Abstract
Background:
The Canadian Network for Mood and Anxiety Treatments (CANMAT) conducted a revision of the 2009 guidelines by updating the evidence and recommendations. The scope of the 2016 guidelines remains the management of major depressive disorder (MDD) in adults, with a target audience of psychiatrists and other mental health professionals.
Methods:
Using the question-answer format, we conducted a systematic literature search focusing on systematic reviews and meta-analyses. Evidence was graded using CANMAT-defined criteria for level of evidence. Recommendations for lines of treatment were based on the quality of evidence and clinical expert consensus. This section is the first of six guidelines articles.
Results:
In Canada, the annual and lifetime prevalence of MDD was 4.7% and 11.3%, respectively. MDD represents the second leading cause of global disability, with high occupational and economic impact mainly attributable to indirect costs. DSM-5 criteria for depressive disorders remain relatively unchanged, but other clinical dimensions (sleep, cognition, physical symptoms) may have implications for depression management. e-Mental health is increasingly used to support clinical and self-management of MDD. In the 2-phase (acute and maintenance) treatment model, specific goals address symptom remission, functional recovery, improved quality of life, and prevention of recurrence.
Conclusions:
The burden attributed to MDD remains high, whether from individual distress, functional and relationship impairment, reduced quality of life, or societal economic cost. Applying core principles of care, including comprehensive assessment, therapeutic alliance, support of self-management, evidence-informed treatment, and measurement-based care, will optimize clinical, quality of life, and functional outcomes in MDD.
Keywords: depressive disorders, clinical practice guidelines, major depressive disorder, systematic reviews, meta-analysis, clinical assessment, diagnosis, phenomenology, evidence-based medicine
In 2009, the Canadian Network for Mood and Anxiety Treatments (CANMAT), a not-for-profit scientific and educational organization, published a revision of evidence-based clinical guidelines for the treatment of depressive disorders.1 CANMAT has updated these guidelines in 2016 to reflect new evidence in the field.
The scope of these guidelines remains the management of adults with unipolar major depressive disorder (MDD), with a target audience of psychiatrists and mental health professionals. CANMAT, in collaboration with the International Society for Bipolar Disorders, has published separate guidelines for bipolar disorder.2 This section on Disease Burden and Principles of Care is the first of six guidelines articles; subsequent sections of the guidelines will expand on psychological, pharmacological, neurostimulation, and complementary and alternative medicine treatments, as well as on special populations (youth, women, and the elderly). The question-answer format has been retained for ease of use. These recommendations are presented as guidance for clinicians who should consider them in context of individual patients and not as standards of care.
Methods
The full methods have been previously described,3 but in summary, relevant studies in English and French published from January 1, 2009, to December 31, 2015, were identified using computerized searches of electronic databases (PubMed, PsychInfo, Cochrane Register of Clinical Trials), inspection of bibliographies, and review of other guidelines and major reports. Each recommendation includes the level of evidence for each graded line of treatment, using specified criteria (Table 1). The level of evidence criteria now reflect the primacy of meta-analysis because of its increasing use in the evaluation of evidence. Note that Level 1 and 2 Evidence refer specifically to treatment studies in which randomized comparisons are available. Recommendations involving epidemiological or risk factors primarily arise from observational studies, and hence the highest level of evidence is usually Level 3. Higher order recommendations (e.g., principles of care) reflect higher-level judgment of the strength of evidence from various data sources and therefore are primarily Level 4 Evidence.
Table 1.
Criteria for Level of Evidencea and Line of Treatment.
| Criteria | |
|---|---|
| Level of evidence | |
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| Line of treatment | |
| First line |
|
| Second line |
|
| Third line |
|
RCT, randomized controlled trial.
aNote that Level 1 and 2 Evidence refer specifically to treatment studies in which randomized comparisons are available. Recommendations involving epidemiological or risk factors primarily arise from observational studies, and hence the highest level of evidence is usually Level 3. Higher order recommendations (e.g., principles of care) reflect higher level judgement of the strength of evidence from various data sources and therefore are primarily Level 4 Evidence.
bClinical support refers to application of expert opinion of the Canadian Network for Mood and Anxiety Treatments committees to ensure that evidence-supported interventions are feasible and relevant to clinical practice. Therefore, treatments with higher levels of evidence may be downgraded to lower lines of treatment due to clinical issues such as side effect or safety profile.
1.1. How Are the Depressive Disorders Classified?
The current classification of depression is based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) or “Recurrent Depressive Episodes” in the International Classification of Diseases, 10th Revision (ICD-10) classification of mental and behavioural disorders.4,5 The DSM-5, introduced in 2013, removed the broad category of mood disorders and classifies depressive disorders separately from bipolar disorder.4 For major depressive episode (MDE), the DSM-5 core symptom and duration criteria (criterion A) are unchanged from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) (Table 2).6 The DSM-IV-TR “bereavement exclusion” criterion was eliminated in the DSM-5, reflecting evidence that bereavement may last longer than 2 months and is not different from other significant stressors or losses that may precipitate an MDE (Table 3). Instead, bereavement with more severe depressive symptomatology has been included in “Conditions for Further Study” as persistent complex bereavement disorder.
Table 2.
DSM-5 Symptom Criteria for Major Depressive Episode.
Five (or more) of the following symptoms have been present during the same 2-week period and represent a change from previous functioning; at least one of the symptoms is either (1) or (2).
|
Note: Do not include symptoms that are clearly due to a general medical condition or mood-incongruent delusions or hallucinations.
Table 3.
Summary of Changes from DSM-IV-TR to DSM-5.
| DSM-IV-TR Item | DSM-5 Item |
|---|---|
MDD episode specifiers
|
New MDD episode specifiers
|
| Bereavement exclusion | Deleted |
Premenstrual dysphoric disorder
|
Premenstrual dysphoric disorder
|
| Dysthymic disorder, “double depression”—MDE superimposed on dysthymic disorder | Persistent depressive disorder
|
DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision; MDD, major depressive disorder; MDE, major depressive episode.
Other important changes in DSM-5 include a new classification of chronic depression as persistent depressive disorder, which comprises the former DSM-IV-TR diagnoses of chronic MDE and dysthymic disorder. This change was in response to evidence showing it was difficult to differentiate the latter diagnoses and the fact that they frequently co-occurred. DSM-5 also includes 2 new depressive disorders. Disruptive mood dysregulation disorder is applicable for children aged 6 to 18 years who exhibit severe and recurrent temper outbursts, uncontrollable behaviour, and persistent irritability. Premenstrual dysphoric disorder recognizes a serious form of premenstrual syndrome characterized by intense emotional symptoms, which may include symptoms of depressed mood, anxiety, mood swings, and irritability, in the final week before menses.
Despite these minor changes in criteria for MDD, the DSM-5 field trials found poor interrater reliability for the diagnosis, and neither DSM-5 nor ICD-10 is based on aetiology or pathophysiology.7 There are renewed efforts to use alternative frameworks, such as the US National Institute of Mental Health Research Domain Criteria Initiative (RDoC), which attempts to align diagnosis with current understanding of brain systems.8
1.2. What Are Important Clinical Specifiers and Dimensions of Depressive Episodes?
It is increasingly recognized that there is a spectrum of clinical presentations that are not captured by the symptom criteria for MDE. These represent important clinical dimensions that have implications for prognosis and treatment and may have different neurobiological substrates. DSM-5 classifies these subtypes and dimensions as episode or course specifiers for MDE. DSM-IV specifiers, including melancholic, atypical, psychotic, and seasonal pattern, have been retained in the DSM-5, but the former postpartum specifier is now termed “with peripartum onset” to reflect evidence that 50% of postpartum depressive episodes have an onset prior to delivery. New specifiers include anxiety and mixed features (Table 4). The DSM-5 “with anxious distress” specifier recognizes that MDE is often accompanied by anxiety symptoms, even when a comorbid anxiety disorder is not present. Anxiety contributes to increased rates of suicide, poor response to treatment, and increased risk of chronicity and recurrence.9
Table 4.
DSM-5 Episode Specifiers and Other Clinical Dimensions Associated with MDE.
| Subtype/Dimension | DSM-5 Specifier | Key Features |
|---|---|---|
| Melancholic depression | With melancholic features | Nonreactive mood, anhedonia, weight loss, guilt, psychomotor retardation or agitation, morning worsening of mood, early morning awakening, excessive or inappropriate guilt |
| Atypical depression | With atypical features | Reactive mood, oversleeping, overeating, leaden paralysis, interpersonal rejection sensitivity |
| Psychotic (delusional) depression | With psychotic features | Hallucinations or delusions |
| Catatonic depression | With catatonic features | Catalepsy (waxy flexibility), catatonic excitement, negativism or mutism, mannerisms or stereotypes, echolalia or echopraxia (uncommon in clinical practice) |
| Anxious depression | With anxious distress | Feeling keyed up or tense, restless, worried, something awful may happen, or afraid of losing control |
| Mixed states | With mixed features | Elevated mood, inflated self-esteem or grandiosity, more talkative, racing thoughts, increased energy and activity, decreased need for sleep, risky and impulsive activities |
| Seasonal affective disorder | Seasonal pattern | Regular onset and remission of depressive episodes during a particular season (usually fall/winter onset) |
| Postpartum and antepartum depression | With peripartum onset | Onset of depressive episode during pregnancy or within 4 weeks postpartum |
| Cognitive dysfunction | NA | Disturbances in attention, memory, processing speed, executive functioning and emotional processing |
| Sleep disturbance | NA | Insomnia or hypersomnia; circadian rhythm disturbance |
| Somatic symptoms | NA | Headaches, body aches, fatigue, anergia |
DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; MDE, major depressive episode; NA, not applicable.
The new DSM-5 specifier “with mixed features” allows for the presence of manic or hypomanic symptoms in individuals diagnosed with unipolar MDEs, as well as the presence of depressive symptoms in patients diagnosed with mania/hypomania. Mixed features are found in up to a third of patients with MDE, although the prevalence rates vary widely depending on the diagnostic criteria employed.10,11 Mixed depressive episodes are more common in younger patients, are more severe, and carry a higher risk for suicide,12 but the specifier is controversial.13
Other clinical dimensions that are not recognized in the DSM-5 may also have important assessment and treatment implications. For example, cognitive symptoms are included as a core diagnostic criterion for MDE, but these do not describe the full spectrum of cognitive dysfunction associated with depressive disorders, including disturbances in attention, memory, processing speed, and executive functioning.14–17 Cognitive deficits can be demonstrated with neuropsychological tests during acute MDEs and are associated with significant impact on daily functioning and quality of life.16,18,19 Moreover, cognitive dysfunction is a common residual symptom during treatment and may continue even after mood symptoms have remitted.20,21 These observations reflect the need and importance for clinical assessment and monitoring of cognitive symptoms during management of MDD.
Other putative clinical dimensions include sleep/circadian rhythms and physical symptoms. Insomnia and hypersomnia can be symptoms of acute MDD, residual symptoms of poor response, or side effects of treatments such as antidepressants. Disruption of social and biological rhythms can also interfere with sleep. There is a bidirectional relationship between sleep problems and depression (i.e., sleep disturbances can be an independent risk factor for onset of an MDE).22 Similarly, somatic symptoms (e.g., painful physical symptoms, fatigue) are commonly associated with depressive episodes and are not well represented in the core MDD criteria.23,24 The presence and severity of somatic symptoms, especially pain, is associated with poor outcomes in depression.25
1.3. How Common Are Depressive Disorders?
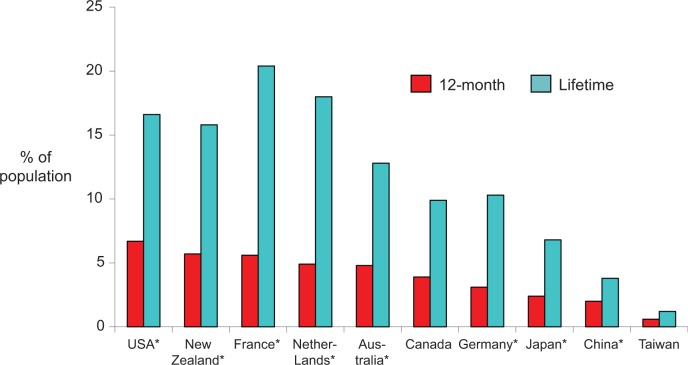
In Canada, the annual prevalence of MDE in the general population is 4.7%, indicating that over 1.5 million Canadians aged 15+ years experienced a current MDE in the past year, and lifetime prevalence is 11.3%.26 Excluding bipolar disorders, the annual and lifetime prevalence of MDD was 3.9% and 9.9%, respectively.26 These rates are intermediary between those in the United States and Asia and similar to those in Europe (Figure 1). Women have a greater annual prevalence of MDD (4.9%) than men (2.8%), and the prevalence has an inverse relationship with age.26
Figure 1.
Prevalence of major depressive disorder by world region. *WMH, World Health Organization’s World Mental Health Surveys, Canada, CCHS113; Taiwan Psychiatric Morbidity Survey.114
The incidence or the risk of developing a depressive disorder can only be estimated from longitudinal studies. There are few large population-based longitudinal studies based on the DSM-IV criteria. The Canadian estimates of incidence proportions of MDE were 2.9% in 2 years and 5.7% in 4 years,27 similar to the 3-year incidence in the Netherlands (4.6%)28 and in the United States (3.3%).29
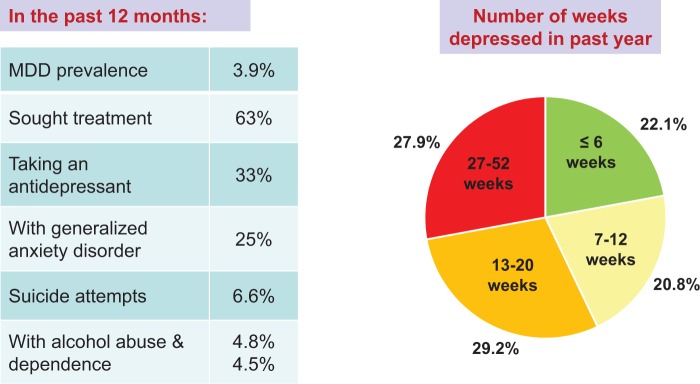
Population-based surveys have shown consistently that about 50% of depressive episodes are brief, with resolution within 3 months. Figure 2 shows Canadian descriptive epidemiology for depressive episodes. Despite increases in mental health service in recent years, there have been no changes in the annual prevalence of MDE in Canada (4.8% in 2002 vs. 4.7% in 2012).26 Similar trends are seen in the United States30 and in Australia.31
Figure 2.
Descriptive epidemiology of depression in Canada, 2012.26 MDD, major depressive disorder.
1.4. What Is the Risk of Relapse or Recurrence?
Depressive disorders often have a chronic and episodic course. In a large American cohort of participants with an MDE, 26.5% were experiencing a chronic episode of ≥2 years’ duration.32 Over the 3-year follow-up, 15.1% had a chronic course for the entire period.33 DSM-5 defines recurrence as a new MDE following a full-episode remission (i.e., 2 months with no significant symptoms).4 In the US general population, of those with MDE at baseline and subsequent episode remission, 34.7% experienced a recurrent MDE in the next 3 years.34 In the Netherlands, a 2-year follow-up of 375 patients with MDD in remission for 3 months found recurrence in 26.8% of patients treated in primary care and 33.5% in specialized mental health care.35
1.5. What Is the Disease Burden Associated with MDD?
Disease burden can be measured by metrics, such as disability-adjusted life years (DALYs) or health-adjusted life years (HALYs), that account for both early mortality and loss of functioning. The Global Burden of Disease Study 2010 found that depressive disorders represented the second leading cause of disability worldwide, and MDD was responsible for 2.5% of global DALYs.36 In Ontario, the largest province in Canada, the disease burden in HALYs with MDD was greater than the combined burden of breast, colorectal, lung, and prostate cancers.37 MDD also is associated with serious impairment in quality of life38 and has major economic impact owing to occupational costs, medical service costs, and suicide-related costs. In Canada, the economic burden of mental illness in 2003 was estimated at C$51 billion39; although there are no Canadian data for MDD specifically, in the United States, the economic cost of MDD in 2010 was estimated at US$210.5 billion.40
1.6. What Is the Occupational Impact of MDD?
MDD is associated with major productivity losses as a result of absenteeism (time away from work) as well as presenteeism (illness-related productivity loss while at work). The World Health Organization (WHO) Mental Health Surveys found that depression accounted for over 5% of the population illness-related productivity loss; participants with depression had a yearly mean of 34.4 “days out of role,” which was largely invariant by country.41 In Canada, workers with MDD, compared to those without depression, were twice as likely to leave work during a 10-year follow-up.42
Increased severity of illness,43 concurrent medical conditions,44 and comorbid anxiety disorders45 result in a higher degree of work disability and greater absenteeism in people with MDD. In addition to overall severity, individual symptoms of MDD can differentially affect workplace performance. Work impairment is most closely associated with impaired concentration and depressed mood, followed by fatigue and insomnia.46 Cognitive dysfunction is also more strongly associated with loss of workplace productivity than ratings of depression severity.19 Depression treatment has a significant positive effect on work productivity.47 Unfortunately, a substantial proportion of depressed workers do not receive evidence-based treatment.48
1.7. What Is the Impact of MDD on Other Domains?
Social factors (e.g., relationships and social activities) have a complex interrelationship with depressive disorders, including a substantial role in the causation of MDD.49 It is therefore unsurprising to observe strong associations between MDD and social impairment, especially in social and close-relation domains.50 Depressed mood, loss of interests, impaired concentration, and self-blame are the symptoms most associated with social impairment.46
Depression in parents may also affect the health of their children. Perinatal maternal depression is associated with multiple adverse outcomes in children, including increased problems with emotional regulation, internalizing disorders, behavioural disorders, hyperactivity, reduced social competence, insecure attachment, adolescent depression, and negative effects on cognitive development.51 Adverse effects in offspring are also observed in the case of paternal depression.52,53 Effective treatment and remission of maternal depression is associated with improved parenting and a reduction in psychiatric symptoms in the offspring.54–56
1.8. What Is the Impact of MDD on Physical Health?
MDD is associated with many chronic medical conditions, including heart disease, arthritis, asthma, back pain, chronic pulmonary disease, hypertension, and migraine.57 Depression is an independent risk factor for ischemic heart disease and cardiovascular mortality,58,59 and vascular risk factors are also associated with onset of depression in later life.60 The presence of depression substantially increases the level of disability61 and reduces quality of life62–64 in individuals with chronic medical illness.
MDD can affect medical conditions via multiple mechanisms. Depression reduces adherence to treatment65,66 and interferes with participation in preventive health care.67,68 Depression is also associated with important risk factors for physical illness, including sedentary lifestyle,69 obesity,70 and cigarette smoking.71 The pathophysiology of depression appears to be related to other fundamental mechanisms of disease (e.g., MDD shares a complex and bidirectional relationship with obesity and associated metabolic problems70,72) and is associated with immune-inflammatory dysfunctions that are implicated in reduced neural plasticity and neuroprogression.73–75
1.9. How Does MDD Typically Present in Clinical Practice?
Depressive disorders have a broad range of presentations in clinical practice, especially in primary care settings. Emotional symptoms are often attributed to stressful work, relationship stress, or life stress, and presenting complaints are often physical symptoms because of the high degree of comorbidity with other medical conditions. Hence, MDD often goes unrecognized and untreated, even in clinical settings.
Screening for depression has been recommended by some agencies76,77 and not by others.78 The value of screening remains controversial because of the limited evidence base on effectiveness,79 although screening is more effective when additional supports (e.g., treatment protocols, care management) are available.80 CANMAT recommends that screening be done in primary and secondary care settings in individuals with risk factors (summarized in Table 5) when there are available resources and services for subsequent diagnostic assessment and management. The quick 2-question screen (“In the last month, have you been bothered by little interest or pleasure in doing things?” and “In the last month, have you been feeling down, depressed or hopeless?”) remains an effective and simple approach for screening in clinical practice.81 An answer of “yes” to either question requires a more detailed assessment.
Table 5.
Risk Factors for Depression Screening (Level 3 and 4 Evidence).
| Clinical Factors | Symptom Factors |
|---|---|
|
|
1.10. What Are the Basic Principles of Clinical Management?
Table 6 summarizes the principles of clinical management of MDD. A comprehensive assessment and management plan, including attention to safety, is the foundation of quality care. A thorough psychiatric assessment should include a comprehensive symptom inquiry, including an evaluation for bipolar disorder, anxiety disorders, and substance use disorders, as these are frequently comorbid with depression. Collateral information should be gathered whenever possible.
Table 6.
Principles of Clinical Management (Level 4 Evidence, Unless Indicated).
|
Stepped care82 and chronic disease management models83 are associated with significant improvements in depression outcomes compared to usual care. These models are consistent with the CANMAT delineation of recommended lines of treatment (see other sections). Common elements of these approaches are applicable to other treatment settings and include systematic monitoring of patient outcomes, patient education,84 and treatment decisions that are evidence-based and responsive to therapeutic goals.
Poor treatment adherence and high discontinuation rates represent a major challenge, particularly for pharmacotherapy. Strategies for enhancing adherence include patient education and supported self-management, as well as use of collaborative care systems by practitioners. Treatment adherence should be discussed at an early stage and monitored frequently during treatment in a collaborative manner. A weak therapeutic alliance predicts poorer treatment adherence.85
Self-management refers to the individual’s ability to manage depression and associated treatments, physical and psychosocial sequelae, and lifestyle modifications. Supported self-management typically includes action planning to change behaviour. Techniques include behavioural activation, communication skills, coping with emotion, patient education, healthy lifestyle, relapse-prevention planning, skill development, and self-monitoring.86 In addition to decreasing patients’ reliance on health care providers, effective self-management also serves to increase empowerment and self-efficacy.86 Peer-support service delivery models are seeing broad uptake and may offer promise, but further research is required to fully evaluate effectiveness.87
1.11. How Do You Assess Suicidal Risk?
Suicidal ideation, plans, and attempts are highly prevalent among people with MDD.88,89 Every clinical encounter with a patient with MDD should include an assessment of suicide risk. Table 7 shows the modifiable and nonmodifiable risk factors for suicide; history of suicide attempt is the strongest risk factor. The low base rate of suicide makes it difficult to predict suicide risk at an individual level.90 Suicide risk assessment tools are available (e.g., SADPERSONS,91,92 Columbia Suicide Severity Rating Scale,93,94 Chronological Assessment of Suicide Risk interview guide95) and, while not particularly reliable in predicting future suicide attempts, can aid systematic assessment and documentation in clinical practice.
Table 7.
Risk Factors for Suicide During a Major Depressive Episode (Level 3 Evidence).
| Nonmodifiable Risk Factors | Modifiable Risk Factors |
|---|---|
|
Symptoms and life events
|
1.12. What Is Measurement-Based Care?
Measurement-based care refers to the systematic use of measurement tools, such as validated rating scales, to monitor outcomes and support clinical decision-making. Using simple rating scales for measurement-based care of depression can improve outcomes such as symptom remission and adherence.96,97
Table 8 shows some clinically useful examples of the many available patient-rated and clinician-rated scales. Symptom scales can be useful tools for screening, diagnosis, and monitoring outcomes. For example, the important distinction between symptom response (usually defined as 50% or greater reduction in baseline score) and remission (a score in the nondepressed range) can be reliably determined using symptom scales.
Table 8.
Examples of Validated Outcome Scales.
| Outcome | Clinician-Rated | Patient-Rated |
|---|---|---|
| Symptoms |
|
|
| Functioning |
|
|
| Side effects |
|
|
| Quality of life |
|
|
Note: See online supplement for references to scales.
Routine monitoring of patient outcomes must go beyond assessing the symptoms of depression and include the ongoing evaluation of functional impairment98 and quality of life.99 These outcomes are more important and relevant to patients, and each may vary independently of symptoms. Assessing functionality should include the evaluation of appropriate domains, such as occupational, social, or educational functioning.100 Quality-of-life assessments, in comparison, offer the opportunity to evaluate patient well-being and overall health satisfaction more broadly.99
Measurement-based care can be incorporated into busy clinical settings using patient-rated questionnaires, which are highly correlated with clinician-rated scales but simpler to use and more efficient. Outcome scales are often used in conjunction with clinical algorithms, such as for decisions about medication adjustment.101 The use of measurement tools should supplement and not replace clinician judgement.
1.13. What Are the Phases of Treatment?
The previous CANMAT guidelines proposed a 2-phase model (acute and maintenance phases)102 for treatment, in contrast to the traditional 3-phase model (acute, continuation, and maintenance).103 The distinction between continuation and maintenance phases was based on a theoretical difference between relapse (symptoms recurring before resolution of the current episode) and recurrence (symptoms that constitute a new episode, after recovery from the previous episode).103 Recent reviews have highlighted the inconsistent use of these terms and lack of evidence to support distinct demarcations between episodes104; hence, CANMAT continues to endorse a single concept of relapse/recurrence and the 2 treatment phases (Table 9).
Table 9.
Phases of Treatments and Activities.
| Treatment Phase | Duration | Goals | Activities |
|---|---|---|---|
| Acute | 8 to 12 weeks |
|
|
| Maintenance | 6 to 24 months, or longer |
|
|
1.14. What Are the Goals of Acute and Maintenance Treatment?
The acute and maintenance treatment phases can be summarized with 2 clinical questions: “How do you get people with depression well?” and “How do you keep them well?” The primary target goals for acute treatment include symptom remission, which implies that signs and symptoms of depression are absent or almost so, and restoration of premorbid psychosocial functioning (Table 9). Full symptom remission is important because residual depressive symptoms are risk factors for relapse and negative predictors of long-term outcome.105,106
For the maintenance phase, a key goal is prevention of recurrence (Table 9). Clinicians should focus on healthy life strategies, personality vulnerabilities, long-term self-management, and clinical strategies to reduce recurrence.104,107 In a significant proportion of patients with MDD, maintenance pharmacological, psychological, complementary and alternative medicine, and neurostimulation treatments have a role in the prevention of recurrence (see other sections).
1.15. Who Needs Longer Term Treatment?
Following successful acute phase treatment (i.e., syndromal remission), clinicians must determine which patients require longer term (maintenance) treatment and for how long. The heterogeneity of MDD results in a varied longitudinal course, but half of patients will have a chronic or recurrent course of depression. Table 10 shows the risk factors for recurrence.104,108
Table 10.
Risk Factors for Chronic or Recurrent Episodes (Level 3 Evidence).
|
Risk-prediction support tools have been developed to estimate risk of recurrence based on individuals’ unique exposure to a key set of risk factors.109 While risk-prediction models may assist clinicians in stratifying baseline risks and making informed decisions about individualized maintenance treatments with patients, they require further validation in different clinical settings and do not replace clinical judgement.
1.16. Can e-Mental Health Help in Management of MDD?
Technology and the Internet have dramatically changed medicine. According to Statistics Canada, 83% of Canadians had Internet access in 2012, and more than 70% use the Internet daily; 62% were smartphone users.110 e-Mental health refers to the use of computers, Internet, and mobile devices for mental health information and care.111 e-Mental health applications are now widely available for information, screening, assessment and monitoring, interactive self-management and psychotherapy (see Psychological Treatments section), and social support. Clinicians should be aware that there are benefits and potential harms to using and recommending e-Mental health applications and that few have good-quality evidence to support effectiveness.111,112 Table 11 lists some examples of e-Mental health resources that are evidence-based and/or come from credible sources.
Table 11.
Examples of e-Mental Health Resources for Depression.
| Purpose | e-Mental Health Application | Website |
|---|---|---|
| Information | Canadian Mental Health Association (CMHA) | www.cmha.ca/mental-health/understanding-mental-illness/depression/ |
| Mental Health Works; CMHA resources focusing on workplace mental health | www.mentalhealthworks.ca | |
| Mood Disorders Society of Canada (MDSC) | www.mooddisorderscanada.ca | |
| Here To Help; self-help information in many languages | www.heretohelp.bc.ca | |
| Screening, assessment and monitoring | MoodFx; online tracking of symptoms (depression, anxiety, cognition) and functioning | www.moodfx.ca |
| What’s My M3; online and mobile app for mood tracking | www.whatsmym3.com | |
| Self-management | MoodGym; evidence-based, interactive online self-help program for depression | https://moodgym.anu.edu.au |
| eCouch; similar to MoodGym with self-help for depression and other diagnoses | https://ecouch.anu.edu.au | |
| Social support | 7 Cups of Tea; access to confidential online text chat to trained listeners | www.7cups.com |
| BlueBoard; online anonymous community for people with depression and anxiety | https://blueboard.anu.edu.au | |
| Depression Support Group; online support groups | http://depression.supportgroups.com |
Note: This is not a comprehensive list but includes examples that are evidence-based and/or from credible sources.
Acknowledgements
The authors thank the contributions of Ms. Cindy Woo and Ms. Trehani Fonseka in the preparation of this manuscript.
Footnotes
Disclosures: The guidelines process and publication were funded entirely by internal CANMAT funds; no external support was sought or received. No honoraria were paid to authors, and no professional editorial assistance was used. All members of the CANMAT Depression Work Group disclosed potential conflicts of interest (available at www.canmat.org). CANMAT is a project-driven organization governed by a volunteer, unpaid advisory board, with no permanent staff or dedicated offices. CANMAT has a conflict of interest policy that includes disclosures by all participants, and all continuing professional development (CPD) projects are accredited by academic institutions. CANMAT has diverse funding, but in the past 5 years (2011-2015), sources of CANMAT revenue (excluding CIHR and research funding) have included national/international scientific conferences (28% of revenue), publications (26%), industry-supported CPD projects (26%), and academic projects (18%).
The CANMAT guidelines are not officially endorsed by the Canadian Psychiatric Association.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article:
RWL has received honoraria for ad hoc speaking or advising/consulting or received research funds from Asia-Pacific Economic Cooperation, AstraZeneca, Brain Canada, Bristol-Myers Squibb, Canadian Institutes of Health Research, Canadian Depression Research and Intervention Network, Canadian Network for Mood and Anxiety Treatments, Canadian Psychiatric Association, Coast Capital Savings, Johnson & Johnson, Lundbeck, Lundbeck Institute, Medscape, Pfizer, St. Jude Medical, Takeda, University Health Network Foundation, and Vancouver Coastal Health Research Institute.
DM has received honoraria for ad hoc speaking or advising/consulting or received research funds from Allergan, Bristol-Myers Squibb, Lundbeck, Janssen-Ortho, Otsuka, Pfizer, Shire, and Sunovion.
JLW has no disclosures.
MWE has no disclosures.
TK has received speaker and/or advisory honoraria from AstraZeneca, Bristol-Myers Squibb, Eli Lilly Canada, Lundbeck, Lundbeck-Otsuka, Janssen-Ortho, Pfizer, and Sunovion.
EEM has received advisory honoraria from Lundbeck.
JS has no disclosures.
WYS has received honoraria for ad hoc speaking or advising/consulting or research funds from AstraZeneca, Bristol-Myers Squibb, Canadian Psychiatric Association, Eli Lilly, Forrest Laboratories, Lundbeck, Ortho-Janssen, Pfizer, and Sunovion.
SHK has received honoraria for ad hoc speaking or advising/consulting or research funds from Allergan, Brain Canada, Bristol-Myers Squibb, Canadian Institutes of Health Research, Janssen, Lundbeck, Ontario Brain Institute, Pfizer, St. Jude Medical, Servier, and Sunovion.
GMM has been on advisory board or speaker for Janssen, Lilly, Lundbeck, and Pfizer.
RVM has received speaker and consultant honoraria or research funds from Allergan, Bristol-Myers Squibb, Canadian Institutes of Health Research, Canadian Network for Mood and Anxiety Treatments, Canadian Psychiatric Association, Eli Lilly, Johnson & Johnson, Lallemand, Lundbeck, Merck, Ontario Brain Institute, Ontario Mental Health Foundation, Otsuka, Paladin, Pfizer, Queen’s University, Sunovion, Takeda, the University Health Network Foundation, and Valeant.
SVP has been a consultant to Bristol Myers Squibb, Lundbeck, and Takeda; has had a research contract with Assurex; and has equity in Mensante.
AVR has received speaker and consultant honoraria or research funds from Bristol-Myers Squibb, Canadian Depression Research and Intervention Network, Canadian Foundation for Innovation and the Ministry of Economic Development and Innovation, Canadian Institutes of Health Research, Grand Challenges Canada, Janssen, Lundbeck, Ontario Mental Health Foundation, Pfizer, and Sunovion.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Kennedy SH, Lam RW, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. Introduction. J Affect Disord. 2009;11(Suppl 1):S1–S2. [DOI] [PubMed] [Google Scholar]
- 2. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord. 2013;15:1–44. [DOI] [PubMed] [Google Scholar]
- 3. Lam RW, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: introduction and methods. Can J Psychiatry. 2016;61(9):506–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Psychiatric Association (APA). Diagnostic and statistical manual of mental disorders. 5th ed Arlington (VA; ): American Psychiatric Association; 2013. [Google Scholar]
- 5. World Health Organization. The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. Geneva (Switzerland): World Health Organization; 1992. [Google Scholar]
- 6. American Psychiatric Association (APA). Diagnostic and statistical manual of mental disorders. 4th ed, text revision. Washington (DC): American Psychiatric Association; 2000. [Google Scholar]
- 7. Regier DA, Narrow WE, Clarke DE, et al. DSM-5 field trials in the United States and Canada, part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170:59–70. [DOI] [PubMed] [Google Scholar]
- 8. Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171:395–397. [DOI] [PubMed] [Google Scholar]
- 9. Seo HJ, Jung YE, Kim TS, et al. Distinctive clinical characteristics and suicidal tendencies of patients with anxious depression. J Nerv Ment Dis. 2011;199:42–48. [DOI] [PubMed] [Google Scholar]
- 10. Perugi G, Angst J, Azorin JM, et al. Mixed features in patients with a major depressive episode: the BRIDGE-II-MIX study. J Clin Psychiatry. 2015;76:e351–e358. [DOI] [PubMed] [Google Scholar]
- 11. McIntyre RS, Soczynska JK, Cha DS, et al. The prevalence and illness characteristics of DSM-5–defined “mixed feature specifier” in adults with major depressive disorder and bipolar disorder: results from the International Mood Disorders Collaborative Project. J Affect Disord. 2014;172C:259–264. [DOI] [PubMed] [Google Scholar]
- 12. Azorin JM, Kaladjian A, Adida M, et al. Self-assessment and characteristics of mixed depression in the French national EPIDEP study. J Affect Disord. 2012;143:109–117. [DOI] [PubMed] [Google Scholar]
- 13. Goldberg JF. Mixed depression: a farewell to differential diagnosis? J Clin Psychiatry. 2015;76:e378–e380. [DOI] [PubMed] [Google Scholar]
- 14. Lee RS, Hermens DF, Porter MA, et al. A meta-analysis of cognitive deficits in first-episode major depressive disorder. J Affect Disord. 2012;140:113–124. [DOI] [PubMed] [Google Scholar]
- 15. Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. 2013;139:81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McIntyre RS, Cha DS, Soczynska JK, et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety. 2013;30:515–527. [DOI] [PubMed] [Google Scholar]
- 17. Papakostas GI. Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. J Clin Psychiatry. 2014;75:8–14. [DOI] [PubMed] [Google Scholar]
- 18. Evans VC, Iverson GL, Yatham LN, et al. The relationship between neurocognitive and psychosocial functioning in major depressive disorder: a systematic review. J Clin Psychiatry. 2014;(12):1359–1370. [DOI] [PubMed] [Google Scholar]
- 19. McIntyre RS, Soczynska JZ, Woldeyohannes HO, et al. The impact of cognitive impairment on perceived workforce performance: results from the International Mood Disorders Collaborative Project. Compr Psychiatry. 2015;56:279–282. [DOI] [PubMed] [Google Scholar]
- 20. Bora E, Harrison BJ, Yücel M, et al. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med. 2013;43:2017–2026. [DOI] [PubMed] [Google Scholar]
- 21. Rock PL, Roiser JP, Riedel WJ, et al. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44:2029–2040. [DOI] [PubMed] [Google Scholar]
- 22. Murphy MJ, Peterson MJ. Sleep disturbances in depression. Sleep Med Clin. 2015;10:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brnabic A, Lin C, Monkul ES, et al. Major depressive disorder severity and the frequency of painful physical symptoms: a pooled analysis of observational studies. Curr Med Res Opin. 2012;28:1891–1897. [DOI] [PubMed] [Google Scholar]
- 24. Fava M, Ball S, Nelson JC, et al. Clinical relevance of fatigue as a residual symptom in major depressive disorder. Depress Anxiety. 2014;31:250–257. [DOI] [PubMed] [Google Scholar]
- 25. Novick D, Montgomery W, Aguado J, et al. Which somatic symptoms are associated with an unfavorable course in Asian patients with major depressive disorder? J Affect Disord. 2013;149:182–188. [DOI] [PubMed] [Google Scholar]
- 26. Patten SB, Williams J, Lavorato DH, et al. Descriptive epidemiology of major depressive disorder in Canada in 2012. Can J Psychiatry. 2015;60:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang J, Williams J, Lavorato D, et al. The incidence of major depression in Canada: the National Population Health Survey. J Affect Disord. 2010;123:158–163. [DOI] [PubMed] [Google Scholar]
- 28. de Graaf R, ten Have M, Tuithof M, et al. First-incidence of DSM-IV mood, anxiety and substance use disorders and its determinants: results from the Netherlands Mental Health Survey and Incidence Study-2. J Affect Disord. 2013;149:100–107. [DOI] [PubMed] [Google Scholar]
- 29. Chou KL, Mackenzie CS, Liang K, et al. Three-year incidence and predictors of first-onset of DSM-IV mood, anxiety, and substance use disorders in older adults: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2011;72:144–155. [DOI] [PubMed] [Google Scholar]
- 30. Mojtabai R, Jorm AF. Trends in psychological distress, depressive episodes and mental health treatment-seeking in the United States: 2001-2012. J Affect Disord. 2015;174:556–561. [DOI] [PubMed] [Google Scholar]
- 31. Jorm AF. Why hasn’t the mental health of Australians improved? The need for a national prevention strategy. Aust N Z J Psychiatry. 2014;48:795–801. [DOI] [PubMed] [Google Scholar]
- 32. Rubio JM, Markowitz JC, Alegria A, et al. Epidemiology of chronic and nonchronic major depressive disorder: results from the national epidemiologic survey on alcohol and related conditions. Depress Anxiety. 2011;28:622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Skodol AE, Grilo CM, Keyes KM, et al. Relationship of personality disorders to the course of major depressive disorder in a nationally representative sample. Am J Psychiatry. 2011;168:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50:1609–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hardeveld F, Spijker J, de Graaf R, et al. Recurrence of major depressive disorder across different treatment settings: results from the NESDA study. J Affect Disord. 2013;147:225–231. [DOI] [PubMed] [Google Scholar]
- 36. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ratnasingham S, Cairney J, Manson H, et al. The burden of mental illness and addiction in Ontario. Can J Psychiatry. 2013;58:529–537. [DOI] [PubMed] [Google Scholar]
- 38. IsHak WW, Balayan K, Bresee C, et al. A descriptive analysis of quality of life using patient-reported measures in major depressive disorder in a naturalistic outpatient setting. Qual Life Res. 2013;22:585–596. [DOI] [PubMed] [Google Scholar]
- 39. Lim KL, Jacobs P, Ohinmaa A, et al. A new population-based measure of the economic burden of mental illness in Canada. Chronic Dis Can. 2008;28:92–98. [PubMed] [Google Scholar]
- 40. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76:155–162. [DOI] [PubMed] [Google Scholar]
- 41. Alonso J, Petukhova M, Vilagut G, et al. Days out of role due to common physical and mental conditions: results from the WHO World Mental Health surveys. Mol Psychiatry. 2011;16:1234–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patten SB, Wang JL, Williams JV, et al. Prospective evaluation of the effect of major depression on working status in a population sample. Can J Psychiatry. 2009;54:841–845. [DOI] [PubMed] [Google Scholar]
- 43. Bouwmans CA, Vemer P, van Straten A, et al. Health-related quality of life and productivity losses in patients with depression and anxiety disorders. J Occup Environ Med. 2014;56:420–424. [DOI] [PubMed] [Google Scholar]
- 44. Rizvi SJ, Cyriac A, Grima E, et al. Depression and employment status in primary and tertiary care settings. Can J Psychiatry. 2015;60:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hendriks SM, Spijker J, Licht CM, et al. Long-term work disability and absenteeism in anxiety and depressive disorders. J Affect Disord. 2015;178:121–130. [DOI] [PubMed] [Google Scholar]
- 46. Fried EI, Nesse RM. The impact of individual depressive symptoms on impairment of psychosocial functioning. PLoS One. 2014;9:e90311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Beck A, Crain LA, Solberg LI, et al. The effect of depression treatment on work productivity. Am J Manag Care. 2014;20:e294–e301. [PMC free article] [PubMed] [Google Scholar]
- 48. Dewa CS, Thompson AH, Jacobs P. The association of treatment of depressive episodes and work productivity. Can J Psychiatry. 2011;56:743–750. [DOI] [PubMed] [Google Scholar]
- 49. Kendler KS, Gardner CO. Sex differences in the pathways to major depression: a study of opposite-sex twin pairs. Am J Psychiatry. 2014;171:426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Druss BG, Hwang I, Petukhova M, et al. Impairment in role functioning in mental and chronic medical disorders in the United States: results from the National Comorbidity Survey Replication. Mol Psychiatry. 2009;14:728–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stein A, Pearson RM, Goodman SH, et al. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384:1800–1819. [DOI] [PubMed] [Google Scholar]
- 52. Kvalevaag AL, Ramchandani PG, Hove O, et al. Paternal mental health and socioemotional and behavioral development in their children. Pediatrics. 2013;131:e463–e469. [DOI] [PubMed] [Google Scholar]
- 53. Gutierrez-Galve L, Stein A, Hanington L, et al. Paternal depression in the postnatal period and child development: mediators and moderators. Pediatrics. 2015;135:e339–e347. [DOI] [PubMed] [Google Scholar]
- 54. Coiro MJ, Riley A, Broitman M, et al. Effects on children of treating their mothers’ depression: results of a 12-month follow-up. Psychiatr Serv. 2012;63:357–363. [DOI] [PubMed] [Google Scholar]
- 55. Weissman MM, Wickramaratne P, Pilowsky DJ, et al. The effects on children of depressed mothers’ remission and relapse over 9 months. Psychol Med. 2014;44:2811–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wickramaratne P, Gameroff JM, Pilowsky DJ, et al. Children of depressed mothers 1 year after remission of maternal depression: findings from the STAR*D-Child study. Am J Psychiatry. 2011;168:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Patten SB, Williams JV, Lavorato DH, et al. Major depression as a risk factor for chronic disease incidence: longitudinal analyses in a general population cohort. Gen Hosp Psychiatry. 2008;30:407–413. [DOI] [PubMed] [Google Scholar]
- 58. Charlson FJ, Moran AE, Freedman G, et al. The contribution of major depression to the global burden of ischemic heart disease: a comparative risk assessment. BMC Med. 2013;11:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Seligman F, Nemeroff CB. The interface of depression and cardiovascular disease: therapeutic implications. Ann N Y Acad Sci. 2015;1345:25–35. [DOI] [PubMed] [Google Scholar]
- 60. Valkanova V, Ebmeier KP. Vascular risk factors and depression in later life: a systematic review and meta-analysis. Biol Psychiatry. 2013;73:406–413. [DOI] [PubMed] [Google Scholar]
- 61. Deschênes SS, Burns RJ, Schmitz N. Associations between depression, chronic physical health conditions, and disability in a community sample: a focus on the persistence of depression. J Affect Disord. 2015;179:6–13. [DOI] [PubMed] [Google Scholar]
- 62. Burgel PR, Escamilla R, Perez T, et al. Impact of comorbidities on COPD-specific health-related quality of life. Respir Med. 2013;107:233–241. [DOI] [PubMed] [Google Scholar]
- 63. Faller H, Brähler E, Härter M, et al. Performance status and depressive symptoms as predictors of quality of life in cancer patients: a structural equation modeling analysis. Psychooncology. 2015;24:1456–1462. [DOI] [PubMed] [Google Scholar]
- 64. Schowalter M, Gelbrich G, Störk S, et al. Generic and disease-specific health-related quality of life in patients with chronic systolic heart failure: impact of depression. Clin Res Cardiol. 2013;102:269–278. [DOI] [PubMed] [Google Scholar]
- 65. Beer L, Skarbinski J. Adherence to antiretroviral therapy among HIV-infected adults in the United States. AIDS Educ Prev. 2014;26:521–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Krass I, Schieback P, Dhippayom T. Adherence to diabetes medication: a systematic review. Diabetes Med. 2015;32:725–737. [DOI] [PubMed] [Google Scholar]
- 67. Price MA, Butow PN, Charles M, et al. Predictors of breast cancer screening behavior in women with a strong family history of the disease. Breast Cancer Res Treat. 2010;124:509–519. [DOI] [PubMed] [Google Scholar]
- 68. Susin N, Boff RM, Ludwig MW, et al. Predictors of adherence in a prevention program for patients with metabolic syndrome. J Health Psychol. 2015. Mar 23. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 69. Brunet J, Sabiston CM, O’Loughlin E, et al. Symptoms of depression are longitudinally associated with sedentary behaviors among young men but not among young women. Prev Med. 2014;60:16–20. [DOI] [PubMed] [Google Scholar]
- 70. Mansur RB, Brietzke E, McIntyre RS. Is there a “metabolic-mood syndrome”? A review of the relationship between obesity and mood disorders. Neurosci Biobehav Rev. 2015;52:89–104. [DOI] [PubMed] [Google Scholar]
- 71. Zvolensky MJ, Bakhshaie J, Sheffer C, et al. Major depressive disorder and smoking relapse among adults in the United States: a 10-year, prospective investigation. Psychiatry Res. 2015;226(1):73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hryhorczuk C, Sharma S, Fulton SE. Metabolic disturbances connecting obesity and depression. Front Neurosci. 2013;7:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Abelaira HM, Réus GZ, Petronilho F, et al. Neuroimmunomodulation in depression: a review of inflammatory cytokines involved in this process. Neurochem Res. 2014;39:1634–1639. [DOI] [PubMed] [Google Scholar]
- 74. Bakunina N, Pariante CM, Zunszain PA. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology. 2015. Jan 10. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Young JJ, Bruno D, Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J Affect Disord. 2014;169:15–20. [DOI] [PubMed] [Google Scholar]
- 76. Siu AL; US Preventive Services Task Force (USPSTF). Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:380–387. [DOI] [PubMed] [Google Scholar]
- 77. National Institute for Health and Care Excellence. Depression in adults: recognition and management. Clinical guideline [Internet] [cited 2016 Mar 8]. Available from: https://www.nice.org.uk/guidance/cg90
- 78. Canadian Task Force on Preventive Health Care, Joffres M, Jaramillo A, et al. Recommendations on screening for depression in adults [published correction appears in CMAJ. 2013;185:1067]. CMAJ. 2013;185:775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Keshavarz H, Fitzpatrick-Lewis D, Streiner DL, et al. Screening for depression: a systematic review and meta-analysis. CMAJ Open. 2013;1:E159–E167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. O’Connor E, Rossom RC, Henninger M, et al. Screening for depression in adults: an updated systematic evidence review for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality; 2016. Report No.: 14-05208-EF-1. Available from: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0085171/ [PubMed] [Google Scholar]
- 81. Bosanquet K, Bailey D, Gilbody S, et al. Diagnostic accuracy of the Whooley questions for the identification of depression: a diagnostic meta-analysis. BMJ Open. 2015;5:e008913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. van Straten A, Hill J, Richards DA, et al. Stepped care treatment delivery for depression: a systematic review and meta-analysis. Psychol Med. 2015;45:231–246. [DOI] [PubMed] [Google Scholar]
- 83. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tursi MF, Baes Cv, Camacho FR, et al. Effectiveness of psychoeducation for depression: a systematic review. Aust N Z J Psychiatry. 2013;47:1019–1031. [DOI] [PubMed] [Google Scholar]
- 85. Pompili M, Venturini P, Palermo M, et al. Mood disorders medications: predictors of nonadherence—review of the current literature. Expert Rev Neurother. 2013;13:809–825. [DOI] [PubMed] [Google Scholar]
- 86. Houle J, Gascon-Depatie M, Bélanger-Dumontier G, et al. Depression self-management support: a systematic review. Patient Educ Couns. 2013;91:271–279. [DOI] [PubMed] [Google Scholar]
- 87. Lloyd-Evans B, Mayo-Wilson E, Harrison B, et al. A systematic review and meta-analysis of randomised controlled trials of peer support for people with severe mental illness. BMC Psychiatry. 2014;14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Borges G, Angst J, Nock MK, et al. A risk index for 12-month suicide attempts in the National Comorbidity Survey Replication (NCS-R). Psychol Med. 2006;36:1747–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bolton JM, Belik SL, Cox BJ, et al. Exploring the correlates of suicide attempts among individuals with major depressive disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69:1139–1149. [DOI] [PubMed] [Google Scholar]
- 90. Sareen J, Isaak C, Katz LY, et al. Promising strategies for advancement in knowledge of suicide risk factors and prevention. Am J Prev Med. 2014;47(3, Suppl 2):S257–S263. [DOI] [PubMed] [Google Scholar]
- 91. Warden S, Spiwak R, Sareen J, et al. The SAD PERSONS scale for suicide risk assessment: a systematic review. Arch Suicide Res. 2014;18:313–326. [DOI] [PubMed] [Google Scholar]
- 92. Bolton JM, Spiwak R, Sareen J. Predicting suicide attempts with the SAD PERSONS scale: a longitudinal analysis. J Clin Psychiatry. 2012;73:e735–e741. [DOI] [PubMed] [Google Scholar]
- 93. Giddens JM, Sheehan KH, Sheehan DV. The Columbia-Suicide Severity Rating Scale (C-SSRS): has the “gold standard” become a liability? Innov Clin Neurosci. 2014;11:66–80. [PMC free article] [PubMed] [Google Scholar]
- 94. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shea SC. The chronological assessment of suicide events: a practical interviewing strategy for the elicitation of suicidal ideation. J Clin Psychiatry. 1998;59(Suppl 20):58–72. [PubMed] [Google Scholar]
- 96. Yeung AS, Jing Y, Brenneman SK, et al. Clinical Outcomes in Measurement-based Treatment (Comet): a trial of depression monitoring and feedback to primary care physicians. Depress Anxiety. 2012;29:865–873. [DOI] [PubMed] [Google Scholar]
- 97. Guo T, Xiang YT, Xiao L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172:1004–1013. [DOI] [PubMed] [Google Scholar]
- 98. Sato S, Yeh TL. Challenges in treating patients with major depressive disorder: the impact of biological and social factors. CNS Drugs. 2013;27(Suppl 1):S5–S10. [DOI] [PubMed] [Google Scholar]
- 99. IsHak WW, Greenberg JM, Balayan K, et al. Quality of life: the ultimate outcome measure of interventions in major depressive disorder. Harv Rev Psychiatry. 2011;19:229–239. [DOI] [PubMed] [Google Scholar]
- 100. Lam RW, Parikh SV, Michalak EE, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) consensus recommendations for functional outcomes in major depressive disorder. Ann Clin Psychiatry. 2015;27:142–149. [PubMed] [Google Scholar]
- 101. Shelton RC, Trivedi MH. Challenges and algorithm-guided treatment in major depressive disorder. J Clin Psychiatry. 2011;72:e14. [DOI] [PubMed] [Google Scholar]
- 102. Patten SB, Kennedy SH, Lam RW, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults: I. Classification, burden and principles of management. J Affect Disord. 2009;117(Suppl 1):S5–S14. [DOI] [PubMed] [Google Scholar]
- 103. Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder: remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48:851–855. [DOI] [PubMed] [Google Scholar]
- 104. Bockting CL, Hollon SD, Jarrett RB, et al. A lifetime approach to major depressive disorder: the contributions of psychological interventions in preventing relapse and recurrence. Clin Psychol Rev. 2015;41:16–26. [DOI] [PubMed] [Google Scholar]
- 105. Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. van der Voort TY, Seldenrijk A, van Meijel B, et al. Functional versus syndromal recovery in patients with major depressive disorder and bipolar disorder. J Clin Psychiatry. 2015;76:e809–e814. [DOI] [PubMed] [Google Scholar]
- 107. Sim K, Lau WK, Sim J, et al. Prevention of relapse and recurrence in adults with major depressive disorder: systematic review and meta-analyses of controlled trials. Int J Neuropsychopharmacol. 2015;19pii:pyv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hardeveld F, Spijker J, de Graaf R, et al. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2010;122:184–191. [DOI] [PubMed] [Google Scholar]
- 109. Wang JL, Patten S, Sareen J, et al. Development and validation of a prediction algorithm for use by health professionals in prediction of recurrence of major depression. Depress Anxiety. 2014;31:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Canadian Internet Registration Authority. Factbook 2013 [Internet] [cited 2016 March 8]. Available from: https://cira.ca/factbook/2013/.9
- 111. Lal S, Adair CE. E-mental health: a rapid review of the literature. Psychiatr Serv. 2014;65:24–32. [DOI] [PubMed] [Google Scholar]
- 112. Karasouli E, Adams A. Assessing the evidence for e-resources for mental health self-management: a systematic literature review. JMIR Ment Health. 2014;1:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24:210–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Liao SC, Chen WJ, Lee MB, et al. Low prevalence of major depressive disorder in Taiwanese adults: possible explanations and implications. Psychol Med. 2012;42:1227–1237. [DOI] [PubMed] [Google Scholar]