Introduction
Identifying populations with high HIV incidence and adequate study retention is necessary to perform HIV vaccine efficacy trials that have sufficient statistical power [1]. The investigators of the International AIDS Vaccine Initiative (IAVI) network in sub-Saharan Africa have been conducting a number of observational studies to assess the suitability and willingness of potential populations to enroll in future HIV vaccine efficacy trials[2]. Volunteers in these observational cohorts have included discordant couples, members of fishing communities, women at high risk, and men who have sex with men (MSM). In these studies, annual HIV incidence ranged from 1.1 to 10.8 per 100 person years of observation (PYO) with one-year study retention of 75% to 97%[2] and HIV prevalence ranged from 8.3% to 16.4%[2]. These populations have also expressed a high willingness to enroll in future efficacy trials [3-5]. Other studies in high risk populations in the same region have found HIV prevalence ranging between 6.1% and 37% [6-10] and annual HIV incidence between 4% and 12.6% per 100 PYO [7, 11, 12]. Microbicide trials among women at high risk of HIV infection identified from sero-discordant relationships residing in areas far from the fishing populations but in the same district observed HIV incidence in the control arm ranging between 3.3 and 4.3 per 100 PYO [13, 14]. Although fishing populations have been identified as possible high risk population for future efficacy trials, no trials have been conducted yet in these populations. These populations have unique characteristics such as high mobility and excessive alcohol consumption that may impact on both HIV incidence and study retention during trials [9, 11]. In such populations, HIV incidence reported from observational cohorts or feasibility studies is usually used to estimate the required sample size for efficacy trials [12]. However, such data may not reflect the incidence in an efficacy trial because of changes in the eligibility criteria for participation and trial procedures such as risk reduction counselling, mandatory use of contraceptives and condom provision, more frequent visits and the difference in duration between observational studies and efficacy trials. Inadvertent selection of lower risk volunteers into a trial could also play a role [12]. In Uganda[13], Nigeria[15] and Ghana [16] microbicide and a Pre Exposure Prophylaxis (PrEP) [17] trials have shown lower HIV incidence in the control arm than that observed in feasibility cohort studies at the planning stage [12, 15-17]. One systematic review [18] reported a number of HIV prevention studies that were unsuccessful or terminated because they found lower HIV incidence and statistical power than what was predicted based on observational data. These under-powered studies expose volunteers to investigational products in experiments of limited clinical value, waste time and financial resources [19, 20]. It is important, therefore, to obtain more accurate estimates of the actual incidence that would occur during trial conditions.
To our knowledge, there is no data that have compared HIV incidence in an observational cohort to that in an efficacy trial using the fishing populations that are potentially being considered for future trials. In this analysis, we compared HIV incidence in a longitudinal HIV vaccine preparedness observational cohort to that in a Simulated Vaccine Efficacy Trial (SiVET) nested within the cohort. These two studies were part of collaboration between the Medical Research Council / Uganda Virus Research Institute (MRC/UVRI) Uganda Research Unit on AIDS and the International AIDS Vaccine Initiative (IAVI) to prepare for future vaccine efficacy trials.
Methods
Study population
We used data from an observational fishing cohort and from a nested Simulated Vaccine Efficacy Trial (SiVET) to compare HIV incidence between the two studies in rural South-Western Uganda. The observational cohort was established to estimate annual HIV incidence and maintain a pool of volunteers for possible recruitment into future efficacy trials. Enrolment for the observational cohort was between January 2012 and February 2013. The SiVET study mimicked an HIV vaccine efficacy trial using a licensed Hepatitis B vaccine as a proxy for an HIV vaccine to assess retention and willingness to participate in future trials. Enrolment into the SiVET started in July 2012; and ended in February 2013 when the estimated sample had been accrued. In this analysis, we included observational cohort volunteers that had been enrolled by the date SiVET completed enrolment. We included data for every volunteer from the 3 month visit date to 12 months later. Each volunteer in the two studies contributed at most 12 months of follow up data or to the point they were last seen or tested HIV positive, if that was shorter.
Observational Cohort Procedures
Volunteers in the observational cohort were recruited from fishing communities located about 40km from the MRC/UVRI research site in Masaka town by trained fieldworkers. Fieldworkers visited each household on the lakeshore, offered HIV counselling and testing (HCT). Male and female adults aged (18-49 years) identified as HIV negative through HCT were screened for high risk of acquiring HIV. High risk was defined as a self-report of any of the following in the previous three months: sexually transmitted infections (STIs), unprotected sex with more than one or a new sexual partner, use of recreational drugs and/or at least weekly alcohol use, and absence from home for at least three consecutive nights per week. Eligible volunteers and at high risk were referred to the MRC/UVRI study clinic for enrolment and subsequent quarterly follow up visits. At the clinic, interviewer-administered case report forms were used to record locator details (physical location and phone contacts), demographics, risk behaviour characteristics, and medical assessments. Medical assessments and HCT were repeated every 3 months. HIV risk was assessed every 6 months and at annual visit, volunteers whose risk profile had changed to low risk were withdrawn from the cohort. Volunteers were reminded by phone call and followed by a home visit if they missed their clinic appointments. Cohort volunteers were considered as lost to follow up and withdrawn from the cohort if they failed to attend two sequential follow up visits. A lost to follow up volunteer was readmitted into the cohort if they came back to the study clinic and still fulfilled the eligibility criteria.
SiVET Procedures
When volunteers presented for their 3 month visits in the observational cohort, they were assessed for recruitment into SiVET. SiVET inclusion criteria included; having spent at least three months in the observational cohort, no contraindications for Hepatitis B vaccine and, if female, willingness to use contraception until 3 months after the last vaccination. Recruitment was stopped at accrual of the estimated sample size when 291 had been screened and 282 enrolled. Of the nine volunteers screened and not enrolled, three were pregnant, two refused to consent and four did not show up for enrolment (figure 1). Volunteers in SiVET continued with their procedures and schedules in the parent observational cohort. SiVET visits were synchronised with observational cohort visits. In addition to the parent observational cohort procedures and schedules SiVET volunteers were administered a licensed Hepatitis B vaccine (ENGERIX-B™ GlaxoSmithKline Biologicals Rixensart, Belgium,) following the standard schedule of 0, 1 and 6 months, akin to what might happen in an HIV vaccine trial. At each vaccination visit, volunteers were kept in the clinic for observation of reactogenicity events for at least 30 minutes after vaccination and asked to return to the clinic after 3 days for further review. Each volunteer was followed for 12 months aligned to the period SiVET was conducted.
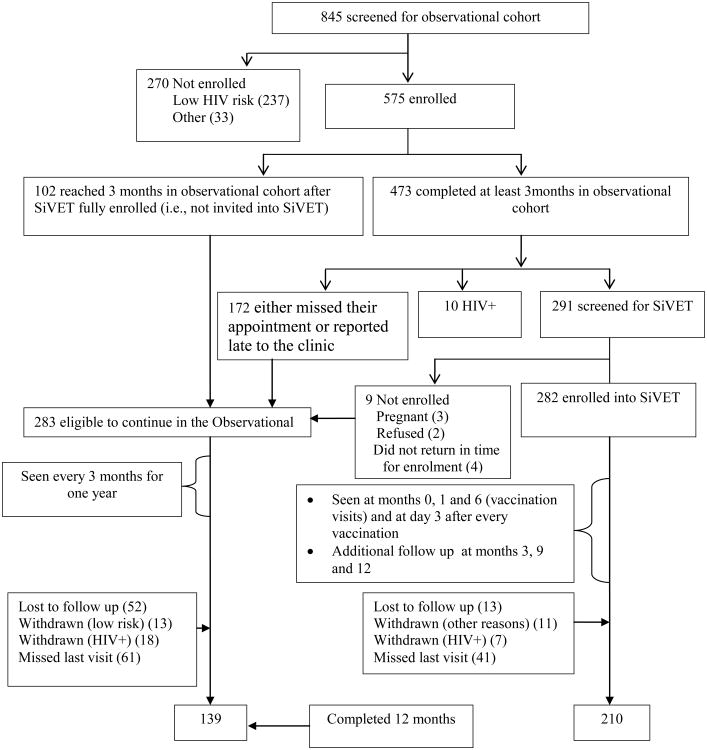
Figure 1.
Study profile of enrolment and follow up of 575 fisher folk enrolled in an Observational cohort and a Simulated Vaccine Efficacy Trial (SiVET) in South Western Uganda
Laboratory methods
Rapid HIV testing was performed by Determine (Alere, Medical Co., Ltd, Matsuhidai, Matsudo-shi, Chiba, Japan) and all positive specimens were confirmed by two ELISA tests (Vironostika HIV Uni-Form II plus 0 microelisa system, Biomerieux, Boxtel, The Netherlands and Murex HIV-1.2.0, Murex, Biotech Limited, Dartford, UK). A western blot was performed if ELISA results were discordant.
Statistical analysis
Data were analysed in Stata 12.0 (StataCorp, College Station, Texas, USA). Volunteer characteristics in the two studies were summarized using frequencies and percentages. Chi-square tests were used to compare demographic and clinical characteristics between the two studies at baseline. Person-years of observation (PYO) were calculated as the sum of the time from baseline to the date of the last HIV-uninfected result, or to the estimated date of HIV infection for each volunteer. Date of HIV infection was estimated as the mid-point of the interval between the last HIV-uninfected and the first HIV-infected result date. The HIV incidence was estimated as number of HIV infections divided by the person time at risk. Hazard ratios with 95% confidence interval (CI) to assess the association between factors and incidence were obtained using cox proportion hazard regression models. To estimate factors associated with HIV incidence, we carried out a bivariate analysis and all factors for which the association attained statistical significance (p< 0.15) on log likelihood test were included in the initial multivariable model. Sex and age were included a priori. Factors were retained in the multivariable model if the log likelihood test p-value of inclusion of a factor was less than 0.05
Ethical Considerations
Both studies were approved by the Uganda Virus Research Institute (UVRI) Research Ethics Committee and the Uganda National Council for Science and Technology. Written informed consent was sought from each volunteer, and a separate informed consent procedure was performed for each study. Volunteer confidentiality was maintained throughout the follow up period. Volunteers that acquired HIV were immediately referred for care to local HIV care service providers of their choice.
Results
Screening and enrolment
A total of 845 individuals were screened for eligibility into the observational cohort from January 2012 to February 2013; of whom, 575 (68.0%) were enrolled (Figure 1). The primary reason for exclusion was low self-reported HIV risk (n=237). After being followed for 3 months in the observational cohort, 172 either missed their appointment for SiVET enrollment or reported late to the clinic, and 10 had sero-converted. By the time SiVET enrollment was completed, an additional 102 volunteers had not yet made their 3 months visit in the observational cohort. Of 575 in the observational cohort, a total of 291 were consecutively screened and 282 enrolled in SiVET between July 2012 and February 2013. Nine volunteers were screened but not enrolled into SiVET because of pregnancy (3), refusal (2) and no show for enrolment (4) Figure 1. Except for the 282 volunteers that enrolled in SiVET and 10 that had sero-converted, the rest (283) remained in the observational cohort. Retention was 82.0% and 72.6%, p=0.02 at the 12 month visit in SIVET and the observational cohort respectively. The reasons for not attending visits were similar in both studies (Figure 1).
Investigating possible differences between volunteers that were screened for SiVET and those that were not, the sub analysis showed that volunteers in the observational cohort that were not screened for SiVET because of either missing or reporting late for their three month visit had higher proportion of females 49.2% vs 28.9%, p<0.01, more persons aged 18-24 years 43.0% vs 31.6%, p=0.04, had more recent immigrants (having less than one year) to the fishing community 29.1% vs 17.5%,p<0.01, had been absent from home for at least three consecutive nights per week 51.7% vs 39.3%, p=0.01 and engaged in other businesses (not fishing or fishing related) 51.5% vs 30.2%,p<0.01. There were no differences in terms of education, tribe, religion and marital status (supplementary table 1).
Baseline characteristics
Table 1 shows the socio-demographic characteristics of the volunteers enrolled in both studies. Although the observational cohort study was the source of enrolment for SiVET, some volunteer characteristics differed between the two studies. While there were equal proportions of men and women enrolled in the observational cohort, more men (72.7% vs 48.4%, p < 0.01) were enrolled in SiVET. Observational cohort included relatively younger volunteers (44.9% vs 31.2% aged under 24 years, p <0.01), those who had no formal education (12.4% vs 6.7%, p=0.02), a lower proportion directly engaged in fishing or fishing related business (47.7% vs 69.9%, p<0.01), had fewer number of years (recent immigrants) spent in the fishing community (34.0% vs 17.0%, p<0.01), more with genital discharge (24.3% vs 11.3%, p<0.01) and more with genital sores (27.6% vs 16.3%, p<0.01). Other characteristics including; religious faith, tribe, marital status, having sex while drunk, number of sexual partners, having a new sexual partner and condom use with a new partner were similar in the two studies.
Table1. Baseline socio-demographic characteristics of 575 volunteers enrolled from a fishing community into an observational cohort and a Simulated Vaccine Efficacy Trial (SiVET) in southwestern Uganda.
| Socio-demographic characteristics | Observational (n=283) n (%) | SiVET (=282) n (%) | P-value |
|---|---|---|---|
| Sex | |||
| Male | 137 (48.4) | 205 (72.7) | <0.01 |
| Female | 146 (51.6) | 77 (27.3) | |
| Age (years) | |||
| 18-24 | 127 (44.9) | 88 (31.2) | <0.01 |
| 25-34 | 115 (40.6) | 127 (45.0) | |
| 35-49 | 41 (14.5) | 67 (23.8) | |
| Tribe | 0.22 | ||
| Baganda | 114 (40.3) | 128 (45.4) | |
| Other | 169 (59.7) | 154 (54.6) | |
| Education | |||
| ≥Primary school | 248 (87.6) | 263 (93.3) | 0.02 |
| None | 35 (12.4) | 19 (6.7) | |
| Religion | |||
| Christian | 216 (76.3) | 217 (77.0) | 0.86 |
| Muslim | 67 (23.7) | 65 (23.0) | |
| Marital status | |||
| Single | 86 (30.4) | 84 (29.8) | 0.15 |
| Married | 125 (44.2) | 143 (50.7) | |
| Separated/widowed | 72 (25.4) | 55 (19.5) | |
| Occupation | |||
| Fishing and fishing related business | 135 (47.7) | 197 (69.9) | <0.01 |
| Other | 148 (52.3) | 82 (29.1) | |
| Residence in fishing community (years) | |||
| 0-1 | 96 (34.0) | 48 (17.0) | <0.01 |
| >1-5 | 132 (46.6) | 121 (42.9) | |
| >5 | 55 (19.4) | 113 (40.1) | |
| Sex while drunk | |||
| Never | 144 (68.6) | 198 (70.2) | 0.72 |
| Always | 66 (31.4) | 84 (29.8) | |
| Genital discharge | |||
| Yes | 51 (24.3) | 32 (11.3) | <0.01 |
| No | 159 (75.7) | 250 (88.7) | |
| Genital sores | |||
| Yes | 58 (27.6) | 46 (16.3) | <0.01 |
| No | 152 (72.4) | 236 (83.7) | |
| Number of partners | |||
| 0-1 | 150 (71.4) | 180 (63.8) | 0.10 |
| 2+ | 60 (28.6) | 102 (36.2) | |
| New partner | |||
| No | 145 (69.0) | 193 (68.4) | 0.90 |
| Yes | 65 (31.0) | 89 (31.6) | |
| Condom use with new partner | |||
| Never | 33 (50.8) | 35 (39.3) | 0.19 |
| Always | 32 (49.2) | 54 (60.7) |
Risky behaviour and clinical characteristics
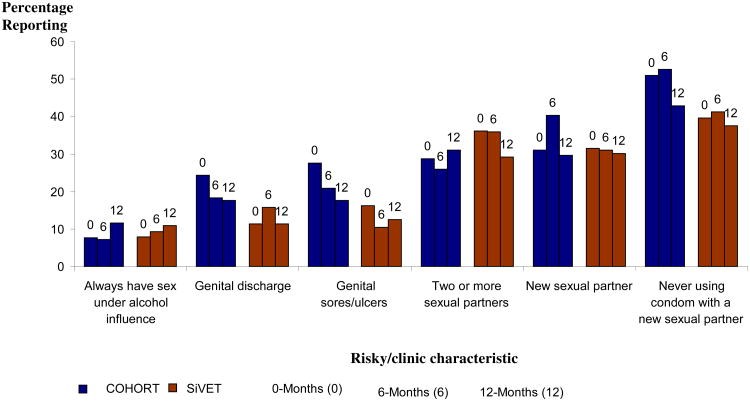
The behavioural and clinical characteristics at baseline, 6 and 12 months of follow-up in both studies are shown in Figure 2. Between 0 and 12 months, there was minimal reduction (<10%) of risky behaviour or self-reported STIs, within each study and this was not statistically significantly different.
Figure 2.
Report of risky behaviour and clinical characteristics of HIV high risk fisher folk enrolled in an Observational cohort and SiVET in south western Uganda
HIV Incidence
During the initial period of at least 3 months of the observational cohort, prior to enrolment of SiVET there were 10 sero-converters over 203.3 PYO of follow-up giving incidence rate (IR) of 4.9 cases per 100 PYO (95% CI: 2.6-9.1). During the subsequent 12 months of follow up in the observational cohort, 20 sero-conversions occurred during 175.5 PYO of follow up [IR= 11.4 cases per 100 PYO (95% CI: 7.4-17.7)] while in SiVET 10 sero-conversions in 264.2 PYO were observed [IR =3.8 cases per 100 PYO (95% CI: 2.0 - 7.0)]. Incidence rate ratio (IRR) comparing observational cohort to SiVET was 3.0 (95% CI: 1.3-7.2, p<0.01). In both studies, women had a higher HIV incidence point estimate than men, though this did not achieve statistical significance: 14.9 per 100 PYO (95% CI: 8.6-25.6) compared to 7.9 per 100 PYO (95% CI: 3.8-16.6) in observational cohort (p=0.09), and 4.2 per 100 PYO (95% CI: 1.4-13.2) versus 3.6 per 100 PYO (95% CI: 1.7-7.6) in SiVET (p=0.18). The HIV incidence among women in the observational cohort was 3 times higher than that among women in SIVET (14.9 per 100 PYO vs 4.2 per 100 PYO, p=0.04); The IR among men in the observational cohort was two times that of men in SIVET (7.9 per 100 PYO vs 3.6 per 100 PYO p=0.05).
Factors associated with HIV incidence
Factors that remained independently associated with increased risk of HIV acquisition in multivariable analysis in the observational cohort included lack of education and shorter length of stay (new immigrants) in the fishing village. In SiVET, only shorter length of stay (new immigrants) in the fishing villages was independently associated with HIV acquisition (table 2).
Table 2. HIV incidence, unadjusted and adjusted factors associated with HIV incidence among 575 people living in the fishing community enrolled in an Observational and SiVET cohorts in South Western Uganda.
| Observational cohort | Simulated Vaccine Efficacy Trial | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Characteristics | Incidence per 100PY | uHR | aHR | Incidence per 100PY | uHR | aHR | |
| Overall | 11.4 | 3.8 | |||||
| Sex | |||||||
| Male | 7.9 | 1 | 1 | 3.1 | 1 | 1 | |
| Female | 14.9 | 1.7 (0.7-4.4) | 1.9 (0.9-4.0) | 5.7 | 1.8 (0.5-6.4) | 1.4 (0.4-5.5) | |
| Age (years) | |||||||
| 18-24 | 9.5 | 1 | 1 | 3.6 | 1 | 1 | |
| 25-34 | 10.5 | 0.9 (0.3-2.8) | 1.5 (0.6-3.7) | 3.4 | 0.9 (0.2-4.1) | 1.3 (0.3-5.9) | |
| 35-48 | 19.0 | 2.0 (0.6-6.2) | 2.5 (0.9-6.5) | 4.9 | 1.3 (0.3-6.7) | 1.9 (0.4-9.9) | |
| Tribe* | |||||||
| Baganda | 13.6 | 1 | 0.8 | 1 | 1 | ||
| Other | 10.1 | 0.7 (0.3-1.7) | 6.3 | 7.5 (1.0-58.8) | 6.6 (0.8 -52.8) | ||
| Education¥ | |||||||
| ≥Primary school | 9.4 | 1 | 1 | 3.8 | 1 | ||
| None | 33.1 | 3.9 (1.4-10.5) | 3.1 (1.3-7.7)* | 5.3 | 1.5 (0.2-11.7) | ||
| Religion | |||||||
| Christian | 11.2 | 1 | 2.4 | 1 | |||
| Muslim | 11.9 | 0.9 (0.3-2.5) | 8.5 | 3.4 (0.9-11.9) | |||
| Marital status | |||||||
| Single | 6.1 | 1 | 5.0 | 1 | |||
| Married | 9.9 | 1.4 (0.4-5.5) | 1.5 | 0.3 (0.1-1.6) | |||
| Separated | 19.5 | 3.2 (0.9-11.6) | 7.8 | 1.5 (0.4-5.9) | |||
| Occupation | |||||||
| Fishing and related business | 10.7 | 1 | 2.6 | 1 | |||
| Other | 12.0 | 1.3 (0.5-3.1) | 6.7 | 2.6 (0.7-9.0) | |||
| Residence in fishing community (years) ¥ | |||||||
| 0-1 | 18.4 | 1 | 1 | 9.4 | 1 | 1 | |
| >1-5 | 8.2 | 0.5 (0.2-1.2) | 0.5 (0.2-1.2)* | 5.3 | 0.6 (0.2-2.0) | 0.5 (0.1-1.9) | |
| >5 | 9.7 | 0.4 (0.1-1.5) | 0.3 (0.1-0.8)* | 0 | - | - | |
| Sex while drunk | |||||||
| Never | 9.7 | 1 | 3.4 | 1 | |||
| Always | 20.6 | 2.2 (0.6-7.6) | 5.0 | 1.4 (0.2-11.2) | |||
| Genital discharge | |||||||
| Yes | 12.5 | 1 | 3.4 | 1 | |||
| No | 10.1 | 0.8 (0.3-2.3) | 3.5 | 1.0 (0.1-8.4) | |||
| Genital sores | |||||||
| Yes | 14.4 | 1 | 7.0 | 1 | |||
| No | 9.4 | 0.7 (0.2-1.8) | 2.8 | 0.4 (0.1-1.8) | |||
| Number of partners | |||||||
| 0-1 | 10.9 | 1 | 3.6 | 1 | |||
| 2+ | 10.3 | 0.9 (0.3-2.7) | 3.3 | 0.9 (0.2-3.8) | |||
| New partner | |||||||
| No | 10.8 | 1 | 3.8 | 1 | |||
| Yes | 12.7 | 1.2 (0.4-3.2) | 2.8 | 0.8 (0.2-4.0) | |||
| Condom use with new partner | |||||||
| Never | 13.6 | 1 | 3.2 | 1 | |||
| Always | 11.9 | 0.8 (0.2-3.9) | 4.3 | 1.4 (0.1-15.0) | |||
uHR unadjusted Hazard ratio, p-likelihood p-value, aHR-adjusted Hazard ratio,
unadjusted p<0.05
Both unadjusted and adjusted p <0.05
Hypothetical sample size estimates for observational cohort and SiVET and effect on study power
To understand the effect of the difference in HIV incidence between observational cohort and SiVET in the context of future vaccine efficacy trials in this community, we did hypothetical power calculations as below. We assumed that an efficacy trial needs to demonstrate a 50% reduction in HIV incidence with 80% power and a two-sided alpha=0.05. Based on incidence from the observational cohort (11.4 per 100 PYO), a sample size of 1,274 (637 in each trial arm) would be required. If we used the SiVET HIV incidence of 3.8 per 100 PYO, we would require a sample size of 4,140 (2070 in each trial arm) which would ultimately decrease the study power of the estimates drawn from the observational cohort to about 30%.
Discussion
We found a threefold greater HIV incidence in the observational cohort than SiVET study. In this comparative analysis, volunteers were drawn from the same population and were followed under similar conditions, although the SiVET had more study visits including vaccination and post vaccination visits. The reported risk behaviours in the two studies were similar, highlighting the potential challenges of relying on self-report for risk data [21]. We did observe that self-reported genital discharge and sores were higher among observational cohort volunteer than SiVET at baseline; GU discharge and sores were not associated with HIV incidence (though our study was not powered to test this association). The large difference in HIV incidence may be in part due to the differences in the volunteer demographic profile at recruitment. SiVET study recruited fewer women, more individuals with formal education and lower proportion of recent immigrants. We also observed that, apart from volunteer sex, which was borderline associated, these factors were independently associated with lower HIV incidence and thus may have contributed to the lower SiVET HIV incidence. Kiwanuka et al, [3] & Seeley et al, [11] in these same communities and communities further north of lake Victoria near Entebbe, have previously shown that recent immigration to these fishing communities is associated with high HIV incidence. The HIV incidence in these studies was 3.4 per 100 Person years at risk (pyar) [3] and 4.9 per 100 pyar [11] respectively. However, these studies did not find association with sex and did not assess the effect of education.
Generally, fishing communities have the highest HIV prevalence and incidence [6, 7, 9, 11] compared to inland agricultural communities [22] in this region. They have retention comparable to stable inland agricultural communities [3, 23]. The fishing communities are characterized by high mobility, multiple sexual partnerships arising from exchange of sex for cash and transactional sex, very fluid marital relationships, high alcohol consumption and a low HIV risk perception due to more imminent dangers such as drowning that are perceived to as the main risk of death [3]. All these factors mediate the HIV risk and raise their risk profile.
The HIV incidence of 3.8 per 100 Person years observed in SiVET is similar to that observed in other microbicide prevention studies in the area (ranging between 3 to 4.5 per 100 person years) [13, 14]. For one microbicide study a preceding feasibility study had observed an HIV incidence of 12.6 per 100 person years [12] but assumed an incidence of 4 per 100 person years similar to our assumptions of risk reduction. The lower incidence observed during the microbicide trial was as anticipated at the design and in agreement with our findings. However our findings do not seem to suggest this difference resulted from risk reduction but rather from the selection criteria at recruitment.
The implication of this finding is that enrolment of volunteers from fishing communities into trials may exclude certain residents through self-selection or other means which may in turn affect the incidence in the trial. In our study, it seems that selection into the study played a greater role as the demographic profile of SiVET study volunteers suggested a lower risk group. Because of consecutive enrolment of the most available individuals into SiVET, volunteers who were likely to enrol were those who were more likely to be on time for the SiVET enrolment visit. They were less likely to miss visits or get lost to follow up. Those that did not enrol were likely to be frequent travellers, younger individuals, women, new immigrants and those engaged in non-fishing related occupation. These have been shown to be associated with high HIV incidence in the previous cohorts [3, 11] and/or in this comparative analysis. Community-based observational studies from which HIV incidence estimates are derived should be considered with this in mind to best avoid risk of low statistical power. Careful consideration of recruitment strategies, source populations, and community outreach and engagement is important to ensure that previous HIV incidence estimates are appropriate for sample size determination. Failure to achieve target parameters used to design a trial could lead to inconclusive results or termination of the trial as observed in previous HIV prevention trials [15, 16].
In a meta-analysis of trials that were terminated or had inconclusive or negative results due to loss of statistical power, risky behaviour reduction and other exceptional HIV prevention procedures performed in the control arm were advanced as reasons for lower incidence of outcome measures [18]. In contrast, our study did not show differences in reduction of reported risky behaviours between the observational cohort and SiVET. In our study, it seems procedures used to select volunteers enrolled into SiVET played a greater role in creating volunteer differences between the two studies. SiVET volunteers were also seen more frequently, including a one-month vaccination visit, and a visit three days following each of the three vaccination visits (enrolment, month 1 and month 6). At the additional non-vaccination visits, volunteers were reassessed for any adverse events. However, these additional visits seem not to have altered the risk profile of the volunteers in the SiVET study.
There are some limitations of our comparative analysis. Volunteers in the SiVET study were recruited from the observational cohort after 3 months of participation in this cohort (i.e., at the cohort's first follow up visit). Similar approach was followed (including data from the month three visit) while analysing the observational cohort data. Although in an actual efficacy trial volunteer enrolment may be delayed after screening, it is unlikely to be up to 3 months. It is unlikely that nearly half of the available population will be enrolled into a given trial like it was the case in this observational cohort where half went into the trial. Volunteers were not randomised to joining SiVET or remaining in the observational cohort. Randomisation could have helped eliminate the imbalance in the demographic profile. However, it could have impaired our ability to rapidly complete SiVET enrolment. We also dropped volunteers from the observational cohort who reported low risk behaviour, and did not do this as part of the SiVET. This may have served to “enrich” the observational cohort for volunteers at greater risk of acquiring HIV, however only 13 (<5% of the cohort) low risk volunteers were dropped from follow up. Additionally, our analysis was not well powered for the multivariable analysis, particularly among the SiVET volunteers, because of the small number of HIV sero-converters in this study. Thus, while we may have observed differences in point estimates, some large differences failed to achieve statistical significance (e.g., HIV incidence across volunteer sex). However, this is a rare example of a simulated trial being conducted alongside an observational cohort drawn from the same source population under study procedures conducted by the same study staff, and it is important to draw what lessons we can.
We have demonstrated that HIV incidence derived from observational cohorts must be considered carefully when estimating the sample size for an efficacy trial to avoid under powering the trial. In our hypothetical example, if you used incidence from the observational cohort to estimate the trial study size, you would have achieved only about 30% power. We suggest two approaches to achieving sufficient power for trials conducted in this community; (i) if you used historical cohorts' data to estimate a study sample size, you would only be off by about 50% or less. Therefore, adjusting the study size to take into consideration the predicted lower incidence for the trial could be a useful strategy. (ii) Using recruitment strategies that focus on known predictors of HIV acquisition, such as recruiting and retaining recent immigrants and volunteers with low education. Another lesson learnt is that the risk assessment questionnaire answers are not reliable.
Supplementary Material
Supplementary Table 1: Comparison of characteristics of 291 volunteers screened for SiVET and 172 that were not screened because of no or late show
Acknowledgments
The Observational cohort is fully funded by IAVI. The SiVET work was partially funded by the Global HIV vaccine Enterprise (grant number 18) as a pilot grant awarded to GA as part of OCTAVE grant training workshop. The SiVET work was also co-funded by IAVI with the generous support of USAID and other donors; a full list of IAVI donors is available at www.iavi.org. The contents of this manuscript are the responsibility of IAVI and co-authors and do not necessarily reflect the views of USAID or the US Government. We wish to acknowledge the support from the University of California, San Francisco's International Traineeships in AIDS Prevention Studies (ITAPS), U.S. NIMH, R25 MH064712. As part of the ITAPS, we thank the helpful reviews of Dr Christina Lindan Krysia, Professor Rhoderick Machekano and peer reviews of Dr Lauren Wong, Zheng Wenjing and Dr Gloria Omosa-Manyonyi. We recognize the support of Dr Jeffrey Mandel, Dr Debbie Brickley and Ritu Sehgal while undertaking a scientific manuscript writing sabbatical at University of San Francisco California.
Footnotes
Competing interests: The authors declare that they have no competing interests
Author contributions: AA: Lead Author, drafted initial draft, carried out data management for both studies, statistical analysis and interpreted the data. GA: designed the SiVET protocol, contributed to study coordination, data analysis and interpretation. MAP: Contributed to the design of both studies, interpreted the data. ER: Contributed to the design of both studies and coordination. FK: contributed to the study coordination, data collection and cleaning. UB: contributed to data collection and cleaning. PEF: Contributed to the design of both studies, interpretation and directed the work. AK contributed to the design of both studies, interpretation and directed the work. All authors critically commented and provided revisions to the manuscript. The authors have approved this final version for submission.
Contributor Information
Gershim Asiki, Email: gershim@gmail.com.
Matthew A. Price, Email: MPrice@iavi.org.
Eugene Ruzagira, Email: Eugene.Ruzagira@mrcuganda.org.
Freddie Kibengo, Email: Freddie.Kibengo@mrcuganda.org.
Ubaldo Bahemuka, Email: Ubaldo.Bahemuka@mrcuganda.org.
Patricia E Fast, Email: PFast@iavi.org.
Anatoli Kamali, Email: Anatoli.Kamali@mrcuganda.org.
References
- 1.Development ICoAa. Understanding the research process for new HIV prevention technologies. 2010 [Google Scholar]
- 2.Kamali A, Price MA, Lakhi S, Karita E, Inambao M, Sanders EJ, Anzala O, Latka MH, Bekker LG, Kaleebu P, et al. Creating an African HIV Clinical Research and Prevention Trials Network: HIV Prevalence, Incidence and Transmission. PLoS One. 2015;10(1):e0116100. doi: 10.1371/journal.pone.0116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiwanuka N, Mpendo J, Nalutaaya A, Wambuzi M, Nanvubya A, Kitandwe PK, Muyanja E, Ssempiira J, Balyegisawa A, Ssetaala A. An assessment of fishing communities around Lake Victoria, Uganda, as potential populations for future HIV vaccine efficacy studies: an observational cohort study. BMC public health. 2014;14(1):986. doi: 10.1186/1471-2458-14-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asiki G, Abaasa A, Ruzagira E, Kibengo F, Bahemuka U, Mulondo J, Seeley J, Bekker LG, Delany S, Kaleebu P, et al. Willingness to participate in HIV vaccine efficacy trials among high risk men and women from fishing communities along Lake Victoria in Uganda. Vaccine. 2013;31(44):5055–5061. doi: 10.1016/j.vaccine.2013.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruzagira E, Wandiembe S, Bufumbo L, Levin J, Price MA, Grosskurth H, Kamali A. Willingness to participate in preventive HIV vaccine trials in a community-based cohort in south western Uganda. Tropical Medicine & International Health. 2009;14(2):196–203. doi: 10.1111/j.1365-3156.2008.02200.x. [DOI] [PubMed] [Google Scholar]
- 6.Opio A, Muyonga M, Mulumba N. HIV Infection in Fishing Communities of Lake Victoria Basin of Uganda—A Cross-Sectional Sero-Behavioral Survey. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0070770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ondondo RO, Waithera Ng‘ang’a Z, Mpoke S, Kiptoo M, Bukusi EA. Prevalence and Incidence of HIV Infection among Fishermen along Lake Victoria Beaches in Kisumu County, Kenya. World Journal of AIDS. 2014;4(02):219. [Google Scholar]
- 8.Smith AD, Tapsoba P, Peshu N, Sanders EJ, Jaffe HW. Men who have sex with men and HIV/AIDS in sub-Saharan Africa. The Lancet. 2009;374(9687):416–422. doi: 10.1016/S0140-6736(09)61118-1. [DOI] [PubMed] [Google Scholar]
- 9.Asiki G, Mpendo J, Abaasa A, Agaba C, Nanvubya A, Nielsen L, Seeley J, Kaleebu P, Grosskurth H, Kamali A. HIV and syphilis prevalence and associated risk factors among fishing communities of Lake Victoria, Uganda. Sexually transmitted infections. 2011;87(6):511–515. doi: 10.1136/sti.2010.046805. [DOI] [PubMed] [Google Scholar]
- 10.Vandepitte J, Bukenya J, Weiss HA, Nakubulwa S, Francis SC, Hughes P, Hayes R, Grosskurth H. HIV and other sexually transmitted infections in a cohort of women involved in high risk sexual behaviour in Kampala, Uganda. Sexually Transmitted Diseases. 2011;38(4):316. [PMC free article] [PubMed] [Google Scholar]
- 11.Seeley J, Nakiyingi-Miiro J, Kamali A, Mpendo J, Asiki G, Abaasa A, De Bont J, Nielsen L, Kaleebu P. High HIV incidence and socio-behavioral risk patterns in fishing communities on the shores of Lake Victoria, Uganda. Sex Transm Dis. 2012;39(6):433–439. doi: 10.1097/OLQ.0b013e318251555d. [DOI] [PubMed] [Google Scholar]
- 12.Nunn A, McCormack S, Crook AM, Pool R, Rutterford C, Hayes R. Microbicides Development Programme: design of a phase III trial to measure the efficacy of the vaginal microbicide PRO 2000/5 for HIV prevention. Trials. 2009;10(1):99. doi: 10.1186/1745-6215-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCormack S, Ramjee G, Kamali A, Rees H, Crook AM, Gafos M, Jentsch U, Pool R, Chisembele M, Kapiga S. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. The Lancet. 2010;376(9749):1329–1337. doi: 10.1016/S0140-6736(10)61086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Damme L, Govinden R, Mirembe FM, Guédou F, Solomon S, Becker ML, Pradeep B, Krishnan A, Alary M, Pande B. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. New England Journal of Medicine. 2008;359(5):463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 15.Feldblum PJ, Adeiga A, Bakare R, Wevill S, Lendvay A, Obadaki F, Olayemi MO, Wang L, Nanda K, Rountree W. SAVVY vaginal gel (C31G) for prevention of HIV infection: a randomized controlled trial in Nigeria. PLoS One. 2008;3(1):0001474. doi: 10.1371/journal.pone.0001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson L, Nanda K, Opoku BK, Ampofo WK, Owusu-Amoako M, Boakye AY, Rountree W, Troxler A, Dominik R, Roddy R, et al. SAVVY (C31G) gel for prevention of HIV infection in women: a Phase 3, double-blind, randomized, placebo-controlled trial in Ghana. PLoS One. 2007;2(12):e1312. doi: 10.1371/journal.pone.0001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, et al. Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women. New England Journal of Medicine. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCoy S, Padian N, Balkus JE. Weighing the gold in the gold standard: challenges in HIV prevention research. 2010 doi: 10.1097/QAD.0b013e328337798a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halpern SD, Karlawish JT, Berlin JA. THe continuing unethical conduct of underpowered clinical trials. JAMA. 2002;288(3):358–362. doi: 10.1001/jama.288.3.358. [DOI] [PubMed] [Google Scholar]
- 20.Janosky JE. THe ethics of underpowered clinical trials. JAMA. 2002;288(17):2118–2119. [PubMed] [Google Scholar]
- 21.Schroder KEE, Carey MP, Vanable PA. Methodological Challenges in Research on Sexual Risk Behavior: II. Accuracy of Self-Reports. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2003;26(2):104–123. doi: 10.1207/s15324796abm2602_03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruzagira E, Wandiembe S, Abaasa A, Levin J, Bwanika A, Bahemuka U, Price MA, Kamali A. Prevalence and Incidence of HIV in a Rural Community-Based HIV Vaccine Preparedness Cohort in Masaka, Uganda. PLoS One. 2011;6(6):e20684. doi: 10.1371/journal.pone.0020684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abaasa A, Asiki G, Mpendo J, Levin J, Seeley J, Nielsen L, Ssetaala A, Nanvubya A, De Bont J, Kaleebu P, et al. Factors associated with dropout in a long term observational cohort of fishing communities around lake Victoria, Uganda. BMC Res Notes. 2015;8(1):015–1804. doi: 10.1186/s13104-015-1804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Comparison of characteristics of 291 volunteers screened for SiVET and 172 that were not screened because of no or late show