Abstract
Considerable neuropsychological and neuroimaging work indicates that the medial temporal lobes are critical for both item and relational memory retrieval. However, there remain outstanding issues in the literature, namely the extent to which medial temporal lobe regions are differentially recruited during incidental and intentional retrieval of item and relational information, and the extent to which aging may affect these neural substrates. The current fMRI study sought to address these questions; participants incidentally encoded word pairs embedded in sentences and incidental item and relational retrieval were assessed through speeded reading of intact, rearranged, and new word-pair sentences, while intentional item and relational retrieval were assessed through old/new associative recognition of a separate set of intact, rearranged, and new word pairs. Results indicated that, in both younger and older adults, anterior hippocampus and perirhinal cortex indexed incidental and intentional item retrieval in the same manner. In contrast, posterior hippocampus supported incidental and intentional relational retrieval in both age groups and an adjacent cluster in posterior hippocampus was recruited during both forms of relational retrieval for older, but not younger, adults. Our findings suggest that while medial temporal lobe regions do not differentiate between incidental and intentional forms of retrieval, there are distinct roles for anterior and posterior medial temporal lobe regions during retrieval of item and relational information, respectively, and further indicate that posterior regions may, under certain conditions, be over-recruited in healthy aging.
Keywords: hippocampus, perirhinal cortex, relational memory, item memory, aging
INTRODUCTION
Intentional memory retrieval is a rich process that involves the conscious recovery of previously experienced events. This phenomenon has been conceptualized in various forms, such as the retrieval of episodic memories (Tulving, 1984), and is thought to depend on the medial temporal lobe (MTL; Squire and Zola-Morgan, 1991). The MTL consists of the hippocampus (HC) and parahippocampal gyrus, and recent models—based on differences in anatomy, connectivity, and function—emphasize distinct roles for MTL regions along the longitudinal axis (Ranganath and Ritchey, 2012; Poppenk et al., 2013). Specifically, anterior HC and perirhinal cortex (PRC)—defined as at, or anterior to, y = −21 in MNI space (Poppenk et al., 2013)—are thought to be more sensitive to item memory (IM), whereas posterior HC and parahippocampal cortex are thought to be more critical for context, or relational, memory (RM), such as the association between two items (e.g., word pair) or the association between an item and a spatial location (Ranganath and Ritchey, 2012).
This distinction between IM and RM can be extended beyond intentional memory retrieval into incidental forms of retrieval (Henke, 2010; Dew and Cabeza, 2011). The incidental retrieval of previously encountered items or associations can be behaviorally indexed during a cognitive task through speeded responses or implicit measures, such as eye movements (for reviews, see Hannula and Greene, 2012; Giovanello and Dew, 2015), and the same MTL regions that subserve intentional IM and RM are also thought to support incidental IM and RM (for review, see Henke, 2010). This assertion, however, remains an open question, as there are also those who propose that MTL primarily mediates intentional forms of memory, whereas other cortical regions subserve incidental forms of memory (Squire and Zola-Morgan, 1991; Squire and Dede, 2015).
Investigating similarities and differences in the neural substrates of incidental and intentional forms of IM and RM retrieval is particularly important given that one hallmark of healthy aging is a deficit in RM. This deficit has largely been observed in intentional RM (e.g., source or associative memory recognition; Johnson et al., 1993; Naveh-Benjamin, 2000) and is thought to be driven, in part, by a deficit in relational binding (associative deficit hypothesis; Naveh-Benjamin, 2000). Many neuroimaging studies of healthy aging have reported differences in MTL activity in older adults (OAs) relative to younger adults (YAs) during intentional RM retrieval, with some finding activity increases in OAs relative to YAs (Morcom et al., 2007; Duverne et al., 2008; Dew et al., 2012), and others reporting the opposite pattern (Kukolja et al., 2009; Tsukiura et al., 2011; Giovanello and Schacter, 2012).
The current fMRI study sought to extend these previous findings by (1) specifying the role of MTL regions in incidental and intentional forms of IM and RM retrieval, and (2) clarifying the effect of aging on these neural substrates. Toward this goal, participants incidentally encoded word pairs embedded in sentences; incidental IM and RM retrieval were tested by examining speeded reading of intact (i.e., old association between old items), rearranged (i.e., old items but novel association), and new (i.e., novel association and items) word-pair sentences, and intentional IM and RM retrieval were tested by examining old/new associative recognition of a separate set of intact, rearranged, and new word pairs. Thus, similar to previous studies (e.g., Rugg et al., 1997), intentional retrieval was assessed using a recognition task that required a purposeful memory search, while incidental retrieval was assessed using a task that did not require intentional memory, yet would still likely elicit the automatic retrieval of previously studied information. In both retrieval tasks, IM was assessed by contrasting rearranged and new trials (i.e., retrieval of old items) and RM was assessed by contrasting intact and rearranged trials (i.e., retrieval of old associations).
Reaction times (RTs), after removing outliers > 3 standard deviations from the mean, are shown in Table 1. Responses were quicker for active baseline trials (i.e., reading a row of X’s) compared to sentences in both age groups (ts > 2.95, Ps < 0.01), indicating that RTs were a valid measure of reading speed. Behaviorally, we assessed incidental IM as the RT difference between rearranged and new trials, and we assessed incidental RM as the RT difference between rearranged and intact trials (Table 1). Both YAs (t[25] = 3.47, P = 0.001) and OAs (t[18] = 2.30, P < 0.05) exhibited significant incidental IM. Similarly, both YAs (t[25] = 2.75, P = 0.01) and OAs (t[18] = 2.02, P = 0.058) exhibited significant incidental RM, although it was only marginally significant in OAs. Critically, however, the incidental memory effects did not significantly differ between age groups (Ps > 0.15). Thus, RTs were quicker as the amount of stimulus novelty decreased for both YAs and OAs.
TABLE 1.
Reaction Times in Milliseconds During Incidental Retrieval of Item and Relational Information
| Younger | Older | ||
|---|---|---|---|
| Intact Trials | 1273 (99) | 1344 (109) | |
| Rearranged Trials | 1352 (109) | 1392 (118) | |
| New Trials | 1460 (125) | 1422 (115) | |
| Active Baseline Trials | 1167 (104) | 997 (64) | |
| Item Priming | New – Rearranged | 108 (31) | 49 (22) |
| Relational Priming | Rearranged – Intact | 79 (29) | 48 (24) |
Standard error is denoted in parentheses.
In the intentional retrieval test, we calculated intentional IM performance as rearranged correct rejections greater than new false alarms and we calculated intentional RM performance as intact hits greater than rearranged false alarms (Table 2). Both YAs (t[25] = 6.79, P < 0.001) and OAs (t[18] = 2.76, P = 0.01) exhibited significant IM, and likewise both YAs (t[25] = 5.93, P < 0.001) and OAs (t[18] = 3.24, P < 0.005) exhibited significant RM. As with the incidental test, there were no differences between age groups (Ps > 0.21).
TABLE 2.
Behavioral Accuracy During Intentional Retrieval of Item and Relational Information
| Younger | Older | ||
|---|---|---|---|
| Intact Trials | Hit | .66 (.03) | .64 (.06) |
| Miss | .34 (.03) | .36 (.06) | |
| Rearranged Trials | Correct Rejection | .62 (.03) | .55 (.05) |
| False Alarm | .38 (.04) | .45 (.05) | |
| New Trials | Correct Rejection | .83 (.03) | .75 (.06) |
| False Alarm | .17 (.03) | .25 (.06) | |
| Item Memory | Rearranged Correct Rejection – New False Alarm | .46 (.07) | .30 (.11) |
| Relational Memory | Intact Hit – Rearranged False Alarm | .28 (.05) | .20 (.06) |
Standard error is denoted in parentheses.
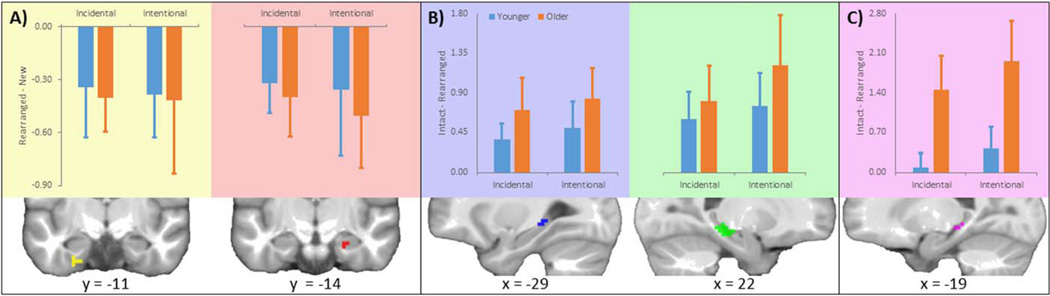
To investigate the neural correlates of incidental and intentional IM retrieval, rearranged and new trials (excluding outliers in the incidental condition and incorrect decisions in the intentional condition) for YAs and OAs were entered into a 2×2×2 (age × retrieval intention × trial type) ANOVA. We focused on the main effect of trial type (i.e., IM retrieval) and interactions with trial type (i.e., interactions with age and/or intention). Two anterior MTL clusters – specifically left PRC (peak MNI coordinates: x = −21, y = −9, z = −39; Cluster Size [CS] = 6; Z score = 3.63) and right anterior HC (x = 24, y = − 18, z = − 21; CS = 6; Z = 3.32) – exhibited a main effect of IM retrieval as there was greater activity for new relative to rearranged trials for both age groups during both intentional and incidental retrieval (Fig. 1A). No suprathreshold clusters exhibited greater activity for rearranged relative to new trials, and no significant interactions were observed. Whole brain results are presented in Supporting Information Table 1.
FIGURE 1.
Medial temporal lobe regions exhibiting item and relational memory effects. A: Anterior hippocampus and perirhinal cortex exhibited activity reductions for rearranged relative to new trials during incidental and intentional retrieval for both age groups. B: Posterior hippocampus exhibited activity increases for intact relative to rearranged trials during incidental and intentional retrieval for both age groups. C: An additional posterior hippocampus cluster exhibited activity increases for intact relative to rearranged trials during both incidental and intentional retrieval for older but not younger adults. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To investigate the neural correlates of incidental and intentional RM retrieval, we set up a similar 2×2×2 ANOVA, but with intact and rearranged trials (again excluding outliers in the incidental condition and incorrect decisions in the intentional condition). In contrast to IM retrieval, posterior HC appeared to be sensitive to RM retrieval (Fig. 1B). Specifically, left (x = − 24, y = − 33, z = − 3; CS = 11; Z = 4.95) and right (x = 24, y = − 27, z = − 12; CS = 40; Z = 4.55) posterior HC exhibited greater activity for intact relative to rearranged trials across both age groups during both incidental and intentional retrieval. Critically, there was also evidence of an age × trial type interaction (Fig. 1C). An additional left posterior HC cluster (x = − 21, y = − 30, z = − 6; CS = 8; Z = 3.73) yielded greater activity in intact than rearranged trials for OAs relative to YAs during both incidental and intentional retrieval. No suprathreshold clusters had greater activity for rearranged relative to intact trials. Whole brain results are presented in Supporting Information Table 2.
Our results illustrate a clear distinction in both age groups between IM and RM; anterior HC and PRC subserved incidental and intentional IM retrieval, and posterior HC subserved incidental and intentional RM retrieval. In addition, posterior HC was over-recruited in OAs during both incidental and intentional RM retrieval. Although we cannot definitively rule out differences in task difficulty as the source of this over-recruitment, behavioral performance was matched and OAs were given ample time to respond. As such, this age effect is unlikely to be driven by differences in task difficulty and certainly not driven by differences in performance. Taken together, these results suggest that anterior and posterior MTL regions, respectively, mediate IM and RM retrieval, and further indicate that posterior HC may be differentially recruited in healthy aging during RM retrieval.
Our results are broadly consistent with the notion that anterior MTL supports item representations, while posterior MTL supports context representations (Ranganath and Ritchey, 2012). First, we observed greater activity for new than rearranged trials indexing both incidental and intentional IM retrieval in anterior HC and PRC. Thus, anterior MTL appears to be sensitive to IM, both when participants are incidentally re-processing previously encountered items and when they are intentionally judging whether these items are old or new. Activity reductions for rearranged relative to new trials may reflect either novelty detection for new items and/or repetition suppression (i.e., familiarity) for old items (Habib, 2001). Our results do not allow us to definitively conclude which of these two processes are mediating the IM retrieval effects, although it has been proposed that anterior MTL regions, such as PRC, may support both processes (Fernandez and Tendolkar, 2006). In either case, our finding is consistent with neuroimaging studies that report activity reductions for old relative to new items during intentional IM retrieval in anterior HC (e.g., Rugg et al., 2003; Daselaar et al., 2006; Johnson et al., 2008; Vilberg and Rugg, 2009; Suzuki et al., 2011) and PRC (e.g., Gonsalves et al., 2005; Henson et al., 2003; Rugg et al., 2003; Daselaar et al., 2006; Montaldi et al., 2006; Vilberg and Rugg, 2009; Wang et al., 2014), as well as during incidental IM retrieval in anterior HC (e.g., Strange et al., 1999; Ganel et al., 2006; Poppenk et al., 2008; Balderston et al., 2011) and PRC (e.g., O’Kane et al., 2005; Ganel et al., 2006; Poppenk et al., 2008; Voss et al., 2012; Heusser et al., 2013; Wang et al., 2014).
Additionally, we observed greater activity for intact than rearranged trials indexing both incidental and intentional RM retrieval in posterior HC. This finding is consistent with studies reporting greater activity for old associations in posterior HC during both intentional (Preston et al, 2004; Prince et al., 2005; Hall et al., 2014) and incidental (e.g., Hannula and Ranganath, 2009; Hall et al., 2014) RM retrieval, and is thought to reflect the reinstatement of previously studied associations (Giovanello, Schnyer, and Verfaellie, 2009). Moreover, these patterns were seen in both age groups, indicating that the differential sensitivity of anterior MTL and posterior HC to IM and RM retrieval, respectively, is present in healthy OAs. We do note, however, that there are instances where only anterior (Giovanello et al., 2004; Kirwan and Stark, 2004) or both anterior and posterior HC (Giovanello et al., 2009; Hannula and Ranganath, 2009; Hannula et al., 2013) are recruited during intentional RM retrieval (although, consistent with our results, only posterior HC was recruited for both incidental and intentional RM retrieval in Hannula and Ranganath, 2009). Thus, it may not be the case that there is a simple dichotomy between anterior and posterior MTL, but rather a continuum (Strange et al., 2014) wherein anterior HC is relatively more sensitive to IM and posterior HC is relatively more sensitive to RM. Consistent with this view, our posterior HC clusters extended into anterior HC at a more liberal statistical threshold (P < 0.005). Moreover, there are methodological differences between our study and those finding intentional RM retrieval effects in anterior HC, such as using cued recall rather than recognition (e.g., Hannula et al., 2013), or contrasting all associative versus all item trials rather than intact versus rearranged trials (e.g., Giovanello et al., 2004). Thus it remains important for future research to examine the conditions under which anterior and posterior HC are recruited during RM retrieval.
One alternative interpretation is that our IM effects in anterior MTL may reflect encoding (of new trials), whereas the RM effects in posterior HC may reflect retrieval. This possibility is consistent with results from a recent meta-analysis (Spaniol et al., 2009) and previous studies (e.g., Lepage et al., 1998). However, no suprathreshold clusters exhibited more activity for rearranged than new trials in posterior MTL (i.e., retrieval of old items) and no suprathreshold clusters exhibited more activity for rearranged than intact trials in anterior MTL (i.e., encoding of new associations). Thus, our results suggest that, at the least, posterior HC is most sensitive to RM retrieval (rather than retrieval in general) and anterior MTL is most sensitive to IM familiarity (rather than encoding in general).
Interestingly, although posterior HC appeared to be sensitive to both incidental and intentional RM retrieval in YAs and OAs, we also observed an additional posterior HC cluster that was sensitive to RM retrieval in OAs, but not in YAs. RM performance was equated between YAs and OAs, yet this cluster exhibited age-related increases when assessing both incidental and intentional RM. This suggests that regardless of memory intention, OAs will have difficulty with RM – consistent with the associative deficit hypothesis (Naveh-Benjamin, 2000)—and the over-recruitment of posterior HC above and beyond the age-invariant effect may either be a direct result of this difficulty (i.e., compensation; Cabeza, 2002; Davis et al., 2008; Park and Reuter-Lorenz, 2009), and/or a byproduct (i.e., neural inefficiency; Morcom et al., 2007; Rypma and D’Esposito, 2000). This over-recruitment may also be related to evidence of decreased posterior relative to anterior HC volume in aging (Raz, 2000; Driscoll et al., 2003). This finding is consistent with prior studies reporting activity increases in HC in OAs relative to YAs (Morcom et al., 2007; Duverne et al., 2008; Dew et al., 2012), but it is not entirely clear why our study elicited over-recruitment of posterior HC in OAs relative to YAs, while other studies observed activity reductions in HC in OAs relative to YAs (Kukolja et al., 2009; Tsukiura et al., 2011; Giovanello and Schacter, 2012). While it is unclear why these studies yielded results inconsistent with our finding, it is notable that, rather than comparing intact and rearranged associative word pairs, these studies either contrasted all associative versus all item trials (Giovanello and Schacter, 2012) or did not use word pairs and tested source or spatial context (Kukolja et al., 2009; Tsukiura et al., 2011). Thus, this remains an inconsistency that must be fully characterized to understand the role of HC in processes such as neural inefficiency and compensation, and obtaining a better understanding of how OAs may differ from YAs in these different types of RM retrieval is a critical first step.
Notably, we found similar IM and RM effects across both incidental and intentional retrieval conditions, even though we restricted responses to correct decisions in the intentional condition and only excluded outliers in the incidental condition. These results suggest that a successful purposeful search is not required to recruit MTL regions during IM or RM retrieval, but it remains important for future work to utilize a dependent measure other than RTs in order to better separate trials with greater incidental retrieval from those with little or no incidental retrieval.
In a broader context, our data are consistent with recent proposals emphasizing shared neural correlates for incidental and intentional forms of memory retrieval (Henke, 2010; Dew and Cabeza, 2011). Additionally, our results also accord with evidence of longitudinal-axis differences in MTL. We found that anterior MTL related to IM retrieval, consistent with its proposed role in item and/or familiarity memory (Ranganath and Ritchey, 2012) and global or relative (i.e., rearranged items from two different associations) representations (Poppenk et al., 2013), whereas posterior HC related to RM retrieval, consistent with its proposed role in recollection and/or context memory (Ranganath and Ritchey, 2012) and fine-grained or precise (i.e., reinstatement) representations (Poppenk et al., 2013). Whether these anterior-posterior distinctions are discrete or continuous (Strange et al., 2014) remains an important question for future studies.
In conclusion, the current study sought to elucidate the role of MTL regions in incidental and intentional retrieval of IM and RM, as well as the effect of aging on these neural substrates. Our results indicated that – across both age groups and both incidental and intentional retrieval conditions – anterior MTL primarily supported IM retrieval, whereas posterior HC primarily supported RM retrieval. Moreover, an additional posterior HC cluster was sensitive to RM retrieval in OAs, but not YAs, possibly reflecting over-recruitment of HC in support of RM in healthy aging. Together these results indicate that MTL regions differentially support IM and RM, irrespective of retrieval intention, and under certain conditions, may be over-recruited in healthy aging.
DETAILED METHODS
Procedures were approved by the UNC – Chapel Hill institutional review board. Twenty-nine YAs and 23 OAs from the university and surrounding communities were recruited and paid for their participation. Three YAs were excluded for excessive in-scanner motion (N = 1) and poor behavioral performance (N = 2). Four OAs were excluded for excessive in-scanner motion (N = 1), poor behavioral performance (N = 2), and an inability to remain in the scanner for the duration of the study (N = 1). Analyses were performed on 26 YAs (M age = 20.6, SD = 4.8; M education = 15.1, SD = 2.3; 14 female) and 19 OAs (M age = 71.3, SD = 4.1; M education = 16.3, SD = 2.8; 15 female). OAs were prescreened in a separate session wherein they completed a battery of neuropsychological tests to confirm the absence of dementia.
Materials composed of 108 one- to three-syllable unrelated noun word pairs (M frequency = 60.3; SD = 53.3) were counterbalanced across retrieval type (incidental and intentional) and retrieval condition (intact, rearranged, and new). A sentence was created for each word pair to link them together (e.g., “The TOOL powered the MACHINE”; “The IRON repaired the EQUIPMENT”). Rearranged word pairs were created by swapping the first word of intact pairs (e.g., “The IRON powered the MACHINE”; “The TOOL repaired the EQUIPMENT”). The sentences allowed us to control the extent to which YAs and OAs associated the word pairs, as there is evidence that providing OAs with elaborative encoding strategies can ameliorate RM deficits (Glisky et al., 2001; Giovanello and Schacter, 2012).
Following informed consent, participants completed three runs of a sentence reading task followed by a single run of an associative memory test in the fMRI scanner. The incidental retrieval task and its encoding task corresponded to the first two runs of the experiment. The third sentence reading run corresponded to the encoding task for the last run: the associative memory test (i.e., the intentional retrieval task). In order to minimize the usage of intentional retrieval strategies in the incidental task, participants were not told that the word pairs corresponded to any sort of memory test and we did not counterbalance the order of the tasks.
In the sentence reading task, participants were presented with word-pair sentences, one at a time, intermixed with active baseline trials consisting of a row of X’s (i.e., “XXXX XXXXX XXXXXX XXXXX XXXX”). For both trial types, participants were instructed to press the key on their right index finger after reading the presented trial in its entirety. In the associative memory test, participants were presented with word pairs, one at a time, and prompted to indicate whether they were “previously seen together.” The right index finger corresponded to “yes” whereas the right middle finger corresponded to “no.” These word pairs were intermixed with active baseline trials consisting of a pair of characters: “&&&&&” and “#####.” Their orders were randomized (e.g., “##### &&&&&”) and participants were prompted to indicate whether the “&&&&&” was presented on the left or the right. Here the right index finger corresponded to “left” whereas the right middle finger corresponded to “right.”
The two encoding runs contained 36 trials each, half intact at retrieval and half rearranged at retrieval. The two retrieval runs contained 54 trials each: 18 intact, 18 rearranged, and 18 new trials (see Supporting Information Table 3 for fMRI condition trial numbers). Each sentence reading or associative memory trial was presented for 6 seconds as initial piloting indicated that OAs required up to 6 seconds to complete the task, particularly in the intentional retrieval condition. This duration allowed us to minimize any behavioral or neural differences due to increased task difficulty or recruitment of effort in OAs because of inadequate processing time. Each active baseline trial was presented for 3 seconds. All trial sequences and timings were optimized for fMRI using the optseq2 algorithm (http://surfer.nmr.mgh.harvard.edu/optseq/).
Imaging data were collected on a 3T Siemens Trio scanner with a 12-channel head coil. Functional images were acquired with a gradient echo-planar imaging (EPI) sequence (TR = 3,000 ms, TE = 23 ms, FOV = 192 mm, flip angle = 80°, matrix size = 64×64). EPI volumes were acquired at an angle parallel to the long axis of the HC and consisted of 46 slices with a 3 mm isotropic voxel size. Additionally, 1 mm isotropic high resolution T1 weighted coplanar structural MPRAGE images were acquired for each participant.
Data were preprocessed and analyzed with Statistical Parametric Mapping 8 (SPM8; Wellcome Trust Centre for Neuroimaging). After the first two scans of each functional run were discarded, the EPI data for each participant were slice-time corrected using sinc interpolation, realigned using a six-parameter, rigid-body transformation, and coregistered to their respective structural images. The magnitude of the six movement parameters did not statistically differ between YAs and OAs (F[1,43] < 1). The structural images were segmented into separate grey and white matter images that were used with the DARTEL toolbox (Ashburner, 2007) to create average grey and white matter templates. These templates were used to normalize the functional and structural data into MNI space. The normalized EPI data were then spatially smoothed with an 8 mm isotropic full-width at half-maximum Gaussian filter.
Statistical analyses were performed in SPM8 using the general linear model (GLM). A high-pass filter of 128 s and grand mean scaling were applied to the data and serial correlations in the time series were accounted for using the autoregressive model (AR[1]). Preprocessed EPIs were modeled with a first-level fixed-effects analysis using a canonical hemodynamic response function with the stimulus onsets serving as event onsets. Intact, rearranged, and new trials from the incidental and intentional retrieval tasks were modeled as conditions of interest. In the intentional retrieval task, these trial types were further broken down into hits and misses for intact trials and correct rejections and false alarms for rearranged and new trials. The active baseline was modeled and subtracted from each of these trial types within each run. Trials in the encoding runs were separately modeled and are considered elsewhere. Additional covariates of no interest included the six motion parameters estimated during realignment, baseline and session effects, and global mean and motion outliers obtained from the Artifact Detection Toolbox (http://www.nitrc.org/projects/artifact_detect/).
Second-level random-effects analyses were conducted in SPM8 on the GLMs and masked with a gray matter mask derived from the mean normalized structural image. Our MTL analysis was further restricted within an MTL ROI defined using the Harvard-Oxford cortical and subcortical atlases in the FSL software package (FMRIB). These atlases provided a probabilistic mask of bilateral HC and parahippocampal gyrus that was thresholded at 20%. The family-wise error rate was corrected for multiple comparisons to P < 0.05 using Monte Carlo simulations with the 3dClustSim program in the AFNI software package (version 16.0.00). This was achieved with a voxel-wide threshold of P < 0.001 and a cluster size ≥ 6 for MTL and ≥ 23 for whole brain.
In the incidental retrieval condition, IM retrieval was defined as the difference between rearranged and new trials (excluding outliers), whereas RM retrieval was defined as the difference between intact and rearranged trials (excluding outliers). In the intentional retrieval condition, IM retrieval was defined as the difference between rearranged and new correct rejections, whereas RM retrieval was defined as the difference between intact hits and rearranged correct rejections. All effects were exclusively masked by higher-order interactions at p < .01 (e.g., main effects were masked by 2-way and 3-way interactions at p < .01).
Supplementary Material
Acknowledgments
Grant sponsor: NIA; Grant numbers: K01AG028774, F32AG049574.
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Balderston NL, Schultz DH, Helmstetter FJ. The human amygdala plays a stimulus specific role in the detection of novelty. Neuroimage. 2011;55:1889–1898. doi: 10.1016/j.neuroimage.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in old adults: The HAROLD Model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza RE. Triple dissociation in the medial temporal lobes: Recollection, familiarity, and novelty. J Neurophysiol. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dew ITZ, Buchler N, Dobbins IG, Cabeza R. Where is ELSA? The early to late shift in aging. Cereb Cortex. 2012;22:2542–2553. doi: 10.1093/cercor/bhr334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dew ITZ, Cabeza R. The porous boundaries between explicit and implicit memory: Behavioral and neural evidence. Annals NY Acad Sci. 2011;1224:174–190. doi: 10.1111/j.1749-6632.2010.05946.x. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, Sutherland RJ. The aging hippocampus: Cognitive, biochemical and structural findings. Cereb Cortex. 2003;13:1344–1351. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- Duverne S, Habibi A, Rugg MD. Regional specificity of age effects on the neural correlates of episodic retrieval. Neurobiol Aging. 2008;29:1902–1916. doi: 10.1016/j.neurobiolaging.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Tendolkar I. The rhinal cortex:‘gatekeeper’ of the declarative memory system. Trends Cogn Sci. 2006;10:358–362. doi: 10.1016/j.tics.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Ganel T, Gonzalez CL, Valyear KF, Culham JC, Goodale MA, Köhler S. The relationship between fMRI adaptation and repetition priming. Neuroimage. 2006;32:1432–1440. doi: 10.1016/j.neuroimage.2006.05.039. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Dew ITZ. Relational memory and its relevance to aging. Wiley Handbook Cogn Neurosci Memory. 2015:371–392. [Google Scholar]
- Giovanello KS, Schacter DL. Reduced specificity of hippocampal and posterior ventrolateral prefrontal activity during relational retrieval in normal aging. J Cogn Neurosci. 2012;24:159–170. doi: 10.1162/jocn_a_00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer D, Verfaellie M. Distinct hippocampal regions make unique contributions to relational memory. Hippocampus. 2009;19:111–117. doi: 10.1002/hipo.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer DM, Verfaellie M. A critical role for the anterior hippocampus in relational memory: evidence from an fMRI study comparing associative and item recognition. Hippocampus. 2004;14:5–8. doi: 10.1002/hipo.10182. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Rubin SR, Davidson PSR. Source memory in older adults: An encoding or retrieval problem? J Exp Psychol. 2001;27:1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005;47:751–761. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Habib R. On the relation between conceptual priming, neural priming, and novelty assessment. Scandinavian J Psychol. 2001;42:187–195. doi: 10.1111/1467-9450.00230. [DOI] [PubMed] [Google Scholar]
- Hall SA, Rubin DC, Miles A, Davis SW, Wing EA, Cabeza R, Berntsen D. The neural basis of involuntary episodic memories. J Cogn Neurosci. 2014;26:2385–2399. doi: 10.1162/jocn_a_00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Greene AJ. The hippocampus reevaluated in unconscious learning and memory: At a tipping point? Front Hum Neurosci. 2012;6:80. doi: 10.3389/fnhum.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Libby LA, Yonelinas AP, Ranganath C. Medial temporal lobe contributions to cued retrieval of items and contexts. Neuropsychologia. 2013;51:2322–2332. doi: 10.1016/j.neuropsychologia.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. The eyes have it: Hippocampal activity predicts expression of memory in eye movements. Neuron. 2009;63:592–599. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke K. A model for memory systems based on processing modes rather than consciousness. Nat Rev Neurosci. 2010;11:523–532. doi: 10.1038/nrn2850. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Cansino S, Herron JE, Robb WGK, Rugg MD. A familiarity signal in human anterior medial temporal cortex? Hippocampus. 2003;13:259–262. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- Heusser AC, Awipi T, Davachi L. The ups and downs of repetition: Modulation of the perirhinal cortex by conceptual repetition predicts priming and long-term memory. Neuropsychologia. 2013;51:2333–2343. doi: 10.1016/j.neuropsychologia.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Muftuler LT, Rugg MD. Multiple repetitions reveal functionally-and anatomically-distinct patterns of hippocampal activity during continuous recognition memory. Hippocampus. 2008;18:975. doi: 10.1002/hipo.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychol Bull. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14:919. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukolja J, Thiel CM, Wilms M, Mirzazade S, Fink GR. Ageing-related changes of neural activity associated with spatial contextual memory. Neurobiol Aging. 2009;30:630–645. doi: 10.1016/j.neurobiolaging.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16:504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age effects on the neural correlates of episodic retrieval: Increased cortical recruitment with matched performance. Cereb Cortex. 2007;17:2491–2506. doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. J Exp Psychol Learn Mem Cogn. 2000;26:1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- O’Kane G, Insler RZ, Wagner AD. Conceptual and perceptual novelty effects in human medial temporal cortex. Hippocampus. 2005;15:326–332. doi: 10.1002/hipo.20053. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annual Rev Psychol. 2009;60:173. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17:230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Walia G, McIntosh AR, Joanisse MF, Klein D, Köhler S. Why is the meaning of a sentence better remembered than its form? An fMRI study on the role of novelty-encoding processes. Hippocampus. 2008;18:909–918. doi: 10.1002/hipo.20453. [DOI] [PubMed] [Google Scholar]
- Preston AR, Shrager Y, Dudukovic NM, Gabrieli JD. Hippocampal contribution to the novel use of relational information in declarative memory. Hippocampus. 2004;14:148–152. doi: 10.1002/hipo.20009. [DOI] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. J Neurosci. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Mahwah, NJ: Erlbaum; 2000. pp. 1–90. [Google Scholar]
- Rugg MD, Fletcher PC, Frith CD, Frackowiak RS, Dolan RJ. Brain regions supporting intentional and incidental memory: A PET study. Neuroreport. 1997;8:1283–1287. doi: 10.1097/00001756-199703240-00045. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Henson RN, Robb WG. Neural correlates of retrieval processing in the prefrontal cortex during recognition and exclusion tasks. Neuropsychologia. 2003;41:40–52. doi: 10.1016/s0028-3932(02)00129-x. [DOI] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Squire LR, Dede AJ. Conscious and unconscious memory systems. Cold Spring Harbor Perspect Biol. 2015;7:a021667-. doi: 10.1101/cshperspect.a021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RNA, Friston KJ, Dolan RJ. Segregating the functions of human hippocampus. Proc Natl Acad Sci USA. 1999;96:4034–4039. doi: 10.1073/pnas.96.7.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Johnson JD, Rugg MD. Recollection-related hippocampal activity during continuous recognition: A high-resolution fMRI study. Hippocampus. 2011;21:575–583. doi: 10.1002/hipo.20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiura T, Sekiguchi A, Yomogida Y, Nakagawa S, Shigemune Y, Kambara T, Kawashima R. Effects of aging on hippocampal and anterior temporal activations during successful retrieval of memory for face-name associations. J Cogn Neurosci. 2011;23:200–213. doi: 10.1162/jocn.2010.21476. [DOI] [PubMed] [Google Scholar]
- Tulving E. Precis of elements of episodic memory. Behav Brain Sci. 1984;7:223–238. [Google Scholar]
- Vilberg KL, Rugg MD. An investigation of the effects of relative probability of old and new test items on the neural correlates of successful and unsuccessful source memory. Neuroimage. 2009;45:562–571. doi: 10.1016/j.neuroimage.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Federmeier KD, Paller KA. The potato chip really does look like Elvis! Neural hallmarks of conceptual processing associated with finding novel shapes subjectively meaningful. Cereb Cortex. 2012;22:2354–2364. doi: 10.1093/cercor/bhr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Ranganath C, Yonelinas AP. Activity reductions in perirhinal cortex predict conceptual priming and familiarity-based recognition. Neuropsychologia. 2014;52:19–26. doi: 10.1016/j.neuropsychologia.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.