Abstract
Introduction
We have previously shown modest weight loss with vildagliptin treatment. Since body weight balance is associated with changes in blood pressure (BP) and fasting lipids, we have assessed these parameters following vildagliptin treatment.
Methods
Data were pooled from all double-blind, randomized, controlled, vildagliptin mono-therapy trials on previously drug-naïve patients with type 2 diabetes mellitus who received vildagliptin 50 mg once daily (qd) or twice daily (bid; n=2,108) and wherein BP and fasting lipid data were obtained.
Results
Data from patients receiving vildagliptin 50 mg qd or bid showed reductions from baseline to week 24 in systolic BP (from 132.5±0.32 to 129.8±0.34 mmHg; P<0.0001), diastolic BP (from 81.2±0.18 to 79.6±0.19 mmHg; P<0.0001), fasting triglycerides (from 2.00±0.02 to 1.80±0.02 mmol/L; P<0.0001), very low density lipoprotein cholesterol (from 0.90±0.01 to 0.83±0.01 mmol/L; P<0.0001), and low density lipoprotein cholesterol (from 3.17±0.02 to 3.04±0.02 mmol/L; P<0.0001), whereas high density lipoprotein cholesterol increased (from 1.19±0.01 to 1.22±0.01 mmol/L; P<0.001). Weight decreased by 0.48±0.08 kg (P<0.001).
Conclusion
This large pooled analysis demonstrated that vildagliptin shows a significant reduction in BP and a favorable fasting lipid profile that are associated with modest weight loss.
Keywords: TG, HDL, LDL, body weight DPP-4 inhibitor, GLP-1
Introduction
We have previously pooled data on vildagliptin monotherapy to compare body weight changes as a function of glycemic control at baseline and showed that, on an average, weight loss is observed with vildagliptin at the glycemic levels at which treatment is often initiated.1 Weight loss is associated with changes in blood pressure (BP) and fasting lipids. Data regarding BP and fasting lipids from the studies on dipeptidyl peptidase-4 (DPP-4) inhibitors are limited and may be confounded by variations among these studies in terms of the variable effects of other oral antidiabetic drugs, baseline adiposity, and insulin resistance that are also associated with changes in BP and fasting lipids.2–6
Here, we aimed to assess the impact of vildagliptin treatment on BP and fasting lipids and correlated these changes with changes in weight, body mass index (BMI), and homeostatic model assessment insulin resistance (HOMA-IR). To address these questions, we employed a large (>2,000) vildagliptin pooled monotherapy database.
Methods
Patients and study design
Data were pooled from eight, double-blind, randomized, controlled, vildagliptin monotherapy trials, including 2,108 previously drug-naïve patients with type 2 diabetes mellitus, who received vildagliptin 50 mg once daily (qd) (n=329) or twice daily (bid) (n=1,779) as a monotherapy and underwent an actual as well as a prespecified study visit, wherein weight, BP, and fasting lipids were assessed at week 24 (studies 1–3, 5, 9, and 12–14 enumerated in Table 1 from a review by Dejager et al7). The data resides in the Novartis vildagliptin database and was extracted and analyzed by Novartis database associates, as directed by the authors.
Table 1.
Mean changes in blood pressure, fasting lipid parameters, and weight with vildagliptin at week 24
| Parameters | Vildagliptin qd/bid n=2,108
|
Mean change | P-value | |
|---|---|---|---|---|
| Baseline value | Endpoint value | |||
| BP parameters, mean ± SE | ||||
| Systolic BP, mmHg | 132.5±0.32 | 129.8±0.34 | −2.70±0.30 | <0.0001 |
| Diastolic BP, mmHg | 81.2±0.18 | 79.6±0.19 | −1.64±0.18 | <0.0001 |
| Fasting lipid parameters, mean ± SE | ||||
| Fasting triglycerides (mmol/L) | 2.00±0.02 | 1.80±0.02 | −0.20±0.02 | <0.0001 |
| VLDL (mmol/L) | 0.90±0.01 | 0.83±0.01 | −0.07±0.01 | <0.0001 |
| LDL (mmol/L) | 3.17±0.02 | 3.04±0.02 | −0.13±0.02 | <0.0001 |
| Total cholesterol (mmol/L) | 5.26±0.02 | 5.08±0.02 | −0.18±0.02 | <0.0001 |
| Non-HDL (mmol/L) | 4.07±0.02 | 3.87±0.02 | −0.21±0.02 | <0.0001 |
| HDL (mmol/L) | 1.19±0.01 | 1.22±0.01 | 0.03±0.004 | <0.001 |
| Body weight (mean ± SE, kg) | 86.16±0.41 | 85.67±0.40 | −0.48±0.08 | <0.001 |
Abbreviations: BP, blood pressure; bid, twice daily; HDL, high density lipoprotein; LDL, low density lipoprotein; qd, once daily; SE, standard error; VLDL, very low density lipoprotein.
Assessments
Laboratory parameters were assessed by central laboratories: Bioanalytical Research Corporation-EU (Ghent, Belgium), Diabetes Diagnostics Laboratory (Columbia, MO, USA), and Covance (Geneva, Switzerland; Singapore; or Indianapolis, IN, USA). HOMA-IR was calculated based on the following formula: HOMA-IR = (fasting insulin [uU/mL]) × (fasting glucose [mmol/L])/22.5.
Data analysis
A linear regression model was applied to analyze the changes in BP and fasting lipid parameters relative to body weight changes, with/without adjusting for BMI and HOMA-IR.
Ethics and good clinical practice
All study participants provided written informed consent. All protocols were approved by the independent ethics committee/institutional review board at each study site or country. All studies were conducted using Good Clinical Practice and in accordance with the Declaration of Helsinki.
Results
This pooled analysis included 2,108 patients (54.6% male) with mean (± standard error) age 54.8±0.3 years, BMI 30.9±0.1 kg/m2, type 2 diabetes mellitus duration 2.1±0.1 years, glycated hemoglobin (HbA1c) 8.4%±0.0%, and fasting plasma glucose 9.9±0.1 mmol/L. Vildagliptin treatment (50 mg qd or bid) for 24 weeks resulted in modest but highly significant reductions in both systolic (2.70 mmHg) and diastolic (1.64 mmHg) BP. Fasting triglycerides (TG), very low density lipoprotein (VLDL) cholesterol, and low density lipoprotein (LDL) cholesterol decreased by 0.2, 0.07, and 0.13 mmol/L, respectively. Similarly, total cholesterol and non-high density lipoprotein (non-HDL) cholesterol decreased by 0.18 and 0.21 mmol/L from baseline, respectively. HDL cholesterol increased by 0.03 mmol/L and weight decreased by 0.48 kg (Table 1).
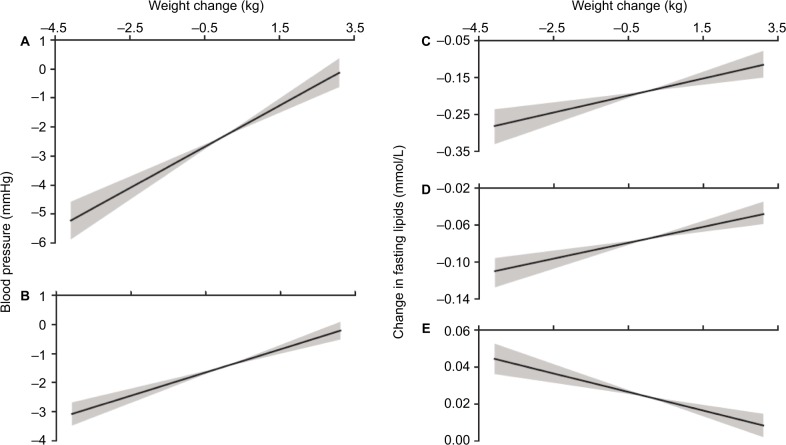
A linear regression model was used to analyze the relationship between the aforementioned BP and fasting lipid levels with weight changes, degree of adiposity as assessed by baseline BMI, and degree of insulin resistance as assessed by HOMA-IR. The changes in systolic (Figure 1A) and diastolic (Figure 1B) BP from baseline to 24 weeks relative to changes in weight showed positive slopes of 0.71 (r2=0.04; P<0.001) and 0.40 (r2=0.03; P<0.001), respectively. These values did not improve by adjusting for BMI and HOMA-IR. Positive slopes of 0.023 (r2=0.01; P<0.0001) and 0.010 (r2=0.01; P<0.0001) were observed for changes in TG (Figure 1C) and VLDL cholesterol (Figure 1D) levels from baseline to 24 weeks relative to changes in weight. These values improved to 0.028 (r2=0.03; P<0.0001) and 0.013 (r2=0.04; P<0.0001), respectively, by adjusting for BMI and HOMA-IR. Total cholesterol, LDL, and non-HDL cholesterol did not correlate with weight change, with or without adjusting for BMI or HOMA-IR. Changes in HDL cholesterol (Figure 1E) at 24 weeks relative to changes in weight showed a negative slope of 0.005 (r2=0.009; P<0.0001), and this value did not improve by adjusting for BMI and HOMA-IR.
Figure 1.
Change in systolic (A) and diastolic (B) blood pressure (mmHg), change in triglycerides (C), VLDL (D), and HDL (E) (mmol/L) as a function of change in body weight (kg).
Notes: Solid lines are the best fit regressions from all 2,108 data points and the shaded areas represent the minimum and maximum slopes calculated from the ±95% confidence limits of the slopes. The X-axis limits are set to two standard deviations around the mean change in weight.
Abbreviations: HDL, high density lipoprotein; VLDL, very low density lipoprotein.
Discussion
We have previously shown that, on an average, weight neutrality is essentially observed at higher baselines with vildagliptin, while a weight loss of ~1 kg was observed in patients with baseline HbA1c <8% (64 mmol/mol).1 The current analysis also demonstrated significant reductions in BP with a favorable fasting lipid profile. The slopes of these parameters versus weight change suggest that these changes are at least partially explained by weight reduction. Because weight reduction is greatest at glycemic levels where patients are most often treated,1 BP and fasting lipid benefits may actually be therapeutically greater than those indicated by the averages in this pooled analysis. There is no mechanistic basis for predicting that similar results would not be seen with other DPP-4 inhibitors.
Both vildagliptin 50 mg qd or bid are indicated as mono-therapy in many countries and clinical trials were carried out with both dosing frequencies. We have previously shown that when baseline HbA1c levels were below 8% there was no additional HbA1c reduction benefit to the twice daily dosing frequency with monotherapy.7 Sixteen percent of the patients in the current analysis were on the 50 mg qd dose; notably the 50 mg qd group was 4 years older and had baseline HbA1c levels 1.2% lower than the 50 mg bid group. Weight loss was greater in the 50 mg qd group (−1.2 kg in the 50 mg qd group versus −0.4 kg in the 50 mg bid group) as predicted previously by the lower baseline HbA1c and presumably lower degree of glycosuria1 in the 50 mg qd group; the reduction in diastolic BP was ~50% greater and the TG reduction was ~50% lower (from a lower TG baseline of 1.8 versus 2.0 mmol/L) in the 50 mg qd group versus the 50 mg bid group; the correlations were not driven by inclusion of the 50 mg qd dose (data not shown). None of the differences were considered notable enough to justify reporting the data in independent pools and thus we have chosen to report the BP and fasting lipids results in a single pool as we did previously with the weight.1
The BP and fasting lipids results are associated with some caveats. The average weight change is small (approximately −0.5 kg), and the measurements of BP and weight in the large clinical trials utilized for the current pool are expected to be associated with a high degree of variability, which presumably explains the reason for low correlation coefficient (r2). In contrast, the n value is very high, hence yielding significant slopes. Partial correlation analysis suggests that the changes in TG and VLDL cholesterol are also partially explained by the degree of adiposity and insulin resistance. These effects of adiposity and insulin resistance or the lack thereof must be interpreted with some caution due to the limitations of the BMI and HOMA-IR surrogates of adiposity and insulin resistance measured in these clinical trials. Furthermore, the studies pooled for this analysis were designed to assess BP and fasting lipids as secondary endpoints, and no data on confounding BP and lipid medications were collected.
Thus, therapeutically, DPP-4 inhibitors yield modest benefits of unknown clinical importance on BP and fasting lipids. Although these BP and fasting lipid benefits of DPP-4 inhibitors have not translated into cardiovascular risk reduction over 3 years,8 it is possible that a primary prevention trial of longer duration might contribute to a cardiovascular benefit after a longer follow-up. Notably, hypotension has not been identified as a side effect with vildagliptin.9
Acknowledgments
This study was funded by Novartis Pharma AG. The authors thank Nihal Maremanda, Novartis Healthcare Pvt. Ltd., Hyderabad, India, for the editorial support.
Footnotes
Disclosure
ME has received honoraria as an advisory panel member and is a speaker for Novo Nordisk, Sanofi Aventis, Novartis, and MSD. JEF and AS are employed by and own shares in Novartis.
References
- 1.Blüher M, Schweizer A, Bader G, Foley JE. Changes in body weight after 24 weeks of vildagliptin therapy as a function of fasting glucose levels in patients with type 2 diabetes. Vasc Health Risk Manag. 2014;10:661–664. doi: 10.2147/VHRM.S73608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doll S, Paccaud F, Bovet P, Burnier M, Wietlisbach V. Body mass index, abdominal adiposity and blood pressure: consistency of their association across developing and developed countries. Int J Obes Relat Metab Disord. 2002;26(1):48–57. doi: 10.1038/sj.ijo.0801854. [DOI] [PubMed] [Google Scholar]
- 3.Flock MR, Green MH, Kris-Etherton PM. Effects of adiposity on plasma lipid response to reductions in dietary saturated fatty acids and cholesterol. Adv Nutr. 2011;2(3):261–274. doi: 10.3945/an.111.000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56(2):320–328. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26(2):19–39. [PMC free article] [PubMed] [Google Scholar]
- 6.Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure a meta-analysis of randomized controlled trials. Hypertension. 2003;42(5):878–884. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 7.Dejager S, Schweizer A, Foley JE. Evidence to support the use of vilda-gliptin monotherapy in the treatment of type 2 diabetes mellitus. Vasc Health Risk Manag. 2012;8:339–348. doi: 10.2147/VHRM.S31758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 9.Galvus® Summary of Product Characteristics. [Accessed July 13, 2016]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000771/WC500020327.pdf.