Abstract
Although numerous neuroimaging studies have examined what happens when individuals are instructed to regulate their emotions, we rarely receive such instruction in everyday life. This study sought to examine what underlies uninstructed modulation of negative affect by examining neural responses when ‘responding naturally’ to negative stimuli—and for comparison—during instructed reappraisal of negative stimuli as well. Two analyses were conducted to identify how variability in negative affect related to neural responses when responding naturally. First, in a within-participant analysis, lower levels of self-reported negative affect on a given trial were associated with recruitment of dorsolateral and dorsomedial prefrontal cortex (PFC)—brain regions also active during instructed reappraisal—whereas higher levels of negative affect were associated with recruitment of the amygdala—a region that responded more strongly overall to negative than neutral stimuli. Second, in a between-participant analysis, lower levels of average self-reported negative affect were associated with recruitment of ventromedial PFC. These results suggest that uninstructed modulation of emotion involves a combination of two types of regulatory processes, with moment-to-moment modulation depending on prefrontal regions that support reappraisal and individual differences in modulation depending on ventromedial PFC, a region involved in fear extinction.
Keywords: affect, emotion regulation, fMRI
INTRODUCTION
In the past decade, there has been a surge of neuroimaging studies examining the psychological and neural bases of emotion regulation. To date, the modal approach to studying emotion regulation has been to examine participants’ neural and behavioral responses to emotional stimuli during an instructed regulation condition (e.g. participants are told to reappraise affective stimuli) in comparison to an uninstructed viewing condition (e.g. Beauregard et al., 2001; Ochsner et al., 2002; Levesque et al., 2003). The basic premise behind such an approach is that only by directing participants to modulate their emotional responses can we clearly identify the neural and psychological mechanisms that underlie emotion regulation.
In our everyday lives, however, our natural responses to emotional stimuli are not driven by external instructions or goals. Yet, even when ‘responding naturally’, our reactions to emotional events can vary significantly according to transient cognitive and contextual factors as well as stable individual differences (Kim et al., 2004; Drabant et al., 2009; Somerville et al., 2013). At the neural level, this variability in negative affect could be supported by differential recruitment of at least three types of neural systems, each of which reflects the contribution to emotional responding of a different kind of process.
The first possibility is that differences in the strength of one’s initial negative affective response are supported by structures involved in the bottom-up generation of emotional reactions. Emotional reactions are supported by a host of subcortical and cortical brain regions that together coordinate physiological, affective and behavioral responses (Kober et al., 2008; Lindquist et al., 2012). Among the most commonly implicated regions is the amygdala, which is involved in the detection and recognition of affectively salient stimuli (Whalen, 1998; Morris et al., 1999; Anderson and Phelps, 2001), coordinating relevant physiological and behavioral responses (LeDoux, 2007), and may correlate with the intensity of affective experience (Canli et al., 2000; Williams et al., 2001; Anderson et al., 2003; Cunningham et al., 2004; Phan et al., 2004a; Ochsner et al., 2009). Given this, we would expect that increases in the strength of affective responding might involve bottom-up emotional processes supported by structures like the amygdala.
The second possibility is that modulation of negative affect is related to recruitment of prefrontal systems that support deliberate and effortful top-down control of emotion. Effortful control of emotion has been studied most commonly by instructing participants to engage in cognitive reappraisal, which involves reinterpreting a stimulus’s meaning so as to alter its emotional impact. Such studies have found that instructed reappraisal tends to recruit dorsal and lateral PFC and posterior parietal cortex (Kalisch, 2009; Diekhof et al., 2011; Buhle et al., 2013), regions commonly implicated in working memory (Wager and Smith, 2003), response inhibition (Robbins, 2007; Simmonds et al., 2008), self-monitoring (Botvinick et al., 2001) and mentalizing (Amodio and Frith, 2006; Olsson and Ochsner, 2008). When regulating emotion, these regions are thought to support the generation, manipulation and selection of appropriate reappraisals with the goal of altering one’s affective response. Reappraisal is also known to modulate activity in brain regions that support emotion generation such as the amygdala (Diekhof et al., 2011; Buhle et al., 2013). If the processes underlying uninstructed emotion modulation are similar to those underlying instructed regulation strategies like reappraisal, then we would expect that negative affect and amygdala responses will be weaker to the extent that dorsal and lateral PFC are activated.
A third possibility is that brain regions such as ventromedial PFC (VMPFC) that are not typically involved in reappraisal (Buhle et al., 2013)—but are critical for other types of regulatory processes—may support uninstructed modulation of negative affect. Prior work has suggested that VMPFC integrates affective appraisals formed by subcortical structures (e.g. the amygdala) with memorial and semantic information stored in the temporal cortex and inputs from other regions that provide information about current behavioral and motivational goals (Ochsner et al., 2002; Ongur et al., 2003; Davachi, 2006; Murray et al., 2007; Cunningham et al., 2011). As such, VMPFC activity has been linked with numerous types of affective learning where the affective value of a stimulus is altered and updated based on feedback, including fear extinction and reversal learning, and more generally appears to scale with the affective value one attributes to a stimulus in a situational and goal-dependent manner (Hare et al., 2009; Schoenbaum et al., 2011; Roy et al., 2012). In the context of negative emotion specifically, it has been suggested that VMPFC activity may serve as a ‘safety signal’ that attenuates amygdala responses (Schiller and Delgado, 2010; Etkin et al., 2011) and anxiety (Wager et al., 2009a; Somerville et al., 2013). If uninstructed emotion modulation relies on these kinds of contextual regulatory process, then we would expect that negative affect and amygdala responses would be weaker to the extent that VMPFC is activated.
In considering these three possibilities, the question arises of whether different types of uninstructed modulation are supported by different neural systems. For example, dorsal and lateral prefrontal systems known to support reappraisal may be associated with transient changes in negative affect within individuals whereas VMPFC may support more general differences in negative affect between individuals. Although it is difficult, if not impossible, to conclude with certainty that uninstructed emotion modulation has occurred, two types of evidence were considered: (i) patterns of brain activity that were associated with less negative affect, and (ii) overlap between brain regions showing such patterns and those known to support other forms of regulation (e.g. instructed reappraisal). With this in mind, this study used four analyses to determine what types of neural responses predict differences in negative affect both within and between individuals. First, we identified patterns of brain activity associated with emotion generation and instructed regulation via reappraisal. Second, we examined what neural responses were associated with transient changes in self-reported negative affect within participants while they responded naturally to aversive stimuli. Third, we examined what neural responses positively or negatively predicted differences in the average levels of negative affect reported between individuals. Finally, we compared neural responses during the uninstructed viewing period with those observed during emotion generation and instructed regulation.
METHODS
Participants
Thirty healthy adults (13 females, mean age = 21.97) took part in the experiment.
All participants gave informed consent, were right-handed, had normal or corrected vision and had no history of diagnosed psychiatric or medical illness as indicated by self-report on a general health questionnaire. A subset of the participants in the present dataset were also included in studies reported elsewhere that focused on instructed reappraisal only (Wager et al., 2008; Denny et al., 2013).
Training procedures
Prior to scanning, participants were told that they would see a series of images preceded by instructional cues that would inform them about the type of stimuli they were about to see and what they were supposed to do while viewing the stimuli. Instructional cues came in the form of geometric shapes and the particular instruction paired with each cue was counterbalanced across participants. In accordance with the instructional cue, participants did one of the following on each trial: (i) look at a neutral image and respond naturally (‘look/neutral’ condition), (ii) look at a negative image and respond naturally (‘look/negative’ condition) or (iii) reappraise a negative image (‘reappraise/negative’ condition). The instructions for look trials were intentionally kept as open-ended as possible and it was assumed that, in responding naturally, participants might respond in a variety of ways. For example, participants may have consciously reappraised images on the look trials, or engaged in ‘reality checking’ by reminding themselves they were inside of a scanner, or they could have simply thought about the images in an entirely unregulated way. The unconstrained nature of the look condition was necessary for examining variability in emotional responding. If participants had been told to respond uniformly on all look trials, there would likely be too little intra- and inter-individual variability to assess uninstructed modulation. In keeping with numerous prior studies (Ochsner et al., 2002, 2004; Urry et al., 2006; Kim and Hamann, 2007; van Reekum et al., 2007a; McRae et al., 2010), on reappraisal trials participants were told to, ‘re-interpret the possible antecedents, outcomes and/or reality of the events you see in such as way that your emotional response is decreased’. Prior to scanning, participants (i) were quizzed on cue–task condition associations (e.g. a triangle cue means a look/negative trial is coming), (ii) completed seven sample trials with the experimenter present to ensure they understood the instructions, and (iii) completed 18 practice trials without receiving feedback from the experimenter. No images used during training were also used during scanning. Participants were asked to not look away from images or to distract themselves with irrelevant or positive thoughts during the task. Participants’ eye motion was monitored live during scanning by experimenters viewing a projected image of the right eye in the scanner control room (I-SCAN, Inc.). No participants averted or closed their eyes during image viewing.
Task design
Participants completed six functional runs, each of which contained 36 trials, for a total of 108 trials. Equal numbers of each condition (look/neutral, look/negative, reappraise/negative) were shown and trials were presented in an event-related fashion. Basic trials began with a 2 s instructional cue followed by a 4 s anticipatory interval during which a fixation cross was presented on the screen. The image stimulus was then presented for 8 s. After image presentation, a fixation cross was presented for a jittered inter-stimulus interval (4–7 s) and then a rating screen appeared for 2.1 s. On the rating screen, participants were asked to rate how strongly negative they felt on a scale of 0–4 (0 = not negative at all, 4 = very negative). The trial concluded after the rating screen with a fixation cross that lasted 4–7 s (the duration was jittered). Self-report was chosen as the primary dependent variable of interest because it provides a unique and relatively direct window into emotional experiences that other measures cannot provide (Gilbert, 2006; Larsen and Prizmic-Larsen, 2006). Peripheral physiological measures have the advantage of being resistant to demand characteristics (e.g. Quirk and Beer, 2006), yet because they only measure gross changes in autonomic arousal, their significance can be ambiguous. For example, changes in skin conductance or pupil dilation may represent shifts in arousal, cognitive effort, or something else altogether (Lang et al., 1993; Siegle et al., 2003; Bradley et al., 2008; Geva et al., 2013; van Steenbergen and Band, 2013).
Basic trials, ‘anticipation only’ trials (trials that did not include image presentation) and ‘stimulus only’ trials (trials that did not include the 4 s anticipation period) were shown with equal frequency. Different trial types were included so as to examine the effects of anticipation on reappraisal and have been reported elsewhere. The focus of this study was on reappraisal of aversive images and therefore, the present analyses exclusively used trial types that included the presentation of an image as well as a rating period (basic and stimulus only trials). Thus, a total of 72 trials were contributed to the present analyses with 24 trials contributing to each condition of interest (look/neutral, look/negative, reappraise/negative). These 48 negative images (mean normative valence = 2.24; mean normative arousal = 6.28) and 24 neutral images (mean normative valence = 5.27; mean normative arousal = 3.51) came from the International Affective Picture Set (Lang et al., 2008).
Imaging acquisition
Whole-brain functional MRI (fMRI) data were acquired on a 1.5 T GE Signa Twin Speed Excite HD scanner (GE Medical Systems). Functional images were acquired with a T2*-sensitive EPI BOLD sequence. Twenty-four axial slices were collected with a repetition time (TR) of 2000 ms (Echo time of 40 ms, flip angle of 60°, field of view of 22 cm and 3.44 × 3.44 × 4.5 mm voxels). Stimuli were presented using E-Prime. Stimuli were displayed using an LCD projector and a back-projection screen mounted in the scanner suite. Participants made their responses using a five-finger-button-response unit with a molded hand brace (Avotec Inc. and Resonance Technologies).
fMRI analysis
Preprocessing and first and second-level analyses
Preprocessing was performed using FSL (FMRIB Center, University of Oxford) and SPM2 (Wellcome Department of Cognitive Neurology, UCL). Functional images were slice-time and motion corrected using FSL. Structural images were coregistered to the first functional image for each subject using an iterative procedure of automated registration using mutual information coregistration in SPM2. Structural images were normalized (spatially warped) to a standard template brain (the MNI avg15T1.img) using SPM2’s default options (7 × 8 × 7 nonlinear basis functions) and warping parameters were applied to functional images for each subject. Normalized functional images were interpolated to 3 × 3 × 3 mm voxels and spatially smoothed with a 6 mm Gaussian filter.
First and second-level analyses were implemented in NeuroElf (http://neuroelf.net). Cue, anticipation, stimulus-viewing and response portions of each trial were modeled as boxcar regressors convolved with a canonical hemodynamic response function. Separate regressors were made for the three trial types: reappraise/negative, look/negative and look/neutral trials. Motion parameters and high-pass filter parameters were included as regressors of no interest. Head motion did not exceed 3 mm in any direction for any participant. Next, a second-level random effects analysis was performed to identify regions of activation at the group level. Results were masked using a gray matter mask created through segmentation of the MNI-T1 template (the ‘Colin’ brain). Significant voxels were identified using joint height (P < 0.005) and extent thresholds determined by AlphaSim, as implemented in NeuroElf so as to control the family-wise-error-rate (FWE) at a < 0.05. AlphaSim was computed for each map separately so as to accurately estimate the inherent smoothness of each contrast, rather than to rely on the size of the Gaussian kernel used during preprocessing which can lead to errors in thresholding (Bennett et al., 2009). Extent thresholds for each contrast are reported in each of the subsequent paragraphs detailing specific analyses. For the neuroimaging, analyses focused on the portion of the trials when the picture was on the screen and participants were implementing the ‘look’ or ‘reappraise’ strategy.
Identifying regions involved in emotion generation and instructed regulation
To identify brain regions that support the generation of emotional responses, the look/negative condition was contrasted with the look/neutral condition (the ‘emotion generation contrast’; thresholded at 277 voxels). To identify brain regions that support instructed emotion regulation, the reappraise/negative condition was contrasted with the look/negative condition (the ‘emotion regulation contrast’; thresholded at 290 voxels). To identify brain regions that supported trial-by-trial changes in negative affect for the instructed regulation condition, trial-by-trial reports of negative affect were used as a modulation parameter for reappraise/negative trials (the ‘instructed regulation parametric analysis’; thresholded at 160 voxels). Given that results identified in this analysis could be somewhat ambiguous to interpret—for example, more negative affect coupled with more neural activity could reflect the generation of stronger negative emotions or failed emotion regulation—a second parametric analysis was conducted that was intended to isolate neural activity associated with greater reappraisal success or failure (i.e. more or less negative affect relative to a normative baseline). In this second analysis, International Affective Picture System (IAPS) normative ratings were rescaled so as to be on the same 0–4 scale used in the task and the difference score between a participant’s self-reported negative affect for a given image and the normative IAPS rating was calculated for each reappraisal trial and used as a parametric modulator. Activation in this second analysis did not yield any clusters that survived FWE correction.
Identifying regions involved in uninstructed emotion modulation
Two analyses were performed to examine the neural bases of differences in negative affect. First, to identify brain regions supporting dynamic, within-participant changes in negative affect, participants’ trial-by-trial self-reports of negative affect were used as a modulation parameter for look/negative trials (the ‘within-participants contrast’; thresholded at 65 voxels). To determine whether trial-by-trial changes in self-reported negative affect were related to normative ratings and whether this may explain differences in neural activation, correlations were calculated for each participant between published normative data on stimulus valence and self-reported negative affect (Lang et al., 2008). The degree to which normative valence predicted self-reported negative affect varied widely between participants (mean r value = 0.35, s.d. = 0.22), but was unrelated to the recruitment of brain regions identified in the within-participants contrast (Ps > 0.34). This suggests not only that participants vary in the degree to which they conform to normative affective data but also that this variability does not drive patterns of activation identified in the within-participants contrast. In a second analysis, neural responses identified in the look/negative > look/neutral fMRI contrast were robustly correlated (a method that reduces the influence of outliers) with participants’ average difference in self-reported negative affect on look/negative and look/neutral trials (the ‘between-participants contrast’; thresholded at 77 voxels).
Finally, exploratory analyses were conducted to characterize the extent of overlap between the within-participants and between-participants contrasts and the emotion generation and emotion regulation contrasts. Specifically, the within-participants and between-participants maps were masked by the emotion generation and regulation contrasts as well as the emotion regulation within-subjects contrast. This was done through a double-masking approach that isolated voxels that were highly significant for both the uninstructed modulation analysis as well as the task-based analysis. For example, to identify brain regions that predicted changes in negative affect within participants that also responded during instructed regulation, the within-subjects contrast map was first masked by itself and then masked by the voxels from the instructed regulation contrast. The extent threshold for the within-subjects map masked by the emotion regulation contrast was set at 16 voxels, whereas the extent threshold for the within-subjects map masked by the emotion generation contrast was set at 7 voxels. No voxels showed any overlap between the between-subjects map and the instructed regulation contrast nor did any voxels show any overlap between the between-subjects map and the emotion generation contrast and thus there were no data to threshold. The extent threshold for the within-subjects map masked by the emotion regulation within-subjects contrast was set at 23 voxels.
RESULTS
Behavioral results
Participants reported significantly stronger negative affect for when responding naturally to negative stimuli (M = 2.62, s.d. = 0.55; minimum, maximum = 0.74, 3.58; skew ± s.e. = −1.21 ± 0.43; kurtosis ± s.e. = 3.72 ± 0.83) than neutral (M = 0.34, s.d. = 0.17; minimum, maximum = 0.05, 0.63; skew ± s.e. = 0.37 ± 0.43; kurtosis ± s.e. = −0.99 ± 0.83) stimuli [t(29) = 22.51, P < 0.001]. Participants reported significantly less negative affect when reappraising negative stimuli (M = 1.68, s.d. = 0.56; minimum, maximum = 0.58, 2.83; skew ± s.e. = −0.16 ± 0.43; kurtosis ± s.e. = −0.21 ± 0.83) than when responding naturally to negative stimuli [t(29) = 8.10, P < 0.001].
Individual differences in trait anxiety
A subset of participants (N = 9, five females, mean age = 24.6 years) completed the trait portion of the State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983) and reported anxiety levels comparable to published norms (M = 34.11, s.d. = 9.75). Anxiety scores did not correlate with self-reported negative affect in any of the task conditions, nor did it correlate with the difference scores between the reappraise/negative and look/negative conditions or the look/negative and look/neutral conditions Ps > 0.25.
Brain regions involved in emotion generation
Relative to neutral stimuli, responding naturally to negative stimuli recruited brain regions involved in both emotional and sensory processing including the amygdala, anterior insula, midbrain and visual cortex. A full list of brain regions identified in the emotion generation contrast can be seen in Table 1.
Table 1.
Brain regions associated with emotion generation (look negative > look neutral)
| MNI coordinates |
||||||
|---|---|---|---|---|---|---|
| Region | Side | Extent | t | x | y | z |
| Midbrain | Medial | 949 | 5.61 | 0 | −26 | −7 |
| Anterior insula | Right | LM | 5.19 | 39 | 25 | −7 |
| Dorsal amygdala | Right | LM | 5.18 | 24 | −5 | −10 |
| Caudate | Right | LM | 3.18 | 15 | 19 | 5 |
| Globus pallidus | Right | LM | 3.57 | 9 | −2 | −10 |
| Globus pallidus | Right | LM | 3.30 | 24 | −11 | 2 |
| Globus pallidus | Right | LM | 3.60 | 15 | −2 | 8 |
| Globus pallidus | Right | LM | 4.14 | 30 | −17 | −7 |
| Putamen | Right | LM | 4.18 | 27 | 7 | −1 |
| Hippocampus | Right | LM | 3.83 | 30 | −29 | −1 |
| Temporal pole | Right | LM | 4.08 | 39 | 22 | −40 |
| Superior temporal gyrus | Right | LM | 3.79 | 48 | 19 | −25 |
| Middle occipital gyrus | Right | 1518 | 9.73 | 54 | −65 | 8 |
| Middle occipital gyrus | Right | LM | 9.47 | 51 | −77 | 8 |
| Fusiform gyrus | Right | LM | 6.46 | 42 | −47 | −22 |
| Middle occipital gyrus | Left | 1446 | 9.01 | −42 | −80 | 2 |
| Middle occipital gyrus | Left | LM | 6.99 | −45 | −65 | 8 |
| Fusiform gyrus | Left | LM | 5.83 | −39 | −65 | −7 |
| Cerebellum | Left | LM | 4.44 | −36 | −71 | −22 |
| Inferior occipital gyrus | Left | LM | 3.48 | −24 | −98 | −16 |
| Posterior insula | Left | 862 | −5.72 | −39 | −41 | 23 |
| Posterior insula | Left | LM | −5.71 | −45 | −29 | 5 |
| Posterior insula | Left | LM | −4.43 | −36 | −14 | 17 |
| Superior temporal gyrus | Left | LM | −4.63 | −63 | −17 | −1 |
| Superior temporal gyrus | Left | LM | −5.06 | −51 | −20 | 5 |
| Middle temporal gyrus | Left | LM | −3.73 | −66 | −26 | −7 |
| Superior temporal gyrus | Right | 801 | −5.99 | 57 | −26 | 8 |
| Superior temporal gyrus | Right | LM | −5.61 | 60 | −2 | 2 |
| Superior temporal gyrus | Right | LM | −5.31 | 69 | −17 | 2 |
| Inferior temporal gyrus | Right | LM | −4.00 | 72 | −26 | −19 |
| Posterior insula | Right | LM | −3.40 | 42 | −26 | 20 |
| Posterior insula | Right | LM | −4.86 | 42 | −14 | 5 |
| Posterior insula | Right | LM | −4.86 | 42 | −14 | 5 |
LM, local maxima. t-values are given for peak voxels in each cluster. Negative t-values indicate brain regions that responded more strongly to neutral stimuli than negative stimuli.
Brain regions involved in emotion regulation
Instructed regulation of negative stimuli recruited large swaths of VLPFC, dorsolateral PFC (DLPFC) and dorsomedial PFC (DMPFC). Although these activations were observed bilaterally, they were more extensive in nature on the left side. Additional activations were observed in left superior temporal cortex and posterior parietal cortex. A full list of brain regions identified in the emotion regulation contrast can be seen in Table 2. Trial-by-trial decreases in negative affect on instructed reappraisal trials were supported by enhanced recruitment of bilateral inferior frontal gyrus (Table 2).
Table 2.
Brain regions associated with instructed regulation (reappraise negative > look negative) and trial-by-trial changes in negative affect on instructed regulation trials
| MNI coordinates |
||||||
|---|---|---|---|---|---|---|
| Region | Side | Extent | t | x | y | z |
| Reappraise/negative > Look/negative | ||||||
| Middle frontal gyrus | Left | 6111 | 7.84 | −42 | 10 | 47 |
| Inferior frontal gyrus | Left | LM | 5.70 | −60 | 28 | 8 |
| Inferior frontal gyrus | Left | LM | 5.00 | −57 | 37 | −1 |
| Inferior frontal gyrus | Left | LM | 5.02 | −54 | 37 | −13 |
| Inferior frontal gyrus | Left | LM | 6.39 | −51 | 28 | 14 |
| Inferior frontal gyrus | Left | LM | 3.92 | −48 | 25 | −19 |
| Inferior frontal gyrus | Left | LM | 4.29 | −45 | 22 | −4 |
| Inferior frontal gyrus | Left | LM | 3.82 | −33 | 34 | −13 |
| Inferior frontal gyrus | Right | LM | 4.42 | 48 | 22 | 17 |
| Inferior frontal gyrus | Right | LM | 3.56 | 48 | 19 | −7 |
| Inferior frontal gyrus | Right | LM | 4.76 | 57 | 28 | −10 |
| Inferior frontal gyrus | Right | LM | 3.57 | 60 | 31 | 2 |
| Middle frontal gyrus | Left | LM | 4.41 | −33 | 52 | 8 |
| Middle frontal gyrus | Right | LM | 4.21 | 24 | 52 | 29 |
| Middle frontal gyrus | Right | LM | 4.15 | 24 | 58 | 20 |
| Middle frontal gyrus | Right | LM | 3.69 | 30 | 16 | 47 |
| Middle frontal gyrus | Right | LM | 5.07 | 45 | 25 | 32 |
| Superior frontal gyrus | Left | LM | 5.02 | −24 | 61 | 11 |
| Superior frontal gyrus | Left | LM | 4.07 | −21 | 28 | 35 |
| Superior frontal gyrus | Left | LM | 3.30 | −15 | 58 | 38 |
| Superior frontal gyrus | Left | LM | 3.59 | −12 | 49 | 38 |
| Superior frontal gyrus | Left | LM | 7.80 | −6 | 28 | 53 |
| Superior frontal gyrus | Right | LM | 4.75 | 12 | 37 | 50 |
| Superior frontal gyrus | Right | LM | 3.41 | 21 | 7 | 68 |
| Superior frontal gyrus | Right | LM | 3.92 | 24 | 46 | 38 |
| Anterior cingulate cortex | Left | LM | 5.14 | −9 | 28 | 32 |
| Medial frontal gyrus | Left | LM | 3.92 | −6 | 52 | 26 |
| Medial frontal gyrus | Medial | LM | 3.70 | 0 | 55 | 11 |
| Anterior insula | Left | LM | 3.66 | −33 | 19 | −4 |
| Anterior insula | Right | LM | 3.26 | 36 | 22 | −4 |
| Middle temporal gyrus | Left | LM | 3.81 | −66 | −41 | 5 |
| Middle temporal gyrus | Left | LM | 4.63 | −63 | −14 | −10 |
| Middle temporal gyrus | Left | LM | 4.42 | −63 | −41 | −4 |
| Middle temporal gyrus | Left | LM | 4.10 | −63 | 1 | −19 |
| Middle temporal gyrus | Left | LM | 4.44 | −54 | −2 | −16 |
| Middle temporal gyrus | Left | LM | 3.61 | −54 | 1 | −40 |
| Middle temporal gyrus | Left | LM | 4.25 | −51 | 10 | −31 |
| Superior temporal gyrus | Left | LM | 4.82 | −69 | −29 | −1 |
| Superior temporal gyrus | Left | LM | 4.47 | −66 | −56 | 17 |
| Superior temporal gyrus | Left | LM | 4.07 | −57 | 19 | −10 |
| Superior temporal gyrus | Left | LM | 4.65 | −48 | −26 | −4 |
| Inferior parietal lobule | Left | LM | 5.10 | −51 | −68 | 44 |
| Inferior parietal lobule | Left | LM | 6.42 | −48 | −56 | 35 |
| Superior parietal lobule | Left | LM | 3.20 | −27 | −77 | 47 |
| Regions associated with less negative affect during reappraisal | ||||||
| Inferior frontal gyrus | Left | 196 | −3.17 | −42 | 7 | 29 |
| Middle frontal gyrus | Left | LM | −2.29 | −42 | 31 | 23 |
| Middle frontal gyrus | Left | LM | −3.08 | −39 | −2 | 41 |
| Middle frontal gyrus | Left | LM | −2.86 | −33 | 40 | 11 |
| Inferior frontal gyrus | Right | 363 | −3.51 | 54 | 13 | 14 |
| Inferior frontal gyrus | Right | LM | −2.41 | 42 | 16 | 29 |
| Inferior frontal gyrus | Right | LM | −2.40 | 48 | 1 | 29 |
| Inferior frontal gyrus | Right | LM | −3.23 | 63 | 13 | 35 |
| Inferior frontal gyrus | Right | LM | −3.41 | 66 | 13 | 11 |
| Regions associated with more negative affect during reappraisal | ||||||
| Cerebellum | Right | 168 | 3.00 | 9 | −83 | −25 |
| Cerebellum | Right | LM | 2.41 | 27 | −77 | −28 |
| Cuneus | Right | LM | 2.57 | 12 | −95 | −16 |
| Cuneus | Right | LM | 2.50 | 15 | −104 | 5 |
| Cuneus | Right | LM | 2.44 | 18 | −98 | −4 |
LM, local maxima. No brain regions responded more strongly for the reverse contrast. t-values are indicated for peak voxels.
Neural bases of within-participant differences in negative affect
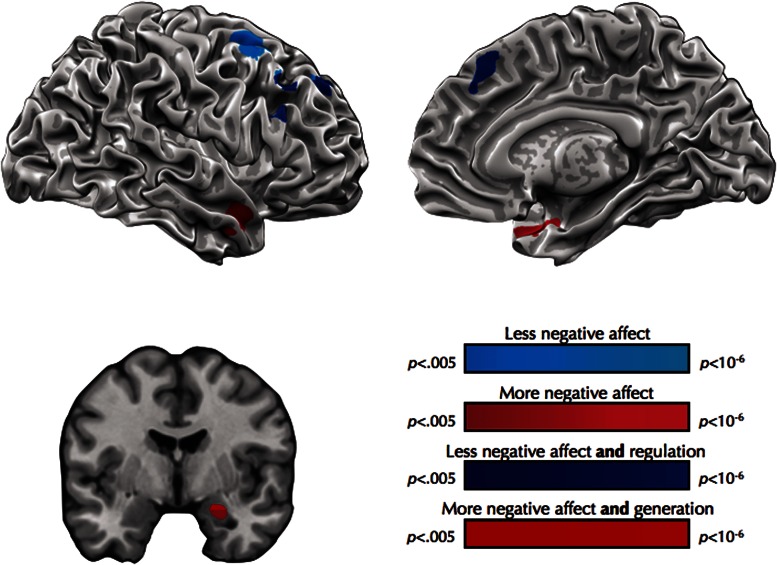
While responding naturally to negative stimuli, relatively lower reports of negative affect on a trial-by-trial basis were associated with increased recruitment of right DLPFC and DMPFC (Table 3; Figure 1). In contrast, relatively greater reports of negative affect were associated with enhanced recruitment of the right medial temporal lobe, including the amygdala (Table 3; Figure 1). The majority of voxels in DMPFC and DLPFC (268/356; 75%) identified as being associated with lower levels of negative affect fell within the emotion regulation mask (Table 4; Figure 1). The dorsal half of the amygdala cluster that was associated with greater levels of negative affect fell within the emotion generation mask (11/26 amygdala voxels; 42%). No brain regions that were associated with lower trial-by-trial reports of negative affect fell within the emotion generation mask nor did any brain regions associated with greater reports of negative affect fall within the emotion regulation mask. In no brain regions did activity track trial-by-trial changes in negative affect on both ‘look’ and ‘reappraise’ trials.
Table 3.
Brain regions associated with within-subject changes in negative affect
| MNI coordinates |
||||||
|---|---|---|---|---|---|---|
| Region | Side | Extent | t | x | y | z |
| Regions associated with less negative affect | ||||||
| Superior frontal gyrus | Right | 133 | −3.74 | 21 | 46 | 38 |
| Medial frontal gyrus | Medial | LM | −3.49 | 0 | 28 | 50 |
| Medial frontal gyrus | Right | LM | −3.45 | 9 | 34 | 47 |
| Middle frontal gyrus | Right | LM | −3.32 | 24 | 52 | 20 |
| Middle frontal gyrus | Right | 152 | −4.31 | 30 | 10 | 56 |
| Superior frontal gyrus | Right | LM | −3.19 | 15 | 10 | 65 |
| Inferior frontal gyrus | Right | 71 | −3.90 | 54 | 28 | 29 |
| Regions associated with more negative affect | ||||||
| Middle temporal gyrus | Right | 155 | 4.86 | 48 | 7 | −28 |
| Temporal pole | Right | LM | 4.26 | 36 | 13 | −31 |
| Amygdala | Right | LM | 3.63 | 27 | −5 | −22 |
t-represents peak t-values for each cluster, negative values indicate regions associated with less negative affect. LM, local maxima.
Fig. 1.
Brain regions associated with within-participant changes in negative affect. Aqua represents brain regions associated with less negative affect and mauve represents brain regions associated with more negative affect. Dark blue represents brain regions associated with less negative affect that also fell within the emotion regulation mask. Red represents brain regions associated with more negative affect that also fell within the emotion generation mask.
Table 4.
Brain regions associated within-subject changes in negative affect, masked by brain regions associated with emotional regulation (reappraise > look negative) and emotion generation (look negative > look neutral)
| MNI coordinates |
||||||
|---|---|---|---|---|---|---|
| Region | Side | Extent | t | x | y | z |
| Regions associated with less negative affect, masked by emotion regulation map | ||||||
| Superior frontal gyrus | Right | 133 | −3.74 | 21 | 46 | 38 |
| Medial frontal gyrus | Medial | LM | −3.49 | 0 | 28 | 50 |
| Medial frontal gyrus | Right | LM | −3.45 | 9 | 34 | 47 |
| Middle frontal gyrus | Right | LM | −3.32 | 24 | 52 | 20 |
| Middle frontal gyrus | Right | 71 | −4.31 | 30 | 10 | 56 |
| Superior frontal gyrus | Right | LM | −3.19 | 15 | 10 | 65 |
| Superior frontal gyrus | Right | LM | −3.44 | 27 | 19 | 59 |
| Inferior frontal gyrus | Right | 64 | −3.90 | 54 | 28 | 29 |
| Middle frontal gyrus | Right | LM | −3.40 | 45 | 31 | 41 |
| Regions associated with more negative affect, masked by emotion generation map | ||||||
| Amygdala | Right | 11 | 3.60 | 24 | −5 | −19 |
| Superior temporal gyrus | Right | 5 | 3.23 | 39 | 19 | −28 |
| Superior temporal gyrus | Right | 6 | 3.68 | 51 | 13 | −22 |
t represents peak t-values for each cluster, negative values indicate regions associated with less negative affect. LM, local maxima. No brain regions associated with less negative affect were revealed within the emotion generation map and no brain regions associated with more negative affect were revealed within the emotion regulation map. No brain regions associated with more or less negative affect fell within the emotion regulation within-subject map.
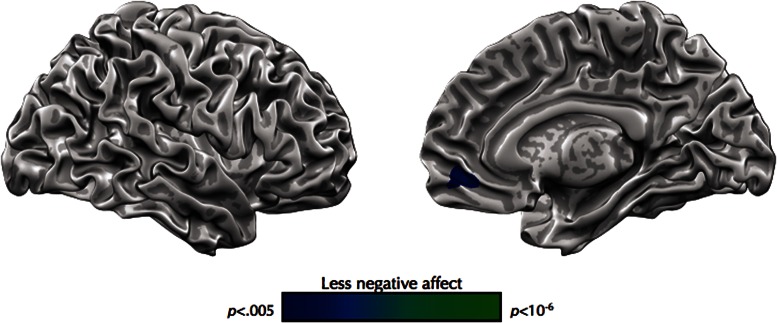
Neural bases of between-participant differences in negative affect
Participants who on average strongly recruited VMPFC when responding to negative vs neutral stimuli reported lower levels of average negative affect (Table 5; Figure 2). No brain regions were associated with relatively greater reports of negative affect in this analysis. No clusters obtained in this analysis fell within the emotion generation or regulation masks, nor within the emotion regulation within-subjects mask. VMPFC responses did not correlate with trait anxiety (r = 0.04, P = .92)
Table 5.
Brain regions associated with between-subject differences in negative affect
| MNI coordinates |
||||||
|---|---|---|---|---|---|---|
| Region | Side | Extent | r | x | y | z |
| Regions associated with less negative affect | ||||||
| Medial frontal gyrus | Right | 127 | −0.60 | 15 | 49 | −7 |
| Medial frontal gyrus | Left | LM | −0.56 | −3 | 49 | −10 |
| Subgenual anterior cingulate cortex | Left | LM | −0.55 | −12 | 37 | −7 |
r-values represent correlation coefficients for the peak voxel identified in each cluster. These regions were identified through a robust correlation between the look negative > look neutral contrast and average self-reported negative affect on look negative vs look neutral trials. LM, local maxima. No brain regions were associated with more negative affect. None of the regions listed below fell within the emotion generation or regulation maps.
Fig. 2.
Brain regions associated with between-participant difference in negative affect. Activation for the look negative > look neutral contrast correlated with participants average self-reported negative affect on look negative vs look neutral trials. None of these brain regions fell within the emotion generation or regulation masks.
DISCUSSION
In our everyday lives, we typically encounter emotion-eliciting stimuli and events without being instructed about how to respond to them. Yet, in ‘responding naturally’ to emotional triggers, our emotional experiences may vary significantly in their intensity. To our knowledge, this study is the first to examine how patterns of neural activity in systems implicated in emotion generation or regulation predict less negative affect during uninstructed viewing of aversive stimuli using analyses to identify both within and between-individuals effects.
Drawing from prior work, we hypothesized that lower levels of negative affect could be associated with (i) less activity in circuitry implicated in affect generation (i.e. amygdala), (ii) more activity in prefrontal regions implicated in effortful regulatory strategies like reappraisal (i.e. dorsal and lateral PFC), and/or (iii) more activity in prefrontal regions that support affective learning, decision making and regulatory processes that do not require explicit regulatory goals (i.e. VMPFC). When examining within-participant variations in negative affect across trials, we found support for our first and second hypotheses: activity in dorsomedial and dorsolateral prefrontal regions associated with reappraisal predicted less negative affect on a trial-by-trial basis whereas activity in an amygdala region associated with emotion generation showed reduced activity. When examining average differences in negative affect between individuals, we found that only activity in VMPFC predicted lower levels of negative affect.
Implications for neural models of emotion regulation
This study has both methodological and conceptual implications for models of emotion generation and regulation. Methodologically speaking, this study points to the value of integrating neuroimaging research on the neural bases of uninstructed emotional responding and instructed emotion regulation. Several studies have examined what brain regions track with increasing emotional intensity during uninstructed emotional responding (Canli et al., 2000; Anderson et al., 2003; Phan et al., 2004b), but few studies have examined what brain regions track with ‘decreasing’ emotional intensity. One exception to this point is work by Heinzel et al. (2005) that examined neural responses that tracked with stimulus valence, as indicated by published normative rating data. However, this study differs from this prior work by (i) using participants own ratings rather than published normative data, (ii) looking at how affective responses vary ‘within’ the context of negative emotion rather than across different types of emotional responses (negative, positive and neutral), and (iii) examining what neural responses are associated with both within and between subject variability in affective experience.
To our knowledge, no other study has directly compared neural recruitment during instructed emotion regulation with neural recruitment during uninstructed modulation of negative affect. In doing this, the present study provides strong evidence to suggest that uninstructed changes in affective experience are supported by recruitment of prefrontal regions that also support cognitive reappraisal. DLPFC and DMPFC are among the most consistently activated brain regions in neuroimaging studies of reappraisal (Kalisch, 2009; Diekhof et al., 2011; Buhle et al., 2013). Given that DLPFC supports working memory (Miller, 2000; Wager and Smith, 2003) and DMPFC supports self-focused processing and mental state attribution (Amodio and Frith, 2006; Olsson and Ochsner, 2008; Denny et al., 2012), it has been suggested that during reappraisal DLPFC facilitates the maintenance and manipulation of candidate reappraisals in working memory whereas DMPFC supports monitoring of regulation success and one’s changing affective states (Ochsner et al., 2012). Although we cannot conclude with certainty whether or not participants were reappraising on trials where they showed lower levels of negative affect, the present data suggest that (i) uninstructed modulation of negative affect and instructed cognitive reappraisal recruit highly overlapping portions of prefrontal cortex, and (ii) uninstructed modulation of negative affect involves recruitment of processes involved in cognitive control and self-monitoring. In contrast to DLPFC and DMPFC, negative affect on look/negative trials tracked ‘positively’ with amygdala activation. There are two potential explanations for this. The first is that amygdala responses were lower on trials where participants simply did not perceive the images to be that aversive in the first place. The second is that amygdala activity was influenced in a top-down manner by recruitment of DLPFC and DMPC, similarly to what has been suggested in prior models and meta-analyses of reappraisal (Diekhof et al., 2011; Ochsner et al., 2012; Buhle et al., 2013). Additional work is needed to tease apart these two possibilities.
The present data also suggest that lateral prefrontal and ventromedial prefrontal regions play complementary roles in managing negative affect. Although dorsal and lateral PFC seemed to support transient modulation of negative affect on given trials, only VMPFC was associated with lower levels of cross-trial averaged negative affect. VMPFC is consistently recruited in placebo, reversal learning and fear extinction paradigms wherein negative affect is reduced because of changes in the contextual meaning of stimuli, or contingencies or expectances about the relationship between behavior and the affective value of stimuli (Schiller and Delgado, 2010; Diekhof et al., 2011). In decision-making and evaluation paradigms, VMPFC recruitment is associated with increased perceived value and diminished perceptions of risk (Wallis and Miller, 2003; Kable and Glimcher, 2007; Hare et al., 2008; Rolls et al., 2008; Xue et al., 2009) whereas other studies examining responses to aversive stimuli have found that VMPFC responses are reduced under stress and threat (Mobbs et al., 2007; Wager et al., 2009b). Taken together, this suggests that VMPFC integrates affective, semantic and contextual knowledge to support the appropriate affective valuation of stimuli for a given individual in a given context (Schoenbaum et al., 2011; Roy et al., 2012).
The present results suggest that VMPFC responses to affective stimuli can vary in meaningful ways between individuals. This notion is supported by prior work demonstrating that individual differences in VMPFC recruitment in response to aversive, stressful or anxiety-provoking contexts scales negatively with self-reported experiences of negative affect, anxiety and discomfort (van Reekum et al., 2007b; Wager et al., 2009b; Eisenberger et al., 2011; Somerville et al., 2013). Additionally, individuals with post-traumatic stress disorder (Etkin and Wager, 2007), anxiety disorders (Etkin et al., 2010) and depression (Johnstone et al., 2007) show dampened VMPFC responses and atypical VMPFC-amygdala connectivity when responding to aversive stimuli across a variety of tasks, including those involving an instructed regulation condition. Taken together, this suggests that greater sustained VMPFC activity may assist in maintaining internalized representations of safety that subsequently buffer individuals against affective triggers that are likely to evoke fear or anxiety.
Limitations and future directions
The present data suggest a novel interpretation of how prefrontal and subcortical regions involved in emotion generation and regulation support dynamic, within-individual changes in affect as well as average differences in the ways that individuals respond to aversive events. Although DLPFC and DMPFC recruitment was associated with less negative affect and amygdala recruitment was associated with more negative affect within individuals, only VMPFC activity was associated with lower levels of average negative affect between individuals. In considering these findings, it is important to consider limitations of the present design as well as to identify important future directions for this line of research.
First, future research may seek to investigate further what strategies (if any) participants are engaging when they report experiencing less negative affect on a trial-by-trial basis. For example, after completing the paradigm, participants could be shown the emotional stimuli a second time and asked to retrospectively report on what thoughts or experiences they had for each one. Such an approach might help elucidate whether the patterns of activation in this study were representative of participants implementing cognitive regulatory strategies or some other type of as yet unidentified process that was incidentally inversely correlated with negative affect.
Second, although VMPFC activation differentiated individuals according to their average negative emotionality on the present task, additional work is needed to establish whether (i) the same pattern would hold across other experimental contexts, and (ii) whether these differences relate to mental health and wellbeing. With regards to the first issue, follow-up work might examine whether individuals’ VMPFC responses to one type of aversive stimuli (e.g. negative images) is predictive of responses to other types of aversive stimuli (e.g. shock) as a means of testing how stable VMPFC activity is across contexts. With regards to the second issue, an important direction for future work will be to use this paradigm in conjunction with additional laboratory and questionnaire measures to better characterize what individual differences relate to VMPFC responses (Bishop et al., 2004; van Reekum et al., 2007b; Urry et al., 2009). Although this study did not find evidence to suggest that trait anxiety levels correlated with VMPFC responses, these results should be interpreted with caution given that only a subset of participants contributed to this analysis. Future work may seek to examine whether individual differences moderate the relationship between VMPFC recruitment and self-reported negative affect. For example, this brain–behavior relationship may be weaker in individuals who are high in trait anxiety, as indexed by the STAI, or stronger in individuals who tend to reappraise more frequently in their everyday lives, as indexed by a measure like the Emotion Regulation Questionnaire.
Finally, it would be worthwhile for future work to attempt to replicate the present findings without the inclusion of an instructed cognitive reappraisal condition. Although the inclusion of this condition in the present paradigm allowed for a direct comparison between uninstructed modulation in negative affect and modulation supported by instructed reappraisal, this methodological choice could also be seen as a limitation given that reappraisal training may have influenced how participants responded naturally to negative stimuli. Prior work has suggested that trait tendencies to reappraise in every day life may impact uninstructed responding to affective stimuli, but no prior work has tested whether a relatively brief introduction to reappraisal may also influence uninstructed responding (Drabant et al., 2009). To address this issue, a follow-up study may adopt a between-subjects design wherein half of the participants engage in reappraisal training prior to completing the task whereas the other half of participants do not.
AUTHOR CONTRIBUTIONS
Design: J.A.S., T.D.W., K.N.O.; analysis: J.A.S., J.W., T.D.W; writing: J.A.S., K.N.O.
Acknowledgments
The authors would like to thank Brent Hughes, Melissa Brandon and Matthew Davidson for their contributions in data collection.
This work was supported by the National Institutes of Health (grant number F31 MH094056 awarded to J.A.S.; R01 MH076137 awarded to K.N.O.; R01 MH076136, awarded to T.D.W.) and the National Science Foundation (grant number 0631637, awarded to K.N.O).
REFERENCES
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411(6835):305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21(18):RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CM, Wolford GL, Miller MB. The principled control of false positives in neuroimaging. [Research Support, U.S. Gov’t, Non-P.H.S.] Social Cognitive and Affective Neuroscience. 2009;4(4):417–22. doi: 10.1093/scan/nsp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. Journal of Neuroscience. 2004;24(46):10364–8. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. [Research Support, N.I.H., Extramural.] Psychophysiology. 2008;45(4):602–7. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex. 2013;24:11, 2981–990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. Journal of Neuroscience. 2000;20(19):RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WA, Johnsen IR, Waggoner AS. Orbitofrontal cortex provides cross-modal valuation of self-generated stimuli. [Research Support, Non-U.S. Gov’t.] Social Cognitive Affective Neuroscience. 2011;6(3):286–93. doi: 10.1093/scan/nsq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Implicit and explicit evaluation: FMRI correlates of valence, emotional intensity, and control in the processing of attitudes. Journal of Cognitive Neuroscience. 2004;16(10):1717–29. doi: 10.1162/0898929042947919. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16(6):693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience. 2012;24(8):1742–52. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Ochsner KN, Weber J, Wager TD. Anticipatory brain activity predicts the success or failure of subsequent emotion regulation. Social Cognitive Affective Neuroscience. 2013;9(4):403–11. doi: 10.1093/scan/nss148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58(1):275–85. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Drabant EM, McRae K, Manuck SB, Hariri AR, Gross JJ. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biological Psychiatry. 2009;65(5):367–73. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Master SL, Inagaki TK, et al. Attachment figures activate a safety signal-related neural region and reduce pain experience. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(28):11721–6. doi: 10.1073/pnas.1108239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. American Journal of Psychiatry. 2010;167(5):545–54. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164(10):1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva R, Zivan M, Warsha A, Olchik D. Alerting, orienting or executive attention networks: differential patters of pupil dilations. Frontiers in Behavioral Neuroscience. 2013;7:145. doi: 10.3389/fnbeh.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. Stumbling on Happiness. New York, NY: Alfred A. Knopf; 2006. [Google Scholar]
- Hare TA, Camerer CF, Knoepfle DT, Rangel A. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. Journal of Neuroscience. 2008;30(2):583–90. doi: 10.1523/JNEUROSCI.4089-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–8. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Heinzel A, Bermpohl F, Niese R, et al. How do we modulate our emotions? Parametric fMRI reveals cortical midline structures as regions specifically involved in the processing of emotional valences. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Brain Research. Cognitive Brain Research. 2005;25(1):348–58. doi: 10.1016/j.cogbrainres.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27(33):8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10(12):1625–33. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neuroscience and Biobehavioral Reviews. 2009;33(8):1215–26. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, et al. Contextual modulation of amygdala responsivity to surprised faces. Journal of Cognitive Neuroscience. 2004;16(10):1730–45. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19(5):776–98. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. 2008. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. (T. C. f. R. i. Psychophysiology, Trans.). Technical Report A-8. University of Florida, Gainsville, FL. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–73. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Larsen RJ, Prizmic-Larsen Z. Measuring emotions: implications of a multimethod perspective. In: Eid M, Diener E, editors. Handbook of Multimethod Measurement in Psychology. Washington, DC: American Psychological Association; 2006. [Google Scholar]
- LeDoux J. The amygdala [Review] Current Biology. 2007;17(20):R868–74. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, et al. Neural circuitry underlying voluntary suppression of sadness. Biological Psychiatry. 2003;53(6):502–10. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. The Behavioral and Brain Sciences. 2012;35(3):121–43. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience. 2010;22(2):248–62. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nature Reviews Neuroscience. 2000;1(1):59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317(5841):1079–83. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating ‘unseen’ fear. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(4):1680–5. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, O’Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. Journal of Neuroscience. 2007;27(31):8166–9. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, et al. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychological Science. 2009;20(11):1322–31. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251(1):E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Ochsner KN. The role of social cognition in emotion. Trends in Cognitive Sciences. 2008;12(2):65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology. 2003;460(3):425–49. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Gao K, Moore GJ, Tancer ME, Posse S. Real-time fMRI of cortico-limbic brain activity during emotional processing. Neuroreport. 2004a;15(3):527–32. doi: 10.1097/00001756-200403010-00029. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004b;21(2):768–80. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16(6):723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philosophical Transactions of the Royal Society of Londlon. 2007 doi: 10.1098/rstb.2007.2097. Series B: Biological Sciences, 362(1481), 917–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, McCabe C, Redoute J. Expected value, reward outcome, and temporal difference error representations in a probabilistic decision task. Cerebral Cortex. 2008;18(3):652–63. doi: 10.1093/cercor/bhm097. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review.] Trends in Cognitive Sciences. 2012;16(3):147–56. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends in Cognitive Sciences. 2010;14(6):268–76. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Takahashi Y, Liu TL, McDannald MA. Does the orbitofrontal cortex signal value? [Research Support, N.I.H., Extramural Review.] Annals of the New York Academy of Sciences. 2011;1239:87–99. doi: 10.1111/j.1749-6632.2011.06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Stenger VA, Konecky R, Carter CS. Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. [Clinical Trial Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.] Neuroimage. 2003;20(1):114–24. doi: 10.1016/s1053-8119(03)00298-2. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46(1):224–32. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Wagner DD, Wig GS, Moran JM, Whalen PJ, Kelley WM. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] Cerebral Cortex. 2013;23(1):49–60. doi: 10.1093/cercor/bhr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushemne R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Davidson RJ. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. Neuroimage. 2009;47(3):852–63. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26(16):4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reekum CM, Johnstone T, Urry HL, et al. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. Neuroimage. 2007a;36(3):1041–55. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- van Reekum CM, Urry HL, Johnstone T, et al. Individual differences in amygdala and ventromedial prefrontal cortex activity are associated with evaluation speed and psychological well-being. Journal of Cognitive Neuroscience. 2007b;19(2):237–48. doi: 10.1162/jocn.2007.19.2.237. [DOI] [PubMed] [Google Scholar]
- van Steenbergen H, Band GP. Pupil dilation in the Simon task as a marker of conflict processing. Frontiers in Human Neuroscience. 2013;7:215. doi: 10.3389/fnhum.2013.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive Affective and Behavioral Neuroscience. 2003;3(4):255–74. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat, Part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009a;47(3):836–51. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: Part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009b;47(3):821–35. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. European Journal of Neuroscience. 2003;18(7):2069–81. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;7(6):177–88. [Google Scholar]
- Williams LM, Phillips ML, Brammer MJ, et al. Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. Neuroimage. 2001;14(5):1070–9. doi: 10.1006/nimg.2001.0904. [DOI] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Weller JA, Li X, Bechara A. Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cerebral Cortex. 2009;19(5):1019–27. doi: 10.1093/cercor/bhn147. [DOI] [PMC free article] [PubMed] [Google Scholar]