Abstract
Background:
Both the United States (US) Food and Drug Administration (FDA) and the European Union (EU) European Medicines Agency (EMA) order pediatric clinical trials as a condition for approval of new compounds. We evaluate clinical value and likelihood of sufficient recruitment for pediatric multiple sclerosis (pMS) studies and discuss US and EU pediatric legislation with pMS as a paradigm.
Methods:
We analyzed pMS clinical trials requested by the FDA and the EMA and industry-sponsored pMS studies registered on www.clinicaltrials.gov and www.clinicaltrialsregister.eu.
Results:
The FDA demands four and the EMA 15 pMS trials
Conclusions:
pMS is rare. Neither FDA nor EMA prioritize compounds for potential benefit in pMS. The EMA in particular orders multiple pMS studies, which will probably not recruit enough patients. Therefore, it is likely that the pMS trial outcomes will not be relevant for evidence-based medicine analyses, clinical practice and a pMS label for the respective drug. EMA requests for multiple pediatric studies have been described in metastasized adolescent melanoma, another very rare pediatric disease. The terms ‘ghost studies’ and ‘therapeutic hostages’ have been proposed for such trials and children whose parents are lured into permitting study participation. Clinical studies are not ethical if the probability is high that they will not provide reasonable outcomes. For now, pMS clinicians will have to continue to use new MS drugs in children off-label. They might consider a more proactive international coordinating role in prioritizing and testing new MS compounds in children.
Keywords: better medicines for children, multiple sclerosis, pediatric clinical trials, pediatric drug development, pediatric legislation
Introduction
Pediatric multiple sclerosis (pMS) occurs in a small fraction of patients with multiple sclerosis. About 2–5% of patients with MS are under 18 years old, less than 1% are under 10 years old, and the incidence is less than one in 100,000 people [Chou et al. 2014; Pena and Lotze, 2013; Suppiej and Cainelli, 2014; Waldman et al. 2014]. No therapies are approved for pMS by the US Food and Drug Administration (FDA), and only limited interferon use by the European Medicines Agency (EMA) [Chitnis et al. 2013]. Modern drug labels result from pharmaceutical legislation in 1962 [Rose, 2012]. This also initiated a discussion on ‘off-label’ use in children and children’s status as ‘therapeutic orphans’ [Rose, 2008, 2014a, 2014b; Shirkey, 1968]. Since 1962, pharmaceutical companies must prove safety and efficacy (S&E) for drug approval in adequate clinical trials, while before, they could make claims for their products without scientific proof. After approval, physicians can use drugs off label in other disorders or patient populations, while pharmaceutical companies must not promote off-label use. However, they may sponsor further trials, and if the outcomes are positive, request expanded approval. This works well in frequent disorders, but is challenging in rare diseases or small patient cohorts like pMS. Newer immunotherapies offer advantages in S&E in adult MS [Coles, 2015]. The International Pediatric Multiple Sclerosis Study Group (IPMSSG) has addressed essential challenges of pediatric clinical studies in new MS drugs in the wake of US and European Union (EU) pediatric legislation [Chitnis et al. 2013], both introduced to change children’s status as ‘therapeutic orphans’. We investigated to what degree the US- and EU-mandated pMS clinical studies are practicable and have clinical value. This is of relevance beyond the US and EU, as pediatric clinical trials recruit patients on a global level today [Pasquali et al. 2010].
Methods
All FDA-approved MS drugs were checked for pediatric study requests in the corresponding FDA approval letters (available on the internet). All EMA requests for pediatric clinical trials for MS drugs within the respective pediatric investigational plan (PIP) were evaluated. PIPs are published in a shortened version on the EMA website. The titles of all mandated clinical studies and other measures are listed towards the end of the published PIP. In addition, we analyzed existing clinical MS trials, registered on ClinicalTrials.gov and ClinicalTrialsRegister.eu.
As all information used for this paper is published or directly available on the internet, no ethics board approval was needed for this paper.
Results
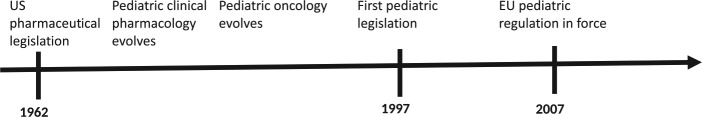
Table 1 and Figure 1 describe key milestones in the development of modern pharmaceutical legislation, consequences for its use in children, including the question of off-label use, and the background of US and EU pediatric legislation. Table 2 provides a schematic overview of US and EU pediatric legislation.
Table 1.
Key dimensions of use of drugs in children.
| 1. US pharmaceutical legislation 1962 led to first modern labels. This had the ‘side effect’ of pediatric disclaimers that the respective drug was not tested in children. In 1963, Shirkey coined the term of children as ‘therapeutic orphans’ [Shirkey, 1968; Rose, 2008] 2. Pediatric clinical pharmacology elaborated that in infants and children absorption, distribution, metabolism and excretion (ADME) differ considerably from adults. Dosing requires careful ADME assessment [Kearns and Reed, 1989; Kearns et al. 2003; ICH E 11, 2000] 3. The debates about therapeutic orphans and ADME led to US pediatric legislation in 1997. Pediatric clinical studies had until then be the domain of academic researchers, with some exceptions, e.g. growth hormone, lung surfactant, vaccines 4. Pediatric oncology evolved by using available drugs without caring for regulatory details. As a precedent for other pediatric disciplines this is not fully appreciated [Niehues, 2015]. 5. Commercial off-label promotion of drugs is prohibited. But all clinical pediatricians treat children responsibly off label [Fratarelli et al., 2014] 6. Medicine is exposed to an upcoming wealth of new medications, which is a special challenge in the question how to use them in children 7. The European Union pediatric legislation is radical and ambitious: a pediatric investigation plan (PIP) is required for all new drugs. No registration in adults or children without PIP |
Figure 1.
Timelines of key dimensions in use of modern drugs in children.
Table 2.
US and EU pediatric legislation.
| US [Rose, 2008, 2014a, 2014b] Introduced 1997 as FDAMA, voluntary Later complemented by mandatory PREA Both reauthorized 2012 as FDASIA Orphan designations are exempt PREA only for same indication as in adults FDA PSPs mandatory after EoP2 meeting Does not threaten to stop adult registration Pediatric and adult registration by respective FDA division |
EU [Rose 2008, 2014a, 2014b] In force since 2007 Strong emphasis on mandatory PIP Refused PIP will block adult registration PIP is an almost 1 year complex procedure Detailed PIP to be submitted end of phase I PDCO represents all EU member states PIP handling by PDCO, not EMA specialists Decision about registration later by other EMA committee: CHMP |
CHMP, Committee on Human Medicinal Products; FDAMA, US Food and Drug Administration Modernization Act; FDASIA, US Food and Drug Administration Safety and Innovation Act; PDCO, pediatric committee; PREA, Pediatric Research Equity Act; PIP, European Union pediatric investigation plan; PSP, US Food and Drug Administration pediatric study plan.
Table 3 lists the studies demanded by the FDA in children and adolescents with MS. S&E trials were mandatory for four compounds. The other MS compounds were introduced when the FDA could not yet demand pediatric trials. The FDA ordered additional pharmacokinetic (PK) data for some compounds for children and in all trials comparison of the respective medication against an appropriate control. The deadline of the last study report of the FDA-demanded pMS trials is in 2021.
Table 3.
FDA-required pediatric MS studies.
| Compound | Abbreviated study title | FSR |
|---|---|---|
| Glatiramer acetate (Copaxone) | R C PG superiority study in 10–17 year olds on S&E compared with an appropriate control for the treatment of relapsing forms of MS | 2021 |
| Teriflunomide (Aubagio) | R C PG superiority trial on single and multiple dose PK, S&E compared with an appropriate control for the treatment of relapsing forms of MS | 2017 |
| Fingolimod (Gilenya) | 24-month R AC PG study on single and multiple dose PK, S&E compared with IFβ1a intramuscularly (Avonex) for the treatment of relapsing remitting MS. E portion of study to be designed to show superiority over AC | 2016 |
| Dimethyl fumarate (Tecfidera) | R C PG superiority trial in 10–17 year olds on PK, S&E compared with an appropriate control for the treatment of relapsing forms of MS | 2020 |
AC, active controlled; C, controlled; E, efficacy; FSR, final study report; IFβ, interferon β; MD, multiple dose; MS, multiple sclerosis; PG, parallel group; PK, pharmacokinetics; R, randomized; S&E, safety and efficacy.
Table 4 shows the PIP-mandated pMS studies. PIP studies for natalizumab and alemtuzumab were waived. One to two comparisons to active control therapies are requested for all other new compounds, and for some compounds, PK data. Most trials have to compare compounds either against an appropriate comparator or interferon β. A placebo-controlled trial is demanded for teriflunomide (Table 3, study 13). The deadline of the last EMA/pediatric committee (PDCO)-demanded clinical trials is in 2024. Negotiations with the authorities to establish a new PIP lasts for approximately 1 year; for a PIP modification, approximately 6 months [Rose, 2012, 2014b].
Table 4.
Multiple sclerosis PIP-requested clinical studies.
| No. | Compound and PIP No. | Abbreviated Study Description | Until |
|---|---|---|---|
| 1 | Alemtuzumab EMEA-001072-PIP01-10 |
R PG S&E T study compared with an appropriate comparator in 10 to <18 year olds with R/R MS w/ prior DMT | 2018 |
| 2 | MAB (LY2127399) EMEA-000802-PIP01-09 |
MC OL R AC (IFβ1a) study on T, PK, S&E in children w/ R/R MS | 2023 |
| 3 | Daclizumab EMEA-001349-PIP01-12-M01 |
OL R AC S&E T study in 10 to <18 year olds w/ R/R MS followed by a 24-month extension study | 2019 |
| 4 | Dimethyl fumarate EMEA-000832-PIP01-10-M02 |
OL R MC MD AC PG S&E study in 10 to <18 year olds w/ R/R MS | 2016 |
| 5 | Fingolimod EMEA-000087-PIP01-07-M03 |
DB R MC MD AC (IFβ1) PG PK S&E study in 10 to <18 year olds, followed by a long-term extension study | 2019 |
| 6 | Laquinimod EMEA-000972-PIP01-10-M04 |
OL SA PK S T single oral dose study in 10 to <18 year olds w/ R/R MS R MC PG AC T S&E AC trial in 10 to <18 year olds w/ R/R MS; blinded MRI assessment |
2018 |
| 7 | Natalizumab EMEA-001095-PIP02-12 |
OL RD PK S study in 10 to <18 year olds w/ R/R MS Meta-analysis of natalizumab S&E in pediatric patients with MS |
2015 |
| 8 | Ocrelizumab EMEA-000310-PIP03-10-M01 |
OL PG SD T PK S&E of three ascending doses in 10 to <18 year olds w/ R/R MS R OL AC (IFβ1a) PG S&E study in 10 to <18 year olds w/ R/R MS |
2023 |
| 9 | Ozanimod EMEA-001710-PIP02-14 |
DB DD R AC S&E trial compared with IFβ1a in 10 to <18 year olds w/ R/R MS Development of a population PK/PD model to support the choice of dose in the S&E study in 10 to <18 year olds w/ R/R MS |
2022 |
| 10 | Pegylated IFβ EMEA-001129-PIP01-11-M1 |
OL R AC S&E trial in 10 to <18 year olds w/ R/R MS | 2021 |
| 11 | Ponesimod EMEA-000798-PIP01-09 |
MC R OL AC (IFβ1a) PG PK PD S&E T study in 10 to <18 year olds w/ R/R MS | 2022 |
| 12 | Siponimod EMEA-000716-PIP01-09-M01 |
OL R MC MD AC PG S&E study in 10 to < 18 year olds w/ R/R MS | 2024 |
| 13 | Teriflunomide EMEA-001094-PIP01-10-M02 |
DB R MC PC PG PK S&E T trial in 10 to <18 year olds w/ R/R MS, followed by a long-term extension | 2020 |
All PIP decisions can be downloaded by googling the respective PIP number (given in column 2).
AC, active controlled; DD, double dummy; DMT, disease-modifying treatment; MAB, monoclonal antibody; IFβ, interferon β; MC, multicenter; MS, multiple sclerosis; OL, open label; PD, pharmacodynamics; PIP, European Union pediatric investigation plan; PK, pharmacokinetics; PG, parallel group; R, randomized; R/R, relapsing/remitting; S, safety; S&E, safety and efficacy; T, tolerability; w/, with.
Table 5 shows the registered industry-sponsored pMS trials on ClinicalTrials.gov. The placebo-controlled trial with teriflunomide (Table 5, number 3) is probably PIP triggered. Studies 2 and 6 in Table 5 might be triggered by both the FDA and EMA. Study 4 in Table 5 compares dimethyl fumarate with placebo, whereas both the FDA and EMA/PDCO ask for an active control. The databases ClinicalTrialsRegistry.eu and ClinicalTrials.gov show four identical trials. No further pMS trials were identified on ClinicalTrialsRegistry.eu.
Table 5.
Industry-sponsored pediatric MS studies on ClinicalTrials.gov.
| No. | Compound | Identifier | Eudra CT No. | Sponsor | Design | Patients, n | Status |
|---|---|---|---|---|---|---|---|
| 1 | IFβ1a | NCT01207648 | EMD Serono | Retrospective cohort study | 307 | Completed | |
| 2 | Betaferon | NCT00963833 | Bayer | Observational | 68; 12–16 years | Active, NR | |
| 3 | DM fumarate | NCT02283853 | 2013-002318-11 | Biogen | versus IFβ-1a | 142; 10–17 years | Recruiting |
| 4 | Teriflunomide | NCT02201108 | 2011-005249-12 | Genzyme | versus placebo | 165; 10–17 years | Recruiting |
| 5 | DM fumarate | NCT02428218 | Biogen | versus placebo | 172; 10–17 years | Not yet recruiting | |
| 6 | NeuroVax | NCT02200718 | IRBP | PhI S&E | 12; 5–17 years | Not yet recruiting | |
| 7 | Fingolimod | NCT01892722 | 2011-005677-23 | Novartis | versus IF | 190; 10–17 years | Recruiting |
| 8 | DM fumarate | NCT02555215 | Biogen | Extension study | 20; 10–17 years | Not yet recruiting | |
| 9 | Natalizumab | NCT01884935 | Biogen | PhI | 13; 10–17 years | Completed | |
| 10 | Natalizumab |
NCT02137109 NCT0213710937109 |
Biogen | Metaanalysis on S&E | 400; up to 18 years | Completed | |
| 11 | DM fumarate | NCT02410200 | 2014-005003-24 | Biogen | Evaluate MRI lesions and PK | 18; 10–17 years | Recruiting |
The search terms ‘multiple sclerosis children’ in ClinicalTrials.gov gave 186 studies on 18 January 2016, of which 11 were industry-sponsored trials in children. Trials without patients’ age were not entered into this table.
The search terms ‘multiple sclerosis AND children’ in ClinicalTrialsRegister.eu (EudraCT) gave 11 results, of which 4 studies corresponded to trials on ClinicalTrials.gov. The other trials were academic trials or trials with adults only
Every single study listed in this table can be looked at directly by googling the NCT number.
DM, fumarate dimethyl fumarate (BG00012); IFβ1a, interferon β1a; IRBP, immune response BioPharma; MRI, magnetic resonance imaging; MS, multiple sclerosis; NR, not reported; PhI, phase I; PK, pharmacokinetics; S&E, safety and efficacy.
Discussion
We showed regulatory complexity and bureaucratic effort for MS compounds that are already approved (FDA) or for which an approval is planned (EMA).
The situation is peculiar in the case of natalizumab. Its use is ‘contraindicated in patients below the age of 18 years’ [Kornek, 2015]; however, only in the EU. In other words, in the EU children with MS have to wait until their 18th birthday before they may receive natalizumab, despite reports on S&E in minors [Kornek, 2013]. Here the EMA perceives everything as forbidden that has not been granted approval. Thus the EMA neglects individual calculations of risk/benefit ratios, the responsibilities of the treating physician and the increasingly well informed patients and parents. There is also a discrepancy of attitudes by the FDA and EMA. The FDA judges the use of natalizumab in children with MS as ‘not indicated’ [FDA, 2012] in contrast to the contraindication by the EMA. In general, both the FDA and the EMA list contraindications as conditions that may represent a lethal threat.
The IPMSSG stated in 2013 that the number of existing pMS cases worldwide would allow concurrent performance of one to two trials only [Chitnis et al. 2013]. Today, we have four FDA- (Table 3) and 15 EMA-demanded pMS clinical trials (Table 4). As a result, recruitment for all these studies is difficult or perhaps may never be completed.
The EMA’s predominant focus on formal regulatory aspects is reflected in numerous documents published by the EU commission, the EMA and members of the EMA PDCO. Key features of this thinking are listed in Table 6.
Table 6.
Theoretical foundations of the European Union pediatric legislation.
| 1. The first official document “Better Medicines For Children” discusses pediatric off-label use without differentiation where this makes medical sense and where it might harm [EU Commission, 2002] 2. An EMA paper (2004) discusses risks of off-label use of drugs in children, without mentioning that off-label use is often necessary and lifesaving [EMA, 2004] 3. The sulfanilamide and thalidomide catastrophes triggered modern pharmaceutical legislation. No catastrophe triggered pediatric legislation [Rose, 2008; Wax, 1995; Taussig, 1962]. The US legislation intended to improve children’s healthcare 4. The EMA website gives a link to Rocchi that addresses a ‘lack of availability of appropriate medicines for children’. This is misleading. Off-label use is characterized as dangerous for children. A medical crisis of pharmaceutic treatment of children in Europe is thus invoked [Rocchi and Tomasi, 2011]. Past and present PDCO members have published comparable statements [Rocchi et al. 2010]. The American Academy of Pediatrics has issued a clear positive statement about off-label use of medicines [Fratarelli et al., 2014] 5. Drug development is moving into rare and neglected diseases. Pediatric multiple sclerosis, oncology and rheumatology are three major areas confronted with new medications [Chitnis et al. 2013; Niehues, 2015; Rose and Walson, 2015]. Pediatricians struggle to deal with this challenge, and the regulatory authorities do not prioritize. 6. The EMA’s approach is activism to enforce as many clinical trials in children as possible. In childhood diseases too rare for multiple trials this results in ‘ghost studies’, described exemplarily for multiple studies requested for metastasized melanoma in adolescents. For patients in ghost studies the term ‘therapeutic hostages’ has been proposed [Rose, 2014c; Rose and Senn, 2014; Rose and Kummer, 2015] 7. Academic research centers compete now with industry-sponsored, questionable clinical studies triggered by EMA/PDCO [Rose and Walson, 2015] |
EMA, European Medicines Agency; EU, European Union; PDCO, pediatric committee.
One conclusion of the IPMSSG summit [Chitnis et al., 2013, p. 1165] was that theoretically ‘placebo-controlled trials (PCTs) are ideal in the current environment as there are no approved treatments for pediatric MS, and placebo-controlled trials typically allow for smaller sample sizes than superiority trials’. In practice, there will be problems in getting a positive ethical vote and recruiting pMS cases for studies that include placebo treatment. There is enough evidence that, for example, interferons and glatiramer acetate are superior to placebo in adult patients with MS [Coles, 2015; Pena and Lotze, 2013; Kornek, 2015]. It would be irresponsible and unethical of the treating physician not to offer ‘off-label’ use with the consent of the patient and the parents. The 18th birthday is a legal limit, but not a biological or medical one. Clinical trials in children and adolescents are necessary, but to demand S&E trials in pMS for every single new compound is questionable, as pMS is too rare. Here the academic clinical community should scrutinize the position of regulatory authorities. The American Academy of Pediatrics has published a clear positive statement on the legitimacy of off-label use in children in general [Fratarelli et al., 2014]. The IPMSSG agreed that ‘a stepwise approach to the launch of clinical trials for the most promising medications is necessary in order to ensure study completion’ [Chitnis et al 2013]. The regulatory authorities do not prioritize the incoming flow of new effective medicines but instead enforce many clinical trials in pMS. Specifically the EMA-mandated trials might become ‘ghost studies’: trials are initiated, study sites opened, protocols submitted to international review boards, but recruitment fails [Rose, 2014c; Rose and Senn, 2014; Rose and Kummer, 2015]. Within 5–10 years, pharmaceutical companies will request a PIP modification from the EMA. An evaluation will then be limited to the few patients whose parents have been lured to allow their children to participate. These study participants may be looked upon as ‘therapeutic hostages’ [Rose, 2014c; Rose and Senn, 2014; Rose and Kummer, 2015].
Conclusion
We have shown that specifically the EMA forces pharmaceutical companies to initiate questionable clinical trials in pMS. Instead, prioritization is needed. This is a new challenge in our increasingly globalized world. Advocacy groups, pMS specialists and parents of children with MS should consider an international initiative to establish a framework for realistic and reasonable pediatric investigations in new powerful MS drugs. It would be desirable for the IPMSSG to play a key role.
Acknowledgments
Klaus Rose: study concept, acquisition of data, first analysis, first draft manuscript. Thomas Müller: study concept, review of data analysis, critical revision of manuscript.
Footnotes
Authors’ note: Klaus Rose has worked for 20 years in pharmaceutical industry in drug development. Independent since 2011, he consults on pediatric drug development, organizes scientific conferences, edits books, and publishes. His clients are small, medium-size and large pharmaceutical companies. He is also father of a daughter with a rare disease. Thomas Müller is chief medical officer. He has been a clinician all his professional life.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors have no conflicts of interest to declare.
Contributor Information
Klaus Rose, Klausrose Consulting, Pediatric Drug Development and More, Aeussere Baselstrasse 308, 4125 Riehen, Switzerland.
Thomas Müller, Department of Neurology, St Joseph Hospital Berlin-Weissensee, Berlin, Germany.
References
- Chitnis T., Tardieu M., Amato M., Banwell B., Bar-Or A., Ghezzi A., et al. (2013) International Pediatric MS Study Group Clinical Trials Summit Meeting report. Neurology 80: 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou I., Whitehouse W., Wang H., Tanasescu R., Constantinescu C. (2014) Diagnostic modalities in multiple sclerosis: perspectives in children. Biomed J 37: 50–59. [DOI] [PubMed] [Google Scholar]
- Coles A. (2015) Newer therapies for multiple sclerosis. Ann Indian Acad Neurol 18(Suppl. 1): S30–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EU Commission (2002) Better medicines for children. Proposed regulatory actions on Paediatric medicinal products Consultation document. Available at: http://ec.europa.eu/health/files/pharmacos/docs/doc2002/feb/cd_pediatrics_en.pdf (accessed 14 June 2016).
- European Medicines Agency (EMA) (2004) Evidence of harm from off-label or unlicensed medicines in children. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2009/10/WC500004021.pdf (accessed 14 June 2016).
- Food and Drug Administration (FDA) (2012) Natalizumab label. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125104s0576lbl.pdf (accessed 14 June 2016).
- Frattarelli D.A., Galinkin J.L., Green T.P., Johnson T.D., Neville K.A., Paul IM., et al. (2014) American Association of Pediatrics: policy statement – off-label use of drugs in children. Pediatrics 133: 563–567. [DOI] [PubMed] [Google Scholar]
- ICH E 11 (2000) Clinical investigation of medicinal products in the pediatric population. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E11/Step4/E11_Guideline.pdf (accessed 14 June 2016). [PubMed]
- Kearns G., Abdel-Rahman S., Alander S., Blowey D., Leeder J., Kauffman R. (2003) Developmental pharmacology – drug disposition, action, and therapy in infants and children.N Engl J Med 349: 1157–1167. [DOI] [PubMed] [Google Scholar]
- Kearns G., Reed M. (1989) Clinical pharmacokinetics in infants and children. A reappraisal. Clin Pharmacokinet 17(Suppl. 1): 29–67. [DOI] [PubMed] [Google Scholar]
- Kornek B. (2013) Treatment of pediatric multiple sclerosis. Neuropediatrics 44: 309–313. [DOI] [PubMed] [Google Scholar]
- Kornek B. (2015) An update on the use of natalizumab in the treatment of multiple sclerosis: appropriate patient selection and special considerations. Patient Prefer Adherence 9: 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornek B., Aboul-Enein F., Rostasy K., Milos R., Steiner I., Penzien J., et al. (2003) Natalizumab therapy for highly active pediatric multiple sclerosis. JAMA Neurol 70: 469–475. [DOI] [PubMed] [Google Scholar]
- Niehues T. (2015) Optimizing treatment in paediatric rheumatology – lessons from oncology. Nat Rev Rheumatol 11: 493–499. [DOI] [PubMed] [Google Scholar]
- Pasquali S., Burstein D., Benjamin D., Jr, Smith P., Li J. (2010) Globalization of pediatric research: analysis of clinical trials completed for pediatric exclusivity. Pediatrics 126: e687–e692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena J., Lotze T. (2013) Pediatric multiple sclerosis: current concepts and consensus definitions. Autoimmune Dis 2013: 673947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi F., Paolucci P., Ceci A., Rossi P. (2010) The European paediatric legislation: benefits and perspectives. Ital J Pediatr 36: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi F., Tomasi P. (2011) The development of medicines for children. Part of a series on Pediatric Pharmacology, guest edited by Gianvincenzo Zuccotti, Emilio Clementi, and Massimo Molteni. Pharmacol Res 64: 169–175. [DOI] [PubMed] [Google Scholar]
- Rose K. (2008) Ethical, regulatory and scientific challenges in paediatric drug development. Pharm Med 22: 221–234. [Google Scholar]
- Rose K. (2012) A paediatric investigation plan case study. Pharm Med 26: 287–295. [Google Scholar]
- Rose K. (2014a) Clinical testing in children. In: Bar-Shalom D., Rose K. (eds), Pediatric Formulations – A Roadmap. New York: Springer, pp. 365–376. [Google Scholar]
- Rose K. (2014b) Pediatric pharmaceutical legislation in the USA and EU and their impact on adult and pediatric drug development. In: Bar-Shalom D., Rose K. (eds), Pediatric Formulations – A Roadmap. New York: Springer, pp. 405–420. [Google Scholar]
- Rose K. (2014c) European Union pediatric legislation jeopardizes worldwide, timely future advances in the care of children with cancer. ClinTherapeut 36: 163–177. [DOI] [PubMed] [Google Scholar]
- Rose K., Kummer H. (2015) A new ethical challenge for institutional review boards (IRBs)/ethics committees (ECs) in the assessment of pediatric clinical trials. Children 2: 198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K., Senn S. (2014) Drug development: EU paediatric legislation, the European Medicines Agency and its Paediatric Committee – adolescents’ melanoma as a paradigm. Pharm Stat 13: 211–213. [DOI] [PubMed] [Google Scholar]
- Rose K., Walson P. (2015) The contributions of the European Medicines Agency and its pediatric committee to the fight against childhood leukemia. Risk Manag Health Policy 8: 185–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirkey H. (1968) Therapeutic orphans. J Pediatr 72: 119–120. [DOI] [PubMed] [Google Scholar]
- Suppiej A., Cainelli E. (2014) Cognitive dysfunction in pediatric multiple sclerosis. Neuropsychiatr Dis Treat 10: 1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig H. (1962) A study of the German outbreak of phocomelia. JAMA 180: 1106–1114. [PubMed] [Google Scholar]
- Waldman A., Ghezzi A., Bar-Or A., Mikaeloff Y., Tardieu M., Banwell B. (2014) Multiple sclerosis in children: an update on clinical diagnosis, therapeutic strategies, and research. Lancet Neurol 13: 936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax P. (1995) Elixirs, diluents, and the passage of the 1938 Federal Food, Drug and Cosmetic Act. Ann Intern Med 122: 456–461. [DOI] [PubMed] [Google Scholar]