Abstract
Previous studies have revealed an increased fractional anisotropy and greater thickness in the anterior parts of the corpus callosum in meditation practitioners compared with control subjects. Altered callosal features may be associated with an altered inter-hemispheric integration and the degree of brain asymmetry may also be shifted in meditation practitioners. Therefore, we investigated differences in gray matter asymmetry as well as correlations between gray matter asymmetry and years of meditation practice in 50 long-term meditators and 50 controls. We detected a decreased rightward asymmetry in the precuneus in meditators compared with controls. In addition, we observed that a stronger leftward asymmetry near the posterior intraparietal sulcus was positively associated with the number of meditation practice years. In a further exploratory analysis, we observed that a stronger rightward asymmetry in the pregenual cingulate cortex was negatively associated with the number of practice years. The group difference within the precuneus, as well as the positive correlations with meditation years in the pregenual cingulate cortex, suggests an adaptation of the default mode network in meditators. The positive correlation between meditation practice years and asymmetry near the posterior intraparietal sulcus may suggest that meditation is accompanied by changes in attention processing.
Keywords: attention, gray matter, default mode network, mindfulness, MRI, VBM
INTRODUCTION
Over the last few years, increasing research has revealed differences in brain anatomy and function between meditators and controls. Structural studies have reported meditation-related alterations in gray matter volume, cortical thickness, gyrification, fractional anisotropy (FA) and other measures throughout the brain (Lazar et al., 2005; Holzel et al., 2008, 2010, 2011; Luders et al., 2009, 2011, 2012a,b, 2013b; Vestergaard-Poulsen et al., 2009; Grant et al., 2010; Tang et al., 2010, 2012a). Functional imaging and electroencephalography (EEG) studies have demonstrated different activation patterns and differences in resting state networks between meditators and controls (Davidson et al., 2003; Lutz et al., 2004; Tei et al., 2009; Brewer et al., 2011; Jang et al., 2011; Xue et al., 2011; Berkovich-Ohana et al., 2012; Keune et al., 2013). Interestingly, many of these studies have observed effects of meditation on the brain that were not expressed equally in both hemispheres, suggesting a potentially different degree or pattern of lateralization in meditators.
Although lateralization in meditation has not been assessed directly in structural studies, functional studies using EEG have reported a leftward bias of frontal brain activity in meditators (Davidson et al., 2003; Moyer et al., 2011). Moreover, structural studies measuring the thickness and FA of the corpus callosum (Luders et al., 2011, 2012b) found significantly higher FA as well as greater callosal thickness in anterior regions of the corpus callosum. These cross-sectional findings are in close correspondence with outcomes from longitudinal studies indicating a meditation-induced FA increase in the frontal part of the corpus callosum (Tang et al., 2010, 2012a). As higher FA and a thicker rostral corpus callosum may indicate a greater number and/or more densely packed axons, these findings suggest stronger inter-hemispheric connectivity in meditators than controls. Consequently, the integration of information across both hemispheres and the degree of functional lateralization may be different in meditation practitioners. This may also be reflected in an altered anatomical asymmetry.
This study sought to follow up on these previous outcomes (and implications) and directly address if hemispheric asymmetry is altered in meditation practitioners. For this purpose, we analyzed high-resolution structural brain images from 50 long-term meditators and 50 matched control subjects with particular focus on hemispheric differences in voxel-wise ‘gray matter’. In addition to assessing differences between meditators and controls with respect to gray matter asymmetry, we examined if there was a link between the degree of gray matter asymmetry and the amount of meditation practice years. As increased FA and greater thickness were observed in the anterior parts of the corpus callosum in previous studies, we hypothesized alterations in gray matter asymmetry, primarily in anterior sections of the brain. Nonetheless, to be able to detect possible effects elsewhere in the brain, we applied an automatic whole-brain voxel-based method allowing an objective assessment of gray matter asymmetry in the framework of meditation.
MATERIALS AND METHODS
Subjects
The study included 50 meditators and 50 control subjects, matched for sex (28 men, 22 women) and for age [meditators (mean ± s.d.): 51.4 ± 12.8 years; controls: 50.4 ± 11.8 years]. Age ranged between 24 and 77 years; the average age difference within a sex-matched pair was 1.8 years. Although scans for the controls were obtained from the ICBM database of normal adults (http://www.loni.usc.edu/ICBM/Databases), meditators were newly recruited from various meditation venues. Years of meditation practice ranged between 4 and 46 years (19.8 ± 11.4 years). The majority of subjects (89%) was right-handed as based on self-reports of hand preference (six meditators and five controls were left-handed). All subjects were required to be free of any neurological and psychiatric disorders and gave informed consent according to UCLA’s Institutional Review Board. Note that the same sample (n = 100) has also been analyzed in two previous studies examining differences between meditators and controls with respect to (i) vertex-wise mean curvature (Luders et al., 2012a) and (ii) voxel-wise gray matter (Luders et al., 2013a). The new aspect of this study is the focus on hemispheric differences (i.e. voxel-wise gray matter asymmetry).
Image acquisition and processing
All subjects (i.e. meditators and controls) were scanned at the same site, using the same scanner and image acquisition protocol. Specifically, magnetic resonance imaging (MRI) data were acquired on a 1.5 T Siemens Sonata scanner (Erlangen, Germany) using an eight-channel head coil and a 3D T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) sequence (TR = 1900 ms, TE = 4.38 ms, flip angle = 15°, 160 contiguous 1 mm sagittal slices, FOV: 256 mm × 256 mm, matrix: 256 × 256, voxel dimensions: 1.0 × 1.0 × 1.0 mm3). All MRI data were processed as further detailed below using SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and the VBM8 Toolbox (http://dbm.neuro.uni-jena.de/vbm.html), where the standard pre-processing steps for voxel-based morphometry (VBM) were carefully adapted to accommodate the analysis of gray matter ‘asymmetries’.
Creating symmetric tissue probability maps
As a first adaptation step, we created symmetric tissue probability maps in MNI space (Luders et al., 2004). For this purpose, the individual high-resolution structural images were registered to the tissue probability maps in MNI space, as provided with the ‘New Segment Toolbox’ (included in SPM8), using 12-parameter affine transformations. Subsequently, the registered images were segmented and flipped in the midsagittal plane to create mirrored images. Original and mirrored segments were then averaged and smoothed with a Gaussian kernel of 4 mm full-width-half-maximum (FWHM). These customized tissue probability maps in symmetric MNI space were utilized in the subsequent affine normalization and tissue segmentation steps.
Affine normalization and tissue segmentation
Using the VBM8 Toolbox, the original individual high-resolution structural images were segmented into gray matter, white matter and cerebrospinal fluid, and registered to the symmetric tissue probability maps using 12-parameter affine transformations. The segmentation algorithm implemented in the VBM8 Toolbox accounts for partial volume effects (Tohka et al., 2004) and uses adaptive maximum a posteriori estimations (Rajapakse et al., 1997), a spatially adaptive non-local means denoising filter (Manjon et al., 2010) and a hidden Markov random field model (Cuadra et al., 2005). This process resulted in normalized gray and white matter segments from each subject in symmetric MNI space which, in turn, were then used to create a customized template and to conduct the non-linear warping procedure (Ashburner, 2007).
Creating a customized template and non-linear warping
Asymmetries in the brain not only encompass shape differences on a larger scale, such as the hemispheric petalia and Yakovlevian torque (LeMay, 1976; Toga and Thompson, 2003), but also on a finer scale, such as the location and dimensions of sulci and gyri (Cunningham, 1892; Toga and Thompson, 2003). Thus, low-dimensional spatial registration to symmetric tissue probability maps may not be sufficient to ensure a point-wise comparability between left and right hemispheres. Therefore, we created an optimized template and performed high-dimensional non-linear warping. More specifically, in order to create the optimized template, the registered gray and white matter segments in symmetric MNI space (described earlier) were flipped in the midsagittal plane. All original and mirrored tissue segments were subsequently used to create a DARTEL template (Ashburner, 2007), which thus represents both original and mirrored tissue segments. The main advantage to using this approach is that the resulting DARTEL template represents a mean (or rather minimal distance) of the original and mirrored segments from all subjects, and thus yields a closer spatial correspondence between left and right hemispheres than just flipping the images subsequent to a non-linear warp.1 The original and mirrored gray matter segments were subsequently warped to the DARTEL template and then modulated (Good et al., 2001). As the template was created from scaled tissue segments, this modulation is for the non-linear portion of the warp only and already accounts for overall differences in brain size.
Calculating the asymmetry index
The asymmetry index (AI) was calculated voxel-wise as the difference between normalized original and mirrored gray matter segments divided by the mean of both (Luders et al., 2004): AI = (original − mirrored)/0.5(original + mirrored). This operation results in individual gray matter asymmetry maps, where positive values in the ‘left hemisphere’ indicate a leftward asymmetry, whereas negative values indicate a rightward asymmetry. Similarly, positive values in the ‘right hemisphere’ indicate a rightward asymmetry, whereas negative values indicate a leftward asymmetry. Given this redundancy in asymmetry information, we masked out the right hemispheres and only kept the left hemispheres.2 The voxel-wise AIs of the remaining left hemisphere were then smoothed with a Gaussian kernel of 8 mm FWHM and constitute the input for the statistical analyses.
Statistical analyses
Voxel-wise gray matter asymmetry differences between meditators and controls were examined via the general linear model, where age was included as co-variate. Similarly, voxel-wise correlations between gray matter asymmetry and number of practice years were examined via the general linear model. However, as age and the number of practice years are highly correlated (Pearson’s r = 0.6, P < 0.001), correlations were performed without including age as co-variate. Instead, we corrected for age by including the age-matched controls (assuming zero meditation years) in the statistical model, in addition to the meditation practitioners (with their respective number of meditation years). All findings resulting from the group comparisons as well as the correlation analyses were corrected for multiple comparisons using threshold-free cluster enhancement (TFCE) (Smith and Nichols, 2009), controlling the false discovery rate at q = 0.05 (Hochberg and Benjamini, 1990). The resulting significance clusters were projected onto the rendered view of the mean brain created from the whole study population (n = 100). Follow-up analyses (detailed in the respective paragraphs of the Results section) were conducted, if applicable, in order to inform about the underlying determinants for the observed effects. For example, a significant group difference indicating that ‘meditators > controls’ might not necessarily be driven by a larger leftward asymmetry in meditators (i.e. higher positive values) but perhaps by a smaller rightward asymmetry in meditators (i.e. lower negative values). Similarly, a significant positive correlation might not be driven by increasing leftward asymmetries with meditation practice years (i.e. higher positive values) but perhaps by decreasing rightward asymmetries with practice years (i.e. lower negative values).
RESULTS
Group differences
As demonstrated in Figure 1, we observed significant differences between meditators and controls with respect to gray matter asymmetry in the medial parietal lobe (Panel A). More specifically, the difference cluster was situated within the precuneus, according to the automated anatomic labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) and at the border between areas SPL 7P and SPL 7M (Scheperjans et al., 2008a,b), according to the anatomy toolbox (Eickhoff et al., 2005). The significance maximum was located at x = −4, y = −72, z = 40 (symmetric MNI space) and at x = −3, y = −74, z = 42 (classic MNI space).
Fig. 1.
Group differences. (Panel A) Significant gray matter (GM) asymmetry differences between meditators and controls. Displayed is the rendered medial view of the mean left hemisphere created from the whole study population (n = 100). The difference cluster located at x = −4, y = −72, z = 40 (symmetric MNI space) is shown at P = 0.001 (uncorrected) and was confirmed when applying corrections for multiple comparisons at q = 0.05. The color bar represents the TFCE statistic. (Panel B) Cluster-specific GM asymmetry (positive values encode leftward asymmetry; negative values encode rightward asymmetry). (Panel C) Cluster-specific GM volume (in ml) in the left hemisphere. (Panel D) Cluster-specific GM volume (in ml) in the right hemisphere. (Panels B–D) The P-values indicate the significance of the group differences (meditators vs controls).
To further explore the determinants of the detected group effect, we extracted the voxel-wise AIs as well as the voxel-wise gray matter volumes from the significance cluster. We then averaged the values over the entire cluster and generated a cluster-specific mean AI as well as the cluster-specific gray matter volumes for the left and right hemispheres. When plotting the cluster-specific ‘mean AI’ separately for both groups, controls showed a prominent rightward asymmetry, whereas meditators showed hardly any differences between the left and right hemispheres (Figure 1, Panel B). When plotting the cluster-specific ‘gray matter volumes’, meditators had significantly more gray matter than that of controls in the left hemisphere (Figure 1, Panel C), whereas both groups had similar gray matter volumes in the right hemisphere (Figure 1, Panel D).
Correlations with meditation practice years
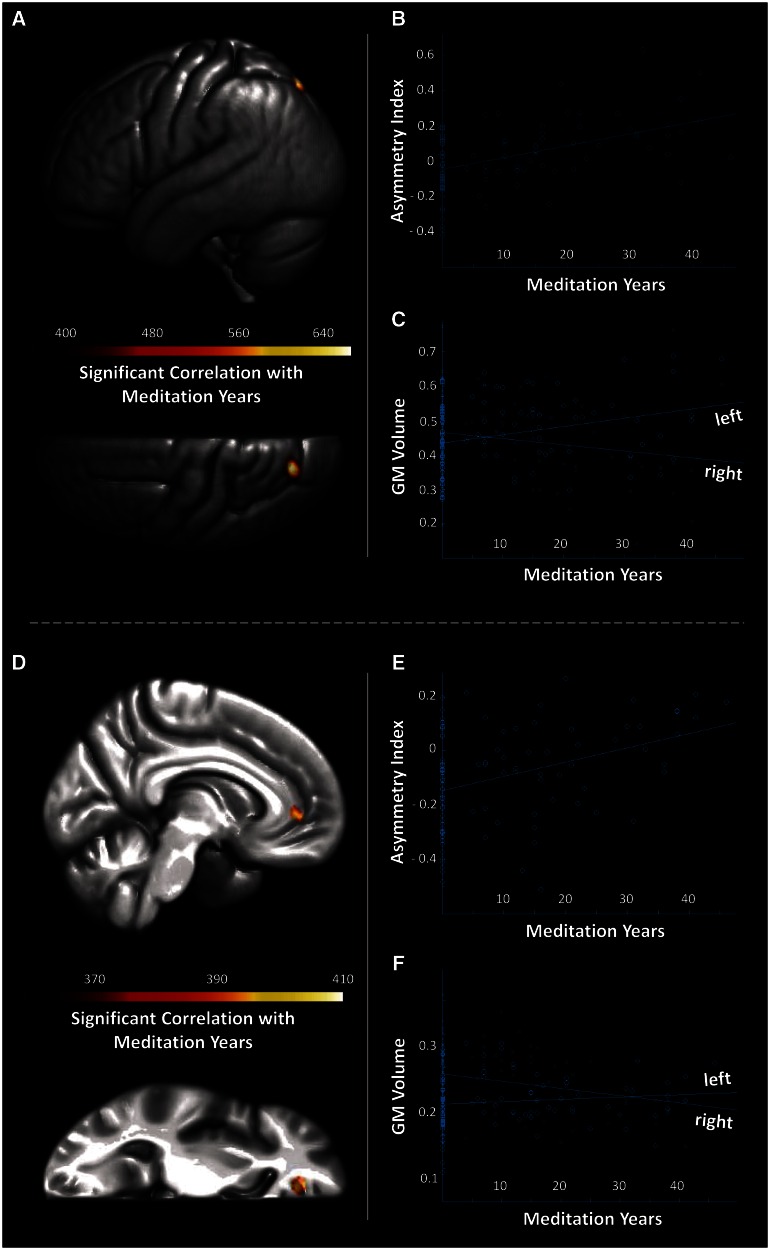
As demonstrated in Figure 2 (top), we observed significant correlations between gray matter asymmetry and the number of meditation practice years in the superior lateral parietal lobe (Panel A). More specifically, the difference cluster was situated in the superior parietal lobe near the posterior part of the intraparietal sulcus, according to the AAL atlas (Tzourio-Mazoyer et al., 2002) and at the lateral border of area SPL 7P (Scheperjans et al., 2008a,b), according to the anatomy toolbox (Eickhoff et al., 2005).
Fig. 2.
Main correlations (top) and exploratory correlations (bottom). (Panel A) Significant correlation between gray matter (GM) asymmetry and meditation practice years. Displayed are the rendered lateral and dorsal views of the mean left hemisphere. The difference cluster located at x = −20, y = −7, z = 52 (symmetric MNI space) is shown at P = 0.001 (uncorrected) and was confirmed when applying corrections for multiple comparisons at q = 0.05. The color bar represents the TFCE statistic. (Panel B) Cluster-specific correlations between AI (positive values encode leftward asymmetry; negative values encode rightward asymmetry) and meditation practice years. (Panel C) Cluster-specific correlations between left and right GM volumes (in ml) and meditation practice years. (Panel D) Significant correlation between GM asymmetry and meditation practice years. Displayed are the rendered medial and dorsal views of the mean left hemisphere. The difference cluster located at x = −12, y = 42, z = 0 (symmetric MNI space) is shown at P = 0.001 (uncorrected) and did not survive corrections for multiple comparisons. The color bar represents the TFCE statistic. (Panel E) Cluster-specific correlations between AI (positive values encode leftward asymmetry; negative values encode rightward asymmetry) and meditation practice years. (Panel F) Cluster-specific correlations between left and right GM volumes (in ml) and meditation practice years.
The significance maximum was located near the posterior part of the intraparietal sulcus at x = −20, y = −7, z = 52 (symmetric MNI space) and at x = −20, y = −76, z = 54 (classic MNI space). To further explore the determinants of the detected correlations, we extracted the voxel-wise AIs as well as the voxel-wise gray matter volumes from the significance cluster. As detailed earlier for the group differences, we then generated a cluster-specific mean AI as well as the cluster-specific gray matter volumes for the left and right hemispheres. When relating the number of meditation practice years to the cluster-specific ‘mean AI’, an increase in practice years was strongly associated with an increase of leftward asymmetry (r = 0.5, P < 0.001; Figure 2, Panel B). When relating the meditation practice years to the cluster-specific ‘gray matter volumes’, an increase in practice years was strongly associated with an increase of gray matter in the left hemisphere (r = 0.3, P < 0.01; Figure 2, Panel C), but decrease of gray matter in the right hemisphere (r = −0.2, P < 0.05; Figure 2, Panel C).
Exploratory analyses
There was a lack of significant effects in frontal regions of the brain when applying statistical corrections for multiple comparisons. However, given the previously reported alterations of inter-hemispheric connectivity in ‘anterior’ sections of the corpus callosum (Tang et al., 2010, 2012a; Luders et al., 2011, 2012b), we repeated the aforementioned analyses using an uncorrected significance threshold at P < 0.001. Interestingly, there were no additional clusters (other than the parietal effect reported earlier) when comparing meditators and controls with respect to gray matter asymmetry. However, as demonstrated in Figure 2 (bottom), we observed an additional cluster indicating correlations between gray matter asymmetry and the number of meditation practice years in the medial frontal cortex (Panel D). The significance maximum was located near the pregenual part of the cingulate sulcus at x = −12, y = 42, z = 0 (symmetric MNI space) and x = −12, y = 44, z = 0 (classic MNI space). To further explore these correlations, we extracted the voxel-wise AIs (and voxel-wise gray matter volumes) and we generated a cluster-specific mean AI (and cluster-specific left/right gray matter volumes). When relating the number of meditation practice years to the cluster-specific ‘mean AI’, an increase in practice years was strongly associated with a decrease of rightward asymmetry (r = 0.4, P < 0.001; Figure 2, Panel E). When relating the number of meditation practice years to the cluster-specific ‘gray matter volumes’, an increase in practice years was strongly associated with a decrease of gray matter in the right hemisphere (r = −0.3, P < 0.01; Figure 2, Panel C), whereas no significant correlation was observed for the left hemisphere (r = 0.1, P > 0.05; Figure 2, Panel F).
DISCUSSION
This study was conducted to address the question if the long-term practice of meditation is associated with altered gray matter asymmetries. We detected a decreased rightward asymmetry in the precuneus in meditators compared with controls. We also observed that a stronger leftward asymmetry near the posterior intraparietal sulcus was positively associated with the number of meditation practice years. In addition, a stronger rightward asymmetry in the pregenual cingulate cortex was negatively associated with the number of meditation practice years.
Asymmetry differences between meditators and controls in the medial parietal lobe
Our results suggest that the two brain hemispheres, at least with respect to the left and right medial parietal lobes, are more similar in meditators than in controls. Functionally, the precuneus has been implicated as part of the default mode network. Although it is not entirely clear if the areas 7P and 7M are included in this network (Raichle et al., 2001; Cavanna and Trimble, 2006; Buckner et al., 2008; Scheperjans et al., 2008b), area 7M has been confirmed to have close connections to posterior cingulate and retrosplenial regions (Buckner et al., 2008). These latter two regions are regularly described as part of the default mode network (Raichle et al., 2001; Buckner et al., 2008). In addition, area 7M has been reported to connect to occipitoparietal and frontal areas which are known as task set regions (Buckner et al., 2008). The conglomerate of connections to task set regions as well as resting state regions may be relevant for meditation in the light of the ‘sentinel hypothesis’. In short, this hypothesis suggests that the precuneus is involved in broadly monitoring the external environment in a non-specific way during the resting state (Buckner et al., 2008). This monitoring is different from classic focused attention to external cues, and has therefore been termed as watchfulness or exploratory state (Shulman et al., 1997; Gilbert et al., 2007). Watchfulness is likely to rely on connectivity to both external task regions (because the external world is monitored) and regions with resting state properties (because monitoring occurs during the resting state and not as a focused task). Our analysis revealed that the diminished gray matter asymmetry in meditators is due to larger gray matter volumes in the left precuneus in meditators than in controls. An increase of gray matter in a region that might be involved in watchfulness and that is linked to both external task regions and resting state regions may therefore be related to engagement in meditation.
Correlations between asymmetry and meditation practice years in the lateral parietal cortex
Examining the correlation between meditation practice years and gray matter asymmetry uncovered a significant correlation in the lateral part of the superior parietal cortex, where more practice years were associated with more left-hemispheric gray matter. The superior parietal region has been largely discussed in attention processing as part of a parietofrontal functional network (Corbetta and Shulman, 2002). As a component of the aforementioned attention network, the superior parietal lobe has extensive connections toward frontal areas via the superior longitudinal fasciculus in humans (Makris et al., 2005). Long-term meditation practitioners were previously reported to have a significantly higher FA in the superior longitudinal fasciculus, with even more pronounced effects in the left than the right hemisphere (Luders et al., 2011). These recent findings seem to agree with the current observation of increased leftward gray matter asymmetries in cortical regions subserved by the superior longitudinal fasciculus.
Although the superior parietal cortex is broadly involved in attention processing, different properties of attention have been attributed to each hemisphere. Corbetta and Shulman (2002), for example, suggest that the ‘left’ superior parietal cortex would assemble ‘associations that link the appropriate stimuli and responses for a given task’. This role in functioning is different from the ‘right’ superior parietal cortex, which seems to be involved in focusing attention on an external task (Corbetta et al., 2008). In this study, we observed that more meditation practice years are associated with more leftward asymmetry (due to an increase of left-hemispheric gray matter). This may imply a shift toward more effective task preparation and task switching in meditators, which one would expect to be beneficial in many executive tasks. Consistent with this interpretation are reports of meditators performing better in different attention and executive function tasks (Tang et al., 2007; van Leeuwen et al., 2009; Prakash et al., 2010; Zeidan et al., 2010). In addition, the reported effortless switching between brain states in experienced meditators (Tang et al., 2012b) may be partly due to an effective task preparation.
Correlations between asymmetry and meditation practice years in the medial frontal cortex
As we hypothesized that alterations in gray matter asymmetry would be primarily in the anterior sections of the brain, we experimentally relaxed the significance threshold to increase sensitivity. In correspondence with our a priori hypothesis, this revealed an additional significance cluster in the pregenual part of the cingulate sulcus, where more meditation practice years were associated with less right-hemispheric gray matter. Functionally, the anterior cingulate has been described as part of the default mode network (Raichle et al., 2001; Buckner et al., 2008). Thus, the currently observed structural link in this particular region may be associated with previously reported functional alterations, such as meditation-induced changes in the default mode network. More specifically, when examining differences in brain activation during active meditation, long-term meditators, but not controls, exhibited a decrease in brain activation in this region (Brewer et al., 2011). In another study, meditators were shown to have a decreased gamma activity over frontal midline areas, which was interpreted as decreased activity in this region (Berkovich-Ohana et al., 2012). Moreover, the presently detected associations between gray matter asymmetry and meditation practice years in anterior sections of the brain closely corroborate prior findings of alterations in anterior sections of the corpus callosum (Tang et al., 2010, 2012a; Luders et al., 2011, 2012b), either observed as cerebral characteristics in long-term meditation practitioners (e.g. higher FA values, larger callosal thickness) or as actual changes due to short-term mindfulness practices (e.g. FA increases). Given these previous callosal findings as well as the negative direction of the correlation (i.e. more meditation practice years are associated with less asymmetry), long-term meditation practices might be associated with an enhanced ‘functional’ integration of both hemispheres over time, at least in anterior/frontal sections of the brain. Although some EEG experiments revealed increased asymmetries of frontal brain activity due to meditation (Davidson et al., 2003; Moyer et al., 2011), these studies examined short-term effects (i.e. after about 5–8 weeks) in meditation beginners. In contrast, this study was conducted in long-term meditators with a mean practice of 19.8 years. Interestingly though, the direction of the effect seems to be comparable in meditation beginners and experts. That is, although the aforementioned two studies reported ‘increasing leftward’ asymmetries, our study revealed ‘decreasing rightward’ asymmetries (i.e. a shift toward the left hemisphere). These asymmetry shifts might be linked to achieving characteristic mental states and/or skills as associated with meditation, either as prerequisite of a successful practice or its consequence (or a powerful interaction of both).
Limitations and future studies
Although the current cross-sectional design is suitable to establish if there are any significant links between the degree of gray matter asymmetry and the practice of meditation, it cannot determine a causal relationship. In other words, the results of this study do not permit an answer to the question if the observed group differences (the correlations with the practice amount) were a product of the meditation practice, or if innate differences in meditators’ brains enabled subjects to successfully meditate and/or keep up this practice for a prolonged period of time. Based on the present data, one might hypothesize that hemisphere-specific gray matter in the pregenual cingulate cortex (i.e. where we observed significant correlations between practice years and degree of asymmetry) was altered through the meditation practice. In contrast, the hemisphere-specific gray matter in the precuneus (i.e. where we observed significant group differences between meditators and controls) may constitute either an innate difference in gray matter asymmetry, a meditation-induced change in gray matter asymmetry (presumably during the first few years of training given the lack of any significant correlation in this region), or a combination of both. Clearly, these are only speculations and future studies are necessary to provide a definite answer.
Moreover, future studies will help revealing the functional implications of an altered structural asymmetry. For example, we observed significant differences in gray matter asymmetry between meditators and controls, and the question arises, if the decreased asymmetry in meditators is linked to an altered inter-hemispheric exchange of information across the corpus callosum and/or an altered intra-hemisphere processing of information in networks related to the precuneus. It is also possibly, that the relatively larger-than-normal gray matter volume in the left precuneus is in itself a marker for a different (perhaps more efficient) processing of information. Either underlying mechanism (or all of them together) might have contributed to certain skills and characteristics of successful meditators. Likewise, as discussed earlier, they might be a consequence of long-term meditation (or even of each other), or developed through a complex interplay between innate prerequisite for meditation and practice-induced consequence.
Acknowledgments
The authors warmly thank all meditators for their participation in our study. For generous support the authors thank the Brain Mapping Medical Research Organization, the Robson Family and Northstar Fund, and the following foundations: Brain Mapping Support, Pierson-Lovelace, Ahmanson, Tamkin, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community, Jennifer Jones-Simon and Capital Group Companies. This study was additionally supported by the National Institutes of Health (P41 EB015922) and by grants from the Human Brain Project (P20-MHDA52176, 5P01-EB001955). Last but not least, the authors are grateful to Trent Thixton who assisted with the acquisition of the image data.
Footnotes
1 Alternatively, one could generate a DARTEL template from the original images (but not the flipped images) and proceed with the flipping after the non-linear warping procedure. Note though, this would generate asymmetric warped segments and any subsequent flipping would result in a spatial mismatch between left and right hemispheres, thus preventing the point-wise correspondence originally intended.
2 The masking step has the additional benefit of not introducing additional bias to the data through the subsequent smoothing procedure. Note that if both hemispheres (and respective asymmetry information) were present the voxel-wise asymmetry indices closer to midline would be compromised due to the blurring effect across hemispheres.
REFERENCES
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Berkovich-Ohana A, Glicksohn J, Goldstein A. Mindfulness-induced changes in gamma band activity—implications for the default mode network, self-reference and attention. Clinical Neurophysiology. 2012;123(4):700–10. doi: 10.1016/j.clinph.2011.07.048. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(50):20254–9. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Transactions on Medical Imaging. 2005;24(12):1548–65. doi: 10.1109/TMI.2005.857652. [DOI] [PubMed] [Google Scholar]
- Cunningham DJ. Contribution to the Surface Anatomy of the Cerebral Hemispheres … With a Chapter Upon Cranio-Cerebral Topography by V. Horsley, etc. Dublin: Academy House, 1892: 1892. [Google Scholar]
- Davidson RJ, Kabat-Zinn J, Schumacher J, et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosomatic Medicine. 2003;65(4):564–70. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW. Comment on “Wandering minds: the default network and stimulus-independent thought”. Science. 2007;317(5834):43; author reply 43. doi: 10.1126/science.317.5834.43. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grant JA, Courtemanche J, Duerden EG, Duncan GH, Rainville P. Cortical thickness and pain sensitivity in zen meditators. Emotion. 2010;10(1):43–53. doi: 10.1037/a0018334. [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Statistics in Medicine. 1990;9(7):811–8. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Evans KC, et al. Stress reduction correlates with structural changes in the amygdala. Social Cognitive and Affective Neuroscience. 2010;5(1):11–7. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Vangel M, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Research. 2011;191(1):36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Gard T, et al. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Social Cognitive and Affective Neuroscience. 2008;3(1):55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JH, Jung WH, Kang DH, et al. Increased default mode network connectivity associated with meditation. Neuroscience Letters. 2011;487(3):358–62. doi: 10.1016/j.neulet.2010.10.056. [DOI] [PubMed] [Google Scholar]
- Keune PM, Bostanov V, Hautzinger M, Kotchoubey B. Approaching dysphoric mood: state-effects of mindfulness meditation on frontal brain asymmetry. Biological Psychology. 2013;93(1):105–13. doi: 10.1016/j.biopsycho.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman R, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16(17):1893–7. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMay M. Morphological cerebral asymmetries of modern man, fossil man, and nonhuman primate. Annals of the New York Academy of Sciences. 1976;280:349–66. doi: 10.1111/j.1749-6632.1976.tb25499.x. [DOI] [PubMed] [Google Scholar]
- Luders E, Clark K, Narr KL, Toga AW. Enhanced brain connectivity in long-term meditation practitioners. Neuroimage. 2011;57(4):1308–16. doi: 10.1016/j.neuroimage.2011.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Gaser C, Jancke L, Schlaug G. A voxel-based approach to gray matter asymmetries. Neuroimage. 2004;22(2):656–64. doi: 10.1016/j.neuroimage.2004.01.032. [DOI] [PubMed] [Google Scholar]
- Luders E, Kurth F, Mayer EA, Toga AW, Narr KL, Gaser C. The unique brain anatomy of meditation practitioners: alterations in cortical gyrification. Frontiers in Human Neuroscience. 2012a;6:34. doi: 10.3389/fnhum.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Kurth F, Toga AW, Narr KL, Gaser C. Meditation effects within the hippocampal complex revealed by voxel-based morphometry and cytoarchitectonic probabilistic mapping. Frontiers in Psychology. 2013a;4:398. doi: 10.3389/fpsyg.2013.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Phillips OR, Clark K, Kurth F, Toga AW, Narr KL. Bridging the hemispheres in meditation: thicker callosal regions and enhanced fractional anisotropy (FA) in long-term practitioners. Neuroimage. 2012b;61(1):181–7. doi: 10.1016/j.neuroimage.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Kurth F, et al. Global and regional alterations of hippocampal anatomy in long-term meditation practitioners. Human Brain Mapping. 2013b;34(12):3369–75. doi: 10.1002/hbm.22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Toga AW, Lepore N, Gaser C. The underlying anatomical correlates of long-term meditation: larger hippocampal and frontal volumes of gray matter. Neuroimage. 2009;45(3):672–8. doi: 10.1016/j.neuroimage.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Greischar LL, Rawlings NB, Ricard M, Davidson RJ. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(46):16369–73. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15(6):854–69. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Manjon JV, Coupe P, Marti-Bonmati L, Collins DL, Robles M. Adaptive non-local means denoising of MR images with spatially varying noise levels. Journal of Magnetic Resonance Imaging. 2010;31(1):192–203. doi: 10.1002/jmri.22003. [DOI] [PubMed] [Google Scholar]
- Moyer CA, Donnelly MP, Anderson JC, et al. Frontal electroencephalographic asymmetry associated with positive emotion is produced by very brief meditation training. Psychological Science. 2011;22(10):1277–9. doi: 10.1177/0956797611418985. [DOI] [PubMed] [Google Scholar]
- Prakash R, Dubey I, Abhishek P, Gupta SK, Rastogi P, Siddiqui SV. Long-term Vihangam Yoga meditation and scores on tests of attention. Perceptual and Motor Skills. 2010;110(3 Pt 2):1139–48. doi: 10.2466/pms.110.C.1139-1148. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Transactions on Medical Imaging. 1997;16(2):176–86. doi: 10.1109/42.563663. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Eickhoff SB, Homke L, et al. Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cerebral Cortex. 2008a;18(9):2141–57. doi: 10.1093/cercor/bhm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cerebral Cortex. 2008b;18(4):846–67. doi: 10.1093/cercor/bhm116. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, et al. Common blood flow changes across visual tasks. 2. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9(5):648–63. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Tang YY, Lu Q, Fan M, Yang Y, Posner MI. Mechanisms of white matter changes induced by meditation. Proceedings of the National Academy of Sciences of the United States of America. 2012a;109(26):10570–4. doi: 10.1073/pnas.1207817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Lu Q, Geng X, Stein EA, Yang Y, Posner MI. Short-term meditation induces white matter changes in the anterior cingulate. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(35):15649–52. doi: 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Wang J, et al. Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(43):17152–6. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Rothbart MK, Posner MI. Neural correlates of establishing, maintaining, and switching brain states. Trends in Cognitive Sciences. 2012b;16(6):330–7. doi: 10.1016/j.tics.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tei S, Faber PL, Lehmann D, et al. Meditators and non-meditators: EEG source imaging during resting. Brain Topography. 2009;22(3):158–65. doi: 10.1007/s10548-009-0107-4. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nature Reviews Neuroscience. 2003;4(1):37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23(1):84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Leeuwen S, Muller NG, Melloni L. Age effects on attentional blink performance in meditation. Consciousness and Cognition. 2009;18(3):593–9. doi: 10.1016/j.concog.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Vestergaard-Poulsen P, van Beek M, Skewes J, et al. Long-term meditation is associated with increased gray matter density in the brain stem. Neuroreport. 2009;20(2):170–4. doi: 10.1097/WNR.0b013e328320012a. [DOI] [PubMed] [Google Scholar]
- Xue S, Tang YY, Posner MI. Short-term meditation increases network efficiency of the anterior cingulate cortex. Neuroreport. 2011;22(12):570–4. doi: 10.1097/WNR.0b013e328348c750. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Johnson SK, Diamond BJ, David Z, Goolkasian P. Mindfulness meditation improves cognition: evidence of brief mental training. Consciousness and Cognition. 2010;19(2):597–605. doi: 10.1016/j.concog.2010.03.014. [DOI] [PubMed] [Google Scholar]