Abstract
Trait impulsivity is characterized by behavioral disinhibition and rash decision-making that contribute to many maladaptive behaviors. Previous research demonstrates that trait impulsivity is related to the activity of brain regions underlying reward sensitivity and emotion regulation, but little is known about this relationship in the context of immediately available primary reward. This is unfortunate, as impulsivity in these contexts can lead to unhealthy behaviors, including poor food choices, dangerous drug use and risky sexual practices. In addition, little is known about the relationship between integration of reward and affective neurocircuitry, as measured by resting-state functional connectivity, and trait impulsivity in everyday life, as measured with a commonly used personality inventory. We therefore asked healthy adults to undergo a functional magnetic resonance imaging task in which they saw cues indicating the imminent oral administration of rewarding taste, as well as a resting-state scan. Trait impulsivity was associated with increased activation during anticipation of primary reward in the anterior cingulate cortex (ACC) and amygdala. Additionally, resting-state functional connectivity between the ACC and the right amygdala was negatively correlated with trait impulsivity. These findings demonstrate that trait impulsivity is related not only to ACC-amygdala activation but also to how tightly coupled these regions are to one another.
Keywords: impulsivity, primary reward, amygdala, anterior cingulate cortex, fMRI
INTRODUCTION
Impulsivity is a personality trait that can be characterized by many disadvantageous behavioral characteristics, including behavioral disinhibition, an inability to delay gratification, and lack of forethought and planning (Cloninger et al., 1994; Kalenscher et al., 2006; Chamorro et al., 2012). Increased trait impulsivity is a hallmark of psychiatric disorders associated with emotional dysregulation and hypersensitivity to reward, including substance abuse disorders (Verdejo-García et al., 2008; Ersche et al., 2010; Hopwood et al., 2011), borderline personality disorder (Laporte et al., 2011; Svaldi et al., 2012) and eating disorders (Waxman, 2009). A deeper understanding of the neurobiological correlates of trait impulsivity may lead to better models of the pathophysiologies underlying these disorders, thereby providing targets for more effective treatments.
A growing body of literature points to a relationship between trait impulsivity and reward and affective neurocircuitry. Evidence suggests that impulsivity is governed by the interaction of regions, such as the amygdala, that underlie the salience of reward cues and associated affective responses, and regions such as the striatum and anterior cingulate cortex (ACC) that represent the hedonic anticipation and cost–benefit analysis of rewards. For instance, adolescent substance abusers exhibit abnormally high amygdala activity with heightened delay discounting (a behavioral facet of trait impulsivity; Stanger et al., 2013) and adults with high trait impulsivity display increased amygdala activation upon receiving monetary rewards (Shao et al., 2013). Likewise, healthy adults with high non-planning (as opposed to motor or attentional) impulsivity show increased D2/3 receptor availability in the ventral striatum (Reeves et al., 2012), which suggests that impulsive individuals have higher dopamine receptor density in this region. This accords with evidence that trait impulsivity is positively associated with amphetamine-induced dopamine release in the ventral striatum (Buckholtz et al., 2010). Dopamine, however is not the only neurotransmitter exerting an influence over trait impulsivity. In addition to dopamine, trait impulsivity appears to be influenced by serotonergic and noradrenaline activity (Passamonti et al., 2006). Perhaps for this reason, the ventral ACC, which is directly influenced within both the dopaminergic and serotonergic pathways (Berger, 1988; Törk, 1990), also influences trait impulsivity. The ACC plays a central role in online cost–benefit computations about reward cues. As a result, individuals with lesions to this area tend to be impaired at weighing the relative values among rewards, and thereby tend to display cognitive impulsivity and an inability to delay gratification (Bechara et al., 1994, 2000b). Similarly, in healthy individuals, the ACC is activated during passive observation of stimuli involving the possibility of immediate, as opposed to delayed, reward (Albrecht et al., 2013).
Heretofore research has examined the relationship between trait impulsivity and reward sensitivity using predominantly secondary, rather than primary, rewards. For example, trait impulsivity is positively correlated with activity in the ventral striatum during positive feedback in a task involving monetary (secondary) reward (Forbes et al., 2007). Similarly, in a monetary incentive delay task, trait impulsivity is associated with activity in the ventral striatum and orbitofrontal cortex (OFC) during reward anticipation (Hahn et al., 2009).
In light of the previous research, there appear to be at least two gaps in our knowledge of the roles played by reward and affective neurocircuitry in trait impulsivity. First, little is known about the relationship between trait impulsivity and activity during the anticipation of ‘primary’ reward. This is not a trivial point. It is important to study the relationship between trait impulsivity and primary reward precisely because individuals are constantly faced with a host of decisions that are associated with adverse outcomes, many of which are made in the presence of immediately available primary rewards, including unhealthy foods, high-risk sexual behaviors and drugs of abuse.
The second gap in our knowledge pertains to the relationship between trait impulsivity and integration of information among the constituent parts of the brain’s reward and affective neurocircuitry. There are few studies of how these regions’ tonic systemic integrity as measured, for example, by resting-state functional connectivity, is related to trait impulsivity in everyday life, although a few prior studies have examined the relationship between trait impulsivity and task-based functional connectivity (e.g. Diekhof and Gruber, 2010; Diekhof et al., 2012a,b). A better understanding of resting functional connectivity among brain regions identified during task-based assessments of reward anticipation would substantially contribute to current knowledge by demonstrating that trait impulsivity is a property of these regions’ intrinsic connectivity, not just their effective connectivity during moments of reward anticipation and receipt.
In order to fill these gaps and evaluate the relationship between trait impulsivity and activity in reward and affective neurocircuitry during anticipation of a primary reward, we assessed healthy adults for their levels of trait impulsivity and then asked them to undergo functional magnetic resonance imaging (fMRI). During the fMRI task, participants saw cues indicating the imminent oral administration of a small amount of sucrose. We then correlated the brain activity during these reward cues with trait impulsivity. Here we show that trait impulsivity is associated with activation during anticipation of a primary reward in the ACC and bilateral amygdala. We then assessed the relationship between trait impulsivity and functional connectivity between these regions during the participants’ independent resting-state scans. Interestingly, higher levels of trait impulsivity, as measured by a commonly used personality inventory, were associated with decreased functional connectivity between the ACC and right amygdala during independent resting-state scans.
MATERIALS AND METHODS
Participants
Eighteen right-handed, native English-speaking healthy participants (10 females; mean age = 31 years; s.d. = 8 years) without a history of head injury or any major medical, psychiatric, or neurological disorder were included in this study. In addition to these criteria, participants reported no use of any psychotropic or other medications that could affect cerebral blood flow within the 3 weeks prior to scanning. Other participation restrictions included general MRI exclusion criteria, including current pregnancy. Participants were excluded from analyses if they had excessive head motion or indicated that they did not experience the sweet tastant as more pleasant than the neutral tastant, as measured by a questionnaire completed immediately after the scan. Resting-state fMRI data were unavailable for one participant. All participants provided informed consent in accordance with the Declaration of Helsinki, and The University of Oklahoma Institutional Review Board approved all procedures.
Materials
Temperament and Character Inventory
Trait impulsivity was assessed using the Impulsivity scale of the Temperament and Character Inventory (TCI) (Cloninger et al., 1994). The TCI is a 240-item, true–false, self-report questionnaire assessing personality. The Impulsivity scale (TCI-Imp) has shown adequate reliability in a community sample (Cronbach’s α = 0.62; Cloninger et al., 1994). In exploratory factor analyses (Whiteside and Lynam, 2001; Flory et al., 2006), this scale loads with other impulsivity scales that measure ‘non-planning’ or ‘lack of premeditation’ [e.g. Revised NEO Personality Inventory R facet of low deliberation (Costa and McCrae, 1992), Barratt Impulsiveness Scale (Patton et al., 1995)].
Tastant delivery
All solutions were delivered via an MRI-compatible tastant delivery system. Solutions were kept in four separate syringe pumps (1 for sweet, 1 for neutral, 2 for wash) and delivered to the participant via medical grade plastic tubes connected to a gustatory manifold. This manifold was anchored to the head coil in the scanner and delivered solutions directly into the participant’s mouth during scanning. The anchoring allowed adjustment of the manifold to a position comfortable for each participant. After adjustment, the manifold was kept in place throughout the rest of the scan. A laptop using LabView software (National Instruments, Austin, TX) enabled precise timing and delivery of all solutions. The sweet tastant consisted of 0.4 ml of a 0.4 or 0.6 M sucrose solution (see Supplementary Data for details regarding the different molarities used), the neutral tastant consisted of 0.4 ml of distilled water, and the wash consisted of 0.8 ml of distilled water.
Stimuli and procedure
Resting-state fMRI scan
Prior to all other tasks in the scanner, participants underwent an 8 min resting-state scan during which a fixation mark (+) was presented on the projection screen. During this time participants were instructed to simply lie in the scanner, clear their minds and try not to think about anything in particular.
Reward cues and tastant delivery
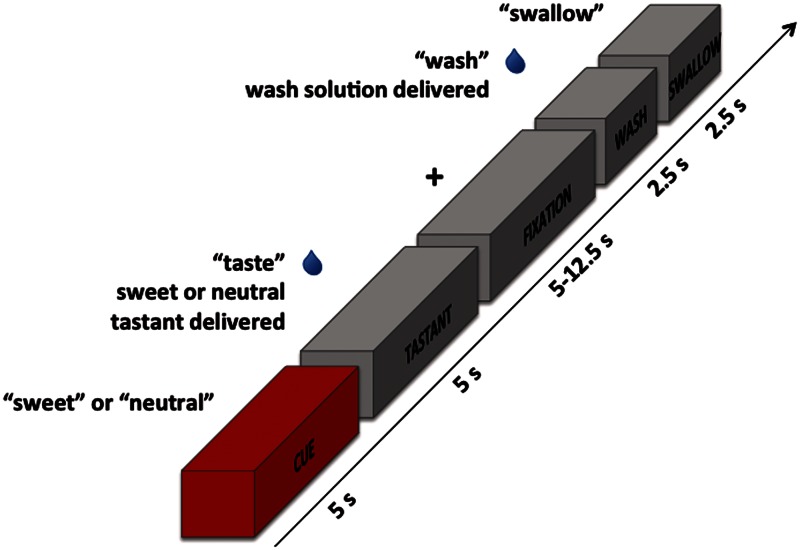
The reward anticipation task consisted of three different types of events: tastant trials, catch trials and wash/swallow trials. Participants were shown cues for 5 s that simply displayed the word ‘sweet’ or ‘neutral’. Catch trials consisted solely of these cues and did not involve administration of any tastants. During tastant trials (shown in Figure 1), cues were followed by delivery of the corresponding tastant (sweet or neutral). While the tastant was being delivered, the word ‘taste’ was shown on the screen for 5 s. Following tastant delivery, subjects held the tastant in their mouths for a variable interval of 5–12.5 s. During this time, a fixation mark (+) was displayed on the screen. This was followed by delivery of a wash solution while the word ‘wash’ was shown for 2.5 s. Finally, the word ‘swallow’ appeared on the screen for 2.5 s while the subject swallowed the solution. Catch and tastant trials were intermingled throughout each run with wash/swallow trials, which included only the wash/swallow procedure without the presentation of any cues or tastants. Each scanning run lasted 10 min and 20 s and consisted of 9 tastant trials, 6 catch trials and 18 independent wash/swallow trials. There was thus a 60% probability that a cue would be followed by delivery of a tastant. Cues from both the catch and tastant trials were included in the reward anticipation analyses. Most participants underwent four scanning runs; however, three participants only completed three runs due to technical issues and time constraints.
Fig. 1.
Reward anticipation task design. While in the scanner, subjects were first shown a cue with either the word ‘sweet’ or ‘neutral’ that was subsequently followed by the oral administration of the corresponding solution (either sweet-tasting or neutral) while the word ‘taste’ appeared on the screen. They then held this solution in their mouths for a variable interval of 5–12.5 s while a fixation mark was shown. This was followed by the oral administration of a wash solution accompanied by the word ‘wash’. Finally, participants were shown the word ‘swallow’ and swallowed the solution in their mouths. In some trials, cues were not followed by delivery of a tastant (catch trials). The cue block is highlighted in red because it is the focus of the current analyses.
MRI data acquisition
A General Electric Discovery MR750 whole-body 3 T MRI scanner was used to obtain all functional and structural brain images. A receive-only 32-element surface coils head array, optimized for parallel imaging (Nova Medical Inc.), was used for MRI signal reception. Blood oxygenation level dependent (BOLD) functional MRI scans utilized a single-shot gradient-recalled echo planar imaging sequence with Sensitivity Encoding (SENSE). For the reward anticipation task, we used the following EPI imaging parameters: field of view/slice/gap = 240/2.9/0 mm, 46 axial slices per volume, acquisition matrix = 96 × 96, repetition/echo time TR/TE = 2500/30 ms, SENSE acceleration factor R = 2 in the phase encoding (anterior–posterior) direction, flip angle = 90°, sampling bandwidth = 250 kHz, number of volumes 248, scan time 10 min 20 s. Identical EPI imaging parameters were used for the resting-state scans, except for: TE = 25 ms, number of volumes 180, scan time 7 min 30 s. The EPI images were reconstructed into a 128 × 128 matrix, resulting in an fMRI voxel volume of 1.875 × 1.875 × 2.9 mm3 for both the task and resting-state scans. Simultaneous with fMRI runs, respiration and pulse oximetry physiological waveforms were recorded (with 40 Hz sampling bandwidth). A pneumatic respiration belt was used for respiration measurements, and for cardiac waveforms, pulse oximetry was measured using an infrared emitter placed under the pad of the participant’s left index finger. An anatomical reference for the fMRI analyses was provided by a T1-weighted magnetization-prepared rapid gradient-echo sequence with SENSE. The anatomical scan used the following parameters: FOV = 240 mm, axial slices per volume = 180, slice thickness = 0.9 mm, image matrix = 256 × 256, voxel volume 0.938 × 0.938 × 0.9 mm3, TR/TE = 5/2.02 ms, SENSE acceleration factor R = 2, flip angle = 8°, inversion time = 725 ms, sampling bandwidth = 31.25 kHz, scan time = 6 min 12 s.
AFNI (http://afni.nimh.nih.gov/afni) was used for image pre-processing prior to statistical analyses. Please see Supplementary Data for details on data pre-processing and analyses.
Statistical analyses
We used a multiple linear regression model to analyze the task data at the participant level. The model included regressors for the sweet cue, neutral cue, sweet tastant, neutral tastant and wash/swallow events. In order to account for the shape and delay of the BOLD hemodynamic response function, the five task regressors were constructed by convolution of a gamma-variate function and a box-car function with a 5 s width beginning at the onset of each occurrence of each type of trial. The regression model also included regressors of non-interest to account for each run’s signal mean, linear, quadratic and cubic signal trends, in addition to six motion parameters (3 translations, 3 rotations) computed during the image registration pre-processing.
Structurally defined regions of interest (ROIs) chosen based on their involvement in reward and affective neurocircuitry were defined a priori. These regions included the OFC, ventral striatum, caudate, putamen, amygdala, ventral ACC, ventromedial prefrontal cortex (PFC), ventral pallidum and ventral tegmental area (see Supplementary Data for ROI anatomical definitions). After setting an uncorrected voxelwise threshold of P < 0.005, corrections for multiple comparisons at a cluster-size threshold of P < 0.05 were made individually for each a priori defined ROI using the AFNI program 3dClustSim. A voxelwise threshold of P < 0.001 and cluster-size correction to P < 0.05 was utilized for brain regions outside the a priori hypothesized ROIs.
The AFNI program 3dTcorr1D was used to determine which brain regions showed activity during the sweet cues that was associated with trait impulsivity. In order to examine activity specific to reward anticipation, the beta coefficients of neutral cues were first subtracted from those of the sweet cues. To ensure the effects were robust to non-normality and the influence of outliers, Spearman correlation analyses were utilized to examine the relationship between raw scores on the TCI-Imp scale and the anticipation of a primary reward. Analyses of the relationship between TCI-Imp and reward receipt as well as the main effects of cue type are reported in the Supplementary Data.
We defined as ‘reward anticipation ROIs’ those voxels where activity during anticipation of primary reward was significantly correlated with trait impulsivity, based on the thresholds described earlier. This resulted in three reward anticipation ROIs. We subsequently conducted resting-state functional connectivity analyses using the reward anticipation ROIs defined in the task data. Please see the Supplementary Data for a description of the resting-state scan pre-processing and physiological noise corrections employed prior to functional connectivity calculations. At the individual participant level, the seed time-series for each cluster was constructed by calculating the average time series during the resting-state scan within all voxels in a given reward anticipation ROI. We then correlated the average time series between each pair of reward anticipation ROIs at the subject-level. These Pearson r-values were then transformed to z-values using Fisher’s r-to-z transformation. Finally, we used Spearman rank correlations to assess the relationship between the resulting z-scores, which represent the correlations of spontaneous fluctuations of the resting-state BOLD signal between regions, with the subjects’ TCI-Imp scores. This provided a measure of the association between trait impulsivity and resting-state functional connectivity among these regions.
RESULTS
Following the scan session, subjects rated the sweet tastant (mean pleasantness t = 7.1, s.d. = 1.5) as more pleasant than the neutral tastant (mean pleasantness t = 3.9, s.d. = 1.8). Importantly, the difference between the pleasantness ratings for the two tastants was statistically reliable (t[17] = 7.13, P < 0.0001).
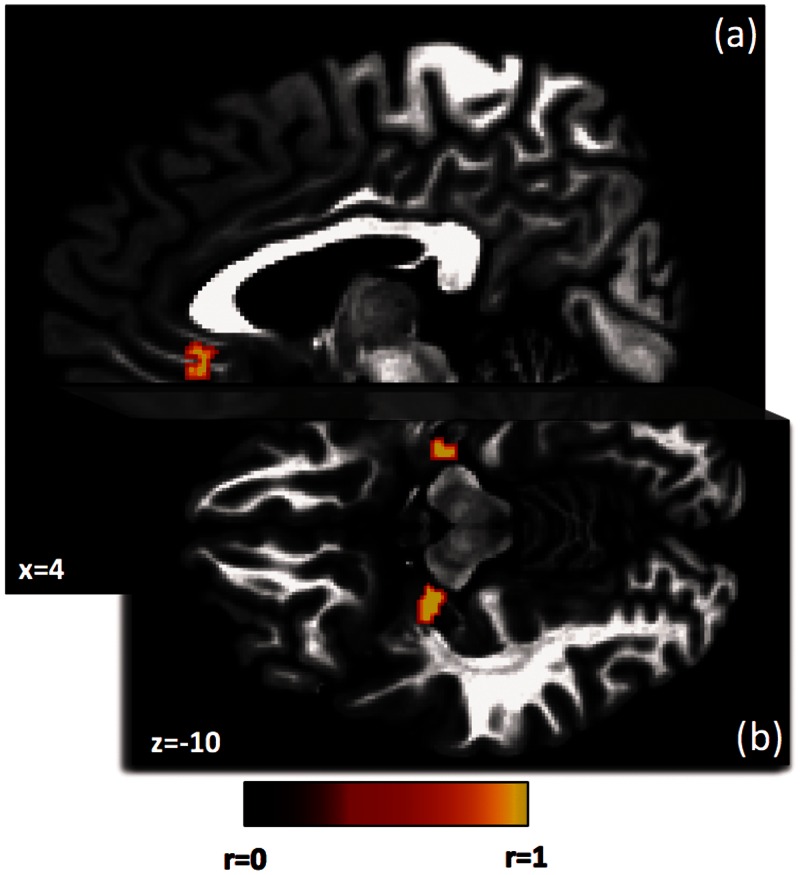
Trait impulsivity was positively correlated with the activity in three brain regions during anticipation of primary reward after correction for multiple comparisons (P < 0.05 corrected). As shown in Figure 2, across individuals, higher levels of trait impulsivity were associated with greater activation during reward anticipation in the ACC and bilateral amygdala (see Supplementary Table S1 for coordinates of these regions). We observed no regions where impulsivity and reward anticipation were significantly negatively correlated.
Fig. 2.
Regions where anticipation of a primary reward is associated with trait impulsivity. The three regions shown include the right ACC (a) and bilateral amygdala (b). All regions met correction for multiple comparisons at P < 0.05. Coordinates are in Talairach space.
Additionally, as a secondary analysis, we also examined the relationship between trait impulsivity and activity during reward receipt. After corrections for multiple comparisons, trait impulsivity was significantly positively correlated during the receipt of primary reward with three clusters in the left caudate, and negatively correlated with a region in the left pallidum (see Supplementary Table S2 and Supplementary Figure S2).
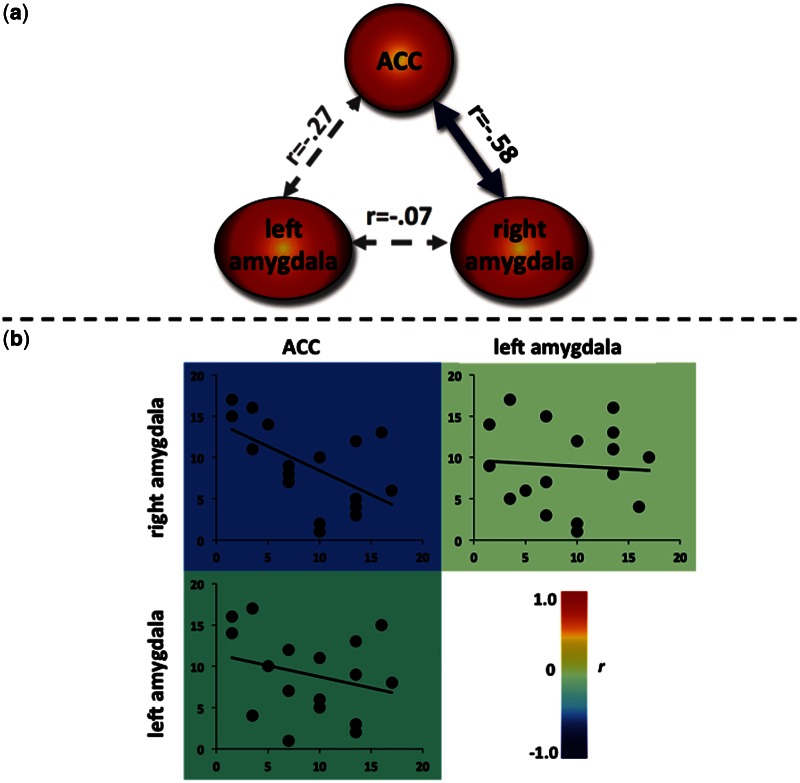
In order to determine if trait impulsivity is related to systemic connectivity between regions demonstrating an association between trait impulsivity and activity in the reward anticipation task data, we performed a functional connectivity analysis using independent resting-state data collected from the same participants prior to their performance of the primary reward task. In this analysis, we correlated trait impulsivity with the functional connectivity between each pair of regions implicated in the reward anticipation task. As displayed in Figure 3, trait impulsivity was negatively associated with resting-state functional connectivity between the ACC and right amygdala regions identified in the task data (P < 0.05).
Fig. 3.
Resting-state functional connectivity between regions from the task-based analysis of impulsivity and reward anticipation. (a) The blue arrow represents the significant negative correlation indicating that high levels of trait impulsivity are associated with decreased correlations in the spontaneous fluctuations at rest between the ACC and right amygdala. Spearman’s r-values represent the relationship between trait impulsivity and the functional connectivity between each pair of regions. (b) Heat map of scatterplots showing low resting-state functional connectivity (y-axis) between the ACC and right amygdala is associated with higher trait impulsivity (x-axis). Values for both trait impulsivity and functional connectivity are shown in ordinally ranked units, as appropriate for Spearman correlations. The background color of each scatterplot depicts the Spearman’s r-value at the group level, which is the same as the correlations shown between regions in panel (a).
DISCUSSION
Heightened trait impulsivity is implicated in a number of psychiatric disorders (Verdejo-García et al., 2008; Waxman, 2009; Ersche et al., 2010; Hopwood et al., 2011; Laporte et al., 2011; Svaldi et al., 2012) as well as risky behaviors in otherwise healthy individuals (Chamorro et al., 2012). Trait impulsivity is thought to be at least partly the result of heightened reward sensitivity and activity of reward and affective neurocircuitry (Cools et al., 2003; Cools, 2008; Buckholtz et al., 2010; Reeves et al., 2012). In this study, trait impulsivity was positively correlated with activity in response to reward cues in the ventral ACC and bilateral amygdala. These findings suggest that individual differences in the functioning of the ventral ACC and amygdala affect reward sensitivity and therefore underlie important differences in personality.
Past research has primarily examined the relationship between trait impulsivity and reward sensitivity using secondary rewards. For example, previous research has reported a negative association between trait impulsivity and activation in a dorsal anterior region of the medial PFC during a monetary incentive delay task (Sripada et al., 2010), and trait impulsivity is positively correlated with activity in the ventral striatum (Forbes et al., 2007). Additionally, in individuals with high trait impulsivity, amygdala activation is increased following the delivery of outcomes indicating a monetary reward (Shao et al., 2013).
This study makes a significant contribution by examining the relationship between trait impulsivity and sensitivity to ‘primary’ rewards. This distinction is important, as demonstrated by a recent meta-analysis showing different patterns of brain activation during the receipt of primary vs secondary rewards (Sescousse et al., 2013). Additionally, the study of reward ‘anticipation’ entails certain implications for the use of a secondary reward such as money. Neuroimaging studies using monetary rewards nearly always provide visual feedback indicating that the participant will receive a certain sum of money post-scan. Activation during anticipation of this visual feedback may, therefore, reflect responses to a stimulus–stimulus association rather than a stimulus–reward association (O’Doherty et al., 2002). In contrast, many potentially consequential decisions are made in the presence of immediately available primary rewards, and impulsivity in these contexts can often lead to unhealthy behaviors, including poor food choices, dangerous drug use and risky sexual practices.
This study also addressed another gap in our knowledge by examining the relationship between trait impulsivity in everyday life and resting-state functional connectivity specifically among reward regions found to be correlated with trait impulsivity during reward anticipation. The reward anticipation task data revealed that the co-activation of the ACC and amygdala during reward anticipation is related to trait impulsivity. We then performed functional connectivity analyses on independent resting-state data from these same subjects, and observed a negative relationship between trait impulsivity and resting-state functional connectivity between the ventral ACC and right amygdala. Evidence suggests that the region of the ventral ACC reported here contributes to cost/benefit computations in the service of estimating overall reward values (Bechara et al., 1994; Kable and Glimcher, 2007; Plassmann et al., 2007; FitzGerald et al., 2009; Glascher et al., 2009; Hare et al., 2009; Wunderlich et al., 2010; Hunt et al., 2012). For example, activity in this region is positively correlated with the expected value of a chosen stimulus in decision-making tasks (FitzGerald et al., 2009; Glascher et al., 2009; Wunderlich et al., 2010), and this region is activated when participants must determine how much money they are willing to pay for different food items (Plassmann et al., 2007). Similarly, in individuals who incorporate both food taste and healthiness in food decision-making, activity in this region is correlated with subjects’ estimates of these factors for visually presented food items (Hare et al., 2009). In addition, patients with lesions to the ventral ACC are often unable to use the emotional value of stimuli in order to guide decision-making in consideration of future consequences (Bechara et al., 1994, 2000b), and thus display cognitive impulsivity and an inability to delay gratification.
The amygdala, which in this study also exhibited activity and functional connectivity related to trait impulsivity, supports emotional coding of stimulus value, providing an emotional context, or arousal, that modulates responses to reward cues (Baxter and Murray, 2002; Anderson et al., 2003; Sander et al., 2003; Small et al., 2003; Murray, 2007; Pessoa, 2010). The amygdala also indexes emotionally salient stimuli, such as reward-predicting cues, for greater attention and subsequent information processing (Peck et al., 2013).
Clearly, both of these regions make a meaningful contribution to the overall representation of a stimulus’ reward value. Previous studies have focused mainly on the relationship between trait impulsivity and anticipatory activity within regions underlying reward and affect. In contrast, this study reveals that trait impulsivity is also related to the functional integrity of connections between reward and affect regions.
Initially it may seem paradoxical that greater trait impulsivity should be associated with higher ACC and amygdala activity during the reward anticipation task but lower functional connectivity between these regions at rest. This paradox may be more obvious than real, however. As reviewed earlier, there is widespread evidence that activity in the ventral ACC and amygdala each contribute important reward-related information that influences an individual’s reward sensitivity. In light of this, the findings reported here suggest at least two routes to trait impulsivity. First, the positive correlation between trait impulsivity and reward anticipation activity in these regions suggests that trait impulsivity is associated with greater reward sensitivity. Alternately, the negative correlation between impulsivity and resting-state functional connectivity between the ACC and amygdala suggests a second route to impulsivity. Trait impulsivity may result from a failure to integrate the ACC’s cost/benefit reward valuations with information from the amygdala concerning the emotional salience of the stimulus. Thus, while activation of the ACC is positively correlated with trait impulsivity, the information from this region concerning cost/benefit reward valuations is not being integrated with the emotional coding of the stimulus value. This second route is supported by evidence that ACC lesions impair reward valuation resulting in increased behavioral impulsivity (Bechara et al., 1994, 2000a,b; Glascher et al., 2012), and highly impulsive individuals exhibit decoupling of the ACC and ventral striatum while performing a task requiring behavioral inhibition (Diekhof et al., 2012b), a finding that bears striking similarity to the ACC resting-state functional connectivity results reported in this study. Similarly, graph theory analyses of functional connectivity within large-scale brain networks (modules) reveal that, in highly impulsive individuals, regulatory structures including the ventral ACC were functionally isolated from subcortical structures including the amygdala (Davis et al., 2013). Lower integrity of frontostriatal white matter tracts is also associated with delay discounting, which is a facet of trait impulsivity (Peper et al., 2013). Likewise, a lack of functional integration may account for impulsivity in adolescence as the brain is still maturing (Luna and Sweeney, 2004; Raznahan et al., 2011). Taken together with these earlier findings, the strength of the relationship between trait impulsivity and right amygdala activation during reward anticipation in this study, along with this study’s finding of a negative relationship between trait impulsivity and resting-state functional connectivity among these regions, suggests that the emotional salience of reward cues coded by the amygdala may be particularly strong in impulsive individuals and not sufficiently tempered by cost/benefit analyses contributed by the ventral ACC.
Our findings emphasize the need to focus not just on activation associated with information processing within brain regions but also on the integration of that information between regions. They suggest that trait impulsivity may result from a combination of heightened reward sensitivity with a lack of integration among reward and affect regions, particularly with respect to reward value information represented in the ventral ACC and emotional salience represented in the amygdala.
A potential limitation of this study is the use of cues that were valid 60% of the time. This percentage enabled us to model the hemodynamic response during anticipation and receipt of tastants separately, but may have slightly attenuated the subjects’ reward expectations. Future research should seek to determine whether reduced functional connectivity within reward and affect regions might underlie psychiatric disorders associated behavioral disinhibition, including substance abuse disorders, bulimia nervosa and binge eating disorder. Because impulsive behaviors are present from childhood and predict important outcomes later in life (Mischel et al., 1989), it may be valuable to explore this phenomenon in pediatric populations at risk for poor health outcomes. For example, pediatric obesity is associated with both greater trait impulsivity (Nederkoorn et al., 2006; van den Berg et al., 2011) and greater activity within reward regions of the OFC and medial PFC (Bruce et al., 2011). It will now be important to also examine whether abnormal integration of reward information with emotional salience, as indexed by decreased functional connectivity between reward and affect regions, may also contribute to impulsive personality traits that in turn predispose individuals to pediatric obesity, impulsive psychiatric disorders and other negative health outcomes.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
The authors thank Rayus Kuplicki for assistance with resting-state data pre-processing, as well as Jessica Santiago, Lisa Kinyon, Jennifer Dobson and Kaiping Burrows for their help with subject assessment, recruitment and data collection. This work was supported by the National Institutes of Mental Health (K01MH096175-01 to W.K.S.); the Oklahoma Center for the Advancement of Science and Technology (OCAST HR10-141 to W.K.S.); the National Alliance for Research on Schizophrenia and Depression (Young Investigator Award to W.K.S.); the Oklahoma Tobacco Research Center (grant to W.K.S.); and The William K. Warren Foundation.
REFERENCES
- Albrecht K, Volz KG, Sutter M, von Cramon DY. What do I want and when do I want it: brain correlates of decisions made for self and other. PLoS One. 2013;8:e73531. doi: 10.1371/journal.pone.0073531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature Reviews Neuroscience. 2002;3:563–73. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000a;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000b;123:2189–202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Berger B, Trottier S, Verney C, Gaspar P, Alvarez C. Regional and laminar distribution of the dopamine and serotonin innervation in the macaque cerebral cortex: a radioautographic study. Journal of Comparative Neurology. 1988;273:99–119. doi: 10.1002/cne.902730109. [DOI] [PubMed] [Google Scholar]
- Bruce AS, Martin LE, Savage CR. Neural correlates of pediatric obesity. Preventive Medicine. 2011;52:S29–35. doi: 10.1016/j.ypmed.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro J, Bernardi S, Potenza MN, et al. Impulsivity in the general population: a national study. Journal of Psychiatric Research. 2012;46:994–1001. doi: 10.1016/j.jpsychires.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger C, Przybeck T, Svrakic D, Wetzel R. The Temperament and Character Inventory. St. Louis: Center for Psychobiology of Personality; 1994. [Google Scholar]
- Cools R. Role of dopamine in the motivational and cognitive control of behavior. The Neuroscientist. 2008;14:381–95. doi: 10.1177/1073858408317009. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. l-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia. 2003;41:1431–41. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Costa PJ, McCrae RR. Revised NEO Personality Inventory Manual. Odessa: Psychological Assessment Resources; 1992. [Google Scholar]
- Davis FC, Knodt AR, Sporns O, et al. Impulsivity and the modular organization of resting-state neural networks. Cerebral Cortex. 2013;23:1444–52. doi: 10.1093/cercor/bhs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Gruber O. When desire collides with reason: functional interactions between anteroventral prefrontal cortex and nucleus accumbens underlie the human ability to resist impulsive desires. Journal of Neuroscience. 2010;30:1488–93. doi: 10.1523/JNEUROSCI.4690-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Keil M, Obst KU, et al. A functional neuroimaging study assessing gender differences in the neural mechanisms underlying the ability to resist impulsive desires. Brain Research. 2012a;1473:63–77. doi: 10.1016/j.brainres.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Nerenberg L, Falkai P, Dechent P, Baudewig J, Gruber O. Impulsive personality and the ability to resist immediate reward: an fMRI study examining interindividual differences in the neural mechanisms underlying self-control. Human Brain Mapping. 2012b;33:2768–84. doi: 10.1002/hbm.21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biological Psychiatry. 2010;68:770–3. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald THB, Seymour B, Dolan RJ. The role of human orbitofrontal cortex in value comparison for incommensurable objects. Journal of Neuroscience. 2009;29:8388–95. doi: 10.1523/JNEUROSCI.0717-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory JD, Harvey PD, Mitropoulou V, et al. Dispositional impulsivity in normal and abnormal samples. Journal of Psychiatric Research. 2006;40:438–47. doi: 10.1016/j.jpsychires.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular Psychiatry. 2007;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J, Adolphs R, Damasio H, et al. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14681–6. doi: 10.1073/pnas.1206608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J, Hampton AN, O’Doherty JP. Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward-related decision making. Cerebral Cortex. 2009;19:483–95. doi: 10.1093/cercor/bhn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T, Dresler T, Ehlis A-C, et al. Neural response to reward anticipation is modulated by Gray’s impulsivity. NeuroImage. 2009;46:1148–53. doi: 10.1016/j.neuroimage.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–8. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hopwood CJ, Morey LC, Skodol AE, et al. Pathological personality traits among patients with absent, current, and remitted substance use disorders. Addictive Behaviors. 2011;36:1087–90. doi: 10.1016/j.addbeh.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt LT, Kolling N, Soltani A, Woolrich MW, Rushworth MFS, Behrens TEJ. Mechanisms underlying cortical activity during value-guided choice. Nature Neuroscience. 2012;15:470–6. doi: 10.1038/nn.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10:1625–33. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalenscher T, Ohmann T, Güntürkün O. The neuroscience of impulsive and self-controlled decisions. International Journal of Psychophysiology. 2006;62:203–11. doi: 10.1016/j.ijpsycho.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Laporte L, Paris J, Guttman H, Russell J. Psychopathology, childhood trauma, and personality traits in patients with borderline personality disorder and their sisters. Journal of Personality Disorders. 2011;25:448–62. doi: 10.1521/pedi.2011.25.4.448. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Annals of the New York Academy of Sciences. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244:933–8. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends in Cognitive Sciences. 2007;11:489–97. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Braet C, Van Eijs Y, Tanghe A, Jansen A. Why obese children cannot resist food: the role of impulsivity. Eating Behaviors. 2006;7:315–22. doi: 10.1016/j.eatbeh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–26. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Fera F, Magariello A, et al. Monoamine oxidase—a genetic variations influence brain activity associated with inhibitory control: new insight into the neural correlates of impulsivity. Biological Psychiatry. 2006;59:334–40. doi: 10.1016/j.biopsych.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Peck CJ, Lau B, Salzman CD. The primate amygdala combines information about space and value. Nature Neuroscience. 2013;16:340–8. doi: 10.1038/nn.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Mandl RCW, Braams BR, et al. Delay discounting and frontostriatal fiber tracts: a combined DTI and MTR study on impulsive choices in healthy young adults. Cerebral Cortex. 2013;23:1695–702. doi: 10.1093/cercor/bhs163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. Emotion and cognition and the amygdala: from “what is it?” to ‘what’s to be done?’. Neuropsychologia. 2010;48:3416–29. doi: 10.1016/j.neuropsychologia.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O’Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. Journal of Neuroscience. 2007;27:9984–8. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Lerch JP, Lee N, et al. Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron. 2011;72:873–84. doi: 10.1016/j.neuron.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves SJ, Polling C, Stokes PRA, et al. Limbic striatal dopamine D2/3 receptor availability is associated with non-planning impulsivity in healthy adults after exclusion of potential dissimulators. Psychiatry Research: Neuroimaging. 2012;202:60–4. doi: 10.1016/j.pscychresns.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences. 2003;14:303–16. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Sescousse G, Caldú X, Segura B, Dreher J-C. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2013;37:681–96. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Shao R, Read J, Behrens TEJ, Rogers RD. Shifts in reinforcement signalling while playing slot-machines as a function of prior experience and impulsivity. Translational Psychiatry. 2013;3:e213. doi: 10.1038/tp.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–11. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Sripada CS, Gonzalez R, Luan Phan K, Liberzon I. The neural correlates of intertemporal decision-making: contributions of subjective value, stimulus type, and trait impulsivity. Human Brain Mapping. 2010;32:1637–48. doi: 10.1002/hbm.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Elton A, Ryan SR, James GA, Budney AJ, Kilts CD. Neuroeconomics and adolescent substance abuse: individual differences in neural networks and delay discounting. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:747–55. doi: 10.1016/j.jaac.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svaldi J, Philipsen A, Matthies S. Risky decision-making in borderline personality disorder. Psychiatry Research. 2012;197:112–8. doi: 10.1016/j.psychres.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Törk I. Anatomy of the serotonergic system. Annals of the New York Academy of Sciences. 1990;600:9–34. doi: 10.1111/j.1749-6632.1990.tb16870.x. [DOI] [PubMed] [Google Scholar]
- van den Berg L, Pieterse K, Malik JA, et al. Association between impulsivity, reward responsiveness and body mass index in children. International Journal of Obesity. 2011;35:1301–7. doi: 10.1038/ijo.2011.116. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience and Biobehavioral Reviews. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Waxman SE. A systematic review of impulsivity in eating disorders. European Eating Disorders Review. 2009;17:408–25. doi: 10.1002/erv.952. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The five factor model and impulsivity: using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–89. [Google Scholar]
- Wunderlich K, Rangel A, O’Doherty JP. Economic choices can be made using only stimulus values. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15005–10. doi: 10.1073/pnas.1002258107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.