Abstract
Background:
The Canadian Network for Mood and Anxiety Treatments (CANMAT) conducted a revision of the 2009 guidelines by updating the evidence and recommendations. The scope of the 2016 guidelines remains the management of major depressive disorder (MDD) in adults, with a target audience of psychiatrists and other mental health professionals.
Methods:
Using the question-answer format, we conducted a systematic literature search focusing on systematic reviews and meta-analyses. Evidence was graded using CANMAT-defined criteria for level of evidence. Recommendations for lines of treatment were based on the quality of evidence and clinical expert consensus. “Pharmacological Treatments” is the third of six sections of the 2016 guidelines. With little new information on older medications, treatment recommendations focus on second-generation antidepressants.
Results:
Evidence-informed responses are given for 21 questions under 4 broad categories: 1) principles of pharmacological management, including individualized assessment of patient and medication factors for antidepressant selection, regular and frequent monitoring, and assessing clinical and functional outcomes with measurement-based care; 2) comparative aspects of antidepressant medications based on efficacy, tolerability, and safety, including summaries of newly approved drugs since 2009; 3) practical approaches to pharmacological management, including drug-drug interactions and maintenance recommendations; and 4) managing inadequate response and treatment resistance, with a focus on switching antidepressants, applying adjunctive treatments, and new and emerging agents.
Conclusions:
Evidence-based pharmacological treatments are available for first-line treatment of MDD and for management of inadequate response. However, given the limitations of the evidence base, pharmacological management of MDD still depends on tailoring treatments to the patient.
Keywords: major depressive disorder, pharmacotherapy, clinical practice guidelines, antidepressants, evidence-based medicine, meta-analysis, antipsychotics, clinical trials, randomized controlled trial
In 2009, the Canadian Network for Mood and Anxiety Treatments (CANMAT), a not-for-profit scientific and educational organization, published a revision of evidence-based clinical guidelines for the treatment of depressive disorders.1 CANMAT has updated these guidelines in 2016 to reflect new evidence in the field.
The scope of these guidelines remains the management of adults with unipolar major depressive disorder (MDD) with a target audience of psychiatrists and other mental health professionals. CANMAT, in collaboration with the International Society for Bipolar Disorders, has published separate guidelines for bipolar disorder.2 This section on “Pharmacological Treatments” is 1 of 6 CANMAT guidelines articles; other sections of the guidelines expand on burden and principles of care, psychological treatments, neurostimulation treatments, complementary and alternative medicine treatments, and special populations. These recommendations are presented as guidance for clinicians who should consider them in the context of individual patients and not as standards of care. Some medications discussed may not be available in Canada or other countries.
Methods
The full methods have been previously described,3 but in summary, relevant studies in English and French published from January 1, 2009, to December 31, 2015, were identified using computerized searches of electronic databases (PubMed, PsychInfo, Cochrane Register of Clinical Trials), inspection of bibliographies, and review of other guidelines and major reports. Each recommendation includes the level of evidence for each graded line of treatment, using specified criteria (Table 1). The level of evidence criteria now reflect the primacy of meta-analysis because of its increasing use in the evaluation of evidence.
Table 1.
Criteria for Level of Evidence and Line of Treatment.
| Criteria | |
|---|---|
| Level of evidencea | |
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| Line of treatment | |
| First line |
|
| Second line |
|
| Third line |
|
RCT, randomized controlled trial.
aNote that Level 1 and 2 Evidence refer specifically to treatment studies in which randomized comparisons are available. Recommendations involving epidemiological or risk factors primarily arise from observational studies, and hence the highest level of evidence is usually Level 3. Higher order recommendations (e.g., principles of care) reflect higher level judgement of the strength of evidence from various data sources and therefore are primarily Level 4 Evidence.
bClinical support refers to application of expert opinion of the CANMAT committees to ensure that evidence-supported interventions are feasible and relevant to clinical practice. Therefore, treatments with higher levels of evidence may be downgraded to lower lines of treatment due to clinical issues such as side effects or safety profile.
Because of the very large number of randomized-controlled trials (RCTs), this section will primarily focus on systematic reviews and individual and network meta-analyses. Although meta-analyses have advantages in summarizing data, they still have limitations that can lead to erroneous or conflicting results depending on the comprehensiveness of the review, criteria for study selection and quality, and generalizability of the included studies.4 We also focus on second-generation antidepressants because there is little new information on the older tricyclic antidepressants (TCAs) and monoamine oxidase (MAO) inhibitors.
3.1. Who Should be Treated with Pharmacotherapy?
Despite earlier reports questioning the efficacy of antidepressants,5 subsequent meta-analyses have continued to support the efficacy of antidepressants in MDD.6 The 2009 CANMAT guidelines identified most second-generation antidepressants as first-line treatments for patients with a major depressive episode (MDE) of moderate or greater severity (as determined by symptom scales and/or functional impairment), and this recommendation is unchanged. First-line treatments for individuals with depression of mild severity include psychoeducation, self-management, and psychological treatments. Pharmacological treatments can be considered for mild depression in some situations, including patient preference, previous response to antidepressants, or lack of response to nonpharmacological interventions.
3.2. Which Antidepressants Are Newly Approved?
Several new antidepressants have been approved in Canada, the United States, and elsewhere since the publication of the 2009 CANMAT guidelines.
Levomilnacipran is an active enantiomer of the racemic drug, milnacipran, a serotonin and noradrenaline reuptake inhibitor (SNRI). Levomilnacipran has greater selectivity for noradrenaline than for serotonin reuptake inhibition compared to other SNRIs. It is available as an extended-release formulation for once-daily administration. There are no published meta-analyses for levomilnacipran, but a pooled analysis of 5 placebo-controlled RCTs (N = 2598) confirmed its efficacy for response and remission.7 One relapse-prevention study did not show significant differences between levomilnacipran and placebo.8 There are no comparison studies of levomilnacipran with other antidepressants.
Vilazodone is a multimodal antidepressant that acts as a serotonin reuptake inhibitor and a partial agonist at 5-HT1A receptors. Published meta-analyses are lacking, but 4 published and 8 unpublished or recently completed RCTs were identified.9–11 A review of the clinical basis for approval has also been published.12 Although 5 early-phase vilazodone trials failed to show efficacy, 4 subsequent studies (phases III and IV) reported efficacy for vilazodone 20 mg and 40 mg over placebo. There are no published relapse-prevention data for vilazodone or comparison studies with other antidepressants. Vilazodone must be taken with food to ensure adequate absorption and a titration dose schedule (10 mg/d for 7 days, 20 mg/d for 7 days, then 40 mg/d if needed) is recommended to avoid adverse gastrointestinal effects.9
Vortioxetine, another multimodal antidepressant, acts as a serotonin reuptake inhibitor, an agonist at 5-HT1A receptors, a partial agonist at 5-HT1B receptors, and an antagonist at 5-HT1D, 5-HT3A, and 5-HT7 receptors. In 1 meta-analysis (12 RCTs, N = 4947), vortioxetine was superior to placebo in standardized mean difference and in odds ratios for response and remission.13 Vortioxetine also has positive effects on neuropsychological performance in multiple cognitive domains in patients with MDD.14–17 A relapse-prevention study showed superiority of vortioxetine over placebo.18 Comparator studies are published for vortioxetine and agomelatine, duloxetine, and venlafaxine.
3.3. How Do You Select an Antidepressant?
General principles of depression management are reviewed in Section 1.3 Table 2 summarizes principles as they apply to pharmacological treatment. The process of selecting an antidepressant should involve both physician expertise and patient perceptions and preferences.
Table 2.
Principles of Pharmacotherapy Management.
| Recommendations (Level 4 Evidence) |
|---|
|
The selective serotonin reuptake inhibitors (SSRIs), SNRIs, agomelatine, bupropion, and mirtazapine remain first-line recommendations for pharmacotherapy for MDD (Table 3). Vortioxetine is also a first-line recommendation. Recommended second-line agents include TCAs, quetiapine and trazodone (owing to higher side effect burden), moclobemide and selegiline (potential serious drug interactions), levomilnacipran (lack of comparative and relapse-prevention data), and vilazodone (lack of comparative and relapse-prevention data and the need to titrate and take with food). Third-line recommendations include MAO inhibitors (owing to higher side effect burden and potential serious drug and dietary interactions) and reboxetine (lower efficacy).
Table 3.
Summary Recommendations for Antidepressants.
| Antidepressant (Brand Name(s)) | Mechanism | Dose Range |
|---|---|---|
| First line (Level 1 Evidence) | ||
|
MT1 and MT2 agonist; 5-HT2 antagonist | 25-50 mg |
|
NDRI | 150-300 mg |
|
SSRI | 20-40 mg |
|
SNRI | 50-100 mg |
|
SNRI | 60 mg |
|
SSRI | 10-20 mg |
|
SSRI | 20-60 mg |
|
SSRI | 100-300 mg |
|
α2-Adrenergic agonist; 5-HT2 antagonist | 60-120 mg |
|
SNRI | 100 mg |
|
α2-Adrenergic agonist; 5-HT2 antagonist | 15-45 mg |
|
SSRI | 20-50 mg 25-62.5 mg for CR version |
|
SSRI | 50-200 mg |
|
SNRI | 75-225 mg |
|
Serotonin reuptake inhibitor; 5-HT1A agonist; 5-HT1B partial agonist; 5-HT1D, 5-HT3A, and 5-HT7 antagonist | 10-20 mg |
| Second line (Level 1 Evidence) | ||
|
TCA | Various |
|
SNRI | 40-120 mg |
|
Reversible inhibitor of MAO-A | 300-600 mg |
|
Atypical antipsychotic | 150-300 mg |
|
Irreversible MAO-B inhibitor | 6-12 mg daily transdermal |
|
Serotonin reuptake inhibitor; 5-HT2 antagonist | 150-300 mg |
|
Serotonin reuptake inhibitor; 5-HT1A partial agonist | 20-40 mg (titrate from 10 mg) |
| Third line (Level 1 Evidence) | ||
|
Irreversible MAO inhibitor | 45-90 mg 20-60 mg |
|
Noradrenaline reuptake inhibitor | 8-10 mg |
5-HT, 5-hydroxytryptamine (serotonin); MAO, monoamine oxidase; MT, melatonin; NDRI, noradrenaline and dopamine reuptake inhibitor; SNRI, serotonin and noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
aNot available in Canada.
bAvailable as sustained-release (SR) and extended-release (XL) versions.
cAvailable as rapid-dissolving (RD) version.
dAvailable as controlled-release (CR) version
eAvailable as extended-release (XR) version.
fNewly approved since the 2009 Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines.
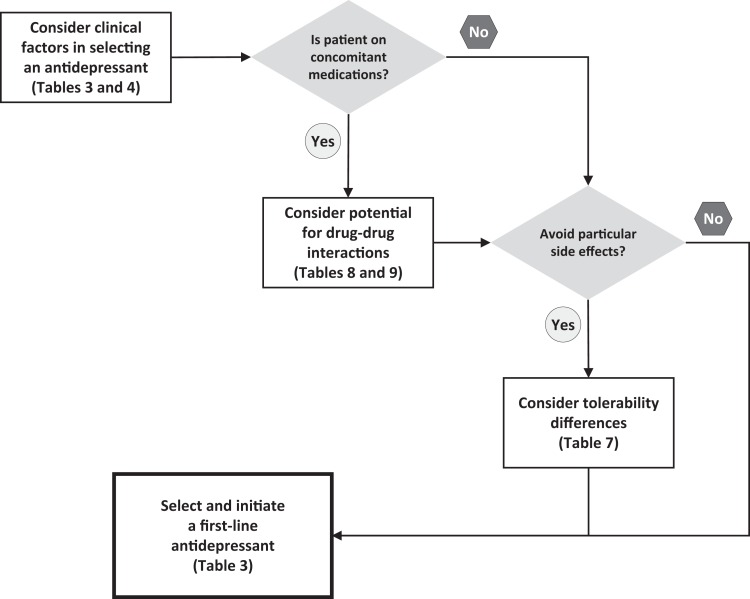
Many clinical features and medication characteristics influence the choice of a first-line antidepressant (Table 4). There are no absolutes, and relative differences between medications are small. Hence, selecting an antidepressant involves an individualized needs assessment for each patient. Figure 1 shows a summary algorithm. The questions that follow summarize the evidence for selection factors.
Table 4.
Factors to Consider in Selecting an Antidepressant.
| Patient Factors | Medication Factors |
|---|---|
|
|
Figure 1.
Summary algorithm for selecting an antidepressant.
3.4. What Clinical Factors Influence Antidepressant Selection?
Several clinical features, including increasing age, presence of anxiety, and long episode duration are associated with poorer response to medications.19–22 However, few clinical features have high-quality evidence to support specific antidepressant recommendations. For example, there is no consistent evidence that age, sex, race, or ethnicity predicts outcomes using specific antidepressants.
The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)23 uses episode and course specifiers to subtype clinical presentations of MDD. Other clinical dimensions, including cognitive dysfunction, sleep disturbance, and somatic symptoms (e.g., pain, fatigue), are proposed.3 Many antidepressants have been studied for these depressive subtypes, but most studies only examine efficacy against placebo, and there are few comparative studies to suggest differential antidepressant efficacy. Table 5 summarizes the recommendations for these specifiers/dimensions.
Table 5.
Recommendations for Clinical Specifiers and Dimensions of Major Depressive Disorder.
| Specifiers/Dimensions | Recommendations (Level of Evidence) | Comments |
|---|---|---|
| With anxious distressa |
|
|
| With catatonic featuresa |
|
|
| With melancholic featuresa |
|
|
| With atypical featuresa |
|
|
| With psychotic featuresa |
|
|
| With mixed featuresa |
|
|
| With seasonal patterna |
|
|
| With cognitive dysfunction |
|
|
| With sleep disturbances |
|
|
| With somatic symptoms |
|
|
MAO, monoamine oxidase; SNRI, serotonin and noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
a DSM-5 specifiers.
bComparisons only with placebo.
Large trials examining response with DSM-IV specifiers (melancholic, atypical, anxious) found no differences in efficacy between escitalopram, sertraline, and venlafaxine XR or between escitalopram and nortriptyline.24,25 The US STAR*D study also did not find differences in remission rates with citalopram in atypical or melancholic subtypes.26,27
For psychotic depression, a Cochrane meta-analysis (12 studies, N = 929) found that an antidepressant-antipsychotic combination was more effective than placebo (2 RCTs), antidepressant monotherapy (3 RCTs), and antipsychotic monotherapy (4 RCTs).28 There is no evidence to address the question of how long individuals should remain on combination treatment once the psychotic depressive episode has remitted.
Mixed features is a new DSM-5 specifier for MDD, and no trials have used these DSM-5 criteria. In studies of MDE with variants of mixed symptoms similar to DSM-5 mixed features, monotherapy with lurasidone and with ziprasidone was efficacious compared with placebo.29,30
For cognitive dysfunction, a systematic review (35 studies) found low-quality evidence that SSRIs, bupropion, duloxetine, moclobemide, and tianeptine (an antidepressant with limited availability) improve cognitive domains such as learning, memory, and executive function.31 In a meta-analysis (17 studies, N = 3653) reviewing the cognitive effects of antidepressants based on neuropsychological tests, vortioxetine had the largest effects on processing speed, executive control, and cognitive control, while duloxetine had the largest effects on delayed recall.17 The quality of these data is limited by small samples sizes and heterogeneity in cognitive testing. There were few differences between individual or classes of antidepressants, but those comparisons were also limited by small sample sizes.
Some antidepressants, including agomelatine, mirtazapine, and trazodone, and the atypical antipsychotic, quetiapine, have shown superior effects on subjective or objective sleep measures. However, mirtazapine, quetiapine, and trazodone also have the highest adverse event rates of somnolence and daytime sedation.32
There are few comparative studies of antidepressants for somatic symptoms such as pain and fatigue.33 SNRIs, especially duloxetine,34 are efficacious for painful conditions, including neuropathic pain and fibromyalgia.35 There are no comparative studies on fatigue or low energy.
3.5. How Do Psychiatric and Medical Comorbidities Influence Antidepressant Selection?
There is limited evidence to guide antidepressant choice in the management of MDD with comorbid conditions. A comprehensive review was conducted by a CANMAT task force in 2012.36 Readers are referred to their summary recommendations for mood disorders and comorbid anxiety,37 attention-deficit/hyperactivity disorder,38 substance use disorders,39 personality disorders,40 metabolic conditions, and common medical conditions.41-43
3.6. How Do Second-Generation Antidepressants Compare in Efficacy?
The 2009 CANMAT guidelines identified that, based on evidence from RCTs and early meta-analyses, some antidepressants had superior efficacy, although differences were small. Since then, meta-analyses with individual comparisons (see Suppl. Table S1) have reported superiority of agomelatine (over sertraline), citalopram (over paroxetine and reboxetine), escitalopram (over citalopram), fluoxetine (over milnacipran), mirtazapine (over SSRIs as a class and venlafaxine), paroxetine (over fluoxetine), and sertraline (over fluoxetine). Unfortunately, many drug comparisons are not represented in these meta-analyses because of lack of head-to-head RCTs.
Network meta-analysis (also known as multiple or mixed-treatments meta-analysis) provides additional comparative information because it uses both direct (comparing 2 drugs head to head) and indirect (comparing 2 drugs based on their comparisons to a common third drug) comparisons.44 Several network meta-analyses have been conducted since 2009 (see Suppl. Table S2). Cipriani and colleagues45 examined 12 second-generation antidepressants in a network meta-analysis and found superior response for escitalopram, mirtazapine, sertraline, and venlafaxine. In direct head-to-head trials, Gartlehner et al.46 found superior response of escitalopram over citalopram, sertraline over fluoxetine, and venlafaxine over fluoxetine. In the indirect treatments analysis, there was superior response to escitalopram over duloxetine and escitalopram over fluoxetine. The differences in response rates were modest, ranging from 5% to 6%.46 A network meta-analysis of only head-to-head trials found that agomelatine, escitalopram, mirtazapine, and venlafaxine were superior to fluoxetine.47 Additionally, mirtazapine and venlafaxine were superior to duloxetine, paroxetine, and sertraline, and agomelatine was superior to sertraline. A multiple-treatments meta-analysis of 10 antidepressants, including only studies conducted in primary care settings, found that escitalopram had superior remission rates.48 In contrast, a network meta-analysis examining only classes of antidepressants in primary care found few differences in response, although SSRIs and TCAs were superior to mianserin/mirtazapine and moclobemide.49
In summary, meta-analyses continue to show that some antidepressants have modest superiority for treatment response, particularly escitalopram, mirtazapine, sertraline, and venlafaxine (Table 6). There is more limited evidence for the superiority of agomelatine and citalopram. Although considered small effects, 5% to 6% differences in response rate may be clinically relevant from a population basis.
Table 6.
Antidepressants with Evidence for Superior Efficacy Based on Meta-Analyses.
| Antidepressant | Level of Evidence | Comparator Medications |
|---|---|---|
| Escitalopram | Level 1 | Citalopram, duloxetine, fluoxetine, fluvoxamine, paroxetine |
| Mirtazapine | Level 1 | Duloxetine, fluoxetine, fluvoxamine, paroxetine, sertraline, venlafaxine |
| Sertraline | Level 1 | Duloxetine, fluoxetine, fluvoxamine, paroxetine |
| Venlafaxine | Level 1 | Duloxetine, fluoxetine, fluvoxamine, paroxetine |
| Agomelatine | Level 2 | Fluoxetine, sertraline |
| Citalopram | Level 2 | Paroxetine |
3.7. How Do Antidepressants Compare on Measures of Functional Outcomes?
CANMAT recommendations for assessment of functional outcomes highlighted the critical impact of depressive symptoms on social, occupational, and physical functioning and that recovery from depression involves both relief of symptoms and improvement of functioning.50 Systematic reviews show that functional outcomes are only modestly correlated with symptom outcomes, and functional improvement may lag behind symptom improvement.51 Few studies of antidepressants assess functional outcomes. A systematic review (247 studies) found that 80% of treatment studies reported only symptom outcomes.52 Another systematic review (35 studies) examined the relationships between antidepressants, cognitive dysfunction, and functional ability.31 Antidepressants were generally associated with improvement in cognitive domains, but there was no conclusive evidence that improved cognition led to improved overall functioning. In the absence of high-quality studies comparing the efficacy of individual antidepressants on functional outcomes in MDD, no medication can be cited as demonstrating superior functional improvement.
3.8. What Is the Comparative Tolerability of Second-Generation Antidepressants?
Comparing tolerability is challenging to assess by RCTs, and meta-analyses have found few differences in tolerability between antidepressants (see Suppl. Tables S1 and S2). CANMAT chose to illustrate differences in side effect profiles of antidepressants by using the summary information contained in product monographs, which is reported in a standard format from the evidence submitted to regulatory authorities. While this information is not placebo-adjusted and is not based on direct comparisons, it can show a qualitative profile of side effects for each antidepressant (Table 7).
Table 7.
Prevalence of Adverse Events among Newer Antidepressants: Unadjusted Frequency (%) of Common Adverse Events as Reported in Product Monographs.
| Nausea | Constipation | Diarrhea | Dry Mouth | Headaches | Dizziness | Somnolence | Nervousness | Anxiety | Agitation | Insomnia | Fatigue | Sweating | Asthenia | Tremor | Anorexia | Increased Appetite | Weight Gain | Male Sexual Dysfunction | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Citalopram | 21 | 8 | 19 | 3 | 3 | 2 | 5 | 11 | 8 | 4 | 9 | ||||||||
| Escitalopram | 15 | 4 | 8 | 7 | 3 | 6 | 4 | 2 | 2 | 8 | 5 | 3 | 2 | 2 | 2 | 10 | |||
| Fluoxetine | 21 | 10 | 13 | 14 | 12 | 16 | 8 | 9 | 10 | 11 | 2 | ||||||||
| Fluvoxamine | 37 | 18 | 6 | 26 | 22 | 15 | 26 | 2 | 2 | 16 | 14 | 11 | 5 | 11 | 15 | 1 | |||
| Paroxetine | 26 | 14 | 11 | 18 | 18 | 13 | 23 | 5 | 5 | 2 | 13 | 11 | 15 | 8 | 1 | 16 | |||
| Sertralinea | 26 | 8 | 18 | 16 | 20 | 12 | 13 | 3 | 3 | 6 | 16 | 11 | 8 | 11 | 3 | 1 | 16 | ||
| Desvenlafaxineb | 22 | 9 | 11 | 13 | 4 | <1 | 3 | 9 | 7 | 10 | 2 | 6 | |||||||
| Duloxetine | 20 | 11 | 8 | 15 | 8 | 7 | 3 | 11 | 8 | 6 | 3 | 10 | |||||||
| Levomilnacipran | 17 | 9 | 10 | 17 | 8 | 2 | 6 | 9 | 11 | ||||||||||
| Milnacipran | 12 | 7 | 9 | 10 | 4 | 7 | 3 | 4 | 3 | ||||||||||
| Venlafaxine IR | 37 | 15 | 8 | 22 | 25 | 19 | 23 | 13 | 6 | 2 | 18 | 12 | 12 | 5 | 11 | 18 | |||
| Venlafaxine XR | 31 | 8 | 8 | 12 | 26 | 20 | 17 | 10 | 2 | 3 | 17 | 14 | 8 | 5 | 8 | 16 | |||
| Agomelatinec | C | C | C | C | C | C | C | C | C | C | |||||||||
| Bupropion SRd | 11 | 7 | 4 | 13 | 28 | 7 | 3 | 5 | 5 | 2 | 8 | 2 | 2 | 3 | |||||
| Bupropion XL | 13 | 9 | 26 | 34 | 6 | 5 | 2 | 16 | 3 | ||||||||||
| Mirtazapine | 13 | 25 | 7 | 54 | 8 | 7 | 17 | 12 | |||||||||||
| Moclobemide | 5 | 4 | 2 | 9 | 8 | 5 | 4 | 4 | 3 | 5 | 7 | 3 | 2 | 1 | 5 | ||||
| Vilazodonee | 24 | 29 | 7 | 14 | 8 | 5 | 6 | 3 | 3 | 2 | 5 | ||||||||
| Vortioxetinef | 23 | 4 | 5 | 6 | 5 | 3 | 3 | 3 | 2 | <1 |
When data from multiple doses were reported separately, the data from the minimum therapeutic dose were used (indicated by footnotes). Data sources and references are available in Supplemental Table S3. Clear cells represent 0% to 9%; shaded cells, 10% to 29%; and black cells, 30% and higher.
aData from all indications.
bData from 50-mg dose.
cC, common effects, ≥1% and <10%.
dData from 100- to 150-mg dose.
eData from 40-mg dose.
fData from 10-mg dose.
Because sexual side effects are inconsistently and inadequately reported, clinical trial data are not reliable for assessing antidepressant-associated sexual dysfunction. A network meta-analysis of second-generation antidepressants (63 studies, N > 26,000)53 found low-quality evidence that bupropion had statistically lower rates of sexual side effects and that escitalopram and paroxetine had higher rates compared to other antidepressants. In studies that used standardized rating scales or interviews, which are more likely to reliably detect sexual side effects, agomelatine, bupropion, mirtazapine, vilazodone, and vortioxetine demonstrated lower risk.54
3.9. Are Antidepressants Associated with Suicidality?
Suicidal ideation and acts are important risks associated with MDD and require diligent assessment, monitoring and management during psychiatric treatment (see Section 13). A signal for increased suicidality in adolescents and young adults in antidepressant clinical trials led many regulatory agencies to issue “black box” warnings in 2004. Since 2009, 3 large meta-analyses have addressed the effect of antidepressants on suicidal ideas or behaviour. The first included data from 372 RCTs comparing 12 antidepressants to placebo and reported a reduced risk of suicidal ideas or acts in those aged 25 to 64 years and a reduced risk of suicidal acts in those older than 65 years.55 A meta-analysis of fluoxetine and venlafaxine showed no difference in suicidality compared to placebo, while another meta-analysis showed a trend toward reduced risk of suicidal ideas or acts with paroxetine versus placebo in the same age groups.56,57 A systematic review of observational studies involving more than 200,000 patients with moderate to severe depression found that exposure to SSRIs reduced the risk of suicide by more than 40% among adults and more than 50% among elderly people.58
In contrast, exposure to SSRIs almost doubled (odds ratio = 1.92) the risk of suicide and suicide attempts among adolescents in these observational studies.58 It is possible that only the most severely ill adolescents would have been prescribed antidepressants, and so this observational sample may well have had a particularly high risk for suicide actions. Nevertheless, caution and close monitoring are recommended when antidepressants are prescribed in this age group (see Section 659). Large observational studies have not shown differences in suicide risk with particular antidepressants or classes of antidepressants, and therefore caution should be exercised for all antidepressants.
3.10. What Are Uncommon but Serious Adverse Effects of Antidepressants?
Prolongation of the corrected QT interval (QTc), a surrogate marker for Torsade de Pointes (TdP) arrhythmia, has been the subject of warnings by regulatory agencies for citalopram, escitalopram, and quetiapine.60 However, TdP is often an idiosyncratic event, and its associations with antidepressants, medication dose, and QTc prolongation remain unclear.61 For example, a systematic review of antidepressants, QTc prolongation, and TdP found that 95% (36 of 38) of published case reports of QTc prolongation associated with antidepressants had 1 or more additional risk factors for TdP.61 Most cases of TdP occurred at therapeutic doses of the antidepressant, and several cases of TdP occurred with QTc interval within the normal range.61 Accordingly, in the absence of other known risk factors for TdP, the use of citalopram, escitalopram, and other antidepressants at therapeutic doses carries only a very low risk of TdP and other arrhythmias.60,61
The long-term use of SSRI antidepressants has been associated with increased risk of falls and fractures that is unrelated to postural hypotension. Systematic reviews and meta-analyses of observational studies indicate a small increased relative risk for fractures associated with SSRIs, with the highest risk in the first 6 weeks of exposure.62–64 Hyponatremia is also associated with SSRI use, primarily in elderly patients with other risk factors for hyponatremia.65
SSRIs can inhibit platelet aggregation by altering platelet serotonin receptors and modestly increase the risk of gastrointestinal bleeding, but this risk may be doubled with concomitant use of nonsteroidal anti-inflammatory drugs (NSAIDs).66 Concomitant use of acid-suppressing drugs can significantly reduce the risk of gastrointestinal bleeding.67
Elevation of liver enzymes is uncommonly seen with most antidepressants, and routine testing is not required. However, regulatory agencies in countries where agomelatine is approved have mandated regular liver function testing owing to the drug’s potential to elevate liver enzymes (1.3%) and sporadic cases of toxic hepatitis.68
3.11. Are There Differences in Formulations of Specific Antidepressants?
A systematic review and network meta-analysis (7 studies for direct comparisons and 68 studies for indirect) found no differences in efficacy or tolerability with extended-release antidepressants compared to immediate-release formulations, although there was some evidence that adherence was lower with the immediate-release agents.69 Extended-release antidepressants should be considered if adherence or compliance to medication is an issue.
Generic substitution for branded medications is a common practice in some countries and may involve alternative drug formulations.70 The Canadian and US regulatory agencies define pharmacokinetic similarity for generics as bioequivalence between 80% and 125% of brand-name agents. Bioinequivalence, which may result in loss of efficacy or increased side effects, can occur and in some cases led to withdrawal of an approved generic agent.71 Although generic medications are safe and reliable for most patients, for some who are well and maintained on a branded medication, a careful risk-benefit assessment (taking into account potential loss of efficacy) should be conducted prior to switching to a generic version.
3.12. What Are Clinically Relevant Drug-Drug Interactions?
Many patients with MDD take other medications for comorbid psychiatric and medical conditions. Drug-drug interactions can potentially reduce the efficacy of an antidepressant or other medications and increase adverse effects. Antidepressants and antipsychotics are primarily metabolized through the cytochrome P450 (CYP) enzyme metabolic pathway.72,73 Most antidepressants are substrates for several CYP enzymes (Tables 8 and 9), but agomelatine and duloxetine are metabolized primarily via the CYP1A2 pathway and should not be coadministered with drugs that potently inhibit CYP1A2, such as cimetidine, ticlopidine, and ciprofloxacin. Similarly, vilazodone is metabolized primarily through CYP3A4 and should be used with caution when prescribed with CYP3A4 inhibitors such as ketoconazole.
Table 8.
Some Clinically Significant Drug-Drug Interactions Resulting from Inhibition of Cytochrome P450 (CYP) Isoenzymes.
| Cytochrome P450 Inhibition of | Increases Serum Levels of These CYP Substrates | |
|---|---|---|
| CYP1A2 |
|
|
| CYP2C19 |
|
|
| CYP2D6 |
|
|
| CYP3A4 |
|
|
This is only a limited selection of interactions. For more comprehensive lists, see references in the text. Psychotropic medications in bold. HIV, human immunodeficiency virus.
Table 9.
Potential Drug-Drug Interactions Involving Newer Antidepressants and Atypical Antipsychotics.
| Potential for Drug-Drug Interaction | Antidepressants | Atypical Antipsychotics |
|---|---|---|
| Minimal or low potential |
|
|
| Moderate potential |
|
|
| Higher potential |
|
|
Moderate and higher potential interactions are noted in parentheses. MAO, monoamine oxidase.
aCoadministration with CYP1A2 inhibitors (e.g., cimetidine, ciprofloxacin and other fluoroquinolone antimicrobials, ticlopidine) should be avoided because serum antidepressant levels will be higher, leading to increased potential for side effects.
bAlso metabolized through the uridine diphosphate glucuronosyltransferase (UGT) pathway.
cPrecautions similar to those of older MAO inhibitors. Avoid coadministration of other antidepressants, serotonergic drugs (e.g., meperidine), and sympathomimetic drugs (e.g., pseudoephedrine, stimulants).
Several antidepressants and atypical antipsychotics act as inhibitors of specific CYP isoenzymes (Table 9). Clinically relevant drug-drug interactions are usually caused by agents that are potent CYP inhibitors, including fluoxetine (CYP2D6), paroxetine (CYP2D6), and fluvoxamine (CYP1A2, 2C19, and 3A4). Drug-drug interactions with moderate CYP inhibitors, including bupropion, duloxetine, and sertraline (CYP2D6), are rarely clinically relevant except at higher doses.
P-glycoprotein is an important component of the blood-brain barrier and the intestinal barrier and affects efflux of medications, including psychotropic, cardiac, and cancer agents.74 However, there is no consistent evidence of clinically relevant P-glycoprotein interactions with antidepressants or antipsychotics.74,75
Although not a pharmacokinetic drug-drug interaction, serotonin syndrome and/or hypertensive crisis can occur when serotonergic or sympathomimetic drugs are combined with MAO inhibitors, including the reversible MAO-A inhibitor, moclobemide, and the irreversible MAO-B inhibitor, selegiline (Table 9). Serotonin syndrome is rare except in cases of overdose, but it can also occur with combination use of multiple serotonergic medications (e.g., SSRIs, SNRIs, tramadol).76
3.13. Can Pharmacogenetic Testing or Therapeutic Drug-Level Monitoring Help to Select or Optimize an Antidepressant?
Pharmacogenetic testing for CYP enzymes is now available in many regions, and comprehensive recommendations for antidepressants have been suggested by the Clinical Pharmacogenetics Implementation Consortium (CPIC).77 Since large-scale RCTs to examine the utility of pharmacogenetic tests are still lacking,78 CANMAT does not recommend routine use of pharmacogenetic testing.
Similarly, CANMAT does not recommend routine therapeutic drug-level monitoring (TDM) for second-generation antidepressants because the poor correlation between blood antidepressant levels and clinical response limits TDM utility. Pharmacogenetic testing and/or TDM may be helpful in individual circumstances, including inability to tolerate minimum doses (i.e., to detect poor metabolizers), repeated failure to respond to high doses (i.e., to detect ultrarapid metabolizers), and to detect nonadherence.
3.14. How Long Do You Wait for a Response from an Antidepressant?
Early improvement (defined as >20%-30% reduction from baseline in a depression rating scale after 2-4 weeks) is correlated with response and remission at 6 to 12 weeks.79 The lack of early improvement at 2 to 4 weeks is also a predictor of later antidepressant nonresponse/nonremission. However, there is only low-quality evidence to support early switching at 2 or 4 weeks for nonimprovers to an initial antidepressant.80,81 CANMAT recommends increasing the antidepressant dose for nonimprovers at 2 to 4 weeks if the medication is tolerated and switching to another antidepressant if tolerability is a problem.
3.15. How Long Do You Continue an Antidepressant?
The CANMAT guidelines identify 2 phases of depression treatment: an acute phase (getting to symptomatic remission) and a maintenance phase (preventing relapse and recurrence) (see Section 13). The 2009 guidelines recommended that patients maintain treatment with antidepressants for 6 to 9 months after achieving symptomatic remission, while those with risk factors for recurrence extend antidepressant treatment to 2 years or more.82 New evidence continues to support this recommendation for antidepressant maintenance. A meta-analysis found significant benefit of antidepressants over placebo in maintenance studies of 1 to 12 months (72 trials, N = 14450) and ≥12 months (35 trials, N = 7253).83 Similarly, a review of all 16 maintenance RCTs (N > 4000) submitted to the Food and Drug Administration (FDA) found a 2-fold difference in recurrence during 24- to 52-week follow-up with antidepressants versus placebo (18% vs 37%, respectively).84 The drug-placebo benefit also narrowed after 6 months, consistent with meta-analyses showing higher relapse/recurrence risk when antidepressants are discontinued within 6 months.85
Few RCTs have specifically evaluated risk factors to guide longer term treatment. In 1 study, patients with recurrent MDD were less likely to experience recurrence and more likely to have improved psychosocial outcomes with 2 years of maintenance treatment with venlafaxine ER versus 1 year.86 The recommendation to extend maintenance treatment to 2 years or beyond in the presence of clinical risk factors (Table 10) is based on Level 3 and 4 Evidence.
Table 10.
Risk Factors to Consider Longer Term (2 Years or Longer) Maintenance Treatment with Antidepressants (Level 3 and 4 Evidence).
|
Discontinuation symptoms, described by the FINISH mnemonic (flu-like symptoms, insomnia, nausea, imbalance, sensory disturbances, hyperarousal), may be experienced by up to 40% of patients when antidepressants are stopped abruptly.87,88 These are generally mild and transient, but more severe symptoms have been described. Immediate-release formulations of paroxetine and venlafaxine are the most likely to be associated with discontinuation effects while long half-life agents such as fluoxetine and vortioxetine are the least likely.89 Unless there are clinical reasons otherwise, we recommend slowly tapering the dose over several weeks when discontinuing antidepressants.
3.16. How Do You Manage Inadequate Response to an Antidepressant?
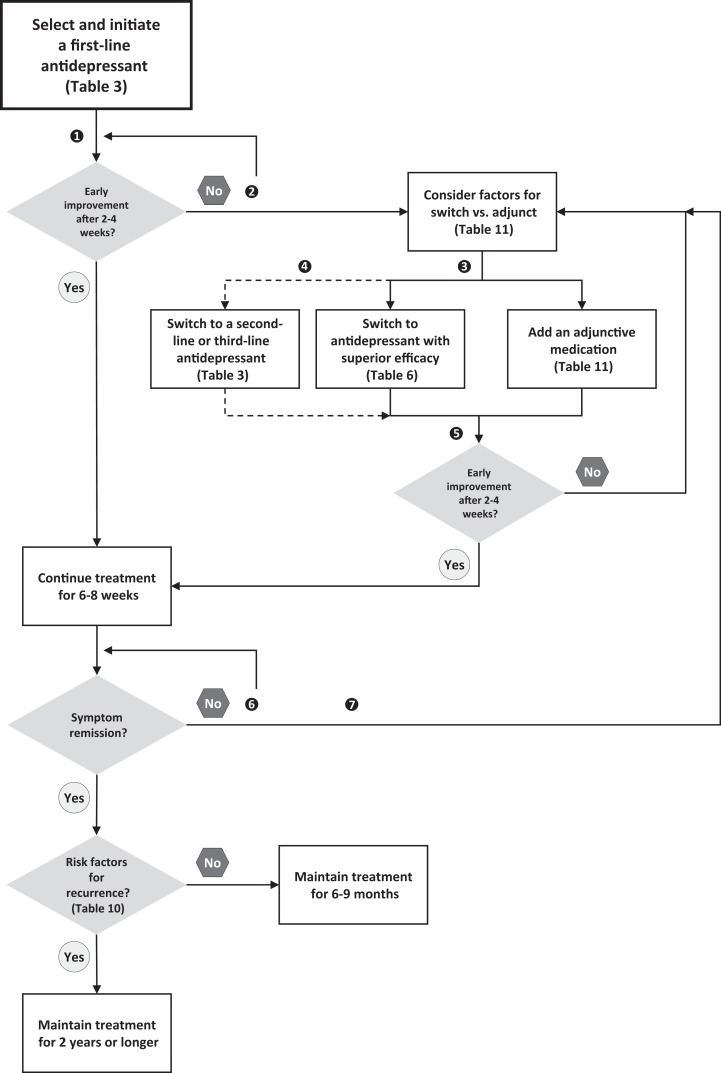
Figure 2 shows an algorithm for inadequate response to an initial antidepressant. If a patient has partial (e.g., 25%-49% reduction in symptom scores) or no response (e.g., <25% reduction) to the initial treatment, clinicians should ensure the treatment is optimized.90,91 There is substantial evidence that many patients receive subtherapeutic doses and/or inadequate duration of treatment, and up to 20% may have poor adherence.92 The clinician should then reevaluate the diagnosis and consider treatment issues that may be affecting response.93 Psychotherapy and neurostimulation approaches should also be considered for patients with an inadequate antidepressant response (see Section 294 and Section 495 respectively).
Figure 2.
Summary algorithm for managing inadequate response to an antidepressant. (1) Monitor outcomes using measurement-based care. (2) Depending on tolerability, first optimize antidepressant by increasing dose. (3) For early treatment resistance, consider adjunctive use of psychological and neurostimulation treatments. (4) After failure of 1 or more antidepressants, consider switch to a second-line or third-line antidepressant. (5) For more resistant depressions, consider longer evaluation periods for improvement. (6) Depending on tolerability, increase dose if not at maximal doses. (7) For more chronic and resistant depressions, consider a chronic disease management approach, with less emphasis on symptom remission and more emphasis on improvement in functioning and quality of life.
Research on strategies for inadequate response to an initial antidepressant has been hampered by a lack of consensus on the concept and definition of treatment-resistant depression (TRD). The most commonly employed definition is inadequate response to 2 or more antidepressants.91 However, this definition does not take into account adjunctive strategies, nor does it differentiate between patients who have had partial response versus those who have had no response. Additionally, few studies address residual symptoms (e.g., ≥50% improvement but symptom score is not in remission range).
In 2012, the United States Agency for Healthcare Research and Quality (AHRQ) published a comparative effectiveness review examining the various strategies to treat depression following inadequate response to an SSRI.96 It concluded there was insufficient evidence to differentiate between monotherapy switch within the SSRI class or switching to a non-SSRI agent. There was low strength of evidence, indicating that augmenting with an atypical antipsychotic was more effective than antidepressant monotherapy. There was also insufficient evidence about the benefits of individual atypical antipsychotics or other adjunctive agents. The following questions summarize subsequent evidence for these strategies.
3.17. How Effective Are Switching Strategies?
The 2009 CANMAT guidelines summarized evidence showing that switching nonresponders to another antidepressant results in good response and remission rates. Studies with newer antidepressants support this finding. Switching has also been studied as a control condition in RCTs of adjunctive treatments, with several studies demonstrating benefit of the switch compared to placebo.97,98 However, there are few RCTs comparing a switch strategy to continuing the same antidepressant. A systematic review identified only 3 RCTs (N = 495), all of which investigated adjunctive strategies as the primary aim but included conditions for switching to a new antidepressant and continuing on the original antidepressant.99 There were no differences in response or remission rates between switch and continuing strategies and no consistent evidence of differential efficacy between switching within class (e.g., from one SSRI to another SSRI) or across classes of antidepressants.99
The value of switching between classes or within classes of antidepressants remains controversial.100 A previous meta-analysis (4 studies, N = 1496) found a modest, but statistically significant, remission advantage for patients on an SSRI switched to an antidepressant in a different class (bupropion, mirtazapine, venlafaxine) versus a second SSRI trial (28% vs. 23.5%, respectively).101 These results are difficult to interpret because specific antidepressants have shown superior efficacy within both SSRI and non-SSRI classes (see 3.6., “How Do Second-Generation Antidepressants Compare in Efficacy?”). Consequently, CANMAT continues to recommend switching to an antidepressant with evidence of superior efficacy (Table 5).
3.18. How Effective Are Adjunctive Strategies?
An adjunctive strategy refers to the addition of a second medication to an initial medication. The term adjunctive is preferred over terms such as combination (adding a second antidepressant to the first) or augmentation (adding another medication that is not an antidepressant, e.g., triiodothyronine) because some augmentation agents (e.g., lithium, quetiapine) also have antidepressant effects as monotherapy.
Recommendations for adjunctive agents are based on efficacy and tolerability (Table 11). A network meta-analysis of RCTs (48 trials, N = 6654) examined the comparative adjunctive effects of aripiprazole, bupropion, buspirone, lamotrigine, lithium, methylphenidate, olanzapine, pindolol, quetiapine, risperidone, and thyroid hormone with each other and with placebo.102 Only aripiprazole, lithium, quetiapine, and triiodothyronine were more effective than placebo, with stronger efficacy estimates for aripiprazole and quetiapine than for lithium and thyroid hormone.102 There were no significant differences between the active treatments, but the network meta-analysis was limited due to few head-to-head comparisons, which reduces the power of indirect comparisons and the reliability of the results. This is apparent when examining the evidence base for lithium and triiodothyronine relative to other agents (summarized below).
Table 11.
Recommendations for Adjunctive Medications for Nonresponse or Partial Response to an Antidepressant.
| Recommendation | Adjunctive Agent | Level of Evidence | Dosing |
|---|---|---|---|
| First line | Aripiprazole | Level 1 | 2-15 mg |
| Quetiapine | Level 1 | 150-300 mg | |
| Risperidone | Level 1 | 1-3 mg | |
| Second line | Brexpiprazolea | Level 1 | 1-3 mg |
| Bupropion | Level 2 | 150-300 mg | |
| Lithium | Level 2 | 600-1200 mg (therapeutic serum levels) | |
| Mirtazapine/mianserin | Level 2 | 30-60 mg | |
| Modafinil | Level 2 | 100-400 mg | |
| Olanzapine | Level 1 | 2.5-10 mg | |
| Triiodothyronine | Level 2 | 25-50 mcg | |
| Third line | Other antidepressants | Level 3 | Various |
| Other stimulants (methylphenidate, lisdexamfetamine, etc.) | Level 3 | Various | |
| TCAs (e.g., desipramine) | Level 2 | Various | |
| Ziprasidone | Level 3 | 20-80 mg bid | |
| Experimental | Ketamine | Level 1 | 0.5 mg/kg, single intravenous doseb |
| Not recommended | Pindolol | Level 1 (lack of efficacy) | Not applicable |
TCA, tricyclic antidepressant.
aNewly approved since the 2009 Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines.
bFor acute treatment.
Atypical antipsychotics
Adjunctive treatment with atypical antipsychotic medications has the most consistent evidence for efficacy in TRD. Four independent meta-analyses103–106 comprising 12 to 17 trials (N = 3208-3807) and a network meta-analysis107 (18 trials, N = 4422) all found superior efficacy when compared to placebo for adjunctive aripiprazole, olanzapine, quetiapine, and risperidone, with small to medium effect sizes. The network meta-analysis did not find evidence for differences in efficacy among the atypical antipsychotics studied.107 Although not included in these meta-analyses, placebo-controlled RCTs have also shown efficacy for adjunctive brexpiprazole108,109 and for ziprasidone.110 All the meta-analyses and RCTs also found evidence for worse tolerability compared to placebo.
Antidepressants
The adjunctive strategy of adding another antidepressant to an existing one for TRD was examined in a systematic review, but only 5 placebo-controlled RCTs (N = 565) were identified: 3 trials with mirtazapine/mianserin and 2 trials with low-dose desipramine added to an SSRI.111 The studies were too heterogeneous to conduct a meta-analysis, but there was a signal for efficacy of adjunctive mirtazapine/mianserin.111 A meta-analysis (23 studies, N = 2435) focusing on adverse effects found that adjunctive antidepressant use was associated with increased side effects compared to monotherapy, especially when adding mirtazapine/mianserin or TCAs to SSRIs.112
Combinations of antidepressants have also been investigated as comedications in the initial treatment of MDD. While initial pilot studies were encouraging,113,114 large-sample RCTs found no differences in efficacy with the combination of bupropion + escitalopram over each agent alone115 or with the combinations of escitalopram + bupropion SR and mirtazapine + venlafaxine XR over escitalopram alone.116 In addition, adverse effects were higher in the combination treatments. A combination of antidepressants at initiation of treatment is not recommended.
Other medications
A systematic review of lithium augmentation trials concluded that it was effective but acknowledged that extant studies mostly involved lithium in combination with TCAs in trials with small sample sizes.117 This was highlighted in a meta-analysis of placebo-controlled RCTs (9 trials, N = 237) that identified only 3 trials (N = 74) of adjunctive lithium with SSRIs118; while the overall comparison and the SSRI-only comparison were both significant, the confidence intervals were wide, indicating Level 2 Evidence for efficacy. There have been no studies of triiodothyronine augmentation since the systematic review in 2008 that identified only 2 placebo-controlled RCTs.119 The STAR*D trial, although not placebo-controlled, is the largest RCT (N = 142) to compare the 2 strategies.120 There were no significant differences in remission rates, but triiodothyronine was better tolerated than lithium and had lower dropout rates.
A meta-analysis of modafanil, an atypical stimulant, in MDD identified 4 trials (N = 568), but only 2 (N = 211) were adjunctive studies.121 After excluding an outlier study, there was only marginal evidence for efficacy in modafinil-treated patients compared to placebo on both response and remission rates. Adverse effects did not appear to differ from placebo.121 Two placebo-controlled RCTs of lisdexamfetamine, a stimulant, found evidence of efficacy as an adjunctive agent for partial responders to SSRIs122,123; however, 2 unpublished phase III trials (N = 830) of adjunctive lisdexamfetamine were negative, and the clinical development program was discontinued.124 To date, other stimulants (e.g., methylphenidate) have only negative studies.125
Several meta-analyses have shown that single doses of intravenous ketamine, which preferentially target N-methyl-D-aspartate (NMDA) receptors, have rapid antidepressant effects in TRD.126–128 However, ketamine is associated with psychotomimetic adverse effects, carries potential for abuse, and still has very limited data on safety and efficacy with longer term use.126,129,130 CANMAT considers ketamine an experimental treatment and recommends its use be limited to academic depression treatment centres.
A meta-analysis (5 trials, N = 154) examined adjunctive use of the beta-blocker pindolol. There was no significant benefit for pindolol versus placebo in combination with SSRI therapy and no differences in tolerability or safety between the 2 groups.131 Pindolol is not recommended as an adjunct treatment.
3.19. How Do you Choose between Switching to Another Antidepressant and Adding an Adjunctive Agent?
An RCT (N = 101) found that adjunctive aripiprazole was superior to antidepressant switch on efficacy outcomes, including response and remission.132 In a retrospective comparison of the STAR*D switch and adjunctive studies, patients who tolerated citalopram and who had partial response were more likely to benefit from adjunctive strategies compared to switching.133 A few studies have addressed residual symptoms, such as fatigue or sexual dysfunction.134,135 However, there is no consistent evidence to support specific adjunctive agents to target specific residual symptoms or side effects.
In summary, given the limited evidence, a pharmacologic approach for TRD would include diagnostic reevaluation, consideration of previous medication trials (including degree of response and tolerability), rational use of adjunctive medications, discontinuation of medications that have not been beneficial, and careful monitoring of symptoms, side effects, and functioning to evaluate outcomes. The decision between switching and adjunctive strategies should be individualized based on clinical factors (Table 12).
Table 12.
Factors to Consider in Choosing between Switching to Another Antidepressant Monotherapy or Adding an Adjunctive Medication (Level 3 Evidence).
Consider switching to another antidepressant when:
|
Consider an adjunctive medication when:
|
aFor the initial antidepressant trial. In subsequent trials, lack of response (<25% improvement) may not be a factor for choosing between switch and adjunctive strategies.
3.20. How Do You Manage Persistent and Chronic Depression?
The DSM-5 has added a new diagnosis of persistent depressive disorder (PDD) that subsumes the DSM-IV diagnoses of dysthymic disorder and chronic MDD (see Section 13). A systematic review and network meta-analysis examined efficacy (response) and acceptability (all-cause discontinuation) of treatments for PDD (depression >2 years’ duration) with a network of 45 RCTs (N = 5804) involving 28 drugs.136 Most of the studied drugs were more effective than placebo, including fluoxetine, paroxetine, sertraline, moclobemide, and imipramine, with no differences in acceptability compared to placebo. The only differences between treatments were superior efficacy of sertraline over imipramine and superior acceptability of moclobemide over fluoxetine.136 These results confirmed a meta-analysis (20 trials, N = 2918) of chronic depression showing that SSRIs were similar in efficacy but superior in tolerability compared with TCAs.137 The network meta-analysis also identified differences in effects between combined psychotherapy + medication and medication-only studies in dysthymia studies compared to studies of chronic MDD, suggesting that the new diagnosis of PDD may not have homogeneous treatment response.136
Although there are positive results in treating chronic depression and PDD with antidepressants, some experts have argued that patients with repeated treatment failures and a chronic course of depression require a chronic disease management approach (i.e., with less emphasis on remission of symptoms and cure, greater emphasis on improving functioning and quality of life, and greater use of psychotherapeutic and nonmedication treatments).138
3.21. What Novel Treatments Are Being Investigated?
The link between the rapid antidepressant effect of ketamine and the glutamate system has stimulated drug development on related compounds, including esketamine (the S-enantiomer of ketamine, delivered intranasally),139 lanicemine, and memantine.140 Other promising compounds include GluN2B antagonists (e.g., CERC-301)141; GLYX-13, which targets the glycine coagonist site on the NMDA receptor142; and basimglurant, which targets the metabotropic glutamate (mGlu) receptors.143 Other potential candidates for antidepressant actions include drugs that target the endocannabinoid system and drugs with neuroplasticity mechanisms, which are thought to play a role in sustained antidepressant effects.144
Preliminary studies have shown promise for several currently available medications with diverse effects. In a meta-analysis (4 studies, N = 150) of adjunctive celecoxib, higher response and remission rates and lower dropout rates were reported with the NSAID compared to placebo.145 In contrast, a subsequent small trial (N = 30 female patients with first episode of MDD) did not demonstrate efficacy of adjunctive celecoxib with sertraline.146 Preliminary studies of pramipexole, a dopaminergic D2, D3, and D4 receptor agonist that has evidence for efficacy in bipolar depression,147 found some benefit in TRD.148,149 Other investigational drugs for MDD include novel atypical antipsychotics such as cariprazine.150
Supplementary Material
Acknowledgements
We thank Cindy Woo and Trehani Fonseka for assisting in preparation of this manuscript.
Footnotes
Disclosures: The guidelines process and publication were funded entirely by internal CANMAT funds; no external support was sought or received. No honoraria were paid to authors, and no professional editorial assistance was used. All members of the CANMAT Depression Work Group disclosed potential conflicts of interest (available at www.canmat.org). CANMAT is a project-driven organization governed by a volunteer, unpaid advisory board, with no permanent staff or dedicated offices. CANMAT has a conflict of interest policy that includes disclosures by all participants, and all continuing professional development (CPD) projects are accredited by academic institutions. CANMAT has diverse funding; in the past 5 years (2011-2015), sources of CANMAT revenue (excluding CIHR and research funding) included national/international scientific conferences (28% of revenue), publications (26%), industry-supported CPD projects (26%), and academic projects (18%).
The CANMAT guidelines are not officially endorsed by the Canadian Psychiatric Association.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article:
SHK has received honoraria for ad hoc speaking or advising/consulting or received research funds from Allergan, Brain Canada, Bristol Myers Squibb, Canadian Institutes of Health Research, Canadian Network for Mood and Anxiety Treatments, Johnson & Johnson, Lundbeck, Lundbeck Institute, Medscape, Ontario Brain Institute, Pfizer, Servier, St. Jude Medical, Sunovion, and Takeda.
RWL has received honoraria for ad hoc speaking or advising/consulting or received research funds from Asia-Pacific Economic Cooperation, AstraZeneca, Brain Canada, Bristol Myers Squibb, Canadian Institutes of Health Research, Canadian Depression Research and Intervention Network, Canadian Network for Mood and Anxiety Treatments, Canadian Psychiatric Association, Coast Capital Savings, Johnson & Johnson, Lundbeck, Lundbeck Institute, Medscape, Pfizer, St. Jude Medical, Takeda, University Health Network Foundation, and Vancouver Coastal Health Research Institute.
RSM has received research grants and/or personal fees and/or nonfinancial support from AstraZeneca, Bristol Myers Squibb, CME Outfitters, Eli Lilly, France Foundation, GlaxoSmithKline, I3CME, Janssen-Ortho, Lundbeck, Merck, National Alliance for Research on Schizophrenia and Depression, National Institutes of Mental Health, Optum Health, Organon, Pfizer, Physicians’ Postgraduate Press, Shire, and Stanley Medical Research Institute,
SVT has received honoraria for ad hoc speaking or advising/consulting or received research funds from Bristol-Myers Squibb, Eli Lilly, Janssen, Lundbeck, Otsuka, Pfizer, Purdue, Shire, Sunovion, and Valeant.
VB has no disclosures.
PB has received research grants, personal fees, and/or nonfinancial support from AstraZeneca, Bristol Myers Squibb, Eli Lilly, Forest, Euthymics, Janssen, Lundbeck, Merck, Otsuka, Pfizer, Pierre Fabre, Servier, Shire, Takeda, and Valeant.
MH has no disclosures.
FJ has no disclosures.
AJL has received research grants, personal fees, and/or nonfinancial support from Eli Lilly, Janssen, Lundbeck, and Sanofi-Aventis,
GMM has received honoraria for ad hoc speaking or advisory/consulting from Janssen, Lilly, Lundbeck, and Pfizer.
SJM has received fellowship funding from Pfizer.
DM has received honoraria for ad hoc speaking or advising/consulting or received research funds from Allergan, Bristol Myers Squibb, Lundbeck, Janssen-Ortho, Otsuka, Pfizer, Shire, and Sunovion.
RVM has received honoraria for ad hoc speaking or advising/consulting or received research funds from Allergan, Bristol Myers Squibb, Canadian Institutes of Health Research, Canadian Network for Mood and Anxiety Treatments, Canadian Psychiatric Association, Eli Lilly, Johnson & Johnson, Lallemand, Lundbeck, Merck, Ontario Brain Institute, Ontario Mental Health Foundation, Otsuka, Paladin, Pfizer, Queen’s University, Sunovion, Takeda, the University Health Network Foundation, and Valeant.
DJM has received research funds from Canadian Institutes of Health Research, Canadian Foundations for Innovation, National Alliance for Research in Schizophrenia and Depression, Ontario Mental Health Foundation, National Institutes of Health, and the University of Toronto.
SVP has been a consultant to Bristol Myers Squibb, Lundbeck, and Takeda; has had a research contract with Assurex; and has equity in Mensante.
NLP has no disclosures.
AVR has received honoraria for ad hoc speaking or advising/consulting or received research funds from Bristol Myers Squibb, Canadian Depression Research and Intervention Network, Canadian Foundation for Innovation and the Ministry of Economic Development and Innovation, Canadian Institutes of Health Research, Grand Challenges Canada, Janssen, Lundbeck, Ontario Mental Health Foundation, Pfizer, and Sunovion.
RU has received research funds from European Commission Framework 6.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: The online tables are available at http://cpa.sagepub.com/supplemental
References
- 1. Kennedy SH, Lam RW, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. Introduction. J Affect Disord. 2009;11(Suppl 1):S1–S2. [DOI] [PubMed] [Google Scholar]
- 2. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord. 2013;15:1–44. [DOI] [PubMed] [Google Scholar]
- 3. Lam RW, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: introduction and methods. Can J Psychiatry. 2016;61(9):506–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lieberman JA, Greenhouse J, Hamer RM, et al. Comparing the effects of antidepressants: consensus guidelines for evaluating quantitative reviews of antidepressant efficacy. Neuropsychopharmacology. 2005;30:445–460. [DOI] [PubMed] [Google Scholar]
- 5. Kirsch I, Deacon BJ, Huedo-Medina TB, et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hieronymus F, Emilsson JF, Nilsson S, et al. Consistent superiority of selective serotonin reuptake inhibitors over placebo in reducing depressed mood in patients with major depression. Mol Psychiatry. 2016;21:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Montgomery SA, Gommoll CP, Chen C, et al. Efficacy of levomilnacipran extended-release in major depressive disorder: pooled analysis of 5 double-blind, placebo-controlled trials. CNS Spectr. 2015;20:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shiovitz T, Greenberg WM, Chen C, et al. A randomized, double-blind, placebo-controlled trial of the efficacy and safety of levomilnacipran ER 40-120mg/day for prevention of relapse in patients with major depressive disorder. Innov Clin Neurosci. 2014;11:10–22. [PMC free article] [PubMed] [Google Scholar]
- 9. Citrome L. Vilazodone for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2012;66:356–368. [DOI] [PubMed] [Google Scholar]
- 10. Hellerstein DJ, Flaxer J. Vilazodone for the treatment of major depressive disorder: an evidence-based review of its place in therapy. Core Evid. 2015;10:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang SM, Han C, Lee SJ, et al. A review of current evidence for vilazodone in major depressive disorder. Int J Psychiatry Clin Pract. 2013;17:160–169. [DOI] [PubMed] [Google Scholar]
- 12. Laughren TP, Gobburu J, Temple RJ, et al. Vilazodone: clinical basis for the US Food and Drug Administration’s approval of a new antidepressant. J Clin Psychiatry. 2011;72:1166–1173. [DOI] [PubMed] [Google Scholar]
- 13. Pae CU, Wang SM, Han C, et al. Vortioxetine: a meta-analysis of 12 short-term, randomized, placebo-controlled clinical trials for the treatment of major depressive disorder. J Psychiatry Neurosci. 2015;40:174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27:215–223. [DOI] [PubMed] [Google Scholar]
- 15. Mahableshwarkar AR, Zajecka J, Jacobson W, et al. A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology. 2015;40:2025–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014; 17:1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosenblat JD, Kakar R, McIntyre RS. The cognitive effects of antidepressants in major depressive disorder: a systematic review and meta-analysis of randomized clinical trials. Int J Neuropsychopharmacol. 2015;19(2):pyv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boulenger JP, Loft H, Florea I. A randomized clinical study of Lu AA21004 in the prevention of relapse in patients with major depressive disorder. J Psychopharmacol. 2012;26:1408–1416. [DOI] [PubMed] [Google Scholar]
- 19. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165:342–351. [DOI] [PubMed] [Google Scholar]
- 20. Gilmer WS, Gollan JK, Wisniewski SR, et al. Does the duration of index episode affect the treatment outcome of major depressive disorder? A STAR*D report. J Clin Psychiatry. 2008;69:1246–1256. [DOI] [PubMed] [Google Scholar]
- 21. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. [DOI] [PubMed] [Google Scholar]
- 22. Uher R, Maier W, Hauser J, et al. Differential efficacy of escitalopram and nortriptyline on dimensional measures of depression. Br J Psychiatry. 2009;194:252–259. [DOI] [PubMed] [Google Scholar]
- 23. American Psychiatric Association (APA). Diagnostic and statistical manual of mental disorders. 5th ed Arlington (VA; ): APA; 2013. [Google Scholar]
- 24. Arnow BA, Blasey C, Williams LM, et al. Depression subtypes in predicting antidepressant response: a report from the iSPOT-D trial. Am J Psychiatry. 2015;172:743–750. [DOI] [PubMed] [Google Scholar]
- 25. Uher R. Genes, environment, and individual differences in responding to treatment for depression. Harv Rev Psychiatry. 2011;19:109–124. [DOI] [PubMed] [Google Scholar]
- 26. Stewart JW, McGrath PJ, Fava M, et al. Do atypical features affect outcome in depressed outpatients treated with citalopram? Int J Neuropsychopharmacol. 2010;13:15–30. [DOI] [PubMed] [Google Scholar]
- 27. McGrath PJ, Khan AY, Trivedi MH, et al. Response to a selective serotonin reuptake inhibitor (citalopram) in major depressive disorder with melancholic features: a STAR*D report. J Clin Psychiatry. 2008;69:1847–1855. [DOI] [PubMed] [Google Scholar]
- 28. Wijkstra J, Lijmer J, Burger H, et al. Pharmacological treatment for psychotic depression. Cochrane Database Syst Rev. 2015;7:CD004044. [DOI] [PubMed] [Google Scholar]
- 29. Suppes T, Silva R, Cucchiaro J, et al. Lurasidone for the treatment of major depressive disorder with mixed features: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2016;173:400–407. [DOI] [PubMed] [Google Scholar]
- 30. Patkar A, Gilmer W, Pae CU, et al. A 6 week randomized double-blind placebo-controlled trial of ziprasidone for the acute depressive mixed state. PLoS One. 2012;7:e34757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219:25–50. [DOI] [PubMed] [Google Scholar]
- 32. Alberti S, Chiesa A, Andrisano C, et al. Insomnia and somnolence associated with second-generation antidepressants during the treatment of major depression: a meta-analysis. J Clin Psychopharmacol. 2015;35:296–303. [DOI] [PubMed] [Google Scholar]
- 33. Thaler KJ, Morgan LC, Van Noord M, et al. Comparative effectiveness of second-generation antidepressants for accompanying anxiety, insomnia, and pain in depressed patients: a systematic review. Depress Anxiety. 2012;29:495–505. [DOI] [PubMed] [Google Scholar]
- 34. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;1:CD007115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Häuser W, Urrútia G, Tort S, et al. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia syndrome. Cochrane Database Syst Rev. 2013;1:CD010292. [DOI] [PubMed] [Google Scholar]
- 36. McIntyre RS, Schaffer A, Beaulieu S. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid conditions. Ann Clin Psychiatry. 2012;24:2–3. [PubMed] [Google Scholar]
- 37. Schaffer A, McIntosh D, Goldstein BI, et al. The CANMAT task force recommendations for the management of patients with mood disorders and comorbid anxiety disorders. Ann Clin Psychiatry. 2012;24:6–22. [PubMed] [Google Scholar]
- 38. Bond DJ, Hadjipavlou G, Lam RW, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid attention-deficit/hyperactivity disorder. Ann Clin Psychiatry. 2012;24:23–37. [PubMed] [Google Scholar]
- 39. Beaulieu S, Saury S, Sareen J, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid substance use disorders. Ann Clin Psychiatry. 2012;24:38–55. [PubMed] [Google Scholar]
- 40. Rosenbluth M, MacQueen G, McIntyre RS, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid personality disorders. Ann Clin Psychiatry. 2012;24:56–68. [PubMed] [Google Scholar]
- 41. McIntyre RS, Rosenbluth M, Ramasubbu R, et al. Managing medical and psychiatric comorbidity in individuals with major depressive disorder and bipolar disorder. Ann Clin Psychiatry. 2012;24:163–169. [PubMed] [Google Scholar]
- 42. Ramasubbu R, Taylor VH, Samaan Z, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and select comorbid medical conditions. Ann Clin Psychiatry. 2012;24:91–109. [PubMed] [Google Scholar]
- 43. Ramasubbu R, Beaulieu S, Taylor VH, et al. The CANMAT task force recommendations for the management of patients with mood disorders and comorbid medical conditions: diagnostic, assessment, and treatment principles. Ann Clin Psychiatry. 2012;24:82–90. [PubMed] [Google Scholar]
- 44. Salanti G, Higgins JP, Ades AE, et al. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17:279–301. [DOI] [PubMed] [Google Scholar]
- 45. Cipriani A, Santilli C, Furukawa TA, et al. Escitalopram versus other antidepressive agents for depression. Cochrane Database Syst Rev 2009;(2):CD006532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gartlehner G, Hansen RA, Morgan LC, et al. Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis. Ann Intern Med. 2011;155:772–785. [DOI] [PubMed] [Google Scholar]
- 47. Khoo AL, Zhou HJ, Teng M, et al. Network meta-analysis and cost-effectiveness analysis of new generation antidepressants. CNS Drugs. 2015;29:695–712. [DOI] [PubMed] [Google Scholar]
- 48. Ramsberg J, Asseburg C, Henriksson M. Effectiveness and cost-effectiveness of antidepressants in primary care: a multiple treatment comparison meta-analysis and cost-effectiveness model. PLoS One. 2012;7:e42003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Linde K, Kriston L, Rücker G, et al. Efficacy and acceptability of pharmacological treatments for depressive disorders in primary care: systematic review and network meta-analysis. Ann Fam Med. 2015;13:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lam RW, Parikh SV, Michalak EE, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) consensus recommendations for functional outcomes in major depressive disorder. Ann Clin Psychiatry. 2015;27:142–149. [PubMed] [Google Scholar]
- 51. McKnight PE, Kashdan TB. The importance of functional impairment to mental health outcomes: a case for reassessing our goals in depression treatment research. Clin Psychol Rev. 2009;29:243–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kamenov K, Cabello M, Coenen M, et al. How much do we know about the functional effectiveness of interventions for depression? A systematic review. J Affect Disord. 2015;188:89–96. [DOI] [PubMed] [Google Scholar]
- 53. Reichenpfader U, Gartlehner G, Morgan LC, et al. Sexual dysfunction associated with second-generation antidepressants in patients with major depressive disorder: results from a systematic review with network meta-analysis. Drug Saf. 2014;37:19–31. [DOI] [PubMed] [Google Scholar]
- 54. Kronstein PD, Ishida E, Khin NA, et al. Summary of findings from the FDA regulatory science forum on measuring sexual dysfunction in depression trials. J Clin Psychiatry. 2015;76:1050–1059. [DOI] [PubMed] [Google Scholar]
- 55. Stone M, Laughren T, Jones ML, et al. Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug Administration. BMJ. 2009;339:b2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gibbons RD, Brown CH, Hur K, et al. Suicidal thoughts and behavior with antidepressant treatment: reanalysis of the randomized placebo-controlled studies of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69:580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Carpenter DJ, Fong R, Kraus JE, et al. Meta-analysis of efficacy and treatment-emergent suicidality in adults by psychiatric indication and age subgroup following initiation of paroxetine therapy: a complete set of randomized placebo-controlled trials. J Clin Psychiatry. 2011;72:1503–1514. [DOI] [PubMed] [Google Scholar]
- 58. Barbui C, Esposito E, Cipriani A. Selective serotonin reuptake inhibitors and risk of suicide: a systematic review of observational studies. CMAJ. 2009;180:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. MacQueen GM, Frey BN, Ismail Z, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 6. special populations: youth, women, and the elderly. Can J Psychiatry. 2016;61(9):588–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vieweg WV, Hasnain M, Howland RH, et al. Citalopram, QTc interval prolongation, and torsade de pointes: how should we apply the recent FDA ruling? Am J Med. 2012;125:859–868. [DOI] [PubMed] [Google Scholar]
- 61. Hasnain M, Vieweg WV. QTc interval prolongation and torsade de pointes associated with second-generation antipsychotics and antidepressants: a comprehensive review. CNS Drugs. 2014;28:887–920. [DOI] [PubMed] [Google Scholar]
- 62. Eom CS, Lee HK, Ye S, et al. Use of selective serotonin reuptake inhibitors and risk of fracture: a systematic review and meta-analysis. J Bone Miner Res. 2012;27:1186–1195. [DOI] [PubMed] [Google Scholar]
- 63. Rabenda V, Nicolet D, Beaudart C. Relationship between use of antidepressants and risk of fractures: a meta-analysis. Osteoporos Int. 2013;24:121–137. [DOI] [PubMed] [Google Scholar]
- 64. Wu Q, Bencaz AF, Hentz JG, et al. Selective serotonin reuptake inhibitor treatment and risk of fractures: a meta-analysis of cohort and case-control studies. Osteoporos Int. 2012;23:365–375. [DOI] [PubMed] [Google Scholar]
- 65. Coupland CA, Dhiman P, Barton G, et al. A study of the safety and harms of antidepressant drugs for older people: a cohort study using a large primary care database. Health Technol Assess. 2011;15:1–202, iii–iv. [DOI] [PubMed] [Google Scholar]
- 66. Anglin R, Yuan Y, Moayyedi P, et al. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:811–819. [DOI] [PubMed] [Google Scholar]
- 67. Jiang HY, Chen HZ, Hu XJ, et al. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2015;13:42–50.e43. [DOI] [PubMed] [Google Scholar]
- 68. Servier Laboratories. Summary of product characteristics: Valdoxan [Internet]. November 2015 [cited 2016 May 3]. Available from: https://www.medicines.org.uk/emc/medicine/21830#DOCREVISION
- 69. Nussbaumer B, Morgan LC, Reichenpfader U, et al. Comparative efficacy and risk of harms of immediate- versus extended-release second-generation antidepressants: a systematic review with network meta-analysis. CNS Drugs. 2014;28:699–712. [DOI] [PubMed] [Google Scholar]
- 70. Carbon M, Correll CU. Rational use of generic psychotropic drugs. CNS Drugs. 2013;27:353–365. [DOI] [PubMed] [Google Scholar]
- 71. Woodcock J, Khan M, Yu LX. Withdrawal of generic budeprion for nonbioequivalence. N Engl J Med. 2012;367:2463–2465. [DOI] [PubMed] [Google Scholar]
- 72. Brandl EJ, Tiwari AK, Zhou X, et al. Influence of CYP2D6 and CYP2C19 gene variants on antidepressant response in obsessive-compulsive disorder. Pharmacogenomics J. 2014;14:176–181. [DOI] [PubMed] [Google Scholar]
- 73. Spina E, Trifirò G, Caraci F. Clinically significant drug interactions with newer antidepressants. CNS Drugs. 2012;26:39–67. [DOI] [PubMed] [Google Scholar]
- 74. O’Brien FE, Dinan TG, Griffin BT, et al. Interactions between antidepressants and P-glycoprotein at the blood-brain barrier: clinical significance of in vitro and in vivo findings. Br J Pharmacol. 2012;165:289–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kennedy WK, Jann MW, Kutscher EC. Clinically significant drug interactions with atypical antipsychotics. CNS Drugs. 2013;27:1021–1048. [DOI] [PubMed] [Google Scholar]
- 76. Abadie D, Rousseau V, Logerot S, et al. Serotonin syndrome: analysis of cases registered in the French Pharmacovigilance Database. J Clin Psychopharmacol. 2015;35:382–388. [DOI] [PubMed] [Google Scholar]
- 77. Hicks JK, Bishop JR, Sangkuhl K, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Drozda K, Müller DJ, Bishop JR. Pharmacogenomic testing for neuropsychiatric drugs: current status of drug labeling, guidelines for using genetic information, and test options. Pharmacotherapy. 2014;34:166–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kudlow PA, McIntyre RS, Lam RW. Early switching strategies in antidepressant non-responders: current evidence and future research directions. CNS Drugs. 2014;28:601–609. [DOI] [PubMed] [Google Scholar]
- 80. Nakajima S, Uchida H, Suzuki T, et al. Is switching antidepressants following early nonresponse more beneficial in acute-phase treatment of depression? a randomized open-label trial. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1983–1989. [DOI] [PubMed] [Google Scholar]
- 81. Romera I, Pérez V, Menchón JM, et al. Early switch strategy in patients with major depressive disorder: a double-blind, randomized study. J Clin Psychopharmacol. 2012;32:479–486. [DOI] [PubMed] [Google Scholar]
- 82. Lam RW, Kennedy SH, Grigoriadis S, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults: III. Pharmacotherapy. J Affect Disord. 2009;117(Suppl 1):S26–S43. [DOI] [PubMed] [Google Scholar]
- 83. Sim K, Lau WK, Sim J, et al. Prevention of relapse and recurrence in adults with major depressive disorder: systematic review and meta-analyses of controlled trials. Int J Neuropsychopharmacol. 2015;19(2):pyv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Borges S, Chen YF, Laughren TP, et al. Review of maintenance trials for major depressive disorder: a 25-year perspective from the US Food and Drug Administration. J Clin Psychiatry. 2014;75:205–214. [DOI] [PubMed] [Google Scholar]
- 85. Baldessarini RJ, Lau WK, Sim J, et al. Duration of initial antidepressant treatment and subsequent relapse of major depression. J Clin Psychopharmacol. 2015;35:75–76. [DOI] [PubMed] [Google Scholar]
- 86. Trivedi MH, Dunner DL, Kornstein SG, et al. Psychosocial outcomes in patients with recurrent major depressive disorder during 2 years of maintenance treatment with venlafaxine extended release. J Affect Disord. 2010;126:420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Berber MJ. FINISH: remembering the discontinuation syndrome. Flu-like symptoms, Insomnia, Nausea, Imbalance, Sensory disturbances, and Hyperarousal (anxiety/agitation). J Clin Psychiatry. 1998;59:255. [PubMed] [Google Scholar]
- 88. Fava GA, Gatti A, Belaise C, et al. Withdrawal symptoms after selective serotonin reuptake inhibitor discontinuation: a systematic review. Psychother Psychosom. 2015;84:72–81. [DOI] [PubMed] [Google Scholar]
- 89. Renoir T. Selective serotonin reuptake inhibitor antidepressant treatment discontinuation syndrome: a review of the clinical evidence and the possible mechanisms involved. Front Pharmacol. 2013;4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Berlim MT, Turecki G. Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can J Psychiatry. 2007;52:46–54. [DOI] [PubMed] [Google Scholar]
- 91. McIntyre RS, Filteau MJ, Martin L, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014;156:1–7. [DOI] [PubMed] [Google Scholar]
- 92. Roberson AM, Castro VM, Cagan A, et al. Antidepressant nonadherence in routine clinical settings determined from discarded blood samples. J Clin Psychiatry. 2016;77:359–362. [DOI] [PubMed] [Google Scholar]
- 93. Trivedi MH, Rush AJ, Gaynes BN, et al. Maximizing the adequacy of medication treatment in controlled trials and clinical practice: STAR(*)D measurement-based care. Neuropsychopharmacology. 2007;32:2479–2489. [DOI] [PubMed] [Google Scholar]
- 94. Parikh SV, Quilty LC, Ravitz P, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 2. psychological treatments. Can J Psychiatry. 2016;61(9):524–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Milev RV, Giacobbe P, Kennedy SH, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 4. neurostimulation treatments. Can J Psychiatry. 2016;61(9):561–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Santaguida PL, MacQueen G, Kashavarz H, et al. Treatment for depression after unsatisfactory response to SSRIs. Rockville (MD): Agency for Healthcare Research and Quality; 2012. Report No.: 12-EHC050-EF. AHRQ Comparative Effectiveness Reviews. [PubMed] [Google Scholar]
- 97. Freeman MP, McKinney A, Bradshaw M, et al. T150. The triple reuptake inhibitor antidepressant effects (triade) trial: Amitifadine for the treatment of major depressive disorder. Neuropsychopharmacology. 2013;38(Suppl 2s):S273–S434. [Google Scholar]
- 98. Trivedi MH, Thase ME, Osuntokun O, et al. An integrated analysis of olanzapine/fluoxetine combination in clinical trials of treatment-resistant depression. J Clin Psychiatry. 2009;70:387–396. [DOI] [PubMed] [Google Scholar]
- 99. Rush AJ, Warden D, Wisniewski SR, et al. STAR*D: revising conventional wisdom. CNS Drugs. 2009;23:627–647. [DOI] [PubMed] [Google Scholar]
- 100. Carvalho AF, Berk M, Hyphantis TN, et al. The integrative management of treatment-resistant depression: a comprehensive review and perspectives. Psychother Psychosom. 2014;83:70–88. [DOI] [PubMed] [Google Scholar]
- 101. Papakostas GI, Fava M, Thase ME. Treatment of SSRI-resistant depression: a meta-analysis comparing within- versus across-class switches. Biol Psychiatry. 2008;63:699–704. [DOI] [PubMed] [Google Scholar]
- 102. Zhou X, Ravindran AV, Qin B, et al. Comparative efficacy, acceptability, and tolerability of augmentation agents in treatment-resistant depression: systematic review and network meta-analysis. J Clin Psychiatry. 2015;76:e487–e498. [DOI] [PubMed] [Google Scholar]
- 103. Komossa K, Depping AM, Gaudchau A, et al. Second-generation antipsychotics for major depressive disorder and dysthymia. Cochrane Database Syst Rev. 2010;(12):CD008121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166:980–991. [DOI] [PubMed] [Google Scholar]
- 105. Spielmans GI, Berman MI, Linardatos E, et al. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10:e1001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wen XJ, Wang LM, Liu ZL, et al. Meta-analysis on the efficacy and tolerability of the augmentation of antidepressants with atypical antipsychotics in patients with major depressive disorder. Braz J Med Biol Res. 2014;47:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhou X, Keitner GI, Qin B, et al. Atypical antipsychotic augmentation for treatment-resistant depression: a systematic review and network meta-analysis. Int J Neuropsychopharmacol. 2015;18:pyv060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry. 2015;76:1224–1231. [DOI] [PubMed] [Google Scholar]
- 109. Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. 2015;76:1232–1240. [DOI] [PubMed] [Google Scholar]
- 110. Papakostas GI, Fava M, Baer L, et al. Ziprasidone augmentation of escitalopram for major depressive disorder: efficacy results from a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2015;172:1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rocha FL, Fuzikawa C, Riera R, et al. Combination of antidepressants in the treatment of major depressive disorder: a systematic review and meta-analysis. J Clin Psychopharmacol. 2012;32:278–281. [DOI] [PubMed] [Google Scholar]
- 112. Galling B, Calsina Ferrer A, Abi Zeid Daou M, et al. Safety and tolerability of antidepressant co-treatment in acute major depressive disorder: results from a systematic review and exploratory meta-analysis. Expert Opin Drug Saf. 2015;14:1587–1608. [DOI] [PubMed] [Google Scholar]
- 113. Blier P, Gobbi G, Turcotte JE, et al. Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. Eur Neuropsychopharmacol. 2009;19:457–465. [DOI] [PubMed] [Google Scholar]
- 114. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167:281–288. [DOI] [PubMed] [Google Scholar]
- 115. Stewart JW, McGrath PJ, Blondeau C, et al. Combination antidepressant therapy for major depressive disorder: speed and probability of remission. J Psychiatr Res. 2014;52:7–14. [DOI] [PubMed] [Google Scholar]
- 116. Rush AJ, Trivedi MH, Stewart JW, et al. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168:689–701. [DOI] [PubMed] [Google Scholar]
- 117. Bauer M, Adli M, Ricken R, et al. Role of lithium augmentation in the management of major depressive disorder. CNS Drugs. 2014;28:331–342. [DOI] [PubMed] [Google Scholar]
- 118. Nelson JC, Baumann P, Delucchi K, et al. A systematic review and meta-analysis of lithium augmentation of tricyclic and second generation antidepressants in major depression. J Affect Disord. 2014;168:269–275. [DOI] [PubMed] [Google Scholar]
- 119. Cooper-Kazaz R, Lerer B. Efficacy and safety of triiodothyronine supplementation in patients with major depressive disorder treated with specific serotonin reuptake inhibitors. Int J Neuropsychopharmacol. 2008;11:685–699. [DOI] [PubMed] [Google Scholar]
- 120. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519–1530. [DOI] [PubMed] [Google Scholar]
- 121. Goss AJ, Kaser M, Costafreda SG, et al. Modafinil augmentation therapy in unipolar and bipolar depression: a systematic review and meta-analysis of randomized controlled trials. J Clin Psychiatry. 2013;74:1101–1107. [DOI] [PubMed] [Google Scholar]
- 122. Madhoo M, Keefe RS, Roth RM, et al. Lisdexamfetamine dimesylate augmentation in adults with persistent executive dysfunction after partial or full remission of major depressive disorder. Neuropsychopharmacology. 2014;39:1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Trivedi MH, Cutler AJ, Richards C, et al. A randomized controlled trial of the efficacy and safety of lisdexamfetamine dimesylate as augmentation therapy in adults with residual symptoms of major depressive disorder after treatment with escitalopram. J Clin Psychiatry. 2013;74:802–809. [DOI] [PubMed] [Google Scholar]
- 124. Shire. Shire reports top-line results from two phase 3 studies for Vyvanse® (lisdexamfetamine dimesylate) capsules (CII) as an adjunctive treatment for adults with major depressive disorder [Internet]. February 2014 [cited 2016 May 3]. Available from: https://www.shire.com/newsroom/2014/february/shire-reports-topline
- 125. Corp SA, Gitlin MJ, Altshuler LL. A review of the use of stimulants and stimulant alternatives in treating bipolar depression and major depressive disorder. J Clin Psychiatry. 2014;75:1010–1018. [DOI] [PubMed] [Google Scholar]
- 126. Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol. 2015;30:152–163. [DOI] [PubMed] [Google Scholar]
- 127. McGirr A, Berlim MT, Bond DJ, et al. A systematic review and meta-analysis of randomized controlled trials of adjunctive ketamine in electroconvulsive therapy: efficacy and tolerability. J Psychiatr Res. 2015;62:23–30. [DOI] [PubMed] [Google Scholar]
- 128. Wan LB, Levitch CF, Perez AM, et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry. 2015;76:247–252. [DOI] [PubMed] [Google Scholar]
- 129. Fond G, Loundou A, Rabu C, et al. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl). 2014;231:3663–3676. [DOI] [PubMed] [Google Scholar]
- 130. Serafini G, Howland RH, Rovedi F, et al. The role of ketamine in treatment-resistant depression: a systematic review. Curr Neuropharmacol. 2014;12:444–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Liu Y, Zhou X, Zhu D, et al. Is pindolol augmentation effective in depressed patients resistant to selective serotonin reuptake inhibitors? A systematic review and meta-analysis. Hum Psychopharmacol. 2015;30:132–142. [DOI] [PubMed] [Google Scholar]
- 132. Han C, Wang SM, Kwak KP, et al. Aripiprazole augmentation versus antidepressant switching for patients with major depressive disorder: a 6-week, randomized, rater-blinded, prospective study. J Psychiatr Res. 2015;66-67:84–94. [DOI] [PubMed] [Google Scholar]
- 133. Gaynes BN, Dusetzina SB, Ellis AR, et al. Treating depression after initial treatment failure: directly comparing switch and augmenting strategies in STAR*D. J Clin Psychopharmacol. 2012;32:114–119. [DOI] [PubMed] [Google Scholar]
- 134. Ravindran AV, Kennedy SH, O’Donovan MC, et al. Osmotic-release oral system methylphenidate augmentation of antidepressant monotherapy in major depressive disorder: results of a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2008;69:87–94. [DOI] [PubMed] [Google Scholar]
- 135. Safarinejad MR. Reversal of SSRI-induced female sexual dysfunction by adjunctive bupropion in menstruating women: a double-blind, placebo-controlled and randomized study. J Psychopharmacol. 2011;25:370–378. [DOI] [PubMed] [Google Scholar]
- 136. Kriston L, von Wolff A, Westphal A, et al. Efficacy and acceptability of acute treatments for persistent depressive disorder: a network meta-analysis. Depress Anxiety. 2014;31:621–630. [DOI] [PubMed] [Google Scholar]
- 137. von Wolff A, Hölzel LP, Westphal A, et al. Selective serotonin reuptake inhibitors and tricyclic antidepressants in the acute treatment of chronic depression and dysthymia: a systematic review and meta-analysis. J Affect Disord. 2013;144:7–15. [DOI] [PubMed] [Google Scholar]
- 138. Keitner GI, Mansfield AK. Management of treatment-resistant depression. Psychiatr Clin North Am. 2012;35:249–265. [DOI] [PubMed] [Google Scholar]
- 139. Segmiller F, Rüther T, Linhardt A, et al. Repeated S-ketamine infusions in therapy resistant depression: a case series. J Clin Pharmacol. 2013;53:996–998. [DOI] [PubMed] [Google Scholar]
- 140. Dale E, Bang-Andersen B, Sánchez C. Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs. Biochem Pharmacol. 2015;95:81–97. [DOI] [PubMed] [Google Scholar]
- 141. Ibrahim L, Diaz Granados N, Jolkovsky L, et al. A Randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J Clin Psychopharmacol. 2012;32:551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Preskorn S, Macaluso M, Mehra DO, et al. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract. 2015;21:140–149. [DOI] [PubMed] [Google Scholar]
- 143. Quiroz JA, Tamburri P, Deptula D, et al. P.2.f.027. The efficacy and safety of basimglurant as adjunctive therapy in major depression: a randomised, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2014;24(Suppl 2):S468. [Google Scholar]
- 144. Connolly KR, Thase ME. Emerging drugs for major depressive disorder. Expert Opin Emerg Drugs. 2012;17:105–126. [DOI] [PubMed] [Google Scholar]
- 145. Na KS, Lee KJ, Lee JS, et al. Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:79–85. [DOI] [PubMed] [Google Scholar]
- 146. Majd M, Hashemian F, Hosseini SM, et al. A randomized, double-blind, placebo-controlled trial of celecoxib augmentation of sertraline in treatment of drug-naive depressed women: a pilot study. Iran J Pharm Res. 2015;14:891–899. [PMC free article] [PubMed] [Google Scholar]
- 147. Dell’Osso B, Ketter TA. Assessing efficacy/effectiveness and safety/tolerability profiles of adjunctive pramipexole in bipolar depression: acute versus long-term data. Int Clin Psychopharmacol. 2013;28:297–304. [DOI] [PubMed] [Google Scholar]
- 148. Cusin C, Iovieno N, Iosifescu DV, et al. A randomized, double-blind, placebo-controlled trial of pramipexole augmentation in treatment-resistant major depressive disorder. J Clin Psychiatry. 2013;74:e636–e641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Pae CU. Pramipexole augmentation in treatment-resistant major depressive disorder. Expert Rev Neurother. 2014;14:5–8. [DOI] [PubMed] [Google Scholar]
- 150. Veselinović T, Paulzen M, Gründer G. Cariprazine, a new, orally active dopamine D2/3 receptor partial agonist for the treatment of schizophrenia, bipolar mania and depression. Expert Rev Neurother. 2013;13:1141–1159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.