences in serious clinical outcomes of infection caused by specific pneumococcal serotypes among adults. Vaccine 2014; 32:2399-405; PMID:24637174; http://dx.doi.org/ 10.1016/j.vaccine.2014.02.096 [DOI] [PubMed] [Google Scholar]
- [5].Centers for Disease Control and Prevention Prevention of pneumococcal disease: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46:1-24. [PubMed] [Google Scholar]
- [6].Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, Adair RK, Clemens JD. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med 1991; 325:1453-60; PMID:1944423; http://dx.doi.org/ 10.1056/NEJM199111213252101 [DOI] [PubMed] [Google Scholar]
- [7].Butler JC, Breiman RF, Campbell JF, Lipman HB, Broome CV, Facklam RR. Pneumococcal polysaccharide vaccine efficacy: An evaluation of current recommendations. JAMA 1993; 270:1826-31; PMID:8411526; http://dx.doi.org/ 10.1001/jama.1993.03510150060030 [DOI] [PubMed] [Google Scholar]
- [8].Fedson DS, Shapiro ED, LaForce FM, Mufson MA, Musher DM, Spika JS, Breiman RF, Broome CV. Pneumococcal vaccine after 15 years of use: another view. Arch Intern Med 1994; 154:2531-5; PMID:7979849 [PubMed] [Google Scholar]
- [9].Maruyama T, Taguchi O, Niederman MS, Morser J, Kobayashi H, Kobayashi T, D'Alessandro-Gabazza C, Nakayama S, Nishikubo K, Noguchi T, et al.. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ 2010; 340:c1004; PMID:20211953; http://dx.doi.org/ 10.1136/bmj.c1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sims RV, Steinmann WC, McConville JH, King LR, Zwick WC, Schwartz JS. The clinical effectiveness of pneumococcal vaccine in the elderly. Ann Intern Med 1988; 108:653-7; PMID:3358567; http://dx.doi.org/ 10.7326/0003-4819-108-5-653 [DOI] [PubMed] [Google Scholar]
- [11].Shapiro ED, Clemens JD. A controlled evaluation of the protective efficacy of pneumococcal vaccine for patients at high risk of serious pneumococcal infections. Ann Intern Med 1984; 101:325-30; PMID:6380367; http://dx.doi.org/ 10.7326/0003-4819-101-3-325 [DOI] [PubMed] [Google Scholar]
- [12].Farr BM, Johnston BL, Cobb DK, Fisch MJ, Germanson TP, Adal KA, Anglim AM. Preventing pneumococcal bacteremia in patients at risk: results of a matched case-control study. Arch Intern Med 1995; 155:2336-40; PMID:7487259; http://dx.doi.org/ 10.1001/archinte.1995.00430210086013 [DOI] [PubMed] [Google Scholar]
- [13].Robbins JB, Austrian R, Lee CJ, Rastogi SC, Schiffman G, Henrichsen J, Mäkelä PH, Broome CV, Facklam RR, Tiesjema RH, et al.. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis 1983; 148:1136-59; PMID:6361173; http://dx.doi.org/ 10.1093/infdis/148.6.1136 [DOI] [PubMed] [Google Scholar]
- [14].Smit P, Oberholzer D, Hayden-Smith S, Koornhof HJ, Hilleman MR. Protective efficacy of pneumococcal polysaccharide vaccines. JAMA 1977; 238:2613-6; PMID:21973; http://dx.doi.org/ 10.1001/jama.1977.03280250039019 [DOI] [PubMed] [Google Scholar]
- [15].Harboe ZB, Thomsen RW, Riis A, Valentiner-Branth P, Christensen JJ, Lambertsen L, Krogfelt KA, Konradsen HB, Benfield TL. Pneumococcal serotypes and mortality following invasive pneumococcal disease: A population-based cohort study. PLoS Med 2009; 6:e1000081; PMID:19468297; http://dx.doi.org/ 10.1371/journal.pmed.1000081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sankilampi U, Herva E, Haikala R, Liimatainen O, Renkonen OV, Leinonen M. Epidemiology of invasive Streptococcus pneumoniae infections in adults in Finland. Epidemiol Infect 1997; 118:7-15; PMID:9042030; http://dx.doi.org/ 10.1017/S0950268896007169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jansen AG, Rodenburg GD, van der Ende A, van Alphen L, Veenhoven RH, Spanjaard L, Sanders EA, Hak E. Invasive pneumococcal disease among adults: Associations among serotypes, disease characteristics, and outcome. Clin Infect Dis 2009; 49:e23-9; PMID:19522653; http://dx.doi.org/ 10.1086/600045 [DOI] [PubMed] [Google Scholar]
- [18].Lexau CA, Lynfield R, Danila R, Pilishvili T, Facklam R, Farley MM, Harrison LH, Schaffner W, Reingold A, Bennett NM, et al.. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 2005; 294:2043-51; PMID:16249418; http://dx.doi.org/ 10.1001/jama.294.16.2043 [DOI] [PubMed] [Google Scholar]
- [19].Rudnick W, Liu Z, Shigayeva A, Low DE, Green K, Plevneshi A, Devlin R, Downey J, Katz K, Kitai I, et al.. Pneumococcal vaccination programs and the burden of invasive pneumococcal disease in Ontario, Canada, 1995–2011. Vaccine 2013; 31:5863-71; PMID:24099873; http://dx.doi.org/ 10.1016/j.vaccine.2013.09.049 [DOI] [PubMed] [Google Scholar]
- [20].Sjöström K, Spindler C, Ortqvist A, Kalin M, Sandgren A, Kühlmann-Berenzon S, Henriques-Normark B. Clonal and capsular types decide whether pneumococci will act as a primary or opportunistic pathogen. Clin Infect Dis 2006; 42:451-9; PMID:16421787; http://dx.doi.org/ 10.1086/499242 [DOI] [PubMed] [Google Scholar]
- [21].van Hoek AJ, Andrews N, Waight PA, George R, Miller E. Effect of serotype on focus and mortality of invasive pneumococcal disease: Coverage of different vaccines and insight into non-vaccine serotypes. PLoS One 2012; 7:e39150; PMID:22815698; http://dx.doi.org/ 10.1371/journal.pone.0039150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mair S, Fiquet A, Meghlaoui G, Thomas S, Ledesma E. Immunogenicity and safety of PNEUMOVAX II manufactured by a new process in older adults. Hum Vaccin 2009; 5:608-13; PMID:19617717; http://dx.doi.org/ 10.4161/hv.9228 [DOI] [PubMed] [Google Scholar]
- [23].Demczuk WHB, Martin I, Griffith A, Lefebvre B, McGeer A, Lovgren M, Tyrrel GJ, Desai S, Sherrard L, Adam H, et al.. Serotype distribution of invasive Steptococcus pneumoniae in Canada after the introduction of the 13-valent pneumococcal conjugate vaccine, 2010–2012. Can J Microbiol 2013; 59:778-88; PMID:24313450; http://dx.doi.org/ 10.1139/cjm-2013-0614 [DOI] [PubMed] [Google Scholar]
- [24].Richter SS, Diekema Dj, Heilmann KP, Dohrn CL, Riahi F, Doern GV. Changes in pneumococcal serotypes and antimicrobial resistance after introduction of the 13-valent pneumococcal conjugate vaccine in the United States. Antimicrobial Agents and Chemotherapy 2014; 58:6484-9; PMID:25136018; http://dx.doi.org/ 10.1128/AAC.03344-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Johnson SE, Rubin L, Romero-Steiner S, Dykes JK, Pais LB, Rizvi A, Ades E, Carlone GM. Correlation of Opsonophagocytosis and Passive Protection Assays Using Human Anticapsular Antibodies in an Infant Mouse Model for Bacteremia for Streptococcus pneumoniae. J Infect Dis 1999; 180:133-40; PMID:10353871; http://dx.doi.org/ 10.1086/314845 [DOI] [PubMed] [Google Scholar]
- [26].Baxendale HE, Johnson M, Stephens RC, Yuste J, Klein N, Brown JS, Goldblatt D. Natural human antibodies to pneumococcus have distinctive molecular characteristics and protect against pneumococcal disease. Clin Exp Immunol 2008; 151:51-60; PMID:17983446; http://dx.doi.org/ 10.1111/j.1365-2249.2007.03535.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Siber GR, Chang I, Baker S, Fernsten P, O'Brien KL, Santosham M, Klugman KP, Madhi SA, Paradiso P, Kohberger R. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine 2007; 25:3816-26; PMID:17368878; http://dx.doi.org/ 10.1016/j.vaccine.2007.01.119 [DOI] [PubMed] [Google Scholar]
- [28].Ekström N, Väkeväinen M, Verho J, Kilpi T, Käyhty H. Functional antibodies elicited by two heptavalent pneumococcal conjugate vaccines in the Finnish Otitis Media Vaccine Trial. Infect Immun 2007; 75:1794-800; PMID:17261612; http://dx.doi.org/ 10.1128/IAI.01673-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Andrews NJ, Waight PA, Burbidge P, Pearce E, Roalfe L, Zancolli M, Slack M, Ladhani SN, Miller E, Goldblatt D. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis 2014; 14:839-46; PMID:25042756; http://dx.doi.org/ 10.1016/S1473-3099(14)70822-9 [DOI] [PubMed] [Google Scholar]
- [30].Manoff SB, Liss C, Caulfield MJ, Marchese RD, Silber J, Boslego J, Romero-Steiner S, Rajam G, Glass NE, Whitney CG, et al.. Revaccination with a 23-valent pneumococcal polysaccharide vaccine induces elevated and persistent functional antibody responses in adults aged ≥65 years. J Infect Dis 2010; 201:525-33; PMID:20088694; http://dx.doi.org/ 10.1086/651131 [DOI] [PubMed] [Google Scholar]
- [31].Hechter RC, Chao C, Jacobsen SJ, Slezak JM, Quinn VP, Van Den Eeden SK, Tseng HF. Clinical effectiveness of pneumococcal polysaccharide vaccine in men: California Men's Health Study. Vaccine 2012; 30:5625-30; PMID:22789510; http://dx.doi.org/ 10.1016/j.vaccine.2012.06.085 [DOI] [PubMed] [Google Scholar]
- [32].Austrian R, Douglas RM, Schiffman G, Coetzee AM, Koornhof HJ, Hayden-Smith S, Reid RD. Prevention of pneumococcal pneumonia by vaccination. Trans Assoc Am Physicians 1976; 89:184-94; PMID:14433 [PubMed] [Google Scholar]
- [33].Austrian R. Prevention of pneumococcal infection by immunization with capsular polysaccharides of Streptococcus pneumoniae: Current status of polyvalent vaccines. J Infect Dis 1977; 136:S38-S42; PMID:19543; http://dx.doi.org/ 10.1093/infdis/136.Supplement.S38 [DOI] [PubMed] [Google Scholar]
- [34].Riley ID, Tarr PI, Andrews M, Pfeiffer M, Howard R, Challands P, Jennison G. Immunisation with a polyvalent pneumococcal vaccine. Reduction of adult respiratory mortality in a New Guinea Highlands community. Lancet 1977; 1:1338-41; PMID:69058; http://dx.doi.org/ 10.1016/S0140-6736(77)92552-1 [DOI] [PubMed] [Google Scholar]
- [35].Vila-Corcoles A, Salsench E, Rodriguez-Blanco T, Ochoa-Gondar O, de Diego C, Valdivieso A, Hospital I, Gomez-Bertomeu F, Raga X. Clinical effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumonia in middle-aged and older adults: a matched case-control study. Vaccine 2009; 27:1504-10; PMID:19171174; http://dx.doi.org/ 10.1016/j.vaccine.2009.01.013 [DOI] [PubMed] [Google Scholar]
- [36].Örtqvist A, Hedlund J, Burman LA, Elbel E, Höfer M, Leinonen M, Lindblad I, Sundelöf B, Kalin M. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Swedish Pneumococcal Vaccination Study Group. Lancet 1998; 351:399-403. [DOI] [PubMed] [Google Scholar]
- [37].Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, Hanson CA, Mahoney LD, Shay DK, Thompson WW. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med 2003; 348:1747-55; PMID:12724480; http://dx.doi.org/ 10.1056/NEJMoa022678 [DOI] [PubMed] [Google Scholar]
- [38].Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2013; 1:CD000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ 2009; 180:48-58; PMID:19124790; http://dx.doi.org/ 10.1503/cmaj.080734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2008; 1:CD000422; PMID:18253977 [DOI] [PubMed] [Google Scholar]
- [41].Mangtani P, Cutts F, Hall AJ. Efficacy of polysaccharide pneumococcal vaccine in adults in more developed countries: the state of the evidence. Lancet Infect Dis 2003; 3:71-8; PMID:12560191; http://dx.doi.org/ 10.1016/S1473-3099(03)00514-0 [DOI] [PubMed] [Google Scholar]
- [42].Ochoa-Gondar O, Vila-Corcoles A, Rodriguez-Blanco T, Gomez-Bertomeu F, Figuerola-Massana E, Raga-Luria X, Hospital-Guardiola I. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against community-acquired pneumonia in the general population aged >=60 years: 3 years of follow-up in the CAPAMIS study. Clin Infect Dis 2014; 58:909-17; PMID:24532544; http://dx.doi.org/ 10.1093/cid/ciu002 [DOI] [PubMed] [Google Scholar]
- [43].Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, et al.. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32-41; PMID:19947881; http://dx.doi.org/ 10.1086/648593 [DOI] [PubMed] [Google Scholar]
- [44].Moore CE, Paul J, Foster D, Mahar SA, Griffiths D, Knox K, Peto TE, Walker AS, Crook DW. Reduction of invasive pneumococcal disease 3 years after the introduction of the 13-valent conjugate vaccine in the oxfordshire region of england. J Infect Dis 2014; 210:1001-11; PMID:24719477; http://dx.doi.org/ 10.1093/infdis/jiu213 [DOI] [PubMed] [Google Scholar]
- [45].Demczuk WH, Martin I, Griffith A, Lefebvre B, McGeer A, Lovgren M, Tyrrell GJ, Desai S, Sherrard L, Adam H, et al.. Serotype distribution of invasive Streptococcus pneumoniae in Canada after the introduction of the 13-valent pneumococcal conjugate vaccine, 2010–2012. Can J Microbiol 2013; 59:778-88; PMID:24313450; http://dx.doi.org/ 10.1139/cjm-2013-0614 [DOI] [PubMed] [Google Scholar]
- [46].Grabenstein JD, Weber DJ. Pneumococcal serotype diversity among adults in various countries, influenced by pediatric pneumococcal vaccination uptake. Clin Infect Dis 2014; 58:854-64; PMID:24344141; http://dx.doi.org/ 10.1093/cid/cit800 [DOI] [PubMed] [Google Scholar]
- [47].Marchese RD, Jain NT, Antonello J, Mallette L, Butterfield-Gerson KL, Raab J, Burke P, Schulman C, Adgate H, Sikkema DJ, et al.. Enzyme-linked immunosorbent assay for measuring antibodies to pneumococcal polysaccharides for the PNEUMOVAX 23 vaccine: Assay operating characteristics and correlation to the WHO International Assay. Clin Vaccine Immunol 2006; 13:905-12; PMID:16893991; http://dx.doi.org/ 10.1128/CVI.00014-06 [DOI] [PMC free article] [PubMed] [Google Scholar]