Abstract
Few studies have examined whether effortful emotion regulation has a protracted impact on subsequent affective appraisal, and even fewer have investigated this effect on a trial-by-trial basis. In this study, we hypothesized that engaging cognitive resources via reappraisal during a trial would result in a subsequent period of increased reactivity on the next trial, as quantified using event-related potentials and oscillations. Forty-eight healthy individuals passively viewed unpleasant and neutral pictures followed by an auditory instruction to either continue viewing normally or reappraise emotional response to pictures. Viewing unpleasant pictures yielded increased late positive potential (LPP) and decreased posterior alpha (8–13 Hz) compared with neutral pictures. A similar pattern was observed on trials that immediately ‘followed’ emotion regulation instructions. Moreover, individuals with increased self-reported depressive symptoms showed greater LPP and alpha modulation following emotion regulation, suggesting that these responses may relate to compromised emotion regulation ability. This study demonstrates that cognitive reappraisal induces subsequent heightened reactivity that may reflect transient resource depletion, and these effects are more pronounced among those with increased depressive symptoms. Interventions that focus on emotion regulation might use these electrocortical markers to track changes in regulatory efficacy.
Keywords: reappraisal; EEG/ERP; LPP; alpha, depression
INTRODUCTION
Emotion regulation is a form of self-control that is critical for goal-directed behavior and refers to the processes that are engaged when individuals try to influence what type or how much emotion is experienced and how it is expressed (see Gross, 1998 for review). One of the most flexible and efficacious emotion regulation strategies is reappraisal, which involves reinterpreting the meaning of emotionally evocative stimuli (Gross, 1998; Goldin et al., 2008). By changing a stimulus’ affective value, reappraisal can effectively up- or down-regulate subjective reports of emotion, facial expression, as well as physiological measures of arousal (Gross, 2002; Aldao et al., 2010). Neuroimaging studies employing cognitive reappraisal have reported increased functional connectivity between regions underlying top-down cognitive control [i.e. the prefrontal cortex (PFC)] and regions that encode the affective properties of stimuli in a bottom-up fashion (e.g. the amygdala), such that increased PFC activity is associated with dampened amygdala response to affective stimuli (Ochsner et al., 2004). Although reappraisal engages prefrontal circuits, it is unclear how this type of regulation impacts subsequent instances of self-control.
Previous studies investigating more protracted consequences of cognitive reappraisal have generally shown favorable outcomes (see Gross, 2002; Richards, 2004 for review), including increased task engagement as a result of reappraisal-mediated deployment of prefrontal resources. For instance, studies have reported improved free recall of reappraised (i.e. both up- and down-regulated) unpleasant pictures (Dillon et al., 2007), sustained down-regulation of unpleasant stimuli even without explicit regulation demands (Thiruchselvam et al., 2011) and improved performance on the flanker task immediately after reappraisal-mediated up-regulation (Moser et al., 2010). These data are consistent with the possibility that reappraisal may increase task engagement. However, other contradicting reports suggest that reappraisal might be cognitively taxing (Sheppes et al., 2009); for instance, reappraisal has been related to subsequent reduction in attention to affective words (Deveney and Pizzagalli, 2008) and an increase in amygdala activity during rest immediately following reappraisal (Walter et al., 2009). Although resource depletion is typically operationalized as a consequence of sustained self-control (Baumeister et al., 1998), these data suggest that engaging in reappraisal ‘might’ temporarily deplete resources even at the single trial level (Sheppes et al., 2009) which could result in transient increases in emotional reactivity. Existing studies, however, have not examined this possibility to date.
The immediate affective consequences of reappraisal might be especially relevant for individuals who struggle with emotion regulation, and utilize interventions employing cognitive reappraisal to promote effective emotion regulation (Barlow et al., 2004). In depressed individuals, for example, amygdala–PFC decoupling leads to amygdala hyperactivity and related PFC hypoactivity, which renders existing negative emotions more salient and thus emotion regulation more difficult (Mayberg, 1997). Indeed, hypersensitivity to negative emotions may limit depressed individuals’ ability to both employ reappraisal (Beck, 2008) and down-regulate amygdala activity (Erk et al., 2010). These deficits may also extend to emotional reactivity following cognitive reappraisal, a possibility which we examine in this study.
Therefore, the primary goal of this study was to better understand the impact of reappraisal on subsequent instances of emotional reactivity and whether these effects vary as a function of depressive symptoms. For this purpose, electroencephalography (EEG), with millisecond temporal resolution, was used to investigate the neural response to visual stimuli. Specifically, the late positive potential (LPP) begins within 300 ms of stimulus onset and is larger throughout the presentation of emotional compared with neutral pictures and words, and was used as objective neural measure of emotional processing (see Hajcak et al., 2010 for review). Moreover, event-related parieto-occipital alpha (i.e. 8–13 Hz) desynchronization (i.e. decrease in alpha power) has also been linked to the synchronized activation of task-relevant cortical regions (Pfurtscheller et al., 1996; Klimesch, 1999; Vuilleumier and Driver, 2007; Parvaz et al., 2012), and has shown to be moderately correlated with the LPP (De Cesarei and Codispoti, 2011). However, the sensitivity of these markers to individual differences in emotion regulation difficulty is currently unknown; to this end in this study, we focused on variations in self-reported depression.
Based on previous reports of reduced post-reappraisal prefrontal regulation at the single trial level (Deveney and Pizzagalli, 2008; Walter et al., 2009), we hypothesized that instructed cognitive reappraisal during a trial (N) would result in increased reactivity on the following trial (N + 1), manifested by increased LPP amplitude and reduced parieto-occipital alpha power. We further hypothesized that instructed emotion regulation would have a greater impact on the emotional reactivity to trial N + 1 among individuals with higher depressive symptoms.
MATERIALS AND METHODS
Participants
For this study, we re-analyzed the data from a previous study (Parvaz et al., 2012) in which 49 students (age: 20.2 ± 2.3 years, 26 females) participated. The study was approved by the Stony Brook University Institutional Review Board. All participants gave informed consent and received course credit.
Task and procedure
Fifty unpleasant and 50 neutral pictures selected from the International Affective Picture System (IAPS; Lang et al., 2005) were displayed for 7000 ms in four blocks of 25 pictures, with an inter-trial interval of 500 ms. Each block contained the pictures of only one type (i.e. unpleasant only or neutral only); thus, there were two blocks for each picture type, and the block order was randomized between participants. Participants received a break after each block of trials. Every trial began with a white fixation cross that was presented in the center of a black background for 1000 ms. Following this, participants viewed an unpleasant or neutral picture, depending on the block; 1000 ms after picture onset, participants received the auditory instruction to either continue viewing (‘normal’ condition) or reduce their emotional response to the picture by ‘making the picture seem less emotional’ by changing either the ‘meaning’ of the picture or their ‘perspective’ on the depicted characters and events (‘reduce’ condition) in an event-related design. Participants received the same instructions for both picture types. Within this same sample, we previously demonstrated left ‘frontal’ alpha desynchronization as well as LPP amplitude reduction ‘following’ emotion reappraisal instructions (Parvaz et al., 2012). In this study, we uniquely examined the effect of the preceding emotion regulation instruction (i.e. reduce vs normal) on ‘subsequent’ neural activity, measured during the first 1000 ms of each trial (i.e. during the passive viewing portion, prior to the presentation of emotion regulation instructions). That is, we examine how emotion regulation instructions on the previous trial impact the spontaneous ‘initial’ processing of stimuli on the current trial. Before undergoing the emotion regulation task, depression (range: 0–21, mean: 6.1 ± 7.0), anxiety (range: 0–21, mean: 6.0 ± 6.5) and stress (range: 0–21, mean: 10.5 ± 8.1) for each participant were quantified using a 21-item version of the Depression, Anxiety and Stress Scale (DASS21; Lovibond and Lovibond, 1995). Although the DASS21 is not intended for diagnosis, three participants exceeded the threshold of severe depression (i.e. depression score > 11).
EEG recording and data reduction
EEG was recorded using a 64-channel ActiveTwo BioSemi system (BioSemi, Amsterdam, The Netherlands) at a sampling rate of 512 Hz. Offline pre-processing was performed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK; www.fil.ion.ucl.ac.uk/spm) and customized MATLAB (The MathWorks, Natick, MA) scripts. Data were band-pass filtered from 0.01 to 30 Hz, and were re-referenced to the averaged activity of all 64 scalp sites. The EEG was segmented beginning 500 ms prior to the picture onset (baseline) and continuing for 1000 ms (i.e. until the onset of the instruction) based on the instruction type of the previous trial (post-reappraised and post-normally viewed) and the picture type (unpleasant and neutral). Each epoch was then corrected for baseline average activity.
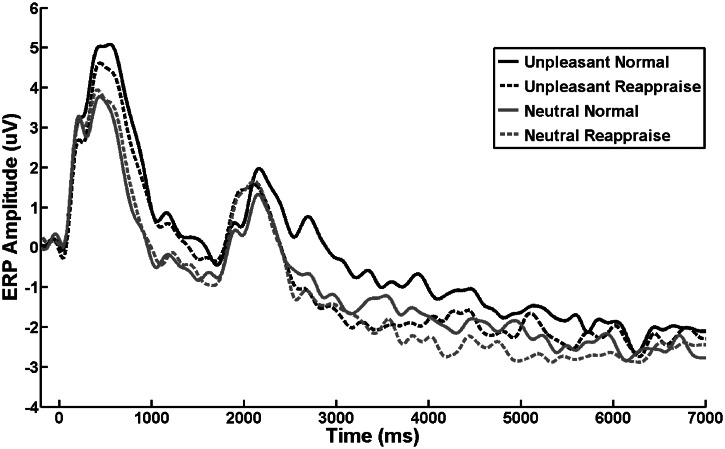
Artifact rejection method included epoch rejections based on the partial signal space projection method for eye blink and ocular corrections (Dong et al., 2010), a voltage step of more than 75 µV between sample points, a peak-to-peak voltage difference of 150 µV within an epoch, as well as visual inspection. Robust averaging was used to create artifact-free Event-related potentials (ERPs) (Dong and Zhou, 2010). Moreover, grand-averaged ERP waveforms for the entirety of trial N, separately for each condition, were also created to assess whether the emotional reactivity in trial N has subsided before the onset of trial N + 1 (Figure 1).
Fig. 1.
ERP waveforms at the centroparietal (Cz, CP1, CPz, CP2 and Pz) electrode cluster during the presentation of unpleasant/neutral stimuli. The normal viewing and reappraisal instructions were presented at 1000 ms. These waveforms show reduced LPP activity for reappraised stimuli as compared with normally viewed stimuli, although this effect was not evident at the end of the 7000 ms trail.
To compute spectral power, the Morlet wavelet transform was applied to the single trial data for alpha band (8–13 Hz) and trials for each condition were then averaged to yield evoked oscillatory alpha power. The Morlet wavelet transform is a convolution of the data with windowed, complex sinusoids, where the width of Gaussian window is coupled with the center frequency. This procedure ensures the adaptability of the window width at higher frequencies to keep the number of cycles under the Gaussian constant (Keil et al., 2003).
Statistical analyses
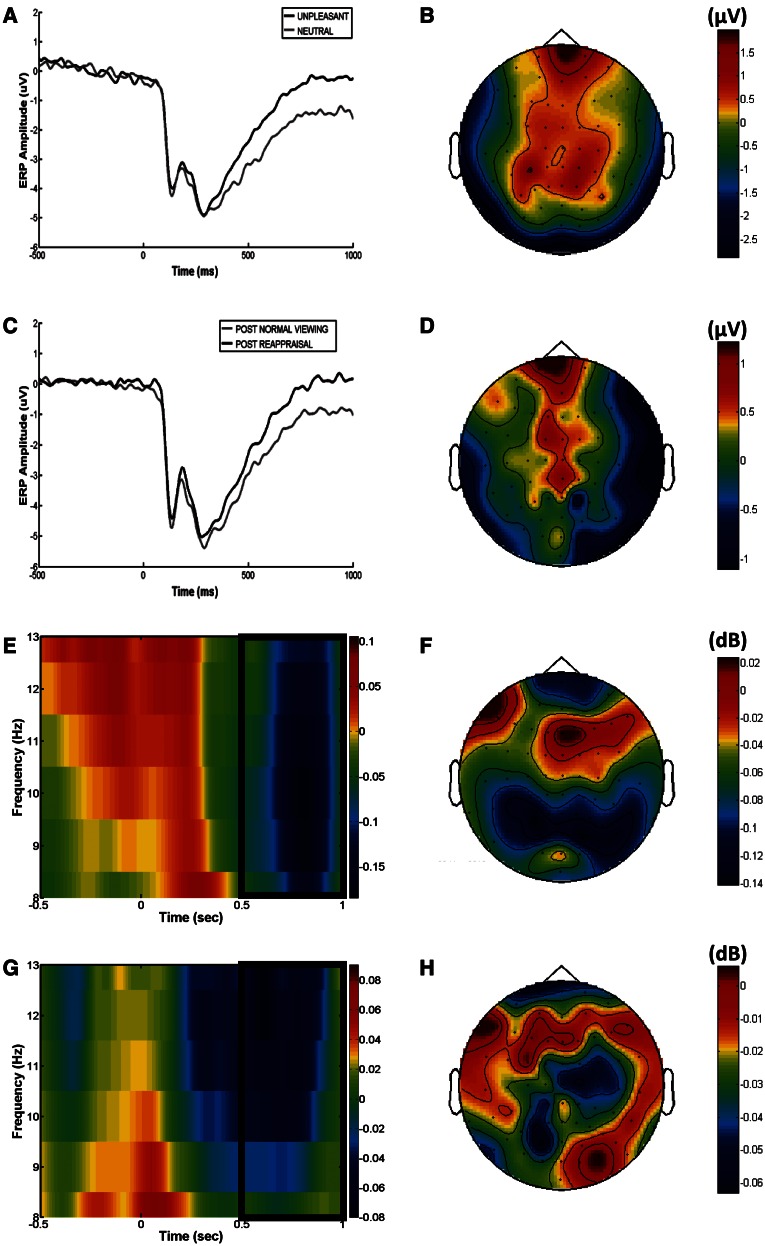
The LPP was scored from 500 to 1000 ms following picture onset (i.e. prior to the presentation of the regulation instruction) as the averaged activity from centroparietal (Cz, CP1, CPz, CP2 and Pz) electrode cluster, based on the scalp distribution of the differential (unpleasant minus neutral) LPP activity1 (Figure 2A–D) as well as to ensure consistency with previous literature (Kliegel et al., 2003; Hajcak et al., 2006). Parieto-occipital alpha power was quantified, for the same temporal window, separately for each hemisphere (left: P7, PO7, PO3, P3 and P5; right: P8, PO8, PO4, P4 and P6; Figure 2E–H), based on previous reports of alpha asymmetry (Heller et al., 1997; Aftanas et al., 2001).
Fig. 2.
ERP waveforms at the centroparietal cluster and scalp topography of their difference for unpleasant and neutral conditions (unpleasant minus neutral) (A and B, respectively) and for post-normal viewing and post-reappraisal conditions (post-reappraisal minus post-normal) (C and D, respectively). Time–frequency (TF) plots for alpha (8–13 Hz) band and scalp topography of the difference between unpleasant and neutral conditions (unpleasant minus neutral) (E and F, respectively) and between post-normal viewing and post-reappraisal conditions (post-reappraisal minus post-normal) (G and H, respectively). The rectangular region on the TF plots shows the region over which alpha power was quantified.
Given the extensive prior literature on gender differences in emotional reactivity and regulation (e.g. Domes et al., 2010), the statistical models for LPP and alpha analyses included gender as a between-subject factor. Thus, the LPP was analyzed with a 2 (Picture Type: unpleasant and neutral) × 2 (Instruction on Previous Trial: post-normally viewed and post-reappraised) × 2 (Gender: male and female) mixed model analysis of variance (ANOVA), separately for frontocentral and centroparietal clusters. However, the posterior-occipital alpha power was analyzed using a 2 (Laterality: left and right) × 2 (Picture Type: unpleasant and neutral) × 2 (Instruction on Previous Trial: post-normally viewed and post-reappraised) × 2 (Gender: male and female) mixed model ANOVA. Moreover, to ensure that post-reappraisal effects are not simply reflecting differential activity in trial N, we also scored and analyzed the LPP activity for the last 500 ms of trials N.
To investigate individual differences in the association between the ability to reappraise an emotionally charged stimulus and the neural reactivity immediately after reappraisal, we quantified the LPP and alpha activity on trial N, at the spatiotemporal regions of interest based on our previous study (Parvaz et al., 2012) and correlated them with the post-reappraisal trial N + 1 LPP and alpha activity, respectively. Finally, all the analyses were repeated by excluding the participants with severe depression scores (N = 3). However, none of the results changed significantly and therefore those results are not reported.
In all analyses, significant interactions were further investigated with post hoc comparisons. Statistically significant LPP and alpha effects were correlated for each of the four task conditions, and also as difference scores (post-normally viewed minus post-reappraised) to explore underlying associations between these electrocortical measures. Finally, LPP amplitude and alpha power were correlated with depression, anxiety and stress scores on the DASS21 (Lovibond and Lovibond, 1995). As the depression scores were non-normally distributed, non-parametric Spearman correlations were used. To protect against Type I error, a significance level of P < 0.01 was required for all correlations, whereas P < 0.05 was considered as trend.
RESULTS
The depression (P = 0.82), anxiety (P = 0.86) and stress (P = 0.31) scores from the DASS21 did not differ between male and female participants.
Late positive potential
The analysis for the LPP activity from last 500 ms of trial N, conducted to ensure that post-reappraisal effects are not simply reflecting differential activity in trial N, did not reveal any significant main effects or interactions (P > 0.22). Thus, there were no significant LPP differences in the last 500 ms of trials N.
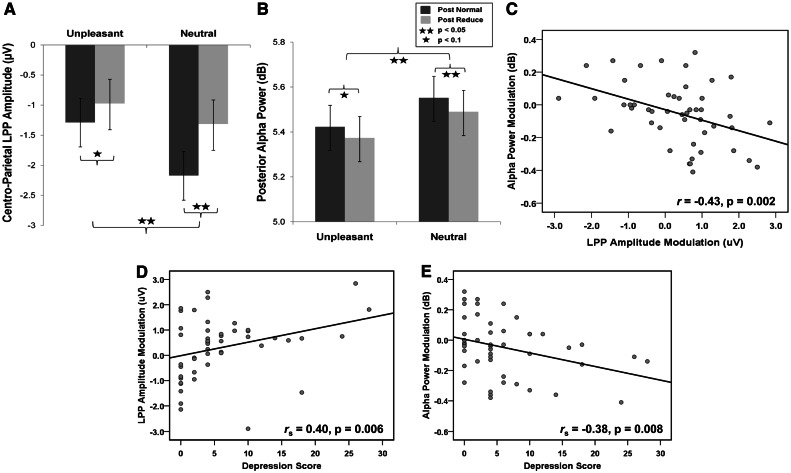
Analyses from the trials N + 1 revealed significant main effects of picture type [F(1,46) = 13.28, P = 0.001, η2 = 0.22, power = 0.95; unpleasant > neutral] and the instruction on the previous trial [F(1,46) = 16.87, P < 0.001, η2 = 0.27, power = 0.98; post-reappraisal > post-normal]. However, the main effect of gender [F(1,46) = 0.02, P = 0.90, η2 = 0.0, power = 0.05] and gender by instruction [F(1,46) = 0.25, P = 0.62, η2 = 0.01, power = 0.08] interactions did not reach significance, whereas the gender by picture type [F(1,46) = 3.05, P = 0.08, η2 = 0.06, power = 0.40] trended toward significance. Thus, the LPP was increased for unpleasant compared with neutral pictures and across both picture types following trials in which subjects were instructed to engage in effortful regulation (Table 1; Figures 2A–D and 3A).
Table 1.
Averaged LPP amplitudes and alpha band power for each condition
| Post-normal | Post-reappraisal | ||
|---|---|---|---|
| LPP (µV) | Unpleasant | −1.28 ± 0.39 | −0.97 ± 0.40 |
| Neutral | −2.17 ± 0.41 | −1.31 ± 0.43 | |
| Left alpha (dB) | Unpleasant | 5.42 ± 0.09 | 5.37 ± 0.09 |
| Neutral | 5.37 ± 0.09 | 5.55 ± 0.10 | |
| Right alpha (dB) | Unpleasant | 5.76 ± 0.08 | 5.77 ± 0.08 |
| Neutral | 5.92 ± 0.09 | 5.89 ± 0.09 |
Values are given as mean ± s.e.
Fig. 3.
LPP amplitude (A) and left-hemispheric posterior alpha power (B) modulations for each trial and picture type show that LPP amplitudes increased and alpha power decreased in trials that followed cognitive reappraisal compared with those followed normal viewing, and these electrocortical modulations (post-normal > post-reappraisal) in unpleasant condition were intercorrelated (C). Depression scores as measured with DASS21 were associated with LPP amplitude (D) and alpha power (E) modulations in response to the unpleasant stimuli. These correlations suggest higher electrocortical modulation with increasing depression scores.
Posterior alpha
The analysis for the posterior alpha activity from last 500 ms of trial N, conducted to ensure that post-reappraisal effects are not simply reflecting differential activity in trial N, also did not reveal any significant main effects or interactions (P > 0.31). Thus, there were no significant alpha differences in the last 500 ms of trials N.
Alpha power in trials N + 1 varied as a function of both laterality [F(1,46) = 66.1, P < 0.001, η2 = 0.59, power = 1.0; left < right] and picture type [F(1,46) = 28.73, P < 0.001, η2 = 0.38, power = 0.99; unpleasant < neutral], but not gender [F(1,46) = 0.83, P = 0.37, η2 = 0.02, power = 0.15], whereas the previous instruction main effect trended toward significance [F(1,46) = 3.34, P = 0.074, η2 = 0.07, power = 0.43]. However, there was a significant interaction between laterality and previous instruction [F(1,46) = 4.75, P = 0.034, η2 = 0.09, power = 0.57]. All gender-related interactions also did not reach significance [F(1,46) < 1.51, P > 0.226, η2 < 0.03, power < 0.23].
To explore this interaction, post hoc analyses were performed between trials that followed reappraisal and normal viewing separately for each hemisphere. A paired t-test yielded a significant difference in the left [t(47) = 2.617, P = 0.012, post-reappraisal < post-normal] but not in the right [t(47) = 0.425, P = 0.67] hemisphere. Thus, there was a bilateral decrease in posterior alpha power in response to unpleasant compared with neutral pictures—consistent with previous studies (De Cesarei and Codispoti, 2011). In addition, we found evidence that alpha power in the left hemisphere was decreased on trials that followed reappraisal instructions compared with those that followed normal viewing instructions (Table 1; Figures 2E–H and 3B).
LPP, alpha and self-report correlations
A larger LPP amplitude difference (post-reappraisal minus post-normal) was related to a larger left alpha power difference (post-normal minus post-reappraisal) for unpleasant (r = −0.43, P = 0.002; Figure 3C) but not neutral stimuli (r = 0.21, P = 0.16). However, the valence-mediated differences in LPP scores (unpleasant minus neutral) did not correlate significantly with the respective modulation in alpha power (left: P = 0.55; right: P = 0.82). The LPP and alpha difference scores (normal-viewing minus down-regulation via reappraisal) on trials N did not significantly correlate with the LPP and alpha difference scores on the trials N + 1 (LPP: P = 0.16; alpha: P = 0.87). The impact of previous instruction on both the LPP and left alpha power correlated with depression scores on the DASS21 (LPP: rs = 0.40, P = 0.006; alpha: rs = −0.38, P = 0.008). These measures were not correlated with anxiety (LPP: P = 0.299; alpha: P = 0.088) or stress (LPP: P = 0.206; alpha: P = 0.222) scores. Thus, greater increase in LPP amplitude and greater desynchronization of alpha power immediately following reappraisal (relative to control condition) in response to unpleasant pictures was uniquely associated with depressive symptoms.
DISCUSSION
We have previously shown that engaging in cognitive reappraisal results in decreased frontal alpha and LPP activity reflecting the activation of frontal regulatory mechanisms in blunting emotional reactivity to both unpleasant and neutral stimuli (Parvaz et al., 2012). In this study, we report increased neural reactivity (i.e. higher LPP amplitude and reduced alpha power) on trials that followed instructed emotion reappraisal compared with trials that followed uninstructed passive viewing, irrespective of valence and gender. Although the post-reappraisal modulation of LPP amplitude and alpha power was correlated, the current results did not show valence specific LPP–alpha correlation, suggesting that these biomarkers nonetheless reflect distinct mechanisms (De Cesarei and Codispoti, 2011). Finally, we showed that the degree to which emotional reactivity increased after reappraisal was larger among individuals with increased depressive symptoms.
These data suggest that appraisal-related processes are temporarily increased immediately following reappraisal. One possibility is that engaging in effortful reappraisal increases task engagement, which in turn results in increased reactivity to the subsequently presented stimulus. Increased engagement following reappraisal was suggested by previous reports of enhanced memory for pictures and emotional conversations under reappraisal instructions to decrease negative emotions (Richards and Gross, 2000; Richards et al., 2003; Dillon et al., 2007), as well as increased cognitive control after up-regulating negative emotions (Moser et al., 2010). Such enhanced performance immediately after reappraisal instructions, regardless of the direction of regulation (i.e. up or down), may reflect a general increase in the deployment of attentional resources as a consequence of engaging in cognitive reappraisal. Unlike previous studies (e.g. Hamann and Canli, 2004) however, the current results did not show gender-related differences in emotional reactivity, which might be due to comparable depression, anxiety and stress symptoms in these healthy and young participants.
The current results might also reflect the transient depletion of cognitive resources following reappraisal (Baumeister et al., 1998). That is, if reappraisal is effortful and depletes limited resources (Sheppes et al., 2009), then it may temporarily decrease self-control (including spontaneous emotion regulation) and thus increase emotional reactivity. Implicating resource depletion raises two critical issues. First, resource depletion is typically operationalized as a consequence of a long and sustained period of self-regulation (i.e. a cumulative effect of a cognitively taxing self-regulation task); the current results, however, would suggest that resource depletion might occur, albeit transiently, at the single-trial level. Second, prior studies have almost always shown that experimentally induced reappraisal effectively reduces both expression and experience of protracted emotion, and is fairly resource-independent (Gross, 1998; Richards and Gross, 2006). Nonetheless, recent evidence has begun to contradict this long-standing notion, with both behavioral (Sheppes and Meiran, 2008) and physiological (Sheppes et al., 2009) studies indicating that reappraisal consumes cognitive resources.
It is important to note that reappraisal instructions in this study were provided after the initial presentation of stimuli (i.e. after a period of initial uninstructed appraisal). Engaging reappraisal in this type of design incurs greater cognitive cost compared with when reappraisal instructions are presented prior to the presentation of an emotional stimulus (Sheppes and Gross, 2011). Indeed, studies showing improved post-reappraisal task-behavior presented the reappraisal instruction before the onset of the stimulus (Moser et al., 2010), whereas those that showed reduced post-reappraisal prefrontal regulatory activity presented the instruction after the onset of the stimulus, after emotional response had already begun (Deveney and Pizzagalli, 2008; Sheppes et al., 2009; Walter et al., 2009). Future studies might further examine or even directly compare electrocortical measures of emotional processing when reappraisal instructions are provided before and after stimulus presentation. To further investigate increased reactivity after cognitive reappraisal, future studies might also compare the impact of reappraisal with a more cognitively fatiguing emotion regulation technique, such as suppression (Richards and Gross, 1999).
A final alternative explanation of the current findings is that increased post-reappraisal emotional reactivity might reflect a contrast between N and N + 1 trials following reappraisal. That is, after down-regulating emotion via reappraisal, the initial processing of the subsequent image may seem relatively more emotionally provocative. To further examine this possibility, future studies could include an emotion up-regulation condition. Following up-regulation, a smaller LPP would argue for a contrast effect, whereas a larger LPP after up-regulation would be consistent with post-reappraisal depletion or task engagement. To disambiguate depletion from task engagement, alternative task designs can be used that either parametrically change the number of consecutive reappraisal trials or provide the reappraisal instruction before the picture onset. Taken together, although the current results unequivocally suggest increased neuronal activity immediately after cognitive reappraisal, it is difficult to adjudicate between the aforementioned neural mechanisms. Rather, future studies that employ alternative task designs are warranted to empirically identify the neurocognitive mechanism responsible for such increased post-reappraisal activity.
Regardless of the specific mechanism, the increase in post-reappraisal neural activity cannot be attributed to carryover effects from the previous trial, as the current results did not show differences in the final 500 ms of the preceding trial as a function of regulation instructions. Also of particular note is the generalized post-reappraisal increase in neural reactivity to both unpleasant as well as neutral pictures. In fact, the LPP to neutral pictures can also be modulated by emotion regulation strategies (MacNamara et al., 2009), and the current findings suggest that post-reappraisal effects generalize to the processing of neutral pictures.
Interestingly, the current results also showed that the increased post-reappraisal emotional reactivity was associated with greater depressive symptoms. Depressed individuals have more difficulty regulating emotions (Scher et al., 2005), and therefore reappraisal might require greater engagement and more cognitive resources among more depressed individuals (Heatherton and Wagner, 2011). Indeed, these results are consistent with previous functional neuroimaging studies that show aberrant connectivity between amygdala and PFC in depressed individuals, which might underlie disinhibited amygdala activity in response to external cues (Johnstone et al., 2007). Of note is the specificity of the correlations between severity of depression symptoms and the post-reappraisal electrocortical modulation to the unpleasant condition, highlighting the vulnerability in these populations in down-regulating negative affect. Future studies might therefore extend these results to investigate if such difficulties also exist when regulating positive affect. Furthermore, these electrocortical markers could also be used to track the efficacy of emotion regulation therapeutic strategies in clinical depression.
In sum, this study highlights the immediate aftereffects of cognitive reappraisal in terms of two distinct electrocortical markers of emotional processing. Moreover, we showed that depressive symptoms correlated with an increased modulation of these cortical measures following reappraisal, suggesting that cognitive self-regulation may potentially predispose more depressed individuals for increased reactivity following emotion regulation. Further exploration of the immediate as well as more long-term aftereffects of cognitive reappraisal is required to better delineate the mechanisms underlying these effects, and to better understand their association with depressive symptoms. Beyond depression, the current findings may be relevant to other psychopathologies characterized by impaired self-regulation and/or affective disturbances, such as drug addiction, aggression and other impulse control disorders.
Acknowledgments
The authors gratefully acknowledge Dr James Gross for his insightful comments on an initial draft of the manuscript.
This study was supported by grants from the National Institute on Drug Abuse (to M.A.P.: 1F32DA033088-01; to S.J.M.: 1F32DA030017-01; and to R.Z.G.: 1R01DA023579 and 1R21DA034954). NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
1 LPPs were also scored from frontocentral (F1, Fz, F2, and FCz) electrode cluster, given some earlier reports showing frontocentral LPP maxima. However, due to similar findings as the more commonly reported centroparietal LPPs, the frontocentral LPP will not be given further consideration in the manuscript.
REFERENCES
- Aftanas L, Varlamov A, Pavlov S, Makhnev V, Reva N. Event-related synchronization and desynchronization during affective processing: emergence of valence-related time-dependent hemispheric asymmetries in theta and upper alpha band. The International Journal of Neuroscience. 2001;110(3–4):197–219. doi: 10.3109/00207450108986547. [DOI] [PubMed] [Google Scholar]
- Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clinical Psychology Review. 2010;30(2):217–37. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Allen LB, Choate ML. Toward a unified treatment for emotional disorders. Behavior Therapy. 2004;35(2):205–30. doi: 10.1016/j.beth.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Ego depletion: is the active self a limited resource? Journal of Personality and Social Psychology. 1998;74(5):1252–65. doi: 10.1037//0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. The American Journal of Psychiatry. 2008;165(8):969–77. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- De Cesarei A, Codispoti M. Affective modulation of the LPP and alpha-ERD during picture viewing. Psychophysiology. 2011;48(10):1397–404. doi: 10.1111/j.1469-8986.2011.01204.x. [DOI] [PubMed] [Google Scholar]
- Deveney CM, Pizzagalli DA. The cognitive consequences of emotion regulation: an ERP investigation. Psychophysiology. 2008;45(3):435–44. doi: 10.1111/j.1469-8986.2007.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Ritchey M, Johnson BD, LaBar KS. Dissociable effects of conscious emotion regulation strategies on explicit and implicit memory. Emotion. 2007;7(2):354–65. doi: 10.1037/1528-3542.7.2.354. [DOI] [PubMed] [Google Scholar]
- Domes G, Schulze L, Bottger M, et al. The neural correlates of sex differences in emotional reactivity and emotion regulation. Human Brain Mapping. 2010;31(5):758–69. doi: 10.1002/hbm.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong GH, Lu QL, Zhou H, Zhao XA. Impulse inhibition in people with Internet addiction disorder: electrophysiological evidence from a Go/NoGo study. Neuroscience Letters. 2010;485(2):138–42. doi: 10.1016/j.neulet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Dong GH, Zhou H. Is impulse-control ability impaired in people with internet addiction disorder: electrophysiological evidence from ERP studies. International Journal of Psychophysiology. 2010;77(3):334–5. [Google Scholar]
- Erk S, Mikschl A, Stier S, et al. Acute and sustained effects of cognitive emotion regulation in major depression. The Journal of Neuroscience. 2010;30(47):15726–34. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63(6):577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: an integrative review. Review of General Psychology. 1998;2(3):271–99. [Google Scholar]
- Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39(3):281–91. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Developmental Neuropsychology. 2010;35(2):129–55. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Simons RF. Attending to affect: appraisal strategies modulate the electrocortical response to arousing pictures. Emotion. 2006;6(3):517–22. doi: 10.1037/1528-3542.6.3.517. [DOI] [PubMed] [Google Scholar]
- Hamann S, Canli T. Individual differences in emotion processing. Current Opinion in Neurobiology. 2004;14(2):233–8. doi: 10.1016/j.conb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences. 2011;15(3):132–9. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W, Nitschke JB, Lindsay DL. Neuropsychological correlates of arousal in self-reported emotion. Cognition & Emotion. 1997;11(4):383–402. [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience. 2007;27(33):8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Stolarova M, Heim S, Gruber T, Muller MM. Temporal stability of high-frequency brain oscillations in the human EEG. Brain Topography. 2003;16(2):101–10. doi: 10.1023/b:brat.0000006334.15919.2c. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Horn AB, Zimmer H. Emotional after-effects on the P3 component of the event-related brain potential. International Journal of Psychology. 2003;38(3):129–37. [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research. Brain Research Reviews. 1999;29(2–3):169–95. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Internation Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual Technical Report. Gainesville, FL: University of Florida; 2005. pp. A–6. [Google Scholar]
- Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Scales. Sydney: Psychology Foundation; 1995. [Google Scholar]
- MacNamara A, Foti D, Hajcak G. Tell me about it: neural activity elicited by emotional pictures and preceding descriptions. Emotion. 2009;9(4):531–43. doi: 10.1037/a0016251. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. The Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9(3):471–81. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Moser JS, Most SB, Simons RF. Increasing negative emotions by reappraisal enhances subsequent cognitive control: a combined behavioral and electrophysiological study. Cognitive Affective and Behavioral Neuroscience. 2010;10(2):195–207. doi: 10.3758/CABN.10.2.195. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Parvaz MA, Macnamara A, Goldstein RZ, Hajcak G. Event-related induced frontal alpha as a marker of lateral prefrontal cortex activation during cognitive reappraisal. Cognitive Affective and Behavioral Neuroscience. 2012;12(4):730–40. doi: 10.3758/s13415-012-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Jr, Neuper C. Event-related synchronization (ERS) in the alpha band—an electrophysiological correlate of cortical idling: a review. International Journal of Psychophysiology. 1996;24(1–2):39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- Richards JM. The cognitive consequences of concealing feelings. Current Directions in Psychological Science. 2004;13(4):131–4. [Google Scholar]
- Richards JM, Butler EA, Gross JJ. Emotion regulation in romantic relationships: the cognitive consequences of concealing feelings. Journal of Social and Personal Relationships. 2003;20(5):599–620. [Google Scholar]
- Richards JM, Gross JJ. Composure at any cost? The cognitive consequences of emotion suppression. Personality and Social Psychology Bulletin. 1999;25(8):1033–44. [Google Scholar]
- Richards JM, Gross JJ. Emotion regulation and memory: the cognitive costs of keeping one’s cool. Journal of Personality and Social Psychology. 2000;79(3):410–24. doi: 10.1037//0022-3514.79.3.410. [DOI] [PubMed] [Google Scholar]
- Richards JM, Gross JJ. Personality and emotional memory: how regulating emotion impairs memory for emotional events. Journal of Research in Personality. 2006;40(5):631–51. [Google Scholar]
- Scher CD, Ingram RE, Segal ZV. Cognitive reactivity and vulnerability: empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clinical Psychology Review. 2005;25(4):487–510. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Catran E, Meiran N. Reappraisal (but not distraction) is going to make you sweat: physiological evidence for self-control effort. International Journal of Psychophysiology. 2009;71(2):91–6. doi: 10.1016/j.ijpsycho.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Gross JJ. Is timing everything? Temporal considerations in emotion regulation. Personality and Social Psychology Review. 2011;15(4):319–31. doi: 10.1177/1088868310395778. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Meiran N. Divergent cognitive costs for online forms of reappraisal and distraction. Emotion. 2008;8(6):870–4. doi: 10.1037/a0013711. [DOI] [PubMed] [Google Scholar]
- Thiruchselvam R, Blechert J, Sheppes G, Rydstrom A, Gross JJ. The temporal dynamics of emotion regulation: an EEG study of distraction and reappraisal. Biological Psychology. 2011;87(1):84–92. doi: 10.1016/j.biopsycho.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2007;362(1481):837–55. doi: 10.1098/rstb.2007.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, von Kalckreuth A, Schardt D, Stephan A, Goschke T, Erk S. The temporal dynamics of voluntary emotion regulation. PLoS One. 2009;4(8):e6726. doi: 10.1371/journal.pone.0006726. [DOI] [PMC free article] [PubMed] [Google Scholar]