Abstract
Purpose
Bevacizumab confers benefits in metastatic breast cancer but may be more effective as adjuvant therapy. We evaluated the cardiac safety of bevacizumab plus dose-dense doxorubicin–cyclophosphamide (ddAC)→nanoparticle albumin−bound (nab)-paclitaxel in human epidermal growth factor receptor 2 normal early-stage breast cancer.
Experimental Design
Eighty patients with normal left ventricular ejection fraction (LVEF) were enrolled. Bevacizumab was administered for 1 year, concurrently with ddAC→nab-paclitaxel then as a single agent. LVEF was evaluated at months 0, 2, 6, 9, and 18. This regimen was considered safe if fewer than three cardiac events or fewer than two deaths from left ventricular dysfunction occurred. Correlative studies of cardiac troponin (cTn) and plasma renin activity (PRA) were conducted.
Results
The median age was 48 years (range, 27−75 years), and baseline LVEF was 68% (53%−82%). After 39 months’ median follow-up (5−45 months): median LVEF was 68% (53%−80%) at 2 months (n=78), 64% (51%−77%) at 6 months (n=66), 63% (48%−77%) at 9 months (n=61), and 66% (42%−76%) at 18 months (n=54). One patient developed symptomatic LV dysfunction at month 15. Common toxicities necessitating treatment discontinuation were hypertension (HTN, 4%), wound-healing complications (4%), and asymptomatic LVEF declines (4%). Neither cTn nor PRA predicted CHF or HTN, respectively.
Conclusions
Bevacizumab with ddAC→nab-paclitaxel had a low rate of cardiac events; cTn and PRA levels are not predictive of CHF or HTN, respectively. The efficacy of bevacizumab as adjuvant treatment will be established in several ongoing phase III trials.
Keywords: breast cancer, adjuvant, bevacizumab, safety, biomarkers
INTRODUCTION
Adjuvant chemotherapy improves outcomes for patients with early-stage breast cancer. After anthracyclines proved beneficial, taxanes demonstrated further advantages (1–5). Sequential regimens and dose-dense administration further increased the benefits of conventional strategies (6–9). Most recently, targeted therapy with trastuzumab (Herceptin®, Genentech Inc, South San Francisco, CA) improved survival when added to chemotherapy in patients with human epidermal growth factor receptor 2 (HER2)–positive early-stage breast cancer, and newer HER2-targeted agents are in development (10, 11). The benefits of targeted therapy have been more modest in patients with HER2-normal breast cancers. Antiangiogenic agents, including bevacizumab (Avastin®, Genentech Inc, South San Francisco, CA), a humanized monoclonal antibody against vascular endothelial growth factor, are active in metastatic disease with 3 large phase III studies of first-line anthracycline and taxane or capecitabine chemotherapy with or without bevacizumab demonstrating consistent progression-free survival benefit (12–14).
A particular advantage for antiangiogenic therapy may be that it can target tumor dormancy. Quiescent tumor cells that seed distant sites may proliferate in part because of changes in their surrounding environment, and neovascularization is thought to be critical (15, 16). Therefore, antiangiogenic agents like bevacizumab may have their greatest impact in the adjuvant setting when being used to treat micrometastatic disease.
Cardiac safety remains a concern when administering bevacizumab to patients with curable disease. Small studies with various chemotherapy agents have raised unresolved questions in this regard, particularly when potentially cardiotoxic anthracycline-based regimens are co-administered (12−14, 17−21).
Further complicating the issue of cardiac safety is the lack of accurate predictors of the well-described clinically relevant toxicities of bevacizumab, such as hypertension (HTN) and possibly congestive heart failure (CHF) when bevacizumab is co-administered with an anthracycline. For example, although HTN develops in 20% to 30% of bevacizumab-treated patients (12, 17, 22), the mechanism has not been clearly elucidated and no accurate clinical predictors have been identified. However, because renin plays a central role in maintaining blood pressure through the renin-angiotensin-aldosterone system, an approach to HTN classification and management based on plasma renin activity (PRA) has been proposed (23, 24). Consequently, serial PRA measurements may identify patients at risk for bevacizumab-mediated HTN. Similarly, cardiac troponins (cTns) are myocardial contractile proteins that are released into the circulation after cardiac injury and are a hallmark of future cardiac events and mortality (25, 26). As highly specific biomarkers of myocardial damage, the assessments of cTns may augment existing cardiac monitoring strategies—whereby serial left ventricular ejection fraction (LVEF) changes are monitored—by providing earlier and more reliable evidence of myocyte damage (27). We hypothesize that a rise in PRA may predict for bevacizumab-mediated hypertension and that cTn elevations may predict subsequent LVEF declines.
Dose-dense chemotherapy was developed with conventional paclitaxel (8). Nanoparticle albumin−bound (nab)-paclitaxel (Abraxane®, Abraxis Oncology, Bridgewater, NJ) may offer toxicity advantages because it is a formulation free of polyoxyethylated castor oil (Cremophor EL; BASF, Ludwigshafen, Germany) and is not associated with the rare but severe hypersensitivity reactions reported with the conventional solvent used with paclitaxel (28). In addition, the administration of nab-paclitaxel every second week may offer an efficacy advantage over administration every third week (29). Previous studies demonstrated the safety of substituting nab-paclitaxel for conventional paclitaxel in the dose-dense adjuvant regimen (30, 31). To inform subsequent and ongoing clinical trials, we built on these observations by testing the cardiac safety and tolerability of bevacizumab added to adjuvant dose-dense doxorubicin–cyclophosphamide followed by nab-paclitaxel and incorporated studies of potential predictors of HTN and CHF.
METHODS
Study and Biostatistical Design
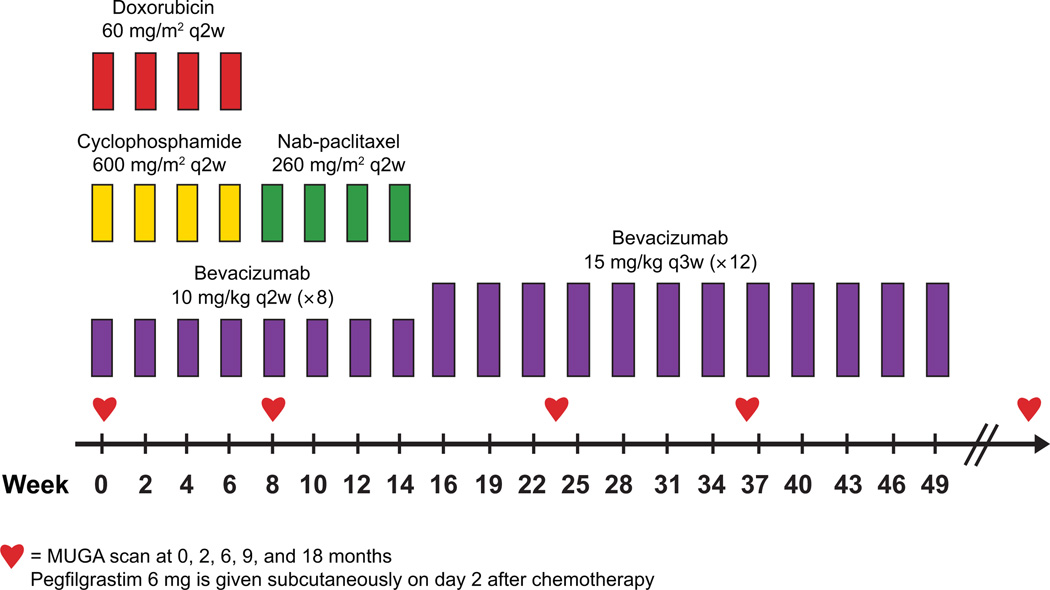
The primary objective was to determine the cardiac safety of adjuvant bevacizumab administered for 1 year (concurrently with dose-dense doxorubicin–cyclophosphamide [ddAC]→nab-paclitaxel and then as monotherapy) as determined by the incidence of cardiac events (Fig. 1). A cardiac event was defined as cardiac death from left ventricular (LV) dysfunction or symptomatic congestive heart failure (CHF, dyspnea with normal activity or at rest and an LVEF of <50%). The secondary end points were non-cardiotoxicity, disease-free survival, and overall survival.
Figure 1.
Treatment schema. Abbreviations: q2w, every 2 weeks; q3w, every 3 weeks.
On the basis of prior studies of targeted therapies such as trastuzumab and bevacizumab with anthracyclines (10, 19, 20), a 15% patient dropout rate due to significant asymptomatic declines in LVEF was anticipated during chemotherapy with co-administered bevacizumab. To study 64 evaluable patients, with “evaluable patients” defined as completing ddAC with bevacizumab and able to continue bevacizumab based on the month 2 multiple-gated acquisition (MUGA) scan or echocardiogram (ECHO), we planned to enroll 75 patients. The trial was to be terminated if we observed three or more (≥ 4.7%) cardiac events among the evaluable patients: three symptomatic CHF events or one cardiac death from LV dysfunction and two symptomatic CHF events. If more than one cardiac death from LV dysfunction was observed, the trial would be terminated. The probability of declaring the regimen safe for a range of true cardiac event rates is shown in Supplemental Table 1 (32). For example, the probability of the trial being stopped if the true cardiac event rate is 8% is 90%.
Because of rapid accrual of patients into the screening phase toward the end of the trial, 80 patients were enrolled. All patients provided informed consent. This study was reviewed and approved by the institutional review boards at both participating institutions (Memorial Sloan-Kettering Cancer Center [MSKCC] and University of California San Francisco Comprehensive Cancer Center [UCSF]).
Patients
Eligible patients were ≥18 years with pathologically confirmed HER2-negative early-stage invasive breast cancer; had completed all planned surgery ≥28 days prior to the start of therapy; had an Eastern Cooperative Oncology Group (ECOG) performance status ≤1; and had adequate hepatic, renal, and hematologic function. Baseline LVEF was within institutional normal limits as measured by MUGA scan or ECHO. Patients were permitted to have received prior therapy for an ipsilateral or contralateral breast cancer but were excluded if they had received a taxane within the preceding year or previously received an anthracycline. Patients with baseline proteinuria (urine protein creatinine ratio [UPC] >1), significant bleeding within 6 months of study entry, baseline blood pressure of >150/100 mm Hg, unstable angina, CHF greater than New York Heart Association (NYHA) class II , myocardial infarction or stroke within 12 months, clinically significant peripheral vascular disease, prior antiangiogenesis therapy, or active full-dose anticoagulation were excluded.
Treatment
Treatment consisted of intravenous ddAC (60/600 mg/m2) every 2 weeks for four cycles followed by intravenous nab-paclitaxel (260 mg/m2) every 2 weeks for four cycles, with pegfilgrastim (6 mg subcutaneously) on day 2 (see Fig. 1). Bevacizumab was administered for a total of 52 weeks (20 doses): eight doses at 10 mg/kg intravenously every 2 weeks concurrent with chemotherapy and 12 doses administered at 15 mg/kg every 3 weeks thereafter. Standard premedications for ddAC and nab-paclitaxel were administered. Patients with estrogen receptor− or progesterone receptor–positive tumors received tamoxifen or aromatase inhibitors, as appropriate, and radiotherapy was recommended per institutional guidelines.
Parameters for holding or discontinuing therapy
Doxorubicin and cyclophosphamide (AC) with nab-paclitaxel
Patients experiencing two episodes of neutropenic fever and/or grade 3 or 4 nonhematologic toxicity had subsequent doses reduced by 20%. A maximum of two dose reductions was permitted. If the platelet count was <100,000/µL, and/or the absolute neutrophil count (ANC) <1,000/µL, and/or nonhematologic toxicities (excluding alopecia) had not recovered to grade 1 or lower on the day that chemotherapy was to be administered, treatment was delayed by 1 week. Complete blood count testing and toxicity grading were repeated weekly. If the platelet count, ANC, and nonhematologic toxicity did not recover, a further delay of up to 1 week was required. Patients were removed from study if treatment delays of >2 consecutive weeks were required. AC was discontinued with symptomatic, confirmed congestive heart failure or myocardial infarction. If grade 3 or greater toxicity recurred after dose adjustments and/or delays, AC was discontinued, and the patient was permitted to proceed with nab-paclitaxel at the discretion of the treating physician.
Bevacizumab
Parameters were set for holding bevacizumab for patients with asymptomatic left ventricular ejection fraction (LVEF) declines (Table 1). Bevacizumab was to be permanently discontinued when two consecutive or three intermittent “holds” occurred. If LVEF was maintained at a “continue” category or improved from a “hold” to a “continue” category, additional multigated acquisition (MUGA) scans (or echocardiograms) could be ordered before the next scheduled MUGA scan at the investigator’s discretion. Bevacizumab administration was not impacted by dose reductions of AC and/or nab-paclitaxel. However, if chemotherapy was delayed, bevacizumab was also delayed to maintain concurrent administration. Chemotherapy was continued alone if bevacizumab was held or discontinued before chemotherapy completion. If chemotherapy was permanently discontinued because of toxicity, patients were permitted to complete therapy with bevacizumab alone. There were no bevacizumab dose modifications for toxicity. The urine protein:creatinine (UPC) ratio was calculated at baseline and prior to every other bevacizumab administration. Any patient developing a UPC ratio >3.5 had bevacizumab held until the UPC recovered to <3.5. Blood pressure was measured at baseline, every 2 weeks during chemotherapy, and every 6 weeks during bevacizumab alone. Bevacizumab was discontinued in patients with grade 4 hypertension, poorly controlled hypertension despite oral medication, or proteinuria requiring a “hold” on bevacizumab administration for >8 weeks. Bevacizumab was administered only if the systolic blood pressure was <150 mm Hg and the diastolic blood pressure was <100 mm Hg. Bevacizumab was discontinued with symptomatic, confirmed congestive heart failure or myocardial infarction. A 4-week maintenance bevacizumab hold was recommended prior to and following delayed breast reconstruction with a maximum 9-week hold permitted.
Table 1.
Guidelines for bevacizumab adjustments based on LVEF results in asymptomatic patients.
| Absolute difference between measured LVEF compared with baseline |
|||
|---|---|---|---|
| LVEF by MUGA scan (or ECHO) |
Absolute decrease of <10 percentage points |
Absolute decrease of 10−15 percentage points |
Absolute decrease of >15 percentage points |
| ≥55% | Continue bevacizumab | Continue bevacizumab | Hold bevacizumab; Repeat LVEF in 4 weeks |
| 50%−54% | Continue bevacizumab; Repeat LVEF in 4 weeks |
Hold bevacizumab; Repeat LVEF in 4 weeks |
Hold bevacizumab; Repeat LVEF in 4 weeks |
| ≤49% | Hold bevacizumab; Repeat LVEF in 4 weeks |
Hold bevacizumab; Repeat LVEF in 4 weeks |
Hold bevacizumab; Repeat LVEF in 4 weeks |
Abbreviations: ECHO, echocardiogram; LVEF, left ventricular ejection fraction; MUGA, multigated acquisition.
Toxicity Assessments Toxicities were assessed using the National Cancer Institute Common Toxicity Criteria (NCI-CTC), version 3.0.
Exploratory Correlative Studies
Physical examination with blood pressure (BP) measurement occurred every 2 weeks during treatment with concurrent bevacizumab with ddAC→nab-paclitaxel and every 6 weeks during treatment with bevacizumab alone. Peripheral blood for PRA was collected at baseline, week 8, week 16, and every 3 months during bevacizumab administration. Baseline BP and PRA values for those who did and did not develop HTN were evaluated by Kruskal-Wallis test. Renin data were censored at the time of the first HTN event, as antihypertensive medications initiated to treat bevacizumab-mediated HTN could cause compensatory changes in PRA.
Peripheral blood for cTnI was collected at baseline, weeks 2, 4, 6, 8, 10, 12, and 14, and months 6, 9, and 18. cTnI values over time were examined as continuous variables by institution and as categorical variables, where cTnI values were categorized as “undetectable” (<0.06 ng/mL MSKCC or <0.05 ng/mL UCSF), “minimally detectable” (0.06–0.31 ng/mL MSKCC or 0.05–0.16 ng/mL UCSF) or “elevated” (>0.31 ng/mL MSKCC or >0.16 ng/mL UCSF). Baseline and maximum cTnI values were compared with the maximum change in LVEF values for all 80 enrolled patients over the study period using analysis of variance or regression. Baseline BP as a predictor of a maximum “minimally detectable” or “elevated” cTnI value was also explored.
RESULTS
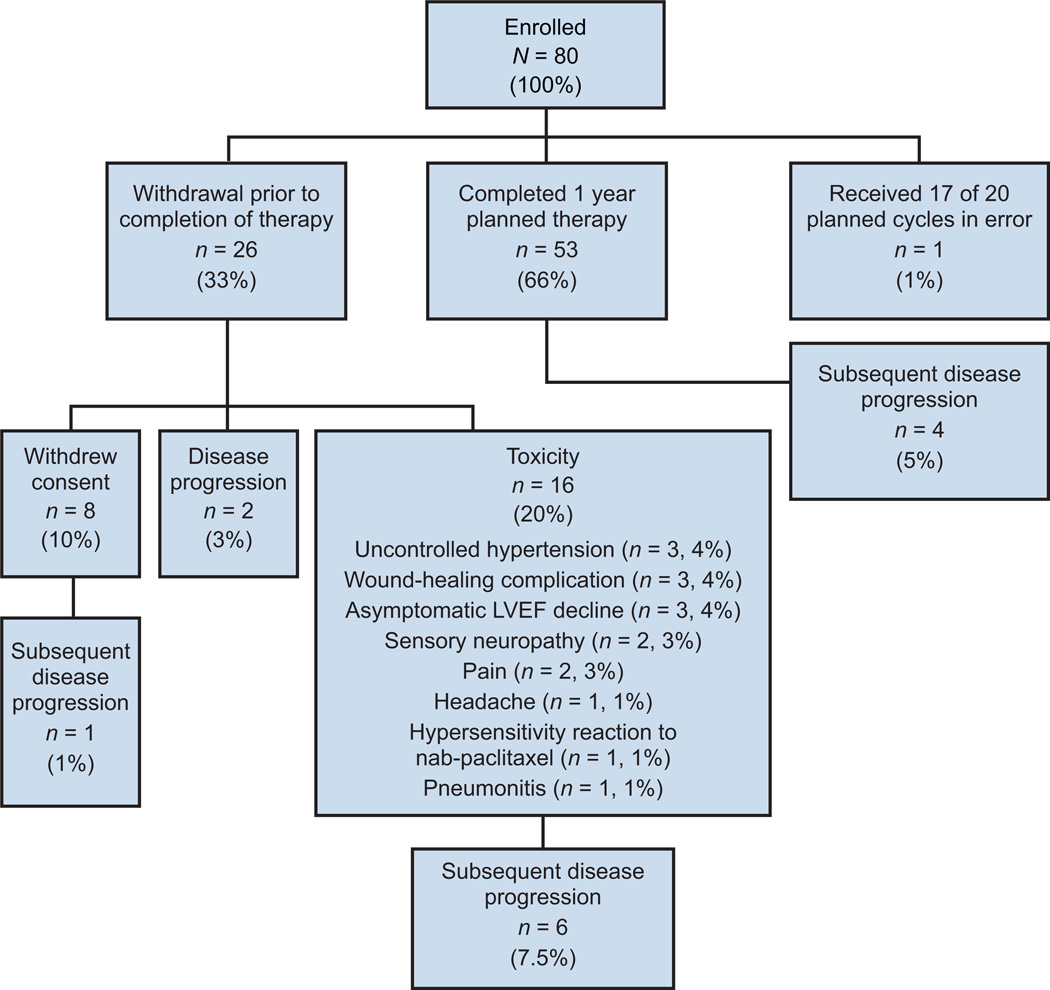
Between July 2006 and August 2007, 80 patients were enrolled. Baseline patient characteristics are outlined in Table 2. The median age was 48 years (range, 27−75 years). As of May 1, 2010, median follow-up was 39 months (range, 5−45 months). The median number of cycles administered was 20 (range, 1−21 with one patient receiving one additional cycle in error). Twenty-seven patients (34%) did not complete the planned course of therapy: eight (10%) withdrew consent, 16 (20%) withdrew from study because of toxicity, two (3%) experienced disease progression on therapy, and one (1%) received only 17 of the planned 20 bevacizumab cycles in error (Fig. 2). As of May 1, 2010, 13 patients (16%) have experienced disease progression: four patients (5%) after completion of therapy (at 13, 23, 24 and 34 months), two (3%) on therapy, one (1%) after withdrawing consent, and six (7.5%) after study withdrawal secondary to toxicity (hypertension, neuropathy, pain/ataxia, wound-healing complication and migraines).
Table 2.
Patient characteristics (N = 80).
| Characteristics | No. | % |
|---|---|---|
| Age, years | ||
| Median | 48 | |
| Range | 27−75 | |
| < 40 | 15 | 19 |
| 40–49 | 30 | 38 |
| 50–59 | 24 | 30 |
| 60–69 | 10 | 13 |
| ≥ 70 | 1 | 1 |
| Tumor size, cm | ||
| ≤ 2.0 | 21 | 26 |
| 2.1–5.0 | 52 | 65 |
| > 5.0 | 7 | 9 |
| Node status | ||
| 0 | 8 | 10 |
| 1–3 | 35 | 44 |
| 4–9 | 20 | 25 |
| > 9 | 17 | 21 |
| Stage* | ||
| I | 2 | 3 |
| IIA | 18 | 23 |
| IIB | 21 | 26 |
| IIIA | 23 | 29 |
| IIIB | 16 | 20 |
| Estrogen receptor status | ||
| Positive | 58 | 73 |
| Negative | 22 | 28 |
| Progesterone receptor status | ||
| Positive | 51 | 64 |
| Negative | 29 | 36 |
| ECOG performance status | ||
| 0 | 67 | 84 |
| 1 | 13 | 16 |
| Surgery | ||
| Resection of ipsilateral recurrence with axillary dissection and adjuvant XRT |
1 | 1 |
| Breast-conserving surgery | 23 | 29 |
| With adjuvant XRT | 21 | 26 |
| With subsequent mastectomy | 1 | 1 |
| Other (diagnosed with metastases post cycle 1) | 1 | 1 |
| Mastectomy | 56 | 70 |
| Without reconstruction | 16 | 20 |
| Immediate prestudy tissue expander | 39 | 49 |
| Tissue expander exchange on study | 12 | 15 |
| Tissue expander exchange post study | 15 | 19 |
| Tissue expander exchange off study | 8 | 10 |
| Failed tissue expansion | 3 | 4 |
| No exchange because of disease progression | 1 | 1 |
| Delayed TRAM | 1 | 1 |
| Post mastectomy XRT | 35 | 44 |
| Median baseline LVEF, % (range) | 68 (53–82) | |
According to the American Joint Committee on Cancer Staging Manual, 6th ed.
Abbreviations: XRT, radiation therapy; TRAM, transverse rectus abdominis musculocutaneous.
Figure 2.
Distribution of enrolled patients.
Cardiac Outcomes
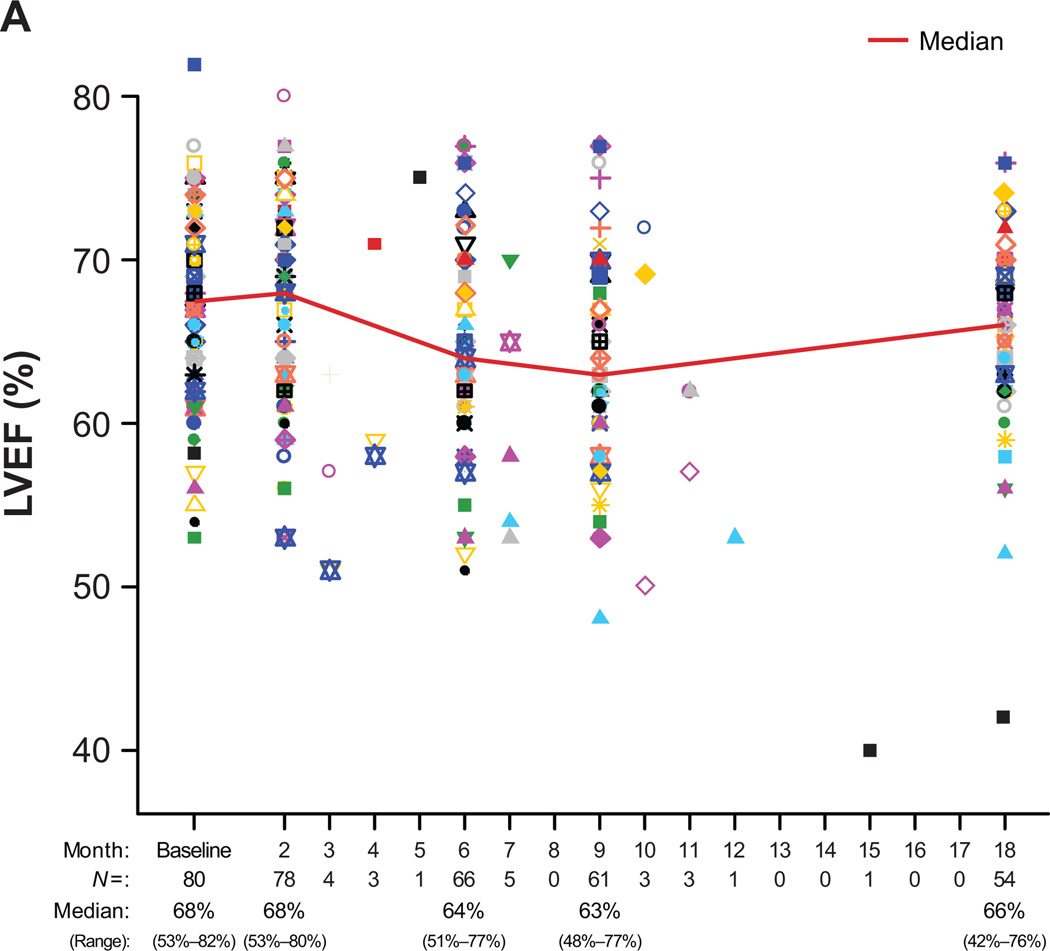
The median baseline LVEF was 68% (range, 53%−82%). Median LVEF was 68% (range, 53%−80%) for the 78 evaluable patients at 2 months, 64% (range, 51%−77%) for the 66 evaluable patients at 6 months, 63% (range, 48%−77%) for the 61 evaluable patients at 9 months, and 66% (range, 42%−76%) for the 54 evaluable patients at 18 months (Fig. 3A).
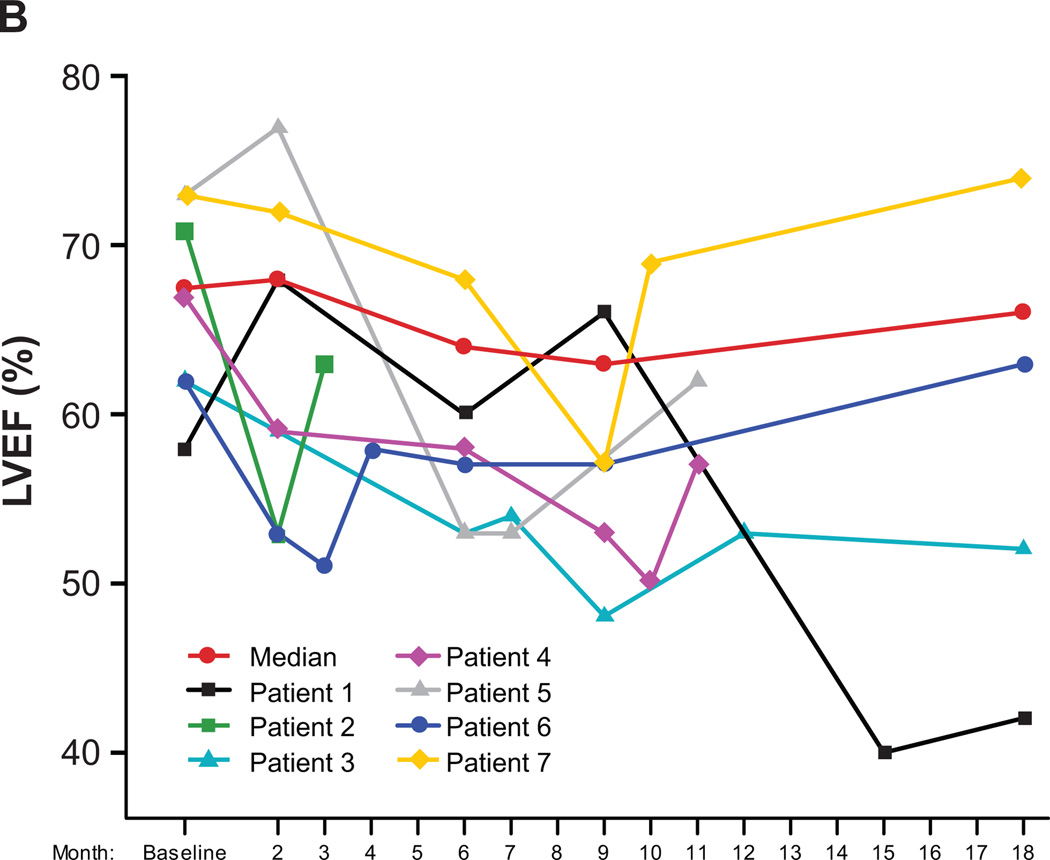
Figure 3.
(A) LVEF data for all study patients over time (N = 80). (B) LVEF data for the seven patients (9%) who experienced a symptomatic LVEF decline or an asymptomatic LVEF decline necessitating bevacizumab hold/discontinuation. Patient 1: Month-15 symptomatic LV dysfunction, cardiac catheterization and left anterior descending stenting; Patient 2: Month-2 bevacizumab hold and subsequent consent withdrawal despite LVEF recovery; Patient 3: Month-6 apical hypokinesis (LVEF, 53%) and subsequent study withdrawal; Patients 4 and 5: Asymptomatic LVEF decline with subsequent recovery post bevacizumab discontinuation; Patients 6 and 7: Month-3 and month-9 bevacizumab hold with subsequent reinstitution.
Seven patients [9%; 95% CI (4%, 18%)] experienced either a symptomatic LVEF decline or an asymptomatic LVEF decline necessitating bevacizumab hold/discontinuation per protocol guidelines (Table 1; Fig. 3B). The median age for these seven patients was 46 years (range, 42−60 years). One experienced symptomatic grade 3 LV dysfunction (nadir LVEF, 40%) 3 months after completing the planned course of therapy and underwent cardiac catheterization with stenting of a 99% left anterior descending artery occlusion; two experienced an asymptomatic grade 1 LVEF decline necessitating bevacizumab discontinuation at months 7 and 10 with subsequent LVEF recovery off therapy; one withdrew consent after a protocol-stipulated bevacizumab hold at month 2 despite subsequent LVEF recovery; one developed apical hypokinesis at month 6 (with an associated LVEF decline to 53%, from 62% at baseline and 59% at month 2) and was withdrawn from study at the treating physician’s discretion—a further decline in LVEF off therapy to 48% (grade 2) was noted at month 9, with LVEF recovery to 53% at month 12 and 52% at month 18; and two had bevacizumab held per protocol. Of these two, one occurred at month 3 secondary to an absolute decrease of 11% from the baseline value of 62% (per protocol, chemotherapy alone was continued for 4 weeks and bevacizumab was subsequently successfully reinstituted and completed after LVEF recovery to 58% at month 4). The second patient had bevacizumab held at month 9 secondary to a >15% LVEF decline from baseline (73% to 57%) with recovery (to 69%) at month 10 and successful reinstitution of bevacizumab. Thus, overall three (4%) patients withdrew from the study because of asymptomatic LVEF declines as specified per protocol, one (1%) withdrew consent after a bevacizumab hold despite LVEF recovery, and two (3%) had bevacizumab reinstituted.
There were no cardiac deaths in this study. Fifteen months after completing bevacizumab, one patient had placement of a defibrillator for arrhythmogenic right ventricular dysplasia, without a documented decline in LVEF. Fourteen months after completing bevacizumab, another patient underwent cardiac catheterization with placement of two stents for unstable angina, and her LVEF at that time was 60%.
Toxicity and Serious Adverse Events
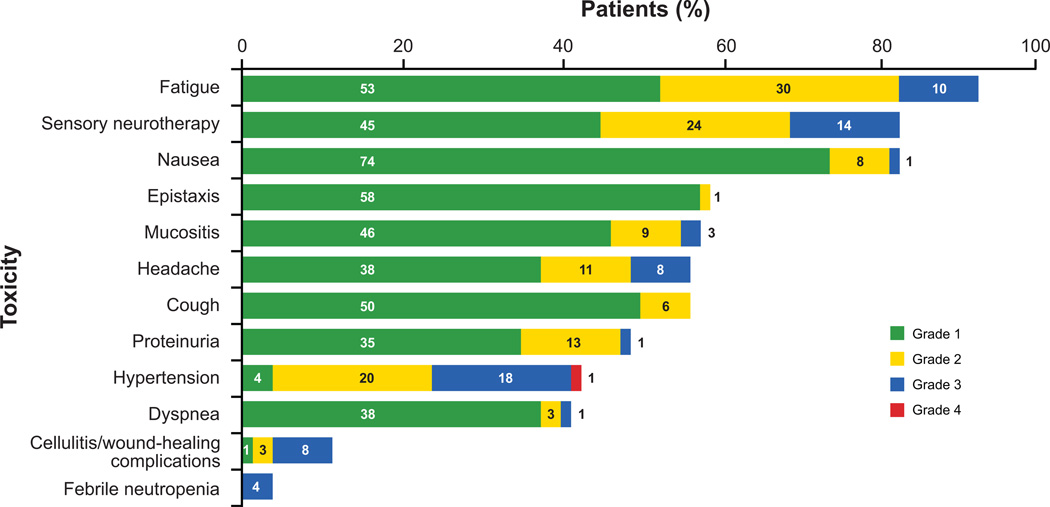
Commonly reported treatment-related toxicities included fatigue, sensory neuropathy, and nausea (Fig. 4). No grade 3 or 4 bone pain was reported. As of May 1, 2010, there were 35 hospitalizations in 26 (33%) of the 80 enrolled patients. Four patients (5%) were hospitalized after completion of the planned course of therapy: one with disease progression, one with biliary colic, one with chest pain requiring left anterior descending stenting as previously described, and one with seizure and a subsequent diagnosis of multiple sclerosis. One patient, off study secondary to HTN, was hospitalized with implant infection 7 months after tissue expander exchange. Among the 21 patients (26%) who were hospitalized during therapy, one patient was hospitalized on four occasions while on therapy (for grade 4 neutropenia, grade 4 febrile neutropenia, tissue expander infection, and tissue expander removal) and on one occasion off study (for persistent wound-healing complications after infected expander removal). Four patients had two hospitalizations during treatment: one was admitted with febrile neutropenia and later anorexia with dehydration, a second with grade 4 neutropenia and later chest pain with negative cardiac evaluation, a third with neuropathy and later pyelonephritis, and a fourth with severe hypothyroidism and then tissue expander exchange complicated by cellulitis 7 days later. One patient was hospitalized once during treatment for hypertension with headache and twice off study for wound-healing complications at 4 and 12 weeks after tissue expander exchange.
Figure 4.
Selected common adverse events. Toxicities were assessed by the NCI-CTC, version 3.0.
The reasons for hospitalization for the remaining 15 patients were infection requiring antibiotics (n = 4), febrile neutropenia (n = 2), chest pain of uncertain etiology (n = 2), nonspecific gastrointestinal complaints (n = 2), grade 2 headache (n = 1), grade 4 hypertension with headache (n = 1), atrial fibrillation (n = 1), supraventricular tachycardia (n = 1), and rash secondary to probable hypersensitivity reaction (n = 1).
Surgery-Related Complications
Of the 56 (70%) study patients who underwent mastectomy, 39 (49%) underwent immediate (prestudy) tissue expander reconstruction, one (1%) underwent an uncomplicated delayed transverse rectus abdominis myocutaneous flap procedure after completion of the planned course of bevacizumab, and 16 (20%) did not undergo reconstruction. Five patients experienced cellulitis or wound-healing complications after mastectomy: one occurred 4 days after tissue expander exchange status after cycle 15 of bevacizumab; one occurred approximately 4 weeks after tissue expander exchange status after cycle 8 of bevacizumab; one occurred approximately 16 weeks after tissue expander exchange, approximately 7 weeks after completion of radiation, and status after cycle 12 of bevacizumab; one occurred after the first cycle of bevacizumab (approximately 2 months after tissue expander placement); and one occurred after mastectomy without reconstruction, 4 days after completion of radiation therapy and status after cycle 11 of bevacizumab. Of the two patients who underwent breast-conserving surgery and experienced wound-healing complications: one experienced grade 2 cellulitis after cycle 2 of bevacizumab (11 weeks after surgery) and one experienced grade 3 cellulitis that necessitated study discontinuation of bevacizumab after cycle 8 (5 months after surgery).
Exploratory Correlative Studies
Baseline median systolic BP predicted grade 3 or 4 HTN (P = 0.045; grade 0 or 1 vs grade 3 or 4), when HTN was evaluated by first grade on study. Neither baseline diastolic BP (P = 0.077) nor PRA (P = 0.862) predicted grade 3 or 4 HTN. Patients with grade 2 and grade 3 or 4 HTN appeared to have an initial decline in PRA before the onset of HTN, but no statistically significant trends were identified (33).
Baseline cTnI was “undetectable” in 79 patients (99%), “minimally detectable” in 1 (1%), and elevated in 0 (0%). Maximum cTnI was “undetectable” in 17 patients (21%), “minimally detectable” in 57 (71%), and elevated in 6 (8%). There was no association between maximum LVEF % change [(max-min)/min] compared with maximum troponin when patients were evaluated by institution (MSKCC P = 0.37 and UCSF P = 0.29). There was no association between maximum categorical cTnl levels for women with significant LVEF declines compared with those without significant LVEF declines. Furthermore, neither systolic nor diastolic baseline BP predicted maximum categorical cTnl levels on study.
DISCUSSION
Bevacizumab plus ddAC→nab-paclitaxel met our predefined feasibility criteria. Only one patient (1%) had symptomatic grade 3 CHF approximately 3 months after completing the planned 1-year course of bevacizumab, and we note that this patient had severe coronary artery disease that required a coronary artery stent. There were no cardiac deaths. Six patients (8%) had asymptomatic LVEF declines resulting in four (5%) withdrawals. Longer-term follow-up for cardiac events is ongoing.
Prior studies of bevacizumab with various chemotherapy agents have raised unresolved questions regarding cardiac safety. In a phase III study of capecitabine plus bevacizumab in women with anthracycline- and taxane-resistant metastatic breast cancer (MBC), the incidence of CHF or cardiomyopathy was significantly greater with the combination than with chemotherapy alone (3.1% versus 0.9%, respectively; ref. 17). In a phase III study (E2100) of first-line weekly paclitaxel with or without bevacizumab in MBC, however, the incidence of grade 3 or 4 LV dysfunction was similar for both the combination and paclitaxel alone arms (0% grade 3 and 0.3% grade 4 events in the paclitaxel alone arm and 0.8% grade 3 and 0% grade 4 events in the combination arm) (12).
The risk of cardiotoxicity is a clinical concern when adjuvant anthracycline-containing regimens are recommended and may be increased when biologic agents are co-administered (21). Specifically, the risk of a grade 3 or 4 cardiac event for adjuvant ddAC→nab-paclitaxel is 1% (8, 34) and the 3-year risk of NYHA class III or IV CHF or death from cardiac causes in the North American multicenter studies of adjuvant AC→paclitaxel with or without trastuzumab was 4.1% versus 0.8%, respectively (10).
In studies in which anthracyclines and bevacizumab have been co-administered, cardiotoxicity rates have been highly variable. For example, in a phase II study of doxorubicin (75 mg/m2 every 3 weeks) plus bevacizumab in 17 patients with metastatic soft tissue sarcoma, two patients (11.8%) developed grade 3 or greater cardiotoxicity (20). Preliminary data from a study of neoadjuvant doxorubicin (50 mg/m2) and docetaxel (75 mg/m2) plus bevacizumab every 3 weeks for six cycles in patients with locally advanced breast cancer indicated a 9.5% rate of asymptomatic LVEF declines (two of 21 patients) with subsequent normalization on follow-up evaluation and no symptomatic CHF (19). However, in a recent report of first-line chemotherapy with or without bevacizumab in MBC (RIBBON 1 study), the incidence of grade 3 or greater LV systolic dysfunction was 0% with anthracycline-based chemotherapy alone versus 3% with the addition of bevacizumab (14). The variability in reported cardiotoxicity rates may reflect differences in specific patient, tumor, and treatment characteristics, including comorbid conditions, prior chemotherapy prescriptions, and differences in anthracycline dosing.
In an indirect comparison, the regimen evaluated in this study had a lower rate of cardiotoxicity than was observed in another phase II adjuvant study, ECOG 2104, wherein breast cancer patients received ddAC→paclitaxel with 1 year of bevacizumab initiated with AC or paclitaxel (35). In ECOG 2104, three of the 223 (1%) patients experienced symptomatic CHF while on therapy. In addition, 13 of 93 patients (14%) who received concurrent ddAC plus bevacizumab and seven of 113 patients (6%) who received ddAC alone followed by sequential administration of bevacizumab (initiated with paclitaxel) had asymptomatic LVEF declines of more than 10% after the first four cycles. Similar to our results, a study of preoperative bevacizumab in combination with doxorubicin (50 mg/m2) and docetaxel (75 mg/m2) every 3 weeks for six cycles, demonstrated that none of the 21 patients experienced symptomatic CHF and only 2 patients had a grade 2 asymptomatic LVEF decline (19).
In our prospectively planned correlative studies, cTnI did not appear to predict LVEF changes with the evaluated regimen, although the analysis was limited as a result of the low cardiac event rate in this study. In addition, we now recognize that the timing of our cTnI measurements may have been suboptimal. Because cTnI peaks immediately after high-dose chemotherapy (27) and because we drew our samples prior to chemotherapy administration (ie, potentially at nadir timepoints) per standard institutional guidelines, we could have missed the maximal impact on cTnI. Therefore, we believe that cTnI as a potential predictor of treatment-mediated cardiotoxicity should be explored further. Exploratory analyses of PRA as a predictor of bevacizumab-mediated HTN were similarly limited by the small number of patients. PRA at time of first HTN event (grade 2 or grade 3/4) appeared to decline before the onset of HTN, but no statistically significant trends were identified. Interestingly, the readily available clinical parameter of baseline systolic blood pressure was the only predictor of grade 3/4 HTN, indicating that vigilance in blood pressure monitoring on bevacizumab is warranted.
Other clinically relevant toxicities reported in phase III trials of bevacizumab plus chemotherapy included HTN, proteinuria, and venous and arterial thromboembolic events (12, 17, 22, 36). The most commonly reported toxicities in our study were attributable to the chemotherapy component of the regimen; namely, fatigue, taxane-associated sensory neuropathy, and nausea. The 14% rate of grade 3 sensory neuropathy in the study is similar to rates reported with ddAC→nab-paclitaxel regimens without bevacizumab (30), suggesting that the addition of bevacizumab is unlikely to exacerbate neurotoxicity. Adverse events likely attributable to bevacizumab include HTN, proteinuria, epistaxis, and wound-healing complications. In this study, the rates of grade 3 and 4 hypertension (19%), proteinuria (1%), and epistaxis (0%) were similar to those reported in a comparable study population (35). Among the nine (11%) patients with cellulitis or wound-healing complications, three experienced the complication after delayed reconstruction (at 4 days, 4 weeks, and 16 weeks). The reported incidence of wound-healing complications after tissue expander or implant reconstruction is highly variable. In a prospective multicenter study, the infection rate after implant reconstruction was 35% in 79 evaluated patients (37). Although it is unknown whether the infection rate in our study was impacted by bevacizumab administration, the 11% incidence is consistent with other reports (38, 39). Furthermore, although a 4-week bevacizumab hold was recommended both before and after delayed reconstruction, the ideal perioperative hold interval is unknown.
Overall, 16 patients (20%) withdrew from this study because of toxicity. Because the consent–withdrawal rate was greater than anticipated at eight patients (10%) and two patients (2.5%) experienced disease progression on therapy, the 26 study withdrawals (33%) prior to completion of the planned therapy were more than expected. By comparison, it is worth noting that in the combined analysis of the North American adjuvant trastuzumab trials, 31% of patients who began treatment with trastuzumab did not complete the planned 1-year course of therapy (10). In terms of feasibility, the statistical plan for this study was originally developed to evaluate 64 patients who completed AC therapy and were able to continue bevacizumab based on their MUGA scan at month 2. This goal was met with 77 patients who had a MUGA scan at month 2 and continued therapy with bevacizumab. Hence, this pilot study demonstrates that bevacizumab with ddAC followed by nab-paclitaxel demonstrates acceptable toxicity. Comparative efficacy information, along with additional cardiac safety data for bevacizumab in combination with adjuvant anthracycline-containing regimens, is anticipated from ongoing phase III studies.
Supplementary Material
Statement of Translational Relevance.
Bevacizumab, a humanized monoclonal antibody that binds vascular endothelial growth factor, prolongs progression-free survival in patients with metastatic breast cancer but may be more effective when targeting minimal residual disease in the adjuvant setting. There is, however, some concern that administration of bevacizumab with adjuvant anthracycline-based therapy will increase the risk of congestive heart failure (CHF). This phase II study indicates that adjuvant anthracycline-based chemotherapy plus bevacizumab is safe and feasible and thus supports ongoing randomized studies. Although the primary end point of this study was clinical cardiac safety, we also explored the use of serum cardiac troponins as predictors of CHF and baseline blood pressure and plasma renin activity as predictors of hypertension. Our results can inform clinical trial design.
Acknowledgments
Supported in part by the Jodi Spiegel Fisher Foundation, Genentech, Inc., and Abraxis. Support for third-party copy editing and graphical design assistance was provided by Genentech, Inc.
Footnotes
The authors attest to the originality of this manuscript. This manuscript has not been published or presented previously.
Data presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 12–16, 2007, Chicago, IL (Abstract #567); the 30th Annual San Antonio Breast Cancer Symposium, December 13–16, 2007, San Antonio, TX (Abstract #3065); the 31st Annual San Antonio Breast Cancer Symposium, December 10–14, 2008, San Antonio, TX (Abstract #4104); the 44th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009, Orlando, FL (Abstract #602); and the 32nd Annual San Antonio Breast Cancer Symposium, December 9-13, 2009, San Antonio, TX (Abstract #3087).
REFERENCES
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 3.Levine MN, Pritchard KI, Bramwell VH, et al. Randomized trial comparing cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer: update of National Cancer Institute of Canada Clinical Trials Group Trial MA5. J Clin Oncol. 2005;23:5166–5170. doi: 10.1200/JCO.2005.09.423. [DOI] [PubMed] [Google Scholar]
- 4.Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23:3686–3696. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 5.Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 6.Bonadonna G, Zambetti M, Valagussa P. Sequential or alternating doxorubicin and CMF regimens in breast cancer with more than three positive nodes Ten-year results. JAMA. 1995;273:542–547. [PubMed] [Google Scholar]
- 7.Burnell M, Levine M, Chapman JA, et al. A randomized trial of CEF versus dose dense EC followed by paclitaxel versus AC followed by paclitaxel in women with node positive or high risk node negative breast cancer, NCIC CTG MA.21: results of an interim analysis; San Antonio, TX. Poster 51 presented at the 29th San Antonio Breast Cancer Symposium.2006. Dec 14–17, [Google Scholar]
- 8.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 9.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 11.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 12.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 13.Miles D, Chan A, Romieu G, et al. Randomized, double-blind, placebo-controlled, phase III study of bevacizumab with docetaxel or docetaxel with placebo as first-line therapy for patients with locally recurrent or metastatic breast cancer (mBC): AVADO [abstract LBA1011] J Clin Oncol. 2008;26(15 suppl):43s. [Google Scholar]
- 14.Robert NJ, Dieras V, Glaspy J, et al. RIBBON-1. Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab (B) for first-line treatment of HER2-negative locally recurrent or metastatic breast cancer (MBC) [abstract 1005] J Clin Oncol. 2009;27(15 suppl):42s. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 15.Demicheli R, Retsky MW, Hrushesky WJM, et al. Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: learning from failures. Nat Clin Pract Oncol. 2007;4:699–710. doi: 10.1038/ncponc0999. [DOI] [PubMed] [Google Scholar]
- 16.Naumov GN, Bender E, Zurakowski D, et al. A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. J Natl Cancer Inst. 2006;98:316–325. doi: 10.1093/jnci/djj068. [DOI] [PubMed] [Google Scholar]
- 17.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 18.Overmoyer B, Silverman P, Leeming R, et al. Phase II trial of neoadjuvant docetaxel with or without bevacizumab in patients with locally advanced breast cancer [abstract 2088] Breast Cancer Res Treat. 2004;88(suppl):s106. [Google Scholar]
- 19.Wedam SB, Low JA, Yang SX, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24:769–777. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- 20.D’Adamo DR, Anderson SE, Albritton K, et al. Phase II study of doxorubicin and bevacizumab for patients with metastatic soft-tissue sarcomas. J Clin Oncol. 2005;23:7135–7142. doi: 10.1200/JCO.2005.16.139. [DOI] [PubMed] [Google Scholar]
- 21.Pinder MC, Duan Z, Goodwin JS, et al. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25:3808–3815. doi: 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 22.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 23.Laragh J. Laragh’s lessons in pathophysiology and clinical pearls for treating hypertension. Am J Hypertens. 2001;14:186–194. doi: 10.1016/s0895-7061(00)01317-0. 296–304, 307–10, 397–404, 491–503, 603–9, 733–42, 837–54. [DOI] [PubMed] [Google Scholar]
- 24.Sealey JE. Plasma renin activity and plasma prorenin assays. Clin Chem. 1991;37(10 pt 2):1811–1819. [PubMed] [Google Scholar]
- 25.Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335:1342–1349. doi: 10.1056/NEJM199610313351802. [DOI] [PubMed] [Google Scholar]
- 26.Sato Y, Yamada T, Taniguchi R, et al. Serum concentration of cardiac troponin T in patients with cardiomyopathy: a possible mechanism of acute heart failure. Heart. 1998;180:209–210. doi: 10.1136/hrt.80.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardinale D, Sandri MT, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109:2749–2754. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 28.Weiss RB, Donehower RC, Wiernik PH, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263–1268. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 29.Gradishar WJ, Tjulandin S, Davidson N, et al. Superior efficacy of albumin-bound paclitaxel, ABI-007, compared with polyethylated castor oil-based paclitaxel in women with metastatic breast cancer: results of a phase III trial. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 30.Robert N, Krekow L, Stokoe C, et al. Adjuvant dose-dense doxorubicin plus cyclophosphamide followed by dose-dense nab-paclitaxel is safe in women with early-stage breast cancer: a pilot study. Breast Cancer Res Treat. 2011;125(1):115–120. doi: 10.1007/s10549-010-1187-2. [DOI] [PubMed] [Google Scholar]
- 31.Burstein HJ, Mayer EL, Peppercorn J, et al. Dose-dense nab-paclitaxel (nanoparticle albumin-bound paclitaxel) in adjuvant chemotherapy for breast cancer: a feasibility study [abstract 594] J Clin Oncol. 2007;25(18s):26s. [Google Scholar]
- 32.Rosner B. 3. Boston, MA: PWS-Kent Publishing Company; 1990. Fundamentals of biostatistics; pp. 82–83. [Google Scholar]
- 33.Dickler MN, McArthur HL, Nulsen B, et al. Evaluation of the correlation of baseline blood pressure (BP) and plasma renin activity (PRA) with bevacizumab (B)-mediated hypertension in patients with early-stage breast cancer. J Clin Oncol. 2009;27(15S) Abstract 602. [Google Scholar]
- 34.Hudis C, Citron M, Berry D, et al. Five year follow-up of INT C9741: dose-dense (DD) chemotherapy (CRx) is safe and effective [abstract 41] Breast Cancer Res Treat. 2005;94(suppl):s20. [Google Scholar]
- 35.Miller KD, O’Neill A, Perez EA, et al. Phase II feasibility trial incorporating bevacizumab into dose-dense doxorubicin and cyclophosphamide followed by paclitaxel in patients with lymph node-positive breast cancer: a trial of the Eastern Cooperative Oncology Group (E2104) [abstract 520] J Clin Oncol. 2008;26(15s):11s. doi: 10.1093/annonc/mdr344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 37.Alderman AK, Wilkins EG, Kim HM, Lowery JC. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2002;109:2265–2274. doi: 10.1097/00006534-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Aitken DR, Minton JP. Complications associated with mastectomy. Surg Clin North Am. 1983;63:1331–1352. doi: 10.1016/s0039-6109(16)43192-0. [DOI] [PubMed] [Google Scholar]
- 39.Vinton AL, Traverso LW, Zehring RD. Immediate breast reconstruction following mastectomy is as safe as mastectomy alone. Arch Surg. 1990;125:1303–1307. doi: 10.1001/archsurg.1990.01410220087012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.