Abstract
Background
Since bone marrow receives innervation from A-delta and C-fibers and since an increase in intramedullary pressure in bone marrow may induce acute pain in orthopedic patients during surgery and chronic pain in patients with bone marrow edema, skeletal pain may partly originate from bone marrow. Intraosseous lesions, such as osteomyelitis and bone cancer, are also known to produce cutaneous hypersensitivity, which might be referred pain from bone. However, little is known about pain perception in bone marrow and referred pain induced by bone disease. Thus, we carried out an in vivo electrophysiological study and behavioral study to determine whether increased intraosseous pressure of the femur induces acute pain and whether increased intraosseous pressure induces referred pain in the corresponding receptive fields of the skin.
Results
Intraosseous balloon inflation caused spontaneous pain-related behavior and mechanical hyperalgesia and allodynia in the lumbosacral region. Single neuronal activities of spinal dorsal horn neurons were extracellularly isolated, and then evoked responses to non-noxious and noxious cutaneous stimuli and intraosseous balloon inflation were recorded. Ninety-four spinal dorsal horn neurons, which had somatic receptive fields at the lower back and thigh, were obtained. Sixty-two percent of the wide-dynamic-range neurons (24/39) and 86% of the high-threshold neurons (12/14) responded to intraosseous balloon inflation, while none of the low-threshold neurons (0/41) responded to intraosseous balloon inflation. Spinally administered morphine (1 µg) abolished balloon inflation-induced spontaneous pain-related behavior and mechanical hyperalgesia in awake rats and also suppressed evoked activities of wide-dynamic-range neurons to noxious cutaneous stimulation and intraosseous balloon inflation.
Conclusions
The results suggest that mechanical stimulation to bone marrow produces nociception, concomitantly producing its referred pain in the corresponding skin fields. These mechanisms might contribute to pain caused by skeletal diseases.
Keywords: Skeletal pain, referred pain, in vivo electrophysiology
Background
Although patients with injury of bone tissues including the periosteum, cortical bone, and bone marrow suffer from skeletal pain, little is known about the mechanisms by which skeletal pain is generated and maintained. It has been believed for a long time that skeletal pain arises predominantly from the periosteum1 since it has been known for more than 50 years that pain sensation accompanies puncture of the periosteum.2 However, recent studies have shown that, in addition to the periosteum, many unmyelinated calcitonin gene-related peptide-labeled fibers innervate bone marrow.3,4 It has also been reported that raising intraosseous pressure greatly increases Fos expression in superficial dorsal horn neurons in the spinal cord.5 Taken together, nociceptors in bone marrow are likely to be excited by increased pressure in bone marrow, possibly resulting in activation of pain pathways including the spinal dorsal horn (SDH).
Patients often perceive skeletal pain when a lesion is confined principally to bone marrow and when there is no obvious periosteal involvement.6 Cement injection into the bone body causes pain, and intraosseous administration of lidocaine reduces this type of acute pain for percutaneous vertebroplasty.7 In addition, it is thought that the degree of chronic osteoarthritic pain is closely related to increased intraosseous pressure.8 Taken together, the results of those studies indicate that mechanical stimulation to bone marrow, which induces an increase in intraosseous pressure, elicits nociceptors located in bone marrow. These nociceptors would be involved in acute and chronic phases of skeletal pain caused by bone injuries and diseases such as bone fracture, osteoarthritis, and bone cancer. Previous studies suggested that intraosseous lesions, such as osteomyelitis and bone cancer, produce cutaneous hypersensitivity, which might be referred pain from skeletal tissues.9–11 From the results of those studies, we hypothesized that nociceptors in bone marrow are activated by mechanical stimuli and produce pain sensation and that activation of the nociceptors in bone marrow is concomitantly associated with occurrence of referred pain.
Thus, the aims of this study were (1) to determine whether increased intraosseous pressure of the femur induces acute pain and (2) to determine whether increased intraosseous pressure induces referred pain in the corresponding receptive fields (RFs) of the skin.
Methods
Animal preparation
All protocols of this study were approved by the Animal Care and Use Committee of Shinshu University School of Medicine, Matsumoto, Japan (No. 230003). Efforts were made to minimize the number of animals used, and the experiments followed the ethical guidelines of the International Association for the Study of Pain. Adult male Sprague-Dawley rats aged six to eight weeks and weighing 200–300 g were housed in groups of four in cages under a 12:12-h day/night cycle. The rats were kept under these conditions from five to 14 days before surgery to up to seven days after surgery for behavioral experiments and electrophysiological experiments.
Chronic implantation of a balloon catheter in the femur
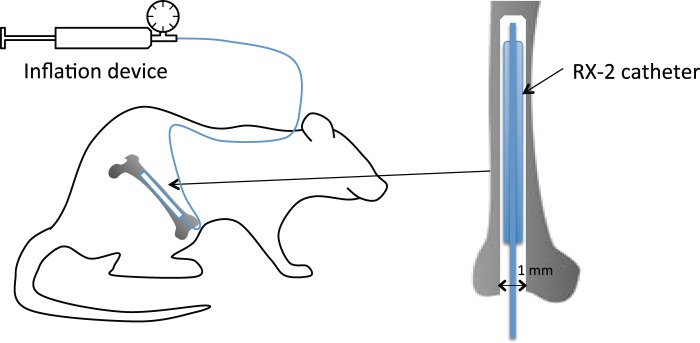
The rats were anesthetized with sevoflurane (5% initially and maintained with 2.5%–3.5% in oxygen) delivered via a face mask and checked for the absence of paw withdrawal in response to noxious pinching before commencing surgery. Rectal temperature was maintained at 36℃–38℃ using a thermo-controlled heating pad. A skin incision of approximately 6–8 mm was made on the medial side of the right knee, and the proximal patellar ligament of the femur was severed, revealing the synovial space of the knee joint, according to a previously described method.12 A 22-gauge needle was used to core between the femoral condyles and into the medullary cavity of the right femur. A diamond drill burr, 1 mm in diameter, was used to prime the opening of the hole made by the 22-gauge needle. A balloon catheter used for coronary angioplasty in patients with coronary artery disease (PTCA catheter, RX-2, 2 × 15 mm, TERUMO Co., Tokyo, Japan) was implanted in the medullary cavity of the femur for increasing intraosseous pressure of the femur. The balloon catheter was then tunneled subcutaneously to emerge at the neck (Figure 1). The wound was closed in two layers. In rats used for behavioral experiments, a PE-10 polyethylene catheter (Becton Dickinson Primary Care Diagnostics, Sparks, MD) was then implanted into the subarachinoid space at the lumbar level after implantation of the balloon catheter according to a previously described method.13 Briefly, the PE-10 catheter was inserted 15 mm cephalad into the lumbar subarachnoid space at the L4/5 intervertebral space with the tip of the catheter being located near the lumbar enlargement of the spinal cord. The catheter was then tunneled subcutaneously to emerge at the neck. The rats were allowed to recover for several hours in an oxygenated perspex box under a heat lamp and were then placed back in the cage. At least six days of postsurgical recovery were allowed before animals were used in the study.
Figure 1.
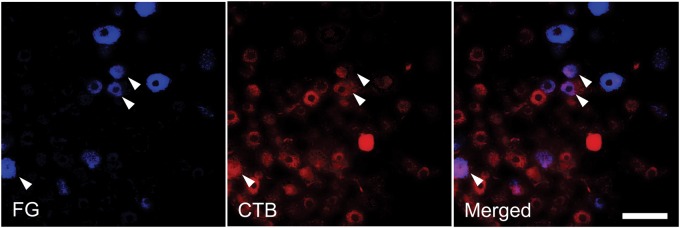
Intraosseous and cutaneous sensory convergence at dorsal root ganglion neurons. Labeled neurons in the L3 dorsal root ganglion (DRG) after application of Fluoro-Gold (FG) to the medullary cavity of the femur and application of cholera toxin subunit B (CTB) to the skin of the lumbosacral region. The arrowheads indicate a neuron double-labeled with FG and CTB. Scale bar, 100 µm.
Behavioral analysis
Seven days after implantation of the balloon catheter and i.t. catheter, the following behavioral tests were performed. Unrestrained rats were placed on a stainless steel mesh floor (openings, 8 × 8 mm) under a clear plastic cage (30 × 25 × 15 cm) and allowed to acclimate for 15 min. After acclimation, the basal value in each behavioral test before inflation of the balloon implanted in the medullary cavity of the femur was assessed. Guarding of the hind paw and spontaneous flinching were assessed as spontaneous pain-related behavior according to a previously described method for assessing ongoing pain in a rat model of bone cancer pain and bone fracture pain.12,14 Briefly, unrestrained rats were placed individually in a plastic cage and the time spent guarding, which was defined as the length of time the balloon-implanted limb was held aloft, and the number of flinches were recorded during a 2-min observation period. First, the balloon was inflated with pressures of 50, 100, 200, and 400 kPa using an inflation device (Encore™ 26, Boston Scientific Japan, Tokyo, Japan), and spontaneous guarding and flinching were assessed in order to determine the relationship between spontaneous pain and intraosseous pressure of the femur. The rats were allowed to rest for 15 min after deflation of the balloon.
In a preliminary study, spontaneous pain-related behavior and withdrawal response to mechanical stimuli of the skin were stably recorded, independently from the duration of balloon inflation at 200 kPa. Thus, spontaneous guarding and flinching with intraosseous stimulation were re-assessed for the first 2 min after balloon inflation, and withdrawal responses to punctate mechanical stimuli and dynamic non-noxious stimuli were then assessed with sustained inflation of the balloon at 200 kPa for 10 min. In such a situation, moderate spontaneous pain-related behavior (guarding and flinching) was seen, but withdrawal responses to cutaneous mechanical stimuli were clearly distinguished from such spontaneous pain-related behavior. Since spontaneous guarding and withdrawal threshold (WT) to punctate mechanical stimuli had returned to the control level at 10 min after the balloon had been deflated in a preliminary study, the rats were allowed to rest for 15 min after deflation of the balloon.
Withdrawal responses to punctate mechanical stimulation were determined using calibrated von Frey filaments (Stoelting Co., Wood Dale, IL) that could produce graded pressure of 1 to 26 g on the skin of the lumbosacral region ipsilateral to the implanted balloon. This method was a modification of a previously reported method for determining an area of mechanical hyperalgesia in the field of the body of rats.15 We determined the 50% WT by the “up-down method” according to a previous report. A series of seven von Frey filaments (1-, 2-, 4-, 6-, 10-, 15-, and 26-g forces) were used. Testing was initiated with 6-g forces. Whenever a positive response occurred, the next weaker von Frey filament was applied. Whenever a negative response occurred, the next stronger one was applied. The test was continued until responses to four stimuli after the first change in response had been obtained or the test reached either end of the spectrum of the von Frey filament set. WT was calculated by using the formula proposed by Chaplan et al.:16 50% WT = (10[Xf + kS])/10,000, where Xf is the value (in log units) of the final von Frey filament used, k is the tabular value for the pattern of positive/negative responses, and S is the mean difference (in log units) between stimuli.
The second test was a modification of a method for evaluating agitation evoked by a dynamic non-noxious stimulation.17 Hairy skin of the lumbosacral region was briskly stroked with a camel hairbrush in a rostral-to-caudal direction on the right and left lumbosacral skin. This test was repeated three times with intervals of approximately 1–2 min between tests, and the response frequency was calculated from the results of the three tests. The responses of the animals to the stimulations in the first and second tests were graded with scores of 0 to 3 according to a previously described method: 0 = no response, 1 = fine movements of the gluteal muscle caused by the stimulation, 2 = movements of the quadriceps or wagging and twisting of the hip and lumbar region to avoid the stimulation, 3 = vigorous efforts to escape from the stimulus such as “shrinking and moving back” movements or vocalization in response to the stimulation.18 Responses with grades of 2 and 3 were defined as withdrawal responses.
One microgram of morphine sulfate (Sankyo Pharm Co., Tokyo, Japan) dissolved in 10 μl of saline was then intrathecally administered through the intrathecal (i.t.) catheter. Fifteen minutes after administration of morphine, a set of behavioral data was obtained, and naloxone (10 µg) dissolved in 10 μl of saline was then intrathecally administered. A set of behavioral data (spontaneous guarding, flinching, and withdrawal responses to punctate mechanical stimuli and dynamic non-noxious stimulation) was again obtained at 15 min after administration of naloxone.
Electrophysiological recording
Seven days after implantation of the balloon catheter, the animals were anesthetized with intraperitoneal urethane (1.2–1.5 g/kg). Laminectomy was performed at the level of vertebrae T12-L1 to expose segments L1-L4 of the spinal cord, and then each rat was placed in a stereotaxic apparatus (Model ST-7, Narishige, Tokyo, Japan). After removing the dura and cutting the pia-arachnoid membrane to make a window large enough for insertion of an electrode, a small reservoir (50–100 μl) overlying the spinal cord was formed with dental impression material (Alflex®, Morita Co., Osaka, Japan) according to a previously described method.19 Body temperature was recorded with a rectal probe and maintained at 36℃–38℃ by an infrared heat lamp and a thermo-controlled heat pad.
In order to record a single neuronal activity from the SDH, a tungsten microelectrode (10–12 MQ) (FHC Inc., Brunswick, ME) was advanced by a hydraulic micromanipulator into the SDH up to a depth of 1000 µm at the level of L1-L4 according to a previously described method.20 In our preliminary study, some of the neurons that exhibited an excitatory response to intraosseous stimulation had cutaneous RFs from the thigh to the lower back. We isolated single activities of neurons that had spontaneous firing and/or cutaneous RFs from the thigh to the lower back and classified the neurons. A neuron was classified as a wide-dynamic-range (WDR) neuron if responses were elicited by both a low-intensity stimulus (light touch and brush) and high-intensity stimulus (pinch). A neuron was classified as an high-threshold (HT) neuron if it did not respond to a low-intensity stimulus but responded to a high-intensity stimulus. A neuron was classified as an low-threshold (LT) neuron if it did not respond to a high-intensity stimulus but responded to a low-intensity stimulus. The proprioceptive neurons that respond to passive joint movement were excluded from analysis in this study. A neuron was classified as an unclassified neuron if it did not respond to low-intensity stimulus, high-intensity stimulus, or passive joint movement. After isolation and classification of the neurons, the LT and HT RFs were then carefully mapped on the shaved skin by a procedure according to a previously described method.20 The edges of the LT RFs were defined as areas in which light touch stimulation with the tip of a 4-g von Frey filament probe elicited a response 50% of the time. The edges of the HT RFs were defined as areas in which high-intensity mechanical stimulation with a tungsten tip attached to a nylon filament (calibrated force of 25 g) evoked a response 50% of the time. The 4-g von Frey filament probe does not elicit painful sensation in examiners, while the 25-g tungsten tip induces pinprick pain in examiners.
We then recorded the spontaneous and evoked activities of the neurons in response to non-noxious and noxious mechanical stimuli to the skin and intraosseous stimuli. Non-noxious mechanical stimuli, including brushing and punctate stimuli, were applied in the most sensitive site of the RF. The responses to brush stimulation using a camel hairbrush were recorded at 1-min intervals. The responses to punctate mechanical stimuli using a 4-g von Frey filament were recorded for 1 s at 1-min intervals. Noxious stimuli were delivered by an arterial clip that exerted a force of 250 g/mm2 for 10 s at 5-min intervals. After completion of recordings in response to non-noxious and noxious cutaneous stimuli, intraosseous stimuli were delivered by sustained inflation of the balloon implanted in the medullary cavity of the femur with pressure of 200 kPa for 10 s at 15-min intervals.
In order to examine the relationship between activities of WDR and HT neurons and sustained pressures of the balloon implanted in the medullary cavity of the femur, the balloon was inflated with pressure from 50 to 400 kPa. In a preliminary study, since the suppressive effect of morphine on firing activity of HT neurons in response to balloon inflation was obscure, the following study was performed only in WDR neurons. After determination of the physiologic characteristics of the last WDR neuron responding to intraosseous stimuli, the following pharmacological trial was performed in that neuron. Stabilization of spontaneous activity and evoked responses to non-noxious and noxious cutaneous stimuli and intraosseous stimuli were confirmed by at least three consistent predrug responses (<10% variation). These values were then averaged before drug administration to generate control values with which to compare the effects of drug administration on the subsequent spontaneous activity and evoked responses. One microgram of morphine sulfate was dissolved in 50 μl of phosphate-buffered saline (PBS), and it was administered into the reservoir and applied directly onto the surface of the spinal cord according to a previously described method.20 Fifteen minutes after administration of morphine, spontaneous and evoked activities of WDR neurons in response to non-noxious and noxious cutaneous stimuli and intraosseous stimuli were recorded again. Then, naloxone (10 µg) dissolved in 50 μl of PBS was applied directly onto the surface of the spinal cord. In this part of the experiments, one neuron was investigated in each animal at the end of each experiment.
At the end of each experiment, laminar locations of the recording sites were estimated from measurements of the depths of the electrodes from the surface of the spinal cord. The animals were then killed with an overdose of potassium chloride.
Retrograde tracing with Fluoro-Gold and cholera toxin subunit B
Fluoro-Gold (FG; Fluorochrome Inc., Denver, CO) and Alexa Fluor 594-conjugated cholera toxin subunit B (CTB; Molecular Probes Inc., Eugene, OR) were used as retrograde tracers to identify neurons in the dorsal root ganglion (DRG) that project to the medullary cavity of the femur or skin. The rats were anesthetized with vaporized sevoflurane (induction 5%, 2%–3% maintenance). An incision of approximately 5 mm was made in the skin, and the proximal patellar ligament of the left femur was severed, revealing the synovial space of the knee joint. A needle was inserted into the medullary cavity of the femur, and 10 μl of 10% FG was injected into the intramedullary space of the femur. The injection site was sealed with a dental amalgam plug to confine the FG within the intramedullary canal and followed by irrigation with sterile water. The wound was closed in two layers. Ten microliters of 0.1% CTB was injected into the skin of the lumbosacral region.
Animals were anesthetized with intraperitoneal urethane (1.2–1.5 g/kg) and perfused through the ascending aorta with heparinized saline followed by 300 ml of 4% paraformaldehyde in 0.1 M PBS (pH 7.40) seven days after FG and CTB had been injected. The DRG L1-4 were identified and removed, post-fixed for 24 h in 20% sucrose in the same fixative at 4℃, and then transferred into 20% in 0.1 M PBS. Serial longitudinal 20-µm-thick frozen sections were cut from the DRGs by a freezing microtome and mounted on poly-L-lysine-coated slides. The sections were viewed and digitized under a fluorescence microscope (Nikon Co., Tokyo, Japan) equipped with a CCD camera using a UV-2 E/C filter (360 nm for excitation and 460 nm for emission) for FG and using a Texas red filter (590 nm for excitation and 620 nm for emission) for CTB. Only DRG-neuronal profiles labeled with a clear nucleus were counted. We determined the number of neurons labeled with either FG or CTB and those double-labeled with FG and CTB.
Data analysis
For evoked firing rates and time spending guarding, number of spontaneous flinches and frequency of withdrawal responses to dynamic cutaneous stimuli, data were expressed as means ± SEMs. Two-way analysis of variance (ANOVA) followed by the Sidak post hoc test was used for comparisons before and after intraosseous stimuli. WT to punctate mechanical stimuli was analyzed with non-parametric tests. The data were expressed as medians and interquartile ranges. Differences were determined by Friedman’s ANOVA followed by Dunn’s post hoc test for comparing WTs before and after intraosseous stimuli.
Activities of isolated single neurons were digitized (CED 1401; Cambridge Electronic Design, Cambridge, UK) and stored in computer format (IBM-AT personal computer, ThinkPad; IBM Japan, Tokyo, Japan). Collected data were analyzed off-line using the computer program Spike 2 (Cambridge Electronic Design). Amplitudes and shapes of the neurons were checked every 30 min after stable recordings had been successfully obtained, and it was confirmed that the recordings were from the same neurons by observation on an oscilloscope and by using the computer software program Spike 2 according to a previously described method.21
Spontaneous firing rates were determined by averaging the activity over 10- to -20-s periods when there was no contact with the RF. To evaluate evoked activities of dorsal horn neurons, prestimulus spontaneous firing rates were subtracted from firing rates in response to non-noxious and noxious stimuli. The effects of morphine are expressed as mean maximum percentage inhibition from the averaged predrug value for each neuron, and results are expressed as means ± SEMs. One-way ANOVA followed by Dunnett’s post hoc test was used for comparisons before and after intraosseous stimuli. All analyses were performed using GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA). A p value < 0.05 was considered statistically significant.
Results
Spontaneous pain induced by balloon inflation in the medullary cavity of the femur
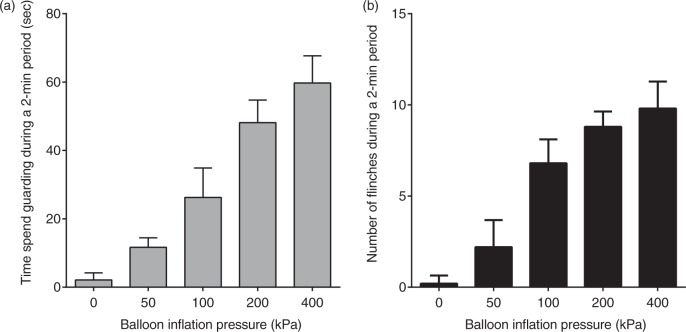
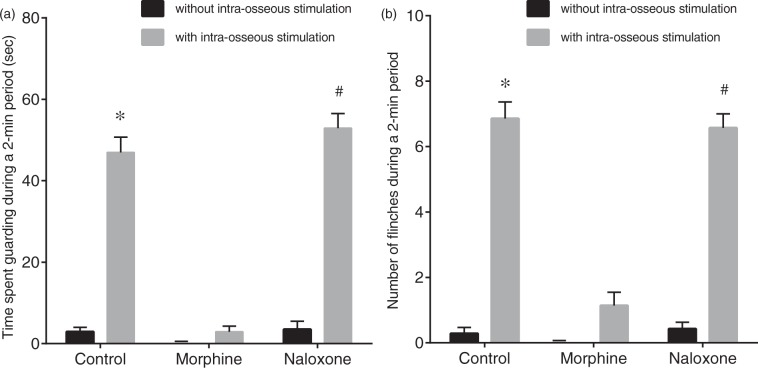
Spontaneous pain-related behavior induced by intraosseous stimulation was evaluated in seven rats. Spontaneous pain-related behavior including guarding and flinching of the hind paw increased with increase in intraosseous balloon pressure (Figure 2). Intraosseous stimulation with pressure of 200 kPa significantly increased the time spent guarding from 3 ± 1 s to 47 ± 4 s (Figure 3, p < 0.01) and the number of flinches from 0.3 ± 0.2 to 6.9 ± 0.5 (p < 0.01). After i.t. administration of morphine (1 µg), intraosseous stimulation did not significantly increase the time spent guarding (from 0 ± 0 s to 3 ± 1 s, p = 0.79) or flinching (from 0 ± 0 to 1.1 ± 0.4, p = 0.10). After i.t. administration of naloxone (10 µg), intraosseous stimulation significantly increased the time spent guarding from 4 ± 2 s to 53 ± 4 s (p < 0.01) and flinching from 0.4 ± 0.2 to 6.6 ± 0.4 (p < 0.01).
Figure 2.
Chronic implantation of a balloon catheter in the femur. A skin incision was made on the medial side of the right knee, and the proximal patellar ligament of the femur was severed, revealing the synovial space of the knee joint. A 22-gauge needle was used to core between the femoral condyles and into the medullary cavity of the right femur. A diamond drill burr, 1 mm in diameter, was used to prime the opening of the hole made by the 22-gauge needle. A balloon catheter used for coronary angioplasty in patients with coronary artery disease (PTCA catheter, RX-2, 2 × 15 mm, TERUMO Co., Tokyo, Japan) was implanted in the medullary cavity of the femur for increasing intraosseous pressure of the femur. The balloon catheter was then tunneled subcutaneously to emerge at the neck. To stimulate intraosseous receptors, the balloon was inflated with saline using an inflation device (Encore™ 26, Boston Scientific Japan, Tokyo, Japan).
Figure 3.
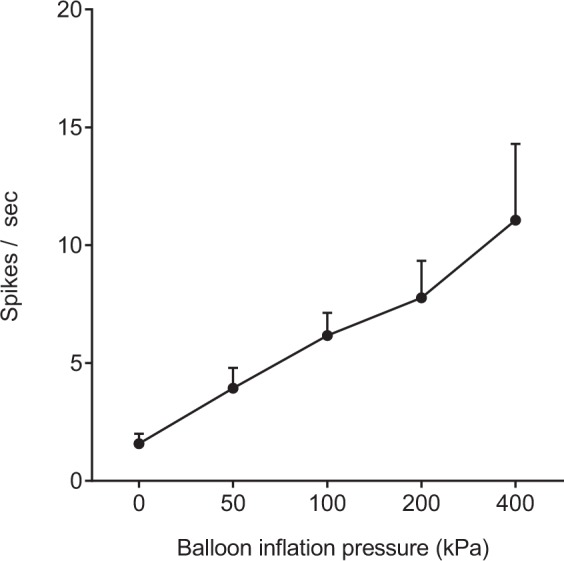
Relationship between spontaneous pain-related behavior and intraosseous pressure of the femur. Guarding behavior and flinches induced by balloon inflation in the medullary cavity of the femur at pressure from 50 to 400 kPa. Guarding behavior (a) and flinches (b) increased in accordance with an increase in balloon inflation pressure. Data are expressed as means ± SEMs.
Mechanical hyperalgesia induced by balloon inflation in the medullary cavity of the femur
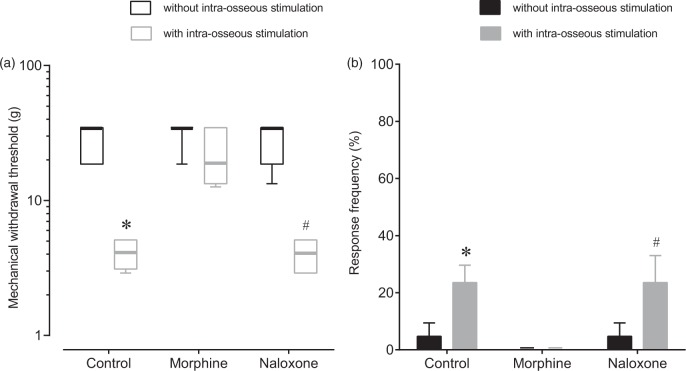
Mechanical hyperalgesia induced by intraosseous stimulation was assessed in seven rats. Intraosseous stimulation significantly decreased the median WT at the skin of the lumbosacral region from 34.6 ± 16 g to 4.1 ± 1.3 g (Figure 4, p = 0.014) and increased the frequency of withdrawal responses to dynamic non-noxious cutaneous stimuli from 4.7 ± 4.7% to 23.6 ± 6.1% (p = 0.036). After i.t. administration of morphine (1 µg), intraosseous stimulation did not significantly decrease the median WT (from 34.6 ±0 g to 18.6 ± 18.6 g, p > 0.99) and did not significantly increase the frequency of withdrawal responses to dynamic non-noxious cutaneous stimuli (from 0 ± 0% to 0 ± 0%, p > 0.99). After i.t. administration of naloxone (10 µg), intraosseous stimulation significantly decreased the median WT from 34.6 ± 16 g to 4.1 ± 1.9 g (p = 0.036) and significantly increased the frequency of withdrawal responses to dynamic non-noxious cutaneous stimuli from 4.7 ± 4.7% to 23.6 ± 9.4% (p = 0.036).
Figure 4.
Effects of intrathecal morphine and naloxone on spontaneous pain-related behavior induced by intraosseous balloon inflation. Effects of intrathecal (i.t.) morphine and i.t. naloxone on spontaneous pain-related behavior assessed as the time spent guarding (a) and the number of flinches (b) induced by balloon inflation in the medullary cavity of the femur (200 kPa). Guarding behavior and flinches without intraosseous stimulation were recorded before injection (control), 15 min after i.t. injection of morphine (morphine, 1 µg), and 15 min after injection of naloxone (naloxone, 10 µg). After recording of pain-related behavior without intraosseous stimulation, guarding behavior and flinches induced by balloon inflation in the medullary cavity of the femur were recorded. I.t. administration of morphine suppressed intraosseous stimulation-induced guarding behavior and flinches. I.t. naloxone antagonized the effect of morphine. Data are expressed as means ± SEMs (n = 7). *p < 0.01 versus control without intraosseous stimulation, #p < 0.01 versus naloxone without intraosseous stimulation.
General properties of recorded SDH neurons
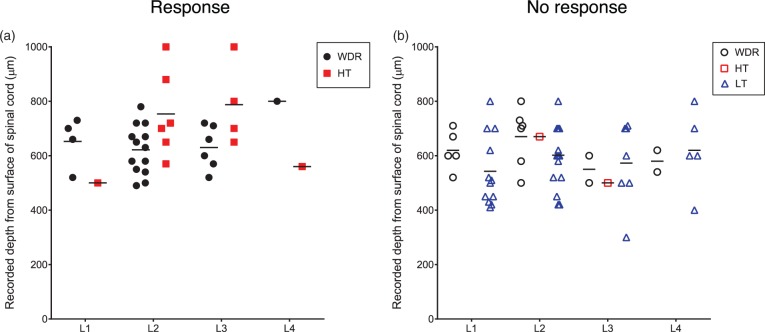
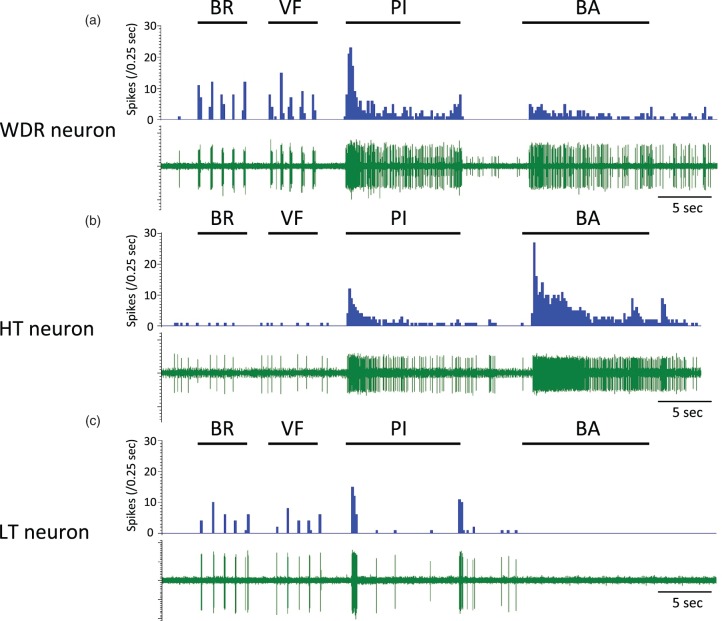
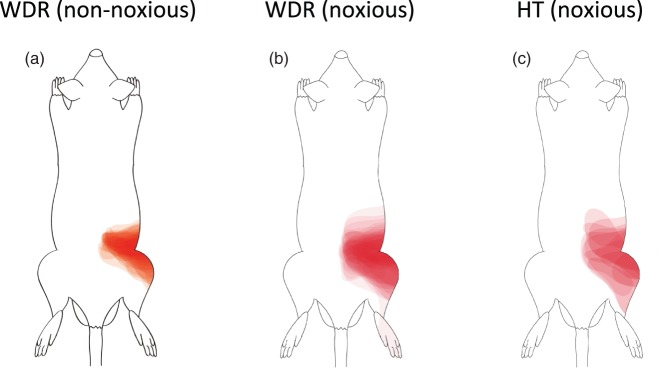
A total of 95 SDH neurons were obtained from 37 rats, and these neurons were identified by spontaneous activity or evoked response to non-noxious cutaneous mechanical stimuli and then classified by using non-noxious and noxious cutaneous stimuli. The neurons were classified into 39 WDR neurons, 14 HT neurons, 41 LT neurons, and one unclassified neuron. All of the WDR, HT, and LT neurons had cutaneous RFs from the hind paw to the lower back, while the unclassified neuron did not have a cutaneous RF. Except for the unclassified neuron, the number of neurons recorded, types of cells and depths of cell location, spontaneous firing rates, and firing rates of responses in the neurons examined to non-noxious and noxious cutaneous stimuli and intraosseous stimulation are shown in Table 1 and Figure 5. The unclassified neuron had a spontaneous firing rate of 0.8 spikes/s and an intraosseous stimuli-evoked firing rate of 8.4 spikes/s. Sixty-two percent of the WDR neurons (24/39) and 86% of the HT neurons (12/14) responded to intraosseous stimulation by balloon inflation, while none of the LT neurons (0/41) responded to such mechanical stimulation by balloon inflation. Typical examples of WDR, HT, and LT neurons are shown in Figure 6. WDR and HT neurons that responded to intraosseous stimulation by balloon inflation had cutaneous excitatory RFs in the lower back, the lumbosacral region, and the thigh (Figure 7).
Table 1.
Physiology of neurons recorded in this study.
| Response to BA | Depth of location (µm) | SP (spikes/s) | BR (spikes/s) | VF4 (spikes/s) | PI (spikes/s) | BA (spikes/s) | |
|---|---|---|---|---|---|---|---|
| WDR (n = 39) | Response (n = 24) | 636 ± 18 | 1.3 ± 0.5 | 15.9 ± 2.9 | 11.5 ± 1.6 | 20.3 ± 2.6 | 10.2 ± 2.4 |
| No response (n = 15) | 625 ± 23 | 1.1 ± 0.2 | 22.1 ± 4.3 | 16.7 ± 2.7 | 19.9 ± 1.9 | — | |
| HT (n = 14) | Response (n = 12) | 642 ± 44 | 1.8 ± 0.9 | — | — | 9.0 ± 1.6 | 11.2 ± 2.2 |
| No response (n = 2) | 585 ± 85 | 1.0 ± 0.3 | — | — | 10.9 ± 3.5 | — | |
| LT (n = 41) | No response (n = 41) | 574 ± 20 | 0.3 ± 0.0 | 13.1 ± 1.0 | 10.0 ± 1.1 | — | — |
Spontaneous activities (SP) and responses to a series of stimuli. BA: response to balloon inflation at 200 kPa in the medullary cavity of the femur; Depth: depths of electrodes from the surface of the spinal cord; BR: response to brush stimulation; VF4: response to punctate stimulation using a 4-g von Frey filament; PI: response to pinch stimulation by an arterial clip that exerts a force of 250 g/mm2; WDR: wide-dynamic-range neurons; HT: high-threshold neurons; LT: low-threshold neurons; —: not recorded. Data are shown as means ± SEMs.
Figure 5.
Effects of intrathecal morphine and naloxone on mechanical hyperalgesia induced by intraosseous balloon inflation. Effects of i.t. morphine and i.t. naloxone on mechanical withdrawal threshold in response to punctate noxious stimuli (a) and dynamic non-noxious stimuli (b) with sustained balloon inflation (200 kPa) in the medullary cavity of the femur. After recording withdrawal responses without intraosseous stimulation, withdrawal responses with intraosseous stimulation were recorded. Then, withdrawal thresholds with intraosseous stimulation (200 kPa) were recorded 15 min after i.t. morphine (morphine) and 15 min after i.t. naloxone (naloxone). Intraosseous stimulation decreased the withdrawal threshold and increased the withdrawal response frequency in the control rats. I.t. administration of morphine reduced intraosseous stimulation-induced mechanical hyperalgesia and allodynia. I.t. naloxone antagonized the effect of morphine on the withdrawal thresholds induced by balloon inflation. Data are expressed as medians (horizontal line) and boxes and whiskers with first and third quartiles (box) and as minimum and maximum (whiskers) or means ± SEMs (n = 7). *p < 0.05 versus control without balloon inflation; p < 0.05 versus naloxone without balloon inflation.
Figure 6.
Recording sites of neurons. Recording sites of neurons that responded to intraosseous stimulation (a) and neurons that did not responded to intraosseous stimulation (b). Black filled circles, wide-dynamic-range (WDR) neurons that responded to inflation of the balloon in the medullary cavity of the femur; black open circles, WDR neurons that did not respond to inflation of the balloon in the medullary cavity of the femur; red filled squares, high-threshold (HT) neurons that responded to inflation of the balloon in the medullary cavity of the femur; red open squares, HT neurons that did not respond to inflation of the balloon in the medullary cavity of the femur; blue open triangle, low-threshold (LT) neurons that did not respond to inflation of the balloon in the medullary cavity of the femur. Bars indicate means of depth of recording sites of each type of the neurons from the dorsal surface of the spinal cord.
Figure 7.
Typical examples of recorded neurons. Typical examples of responses of wide-dynamic-range (WDR), high-threshold (HT), and low-threshold (LT) neurons to mechanical stimuli (a, b, and c). BR: brush stimulation; VF: stimulation using a 4-g von Frey filament; PI: pinch stimulation using an arterial clip with a force of 250 g/mm2; BA: sustained balloon inflation in the medullary cavity of the femur at 200 kPa.
Response of neurons to balloon inflation in the medullary cavity of the femur
A total of five WDR neurons were examined for the relationship between neuronal activity and increase in balloon inflation pressure. As shown in Figure 8, the WDR neurons responded linearly to increase in balloon inflation pressure from 50 to 400 kPa. Only one HT neuron was examined for the relationship between neuronal activity and increase in balloon inflation pressure. The neuron responded linearly to balloon inflation pressures at 50 (0.8), 100 (1.6), 200 (2.1), and 400 kPa (4.2 spikes/s).
Figure 8.
Cutaneous mechanical receptive fields of neurons that responded to intraosseous balloon inflation. Cutaneous mechanical receptive fields (RFs) of the dorsal horn neurons that responded to balloon inflation in the medullary cavity of the femur at 200 kPa. The edge of the low-threshold RF was defined as the area in which light touch stimulation with the tip of a 4-g von Frey filament elicited a response 50% of the time. The edge of the high-threshold RF was defined as the area in which high-intensity mechanical stimulation with a tungsten tip attached to a nylon filament (calibrated force of 25 g) elicited a response 50% of the time (see text). Wide-dynamic-range (WDR) neurons had low-threshold and high-thresholds RFs, while HT neurons had a high-threshold RF. Both WDR and HT neurons had cutaneous RFs at the lower back, lumbosacral region, and thigh. The schemas were made by overlaying the map of the RF area of each neuron repeatedly. HT: high-threshold.
Effects of spinal administration of morphine on evoked responses of the neurons
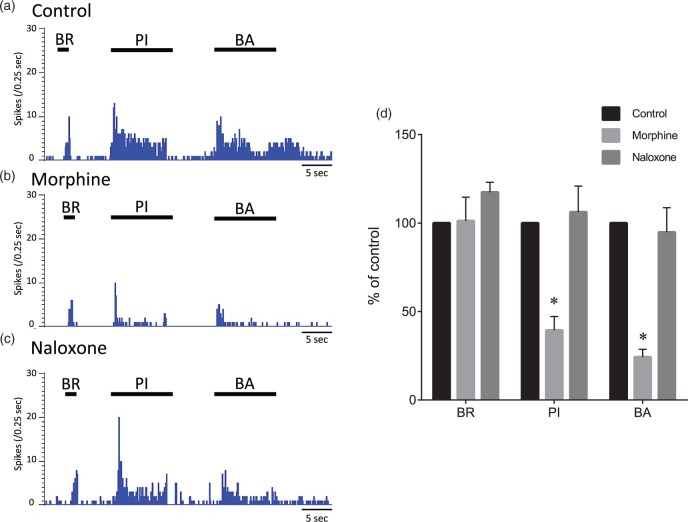
A total of five WDR neurons responding to intraosseous stimuli were obtained from five rats. The effects of spinal administration of morphine (1 µg/50 μl) and naloxone (10 µg/50 μl) on evoked responses of the neurons to stimuli by brushing, pinching, and intraosseous balloon inflation were recorded. Maximal effects were seen 10–20 min after spinal administration of morphine. After spinal administration of morphine, the evoked firing rates by pinching and intraosseous balloon inflation were significantly decreased from 100% and 100% to 39 ± 8% and 24 ± 4%, respectively (Figure 9, p < 0.01). After spinal administration of naloxone, the evoked firing rates by pinching and intraosseous balloon inflation were 106 ± 15% and 95 ± 14%, respectively, and were not significantly different from predrug values (p = 0.94 and p = 0.97, respectively).
Figure 9.
Relationship between intraosseous stimuli-evoked firing rate of neurons and intraosseous pressure of the femur. Responses of wide-dynamic-range (WDR) neurons to balloon inflation in the medullary cavity of the femur at pressures from 50 to 400 kPa. Data are expressed as means ± SEMs.
Intraosseous and cutaneous sensory convergence at dorsal root ganglion neurons
Data were obtained from three rats. Neurons labeled with FG transported from the medullary cavity of the femur were distributed in the ipsilateral L1 to L4 DRGs and predominated in L2 to L3 DRGs (Table 2). DRG neurons labeled with CTB transported from the skin were in the ipsilateral L1 to L4 DRGs. DRG neurons double-labeled with FG and CTB were found in L2-3 DRGs. The percentage of double-labeled neurons among FG-labeled neurons in the L3 DRG was 6.6%, and the percentage of double-labeled neurons among CTB-labeled neurons in the L3 DRG was 5.6% (Figure 10). No FG-labeled or CTB-labeled neuron was detected on the contralateral side of the DRGs.
Table 2.
Total numbers of FG- and CTB-labeled neurons in L1-L4 DRGs.
| DRG | Number of FG-labeled neurons | Number of CTB-labeled neurons | Number of FG/CTB double-labeled neurons |
|---|---|---|---|
| L1 | 43 | 62 | 0 |
| L2 | 142 | 172 | 5 |
| L3 | 121 | 143 | 8 |
| L4 | 16 | 39 | 0 |
Fluoro-Gold (FG) and cholera toxin subunit B (CTB) were applied to the bone marrow and skin of the lumbosacral region, respectively, and total numbers of FG- and CTB-labeled neurons in L1-L4 dorsal root ganglions (DRGs) were counted seven days later (see text).
Figure 10.
Effects of morphine and naloxone on evoked responses of wide-dynamic-range neurons. Typical examples of evoked responses of a wide-dynamic-range (WDR) neuron to mechanical stimuli to the skin and balloon inflation before administration (control), 15 min after spinal administration of morphine, and 15 min after spinal administration of naloxone (a, b, and c). The cutaneous stimuli were applied in the most sensitive site of the receptive field. Mean changes in responses of WDR neurons (n = 5) to mechanical stimuli (d). BR: brush stimulation; PI: pinch stimulation using an arterial clip with a force of 250 g/mm2; BA: balloon inflation (200 kPa) in the medullary cavity of the femur. Data are expressed as means ± SEMs. *p < 0.01 versus control.
Discussion
The results of this study showed that (1) intraosseous balloon inflation elicited spontaneous pain-related behavior and induced cutaneous mechanical hyperalgesia and allodynia, (2) i.t. administration of morphine suppressed such spontaneous pain-related behavior and cutaneous mechanical hyperalgesia and allodynia, (3) intraosseous balloon inflation elicited a barrage of firing of SDH neurons that was linearly graded with balloon inflation pressure, (4) the majority of WDR neurons and HT neurons, but not LT neurons, that transmitted cutaneous perceptive information were activated by acute intraosseous mechanical stimuli, (5) spinal administration of morphine suppressed the evoked response to intraosseous balloon inflation in WDR neurons, and (6) intraosseous and cutaneous sensory convergence was seen in some DRG neurons.
Intraosseous nociception
Previous studies have shown that both electrical stimulation and increase in pressure within bone marrow generate a blood pressure increase that may be indicative of nociceptive activation.22 An increase in intraosseous pressure has been shown to activate fine-diameter afferent nerve fibers arising from bone marrow, as do irritant and inflammatory agents such as H+and K+ ions and histamine and bradykinin.23 These findings suggest that intraosseous nociceptors are activated by various kinds of stimuli including an increase in intraosseous pressure. It has been shown that vertebral intraosseous pressure with bone marrow edema is closely related to back pain and vertebral perforation and that decrease in intraosseous pressure is effective for reducing pain in patients with vertebral compression fracture.24,25 Abrupt intraosseous pressure changes caused by bone marrow aspiration are also known to cause acute pain.26 Taken together with results of these previous studies, our results suggest that intraosseous pressure is an important factor for skeletal pain.
Little is known about how SDH neurons receive nociceptive information from skeletal tissues including bone marrow. In this study, the majority of WDR neurons and HT neurons, which had RFs of the skin around the lower back, lumbosacral region, and thigh, responded to intraosseous balloon inflation. The evoked response of WDR neurons increased in a pressure-dependent manner (Figure 8). Interestingly, none of the LT neurons responded to intraosseous balloon inflation. These results suggest that mechanical stimuli, which increases intraosseous pressure, produces only pain sensation as sensory perception of bone marrow. Since afferent fibers innervating the medullary cavity of the bone are confined to A-delta and C-fibers,27 the afferent fibers will specifically sense noxious stimuli. The pressure of bone marrow of the femur could not be measured when the balloon was inflated at various pressures in this study. This was because the intraosseous space was so narrow that another catheter could not be inserted to monitor the intraosseous pressure. However, since spontaneous pain-related behavior such as guarding and flinching of the hind paw increased in association with the degree of balloon inflation pressure, the degree of balloon inflation pressure seems to be related to that of intraosseous pressure. Evoked response of WDR neurons was also linearly increased in relation to the increase in balloon inflation pressure. The intraosseous afferent fibers will sense the increase in pressure in the medullary cavity as nociceptive stimuli. In this study, we could not determine whether C-fibers or A-delta fibers or both were activated by intraosseous balloon inflation. However, WDR neurons receive monosynaptic input from nociceptive A-delta fibers and polymodal C-fibers,28 and both A-delta fiber-evoked excitation and C-fiber-evoked excitation in SDH neurons are suppressed by the μ agonist [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO).29 In this study, pain-related behavior and evoked response of WDR neurons by balloon inflation were abolished by i.t. administration and spinal application of morphine (1 µg) in the behavioral study and electrophysiological study, respectively. Thus, it was suggested that acute intraosseous mechanical stimulation activates nociceptors including C-fibers and A-delta fibers located in bone marrow, sends the signal to SDH neurons, and elicits nociception, which is suppressed by spinal morphine.
Referred pain induced by intraosseous stimuli
In this study, it was shown that balloon inflation in the medullary cavity of the femur significantly decreased WTs in response to punctate nociceptive stimuli and dynamic non-nociceptive stimuli in the lumbosacral region and that i.t. morphine reversed such decreases in WTs. These results suggest that activation of nociceptors located in bone marrow induces referred pain of the corresponding areas of the skin. In the case of bone marrow of the femur, the referred pain seems to occur in the lumbosacral region, lower back, and thigh.
Numerous studies have shown convergence of cutaneous, deep tissue and visceral afferents at both the spinal and supraspinal levels, and it is believed that convergence of visceral and somatic afferents on the same spinal dorsal neuron results in referred pain and referred hyperalgesia.30–32 In this study, the majority of WDR neurons (62%) and HT neurons (86%) received noxious stimuli from both cutaneous and intraosseous nociceptive afferents. The results showed convergence of cutaneous and intraosseous afferent nociceptive fibers at the SDH level. Unfortunately, the retrograde tracing experiment conducted in this study could not show quantitative somatoskeletal convergence rates at the levels of the DRGs and SDH. However, the results of this study have shown that convergence of afferent nerve fibers from bone marrow of the femur and skin of the lumbosacral region occurs at least in part in DRG neurons at the L2–L3 level. Similarly, convergence of splanchnic nerves and intracostal nerves onto DRG neurons33 and convergence of vertebral disk and skin afferents onto DRG neurons have been reported.34 Thus, neural convergence in the DRG and/or SDH might cause referred pain, hyperalgesia, and allodynia to corresponding areas of the skin in patients with skeletal diseases as well as patients with visceral diseases.
Clinical implications
Since the bone, muscle, and skin are damaged by surgical manipulations in patients undergoing orthopedic surgery, bone injury such as injury caused by intramedullary rod insertion might amplify afferent inputs from the skin and muscles during surgery and worsen postoperative pain. In addition, from the results of this study, intraosseous noxious stimuli of the femur causes referred pain and referred hyperalgesia in the corresponding skin areas, that is, the lower back, lumbosacral region, and lateral part of the thigh, where an incision is commonly made for surgery of the femur. Thus, if pain signals persistently originate from primary afferents in the cavity of the femur even after surgery, such painful signals may contribute to cutaneous spontaneous pain, hyperalgesia, and allodynia as components of postoperative pain.
Previous studies suggested that the dose of morphine required to alleviate bone cancer pain was greater than that required to alleviate inflammatory pain.35,36 In this study, pain induced by intraosseous balloon inflation was almost completely abolished by spinally administered morphine (1 µg). The difference in the results may be explained as follows. Intramedullary balloon inflation used in this study might increase intraosseous pressure. On the other hand, bone cancer pain is caused by not only increased intraosseous pressure but also the release of chemical mediators and microfractures.37 Various factors might worsen bone cancer pain and make treatment of bone cancer pain difficult.
Limitations
There are several limitations in this study. First, when intraosseous pressure is increased in bone marrow, it propagates through the foramen nutricium and transits to the periosteum. Thus, balloon inflation would have increased intraosseous pressure and might have stimulated not only intraosseous nociceptors located in bone marrow but also nociceptors located in the periosteum and cortical bone in this study. However, such a situation is similar to most skeletal injuries and diseases including bone fracture, osteomyelitis, and bone cancer, in which intraosseous pressure increases and propagates through the foramen nutricium and transits to the periosteum.
Second, single neuronal activity of SDH neurons, which had RFs in the lower back, lumbosacral region, and thigh, were first isolated, and then intraosseous mechanical stimulation was applied to these neurons in this study. Therefore, we could find only one neuron that did not have any cutaneous RF but responded to intraosseous stimulation. However, a skeletal pain pathway apart from somatosensory pain processing of the skin must also be present. In such a skeletal pain pathway, SDH neurons would specifically respond to intraosseous mechanical stimulation but would not have any cutaneous RFs. Further study is thus needed to elucidate the nociception pathway specific to intraosseous stimuli.
Third, the in vivo nature of the present electrophysiological study could not directly show whether the recorded neurons were excitatory or inhibitory neurons and whether the recorded neurons were involved in intraosseous nociception. However, spinal administration of morphine attenuated the evoked response of WDR neurons to intraosseous balloon inflation. This result suggests that the majority of the recorded neurons would be excitatory neurons and involved in intraosseous nociception.
Conclusions
In conclusion, nerve fibers in the medullary cavity sense noxious stimulation and send signals to the SDH. Bone marrow afferent input converges onto SDH neurons responding to somatic mechanical stimuli. The results support the concept that activation of bone marrow afferents might lead to the production of referred pain in somatic fields, which may contribute to pain caused by skeletal diseases.
Author Contributions
TI carried out the electrophysiological recording, behavioral study, and retrograde tracing study; conceived and designed the research; and wrote the manuscript. ST assisted in the electrophysiological recording and helped to write the manuscript. TS and DS carried out the electrophysiological recording. MK conceived and designed the research and helped to write the manuscript. All authors have read and approved the final version of the manuscript.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declare that they have no competing interests.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research Grant Number 24791587 (TI) and 21390432 (MK).
References
- 1.Forley KM. Pain assessment and cancer pain syndromes. In: Doyle D, Hanks GW, MacDonald N. (eds). Oxford textbook of palliative medicine, 2nd ed Oxford: Oxford Medical Publications, 1998, pp. 310–331. [Google Scholar]
- 2.Bazett HC, McGlone B. Note on the pain sensations which accompany deep punctures. Brain 1928; 51: 18–23. [Google Scholar]
- 3.Tabarowski Z, Gibson-Berry K, Felten SY. Noradrenergic and peptidergic innervation of the mouse femur bone marrow. Acta Histochem 1996; 98: 453–457. [DOI] [PubMed] [Google Scholar]
- 4.Mach DB, Rogers SD, Sabino MC, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 2002; 113: 155–166. [DOI] [PubMed] [Google Scholar]
- 5.Ivanusic JJ. The pattern of Fos expression in the spinal dorsal horn following acute noxious mechanical stimulation of bone. Eur J Pain 2008; 12: 895–899. [DOI] [PubMed] [Google Scholar]
- 6.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006; 12: 6243 s–6249 s. [DOI] [PubMed] [Google Scholar]
- 7.Sesay M, Dousset V, Liguoro D, et al. Intraosseous lidocaine provides effective analgesia for percutaneous vertebroplasty of osteoporotic fractures. Can J Anaesth 2002; 49: 137–143. [DOI] [PubMed] [Google Scholar]
- 8.Rice JR, Pisetsky DS. Pain in the rheumatic diseases. Practical aspects of diagnosis and treatment. Rheum Dis Clin North Am 1999; 25: 15–30. [DOI] [PubMed] [Google Scholar]
- 9.Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol 2010; 10: 599–606. [DOI] [PubMed] [Google Scholar]
- 10.Schwei MJ, Honore P, Rogers SD, et al. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci 1999; 19: 10886–10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang CJ, Li Q, Wu GC, et al. A practical model of osteomyelitis-induced bone pain by intra-tibial injection of Staphylococcus aureus in rats. Neurosci Lett 2012; 513: 198–203. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez-Andrade JM, Martin CD, Koewler NJ, et al. Nerve growth factor sequestering therapy attenuates non-malignant skeletal pain following fracture. Pain 2007; 133: 183–196. [DOI] [PubMed] [Google Scholar]
- 13.Kawamata T, Omote K, Toriyabe M, et al. Intracerebroventricular morphine produces antinociception by evoking gamma-aminobutyric acid release through activation of 5-hydroxytryptamine 3 receptors in the spinal cord. Anesthesiology 2002; 96: 1175–1182. [DOI] [PubMed] [Google Scholar]
- 14.Sabino MA, Luger NM, Mach DB, et al. Different tumors in bone each give rise to a distinct pattern of skeletal destruction, bone cancer-related pain behaviors and neurochemical changes in the central nervous system. Int J Cancer 2003; 104: 550–558. [DOI] [PubMed] [Google Scholar]
- 15.Yaksh TL. Behavioral autonomic correlates of the tactile evoked allodynia produced by spinal glycine: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain 1989; 37: 111–123. [DOI] [PubMed] [Google Scholar]
- 16.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 17.Hao JX, Xu XJ, Aldskogius H, et al. Allodynia-like effects in the rat after ischaemic spinal cord injury photochemically induced by laser irradiation. Pain 1991; 45: 175–185. [DOI] [PubMed] [Google Scholar]
- 18.Kawamata M, Koshizaki M, Shimada SG, et al. Changes in response properties and receptive fields of spinal dorsal horn neurons in rats after surgical incision in hairy skin. Anesthesiology 2005; 102: 141–151. [DOI] [PubMed] [Google Scholar]
- 19.Light AR, Willcockson HH. Spinal laminae I-II neurons in rat recorded in vivo in whole cell, tight seal configuration: properties and opioid responses. J Neurophysiol 1999; 82: 3316–3326. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Kawamata M, Namiki A. Changes in properties of spinal dorsal horn neurons and their sensitivity to morphine after spinal cord injury in the rat. Anesthesiology 2005; 102: 152–164. [DOI] [PubMed] [Google Scholar]
- 21.Hillsley K, Kirkup AJ, Grundy D. Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. J Physiol 1998; 506: 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shevelev OA, Sokov EL, Khodorovich NA. Role of intraosseous receptors in afferent and motor reaction modulation. Bull Exp Biol Med 1995; 120: 685–687. [PubMed] [Google Scholar]
- 23.Seike W. Electrophysiological and histological studies on the sensibility of the bone marrow nerve terminal. Yonago Acta Med 1976; 20: 192–211. [PubMed] [Google Scholar]
- 24.Moore MR, Brown CW, Brugman JL, et al. Relationship between vertebral intraosseous pressure, pH, pO2, pCO2, and magnetic resonance imaging signal inhomogeneity in patients with back pain. An in vivo study. Spine 1991; 16: s239–s242. [DOI] [PubMed] [Google Scholar]
- 25.Yokoyama K, Kawanishi M, Yamada M, et al. Comparative study of percutaneous vertebral body perforation and vertebroplasty for the treatment of painful vertebral compression fractures. AJNR Am J Neuroradiol 2012; 33: 685–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanhelleputte P, Nijs K, Delforge M, et al. Pain during bone marrow aspiration: prevalence and prevention: prevalence and prevention. J Pain Symptom Manage 2003; 26: 860–866. [DOI] [PubMed] [Google Scholar]
- 27.Ivanusic JJ, Mahns DA, Sahai V, et al. Absence of large-diameter sensory fibers in a nerve to the cat fumers. J Anat 2006; 208: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dostrovsky JO, Craig AD. Ascending projection system. In: McMahon SB, Koltzenburg M. (eds). Wall and Melzack’s textbook of pain, 5th ed Philadelphia: Elsevier Churchill Livingstone, 2006, pp. 187–203. [Google Scholar]
- 29.Ikoma M, Kohno T, Baba H. Differential presynaptic effects of opioid agonists on Adelta- and C-afferent glutamatergic transmission to the spinal dorsal horn. Anesthesiology 2007; 107: 807–812. [DOI] [PubMed] [Google Scholar]
- 30.Fields HL, Meyer GA, Partridge LD., Jr Convergence of visceral and somatic input onto spinal neurons. Exp Neurol 1970; 26: 36–52. [DOI] [PubMed] [Google Scholar]
- 31.Woolf CJ. Evidence for a central component of post-injury hypersensitivity. Nature 1983; 306: 686–688. [DOI] [PubMed] [Google Scholar]
- 32.Hoheisel U, Mense S. Response behaviour of cat dorsal horn neurones receiving input from skeletal muscle and other deep somatic tissues. J Physiol 1990; 426: 265–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pierau FK, Fellmer G, Taylor DC. Somato-visceral convergence in cat dorsal root ganglion neurones demonstrated by double-labelling with fluorescent tracers. Brain Res 1984; 321: 63–70. [DOI] [PubMed] [Google Scholar]
- 34.Sameda H, Takahashi Y, Takahashi K, et al. Dorsal root ganglion neurones with dichotomising afferent fibres to both the lumbar disc and the groin skin. J Bone Joint Sureg Br 2003; 85: 600–603. [DOI] [PubMed] [Google Scholar]
- 35.Luger NM, Sabino MA, Schwei MJ, et al. Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs inflammatory pain. Pain 2002; 99: 397–406. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto J, Kawamata T, Niiyama Y, et al. Down-regulation of mu opioid receptor expression within distinct subpopulations of dorsal root ganglion neurons in a murine model of bone cancer pain. Neuroscience 2008; 151: 843–853. [DOI] [PubMed] [Google Scholar]
- 37.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain 1997; 69: 1–18. [DOI] [PubMed] [Google Scholar]