Abstract
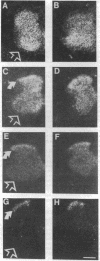
Quantitative receptor autoradiography was used to map the distribution of N-methyl-D-aspartate (NMDA) receptors in the developing rat spinal cord. Three different specific ligands, which label partially overlapping subpopulations of NMDA receptors, were used: an agonist (L-[3H]glutamate), a noncompetitive antagonist ([3H]MK-801), and a competitive antagonist ([3H]CGP-39653). In the adult, NMDA receptors labeled with all three ligands are restricted to the substantia gelatinosa in the spinal dorsal horn. In marked distinction, at postnatal day 7 NMDA receptors labeled with L-[3H]glutamate and [3H]MK-801 are present throughout the spinal gray matter. NMDA receptors in the neonatal spinal ventral horn have a higher affinity for L-[3H]glutamate than those in the adult substantia gelatinosa. Over the second and third postnatal weeks, NMDA receptors are lost from all areas of the spinal gray matter except for the substantia gelatinosa. Neonatal NMDA receptors identified with [3H]CGP-39653 are restricted to the substantia gelatinosa. These results show that the immature ventral horn contains a subpopulation of NMDA receptors and raise the possibility that motor neurons transiently express NMDA receptors in early postnatal life. Ventral horn NMDA receptors may be a component of the mechanisms by which the mature phenotype of motor neurons is acquired through activity-dependent processes. The loss of NMDA receptors over the course of development may play a role in limiting the period of motor neuron plasticity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bode-Greuel K. M., Singer W. The development of N-methyl-D-aspartate receptors in cat visual cortex. Brain Res Dev Brain Res. 1989 Apr 1;46(2):197–204. doi: 10.1016/0165-3806(89)90283-6. [DOI] [PubMed] [Google Scholar]
- Cline H. T., Constantine-Paton M. NMDA receptor agonist and antagonists alter retinal ganglion cell arbor structure in the developing frog retinotectal projection. J Neurosci. 1990 Apr;10(4):1197–1216. doi: 10.1523/JNEUROSCI.10-04-01197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline H. T., Debski E. A., Constantine-Paton M. N-methyl-D-aspartate receptor antagonist desegregates eye-specific stripes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4342–4345. doi: 10.1073/pnas.84.12.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradi S., Ronnevi L. O. Spontaneous elimination of synapses on cat spinal motoneurons after birth: do half of the synapses on the cell bodies disappear? Brain Res. 1975 Jul 18;92(3):505–510. doi: 10.1016/0006-8993(75)90338-8. [DOI] [PubMed] [Google Scholar]
- Cummings J. P., Stelzner D. J. Prenatal and postnatal development of lamina IX neurons in the rat thoracic spinal cord. Exp Neurol. 1984 Jan;83(1):155–166. doi: 10.1016/0014-4886(84)90054-2. [DOI] [PubMed] [Google Scholar]
- D'Amato R. J., Blue M. E., Largent B. L., Lynch D. R., Ledbetter D. J., Molliver M. E., Snyder S. H. Ontogeny of the serotonergic projection to rat neocortex: transient expression of a dense innervation to primary sensory areas. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4322–4326. doi: 10.1073/pnas.84.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdö S. L., Wolff J. R. Postnatal development of the excitatory amino acid system in visual cortex of the rat. Changes in ligand binding to NMDA, quisqualate and kainate receptors. Int J Dev Neurosci. 1990;8(2):199–204. doi: 10.1016/0736-5748(90)90011-p. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Comparison of L-[3H]glutamate, D-[3H]aspartate, DL-[3H]AP5 and [3H]NMDA as ligands for NMDA receptors in crude postsynaptic densities from rat brain. Eur J Pharmacol. 1987 Jan 20;133(3):291–300. doi: 10.1016/0014-2999(87)90025-2. [DOI] [PubMed] [Google Scholar]
- Fox K., Sato H., Daw N. The location and function of NMDA receptors in cat and kitten visual cortex. J Neurosci. 1989 Jul;9(7):2443–2454. doi: 10.1523/JNEUROSCI.09-07-02443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenamyre J. T., Olson J. M., Penney J. B., Jr, Young A. B. Autoradiographic characterization of N-methyl-D-aspartate-, quisqualate- and kainate-sensitive glutamate binding sites. J Pharmacol Exp Ther. 1985 Apr;233(1):254–263. [PubMed] [Google Scholar]
- Gu Q. A., Bear M. F., Singer W. Blockade of NMDA-receptors prevents ocularity changes in kitten visual cortex after reversed monocular deprivation. Brain Res Dev Brain Res. 1989 Jun 1;47(2):281–288. doi: 10.1016/0165-3806(89)90183-1. [DOI] [PubMed] [Google Scholar]
- Guimarães A., Zaremba S., Hockfield S. Molecular and morphological changes in the cat lateral geniculate nucleus and visual cortex induced by visual deprivation are revealed by monoclonal antibodies Cat-304 and Cat-301. J Neurosci. 1990 Sep;10(9):3014–3024. doi: 10.1523/JNEUROSCI.10-09-03014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore T., Drejeŕ J., Nielsen E. O., Nielsen M. Non-NMDA glutamate receptor antagonist 3H-CNOX binds with equal affinity to two agonist states of quisqualate receptors. Biochem Pharmacol. 1989 Oct 1;38(19):3207–3212. doi: 10.1016/0006-2952(89)90615-1. [DOI] [PubMed] [Google Scholar]
- Honoré T., Drejer J., Nielsen M. Calcium discriminates two [3H]kainate binding sites with different molecular target sizes in rat cortex. Neurosci Lett. 1986 Mar 28;65(1):47–52. doi: 10.1016/0304-3940(86)90118-7. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Yoshioka K. Ia afferent excitation of motoneurones in the in vitro new-born rat spinal cord is selectively antagonized by kynurenate. J Physiol. 1986 Jan;370:515–530. doi: 10.1113/jphysiol.1986.sp015948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt D. C., Zukin S. R. Biexponential kinetics of [3H]MK-801 binding: evidence for access to closed and open N-methyl-D-aspartate receptor channels. Mol Pharmacol. 1989 Apr;35(4):387–393. [PubMed] [Google Scholar]
- Kalb R. G., Hockfield S. Induction of a neuronal proteoglycan by the NMDA receptor in the developing spinal cord. Science. 1990 Oct 12;250(4978):294–296. doi: 10.1126/science.2145629. [DOI] [PubMed] [Google Scholar]
- Kalb R. G., Hockfield S. Large diameter primary afferent input is required for expression of the Cat-301 proteoglycan on the surface of motor neurons. Neuroscience. 1990;34(2):391–401. doi: 10.1016/0306-4522(90)90148-w. [DOI] [PubMed] [Google Scholar]
- Kalb R. G., Hockfield S. Molecular evidence for early activity-dependent development of hamster motor neurons. J Neurosci. 1988 Jul;8(7):2350–2360. doi: 10.1523/JNEUROSCI.08-07-02350.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt A., Bear M. F., Singer W. Blockade of "NMDA" receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987 Oct 16;238(4825):355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T., Kashiwabuchi N., Mori H., Sakimura K., Kushiya E., Araki K., Meguro H., Masaki H., Kumanishi T., Arakawa M. Molecular diversity of the NMDA receptor channel. Nature. 1992 Jul 2;358(6381):36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Lidow M. S., Goldman-Rakic P. S., Rakic P., Gallager D. W. Differential quenching and limits of resolution in autoradiograms of brain tissue labeled with 3H-, 125I- and 14C-compounds. Brain Res. 1988 Aug 30;459(1):105–119. doi: 10.1016/0006-8993(88)90290-9. [DOI] [PubMed] [Google Scholar]
- Lidow M. S., Goldman-Rakic P. S., Rakic P. Synchronized overproduction of neurotransmitter receptors in diverse regions of the primate cerebral cortex. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10218–10221. doi: 10.1073/pnas.88.22.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London E. D., Coyle J. T. Specific binding of [3H]kainic acid to receptor sites in rat brain. Mol Pharmacol. 1979 May;15(3):492–505. [PubMed] [Google Scholar]
- Meguro H., Mori H., Araki K., Kushiya E., Kutsuwada T., Yamazaki M., Kumanishi T., Arakawa M., Sakimura K., Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992 May 7;357(6373):70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985 Nov;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Identification and properties of N-methyl-D-aspartate receptors in rat brain synaptic plasma membranes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7532–7536. doi: 10.1073/pnas.83.19.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D. T., Olverman H. J., Nguyen L., Watkins J. C., Cotman C. W. Two classes of N-methyl-D-aspartate recognition sites: differential distribution and differential regulation by glycine. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9836–9840. doi: 10.1073/pnas.85.24.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P. H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992 May 22;256(5060):1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Ronnevi L. O., Conradi S. Ultrastructural evidence for spontaneous elimination of synaptic terminals on spinal motoneurons in the kitten. Brain Res. 1974 Nov 15;80(2):335–339. doi: 10.1016/0006-8993(74)90696-9. [DOI] [PubMed] [Google Scholar]
- Sakurai S. Y., Cha J. H., Penney J. B., Young A. B. Regional distribution and properties of [3H]MK-801 binding sites determined by quantitative autoradiography in rat brain. Neuroscience. 1991;40(2):533–543. doi: 10.1016/0306-4522(91)90139-f. [DOI] [PubMed] [Google Scholar]
- Scherer W. J., Udin S. B. N-methyl-D-aspartate antagonists prevent interaction of binocular maps in Xenopus tectum. J Neurosci. 1989 Nov;9(11):3837–3843. doi: 10.1523/JNEUROSCI.09-11-03837.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz C. J. Impulse activity and the patterning of connections during CNS development. Neuron. 1990 Dec;5(6):745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Sur M., Frost D. O., Hockfield S. Expression of a surface-associated antigen on Y-cells in the cat lateral geniculate nucleus is regulated by visual experience. J Neurosci. 1988 Mar;8(3):874–882. doi: 10.1523/JNEUROSCI.08-03-00874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel T. N. Postnatal development of the visual cortex and the influence of environment. Nature. 1982 Oct 14;299(5884):583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A., Priestley T., Knight A. R., Woodruff G. N., Iversen L. L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaremba S., Guimaraes A., Kalb R. G., Hockfield S. Characterization of an activity-dependent, neuronal surface proteoglycan identified with monoclonal antibody Cat-301. Neuron. 1989 Mar;2(3):1207–1219. doi: 10.1016/0896-6273(89)90305-x. [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L. NMDA receptors mediate poly- and monosynaptic potentials in motoneurons of rat embryos. J Neurosci. 1990 Jan;10(1):125–135. doi: 10.1523/JNEUROSCI.10-01-00125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivin J. A., Waud D. R. How to analyze binding, enzyme and uptake data: the simplest case, a single phase. Life Sci. 1982 Apr 26;30(17):1407–1422. doi: 10.1016/0024-3205(82)90554-9. [DOI] [PubMed] [Google Scholar]