Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is an inflammatory central nervous system disease that preferentially affects the optic nerve and spinal cord [Wingerchuk et al. 2015]. Up to 70% of patients with NMOSD have antibodies to aquaporin-4 (AQP4-IgG). AQP4 is expressed in astrocytes of the optic nerve and Müller cells in the eye. A subgroup of AQP4-IgG-seronegative patients has antibodies to myelin oligodendrocyte glycoprotein (MOG-IgG), and optic neuritis (ON) relapses are also frequent in these patients [Höftberger et al. 2015].
We hypothesize that retinal injury may be additionally driven by Müller cells dysfunction in patients with AQP4-IgG. This condition, in contrast with those patients who harbour MOG-IgG, may induce differential changes in the outer retinal layers. In this brief series of cases, we aim to investigate if optical coherence tomography (OCT) may distinguish ON associated with AQP4-IgG or MOG-IgG in NMOSD.
Methods
We included 15 ON eyes from five patients diagnosed with NMOSD and AQP4-IgG and four patients who fulfilled the new diagnostic criteria for NMOSD without AQP4-IgG [Wingerchuk et al. 2015] and who had MOG-IgG. AQP4-IgG and MOG-IgG were tested by cell-based assays as reported [Höftberger et al. 2015]. The presence of prior ON was assessed following international guidelines [Petzold et al. 2014]. Patients suffered bilateral ON, except for one patient in each group who only developed a unilateral ON. In addition, one ON eye of a patient with MOG-IgG was excluded due to the low quality of the OCT scan. Thus, we included 15 NMOSD–ON eyes (nine ON eyes with AQP4-IgG and six ON eyes with MOG-IgG). As a group of comparison, we selected 15 ON eyes of 15 MS patients with at least one prior ON episode (MS–ON eyes). We retrieved the MS patients whose age better matched NMOSD patients’ age from our general data set of MS patients (nine matched with AQP4-IgG-seropositive patients and six with MOG-IgG-seropositive patients). The period of time from the last ON episode to the visual examination was at least six months for all cases. Patients gave consent to participate, and the institutional review board of the Hospital Clinic, University of Barcelona, Spain, approved the study.
We evaluated visual acuity, colour vision and visual fields as previously described [Martínez-Lapiscina et al. 2014]. The retinal scans were performed using a Spectralis® SD-OCT device (Spectralis® Heidelberg Engineering, Heyex 5.30) by a trained technician under standard ambient light conditions and without pupillary dilatation, using eye-tracking modality. Correction for spherical errors was adjusted prior to each measurement. The peripapillary retinal nerve fibre layer (pRNFL) thickness (μm) was measured using a ring scan of 12 degrees of diameter, automatically centred on the optic nerve head (100 ART; 1536 A scans per B scan). The macular scan protocol was a 20 × 20 degree raster scan (horizontal orientation) centred on the fovea, including 25 high-resolution B scans (ART⩾9; 512 A scans per B scan). A single masked grader performed intraretinal layer segmentation using the same standard 6.0c version of the Spectralis segmentation algorithm in a semi-automated fashion, with manual correction of obvious errors to quantify macular ganglion cell complex (mGCC) including the retinal nerve fibre; ganglion cell and inner plexiform layers; inner nuclear layer (INL); outer plexiform layer (OPL); outer nuclear layer (ONL) and photoreceptors (PR) including retinal pigment epithelium retinal and Bruch membrane.
We separately included both NMOSD–ON eyes of each patient, rather than using the mean of both eyes, because even though some studies showed a mild retinal injury in non-ON eyes in NMOSD [Monteiro et al. 2012], a recent review suggested that the visual impairment in NMOSD is basically attributable to the ON episodes [Bennett et al. 2015]. Using the Mann–Whitney U test, we compared visual outcomes in the two groups and thereafter, we repeated analyses stratifying by antibody status. Two-tailed p values <0.05 were considered significant. Analyses were performed with the statistical package IBM-SPSS 20.0 software.
Results
We did not find significant differences in sex, age, number of ON episodes, and median time between last ON and visual examination in NMOSD–ON and MS–ON eyes in our study. NMOSD–ON eyes displayed poorer visual outcomes compared with MS–ON, although differences were only significant for low-contrast visual acuity. NMOSD–ON eyes displayed thinner pRNFL compared with MS–ON eyes. NMOSD–ON eyes showed thinner OPL and thicker ONL compared with MS–ON eyes (data not shown). We did not find microcystic macular oedema in any of the ON eyes.
Table 1 shows visual outcomes for NMOSD–ON eyes. Patients with MOG-IgG were older than patients with anti-AQP4 antibodies. Therefore, to avoid the influence of the age in the visual outcomes, both groups of NMOSD patients, according to the antibody serostatus, were age-matched with MS–ON eyes. Compared with MS–ON eyes, only ON eyes of patients with AQP4-IgG displayed thinner pRNFL. Additionally, they showed thicker INL, thinner OPL and thicker ONL. However, we did not find differences in OCT thicknesses between ON eyes of patients with MOG-IgG and MS–ON eyes.
Table 1.
Comparison of visual outcomes between NMOSD–ON eyes with AQP4-IgG and MOG-IgG and age-matched MS–ON eyes.
| AQP4+ NMOSD–ON eyes [1] |
MS–ON eyes [2] |
p value [1–2] |
MOG+ NMOSD–ON eyes [3] |
MS–ON eyes [4] |
p value [3–4] |
|
|---|---|---|---|---|---|---|
| Sex n [%] (female) | 6 [66%] | 7 [77%] | 0.910 | 3 [50%] | 2 [33%] | 0.558 |
| Age (years) | 34.9 [19.4–43.8] | 32.6 [27.6–36.7] | 0.965 | 54.4 [53.4–58.1] | 49.7 [42.2–61.5] | 0.240 |
| Number of ON | 2 [1–3.5] | 2 [1–2] | 0.352 | 1.5 [1–4] | 2 [1–2.25] | 0.932 |
| Time since last ON (months) | 86.4 [15.2–107.3] | 28.1 [14.1–100.2] | 0.436 | 99.0 [21.4–185.8] | 139.1 [69.1–169.6] | 0.485 |
| HCVA (#0-70 letters) | 45 [28–64] | 58 [50–60] | 0.565 | 47 [40–56] | 55 [41–56] | 0.310 |
| 2.5% LCVA (#0-70 letters) | 0 [0–20] | 21 [3–30] | 0.159 | 13 [0–30] | 27 [0–30] | 0.662 |
| 1.25% LCVA (#0-70 letters) | 0 [0–0] | 4 [0–20] | 0.031 | 0 [0–6] | 10 [0–18] | 0.247 |
| HRR (#0-36 symbols) | 21 [6–36] | 36 [29–36] | 0.052 | 35 [17–36] | 31 [12–35] | 0.329 |
| Visual field (MD) (dB) | −5.9 [−12.5 to −0.5] | −4.3 [−6.5 to −2.2] | 0.831 | −2.7 [−4.7 to −1.9] | −2.5 [−6.6 to −1.7] | 0.841 |
| pRNFL thickness (µm) | 50 [40–77] | 80 [72–90] | 0.038 | 68 [48–78] | 74 [65–78] | 0.310 |
| mGCC thickness (µm) | 67 [62–100] | 91 [78–99] | 0.112 | 82 [62–93] | 83 [65–92] | 0.872 |
| INL thickness (µm) | 43 [42–46] | 39 [37–42] | 0.021 | 40 [37–41] | 40 [40–42] | 0.565 |
| OPL thickness (µm) | 30 [29–33] | 35 [32–38] | 0.008 | 30 [28–37] | 32 [30–36] | 0.626 |
| ONL thickness (µm) | 80 [78–81] | 71 [65–75] | 0.021 | 75 [68–80] | 76 [71–81] | 0.872 |
| PR thickness (µm) | 80 [78–81] | 82 [80–84] | 0.190 | 81 [77–83] | 82 [80–86] | 0.416 |
Data represents median [P25–P75] unless otherwise indicated. Chi-squared Pearson test for categorical variables and Mann–Whitney U test for quantitative variables.
AQP4+: antibodies against Aquaporin 4; HCVA: high contrast visual acuity; HRR: Hardy, Rand and Rittler pseudoisochromatic plates; INL: macular inner nuclear layer; LCVA: low contrast visual acuity; MD: mean deviation; mGCC: macular ganglion cell complex; MOG+: antibodies against myelin oligodendrocyte glycoprotein; MS: multiple sclerosis; NMOSD: neuromyelitis optica spectrum disorder; OCT: optical coherence tomography; ON: optic neuritis; ONL; macular outer nuclear layer; OPL: macular outer plexiform layer; PR: photoreceptors including retinal pigment epithelium and Bruch membrane; pRNFL: peripapillary retinal nerve fibre layer.
Discussion
The OCT findings of our comparative study highlight important observations. First, serostatus stratification in NMOSD is important, because a different type of retinal cell damage may occur in patients with NMOSD who share a similar clinical phenotype but have a different antibody profile. Second, ON eyes of patients with AQP4-IgG displayed a distinguished outer retinal OCT macular phenotype (OPL thinning and ONL thickening). Third, OCT results of ON eyes of patients with MOG-IgG were similar to those found with MS–ON eyes.
Previous studies found that NMOSD–ON eyes typically displayed thinner pRNFL and mGCC as compared with MS–ON eyes [Bennett et al. 2015]. However, the evidence of involvement of other layers was scarce. Previous studies have described INL thickening in patients with NMOSD compared with MS [Fernandes et al. 2013] and OPL thinning with ONL thickening compared with isolated ON eyes and MS eyes [Park et al. 2014]. However, none of these studies analyzed these findings according to the antibody serostatus in patients with NMOSD.
The novelty of this brief study is that we addressed differences in NMOSD according to serostatus and the results suggest that even the clinical profile is similar, pathophysiological mechanisms underlying neuroaxonal damage are different in these two entities. In Figure 1, we discuss several biological processes that may explain the changes in outer retinal layers in ON eyes when associated with AQP4-IgG. INL thickening has been described in ON eyes of patients with MS. Some authors have proposed that INL thickening and microcystic macular oedema may be a continuum and represents trans-syntactic degeneration in the retina [Saidha et al. 2012]. However, we suggest that antibody-mediated damage of Müller cells is likely a key and specific contributor to the outer retinal damage observed in patients with AQP4-IgG. Moreover, the absence of differences between patients with ON associated with MOG-IgG or MS suggests that the physiopathological mechanism involved in both disorders may be similar. In fact, a MS-type pattern II was found in a recent histopathological study of one patient with MOG-IgG [Spadaro et al. 2015]. Altogether, this suggests that the neuroaxonal injury may be driven by different mechanisms: astrocytopathy for AQP4+ NMOSD and oligodendropathy for MOG+ NMOSD and MS patients.
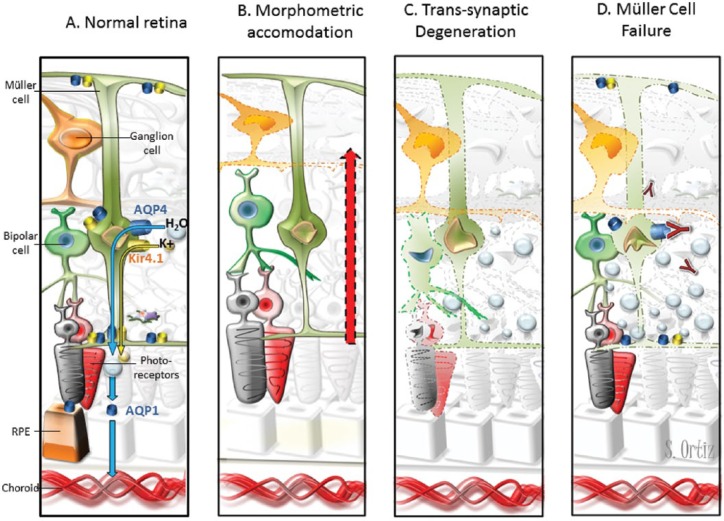
Figure 1.
Pathogenic models of retinal damage in NMOSD–ON eyes.
(A) Water transportation through the retina. Extracellular fluid accumulates in the neural retina and subretinal space. Müller cells and RPE cells promote fluid clearance towards the vessels using aquaporins receptors. Müller cell express AQP4, and RPE cells express AQP1. Moreover, water transportation is coupled to the transport of potassium through Kir4.1 channels. Müller cells display AQP4 and Kir4.1 surrounding the vessels of INL and at both limiting membranes. (B) The hypothesis of morphometric accommodation. Müller cells may promote thickening of other layers as compensatory effect to mGCC thinning after AON. We would expect proportional or greater thickening in adjacent layers (INL/OPL) and we found thickening of INL and ONL but OPL thinning. (C) The hypothesis of trans-synaptic degeneration. Retrograde degeneration of ganglion cells may induce trans-synaptic degeneration that finally lead to neuronal loss in the retina. Neuronal loss would translate in layer thinning instead of thickening. However, trans-synaptic degeneration may promote Müller cell dysfunction with fluid accumulation leading to retinal thickening that mask neuronal loss. (D) The hypothesis of Müller cell dysfunction. Inflammation in the acute phase of optic neuritis may induce Kir4.1 down regulation leading to water accumulation and transient thickening of outer layers [Gabilondo et al. 2015]. In patients with AQP4-antibodies, the immune-mediated damage may promote Müller cells loss. This would likely produce water accumulation in the neural retina but not in the subretinal space because RPE cells express AQP1. In our study, NMOSD–ON eyes patients with AQP4-IgG displayed INL and ONL thickening but not RPE thickening.
AQP, aquaporin; INL, inner nuclear layer; NMOSD, neuromyelitis optica spectrum disorder; ON, optic neuritis; RPE, retinal pigment epithelium; AON, acute optic neuritis; mGCC, macular ganglion cell complex; ONL, outer nuclear layer; OPL, outer plexiform layer.
In conclusion, OCT seems to be a useful tool to evaluate the underlying retinal damage related to the different serostatus in patients with NMOSD; however, larger and longitudinal studies are needed to confirm the results of the current exploratory study.
Acknowledgments
We thank Erika J Lampert for linguistic revision of the manuscript.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Instituto de Salud Carlos III, Spain: PS09/00259 and RD07/0060/01 to PV and RD12/0032/0002 to AS, and by Marató de TV3 20141830 to AS. EHML was supported by a fellowship from the Instituto de Salud Carlos III, Spain (Rio Hortega program: CM13/00150). The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Pablo Villoslada received consultancies fees from Heidelberg Engineering.
Contributor Information
Elena H. Martinez-Lapiscina, Center of Neuroimmunology and Service of Neurology, August Pi Sunyer Institute of Biomedical Research, Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain.
Maria Sepulveda, Center of Neuroimmunology and Service of Neurology, August Pi Sunyer Institute of Biomedical Research, Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain.
Ruben Torres-Torres, Service of Ophthalmology, Institut d’Investigacions Biomèdiques August Pi Sunyer (IDIBAPS), Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain.
Salut Alba-Arbalat, Center of Neuroimmunology and Service of Neurology, August Pi Sunyer Institute of Biomedical Research, Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain.
Sara Llufriu, Center of Neuroimmunology and Service of Neurology, August Pi Sunyer Institute of Biomedical Research, Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain.
Yolanda Blanco, Center of Neuroimmunology and Service of Neurology, August Pi Sunyer Institute of Biomedical Research, Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain.
Ana M. Guerrero-Zamora, Center of Neuroimmunology and Service of Neurology, August Pi Sunyer Institute of Biomedical Research, Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain
Nuria Sola-Valls, Center of Neuroimmunology and Service of Neurology, August Pi Sunyer Institute of Biomedical Research, Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain.
Santiago Ortiz-Perez, Service of Ophthalmology, Institut d’Investigacions Biomèdiques August Pi Sunyer (IDIBAPS), Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain.
Pablo Villoslada, Center of Neuroimmunology and Service of Neurology, August Pi Sunyer Institute of Biomedical Research, Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain; Multiple Sclerosis Center, Department of Neurology, University of California, San Francisco (UCSF), San Francisco, CA, USA.
Bernardo Sanchez-Dalmau, Service of Ophthalmology, Institut d’Investigacions Biomèdiques August Pi Sunyer (IDIBAPS), Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain.
Albert Saiz, Center of Neuroimmunology and Service of Neurology, August Pi Sunyer Institute of Biomedical Research, Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain.
References
- Bennett J., de Seze J., Lana-Peixoto M., Palace J., Waldman A., Schippling S., et al. (2015) Neuromyelitis optica and multiple sclerosis: seeing differences through optical coherence tomography. Mult Scler 21: 678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes D., Raza A., Nogueira R., Wang D., Callegaro D., Hood D., et al. (2013) Evaluation of inner retinal layers in patients with multiple sclerosis or neuromyelitis optica using optical coherence tomography. Ophthalmology 120: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabilondo I., Martínez-Lapiscina E., Fraga-Pumar E., Ortiz-Perez S., Torres-Torres R., Andorra M., et al. (2015) Dynamics of retinal injury after acute optic neuritis. Ann Neurol 77: 517–528. [DOI] [PubMed] [Google Scholar]
- Höftberger R., Sepulveda M., Armangue T., Blanco Y., Rostásy K., Cobo Calvo A., et al. (2015) Antibodies to MOG and AQP4 in adults with neuromyelitis optica and suspected limited forms of the disease. Mult Scler 21: 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Lapiscina E., Ortiz-Pérez S., Fraga-Pumar E., Martinez-Heras E., Gabilondo I., Llufriu S., et al. (2014) Colour vision impairment is associated with disease severity in multiple sclerosis. Mult Scler 20: 1207–1216. [DOI] [PubMed] [Google Scholar]
- Monteiro M., Fernandes D., Apóstolos-Pereira S., Callegaro D. (2012) Quantification of retinal neural loss in patients with neuromyelitis optica and multiple sclerosis with or without optic neuritis using Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci 53: 3959–3966. [DOI] [PubMed] [Google Scholar]
- Park K., Kim J., Oh S. (2014) Analysis of spectral domain optical coherence tomography measurements in optic neuritis: differences in neuromyelitis optica, multiple sclerosis, isolated optic neuritis and normal healthy controls. Acta Ophthalmol 92: e57–65. [DOI] [PubMed] [Google Scholar]
- Petzold A., Wattjes M., Costello F., Flores-Rivera J., Fraser C., Fujihara K., et al. (2014) The investigation of acute optic neuritis: a review and proposed protocol. Nat Rev Neurol 10: 447–458. [DOI] [PubMed] [Google Scholar]
- Saidha S., Sotirchos E., Ibrahim M., Crainiceanu C., Gelfand J., Sepah Y., et al. (2012) Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol. 11: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadaro M., Gerdes L., Mayer M., Ertl-Wagner B., Laurent S., Krumbholz M., et al. (2015) Histopathology and clinical course of MOG-antibody-associated encephalomyelitis. Ann Clin Transl Neurol 2: 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuk D., Banwell B., Bennett J., Cabre P., Carroll W., Chitnis T., et al. (2015) International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]