Abstract
Currently available combination chemotherapy for acute myeloid leukemia (AML) often fails to result in long-term remissions, emphasizing the need for novel therapeutic strategies. We reasoned that targeted inhibition of a prominent nuclear exporter, XPO1/CRM1, could eradicate self-renewing leukemia-initiating cells (LICs) whose survival depends on timely XPO1-mediated transport of specific protein and RNA cargoes. Using an immunosuppressed mouse model bearing primary patient-derived AML cells, we demonstrate that selinexor (KPT-330), an oral antagonist of XPO1 that is currently in clinical trials, has strong activity against primary AML cells while sparing normal stem and progenitor cells. Importantly, limiting dilution transplantation assays showed that this cytotoxic activity is not limited to the rapidly proliferating bulk population of leukemic cells but extends to the LICs, whose inherent drug resistance and unrestricted self-renewal capacity has been implicated in the difficulty of curing AML patients with conventional chemotherapy alone.
INTRODUCTION
Acute myeloid leukemia (AML) is a heterogeneous hematological malignancy characterized by uncontrolled proliferation of immature myeloid cells.1,2 Currently available combination chemotherapy often leads to complete remission, but a subset of patients develop recurrent disease, depending upon the presence or absence of defined prognostic risk factors.1 Similar to the organization of the normal hematopoietic system, where self-renewing, multipotent stem cells provide the capacity for the generation of all blood cell lineages, AML is organized as a cellular network with leukemia-initiating cells (LICs) at the apex of the hierarchy.3–5 LICs have the functional capability to self-renew and replenish AML blasts.3 The disease relapse that is observed in patients with AML who are treated with currently available chemotherapy is thought to occur because of the inability of the existing drugs to target the self-renewing LICs in AML.6 Thus novel therapies that eliminate the LICs in addition to the bulk leukemia cells are needed to prevent leukemic relapse in AML patients.
An attractive new target for AML therapy is the nuclear export protein CRM1, also called exportin 1 (XPO1). Leukemic cells require the continuous nuclear export of one or more onco-requisite proteins or RNAs and the removal of tumor-suppressor proteins that require nuclear localization for their functions.7–10 XPO1, a member of the karyopherin β family, is a major eukaryotic nuclear-cytoplasmic transporter that mediates the transport of certain proteins and selected RNA molecules from the nucleus to the cytoplasm.7–9,11 XPO1 regulates nuclear export of proteins that contain leucine-rich nuclear export signals, including protein adaptors that transport RNA molecules.12,13 Nuclear export by XPO1 is regulated by Ran-GTP binding in the nucleus, with XPO1 cargo being released in the cytoplasm following Ran-GTP hydrolysis by Ran-GAP.14–18 XPO1 cargoes comprise ~ 220 eukaryotic proteins, including the tumor-suppressor proteins p53, p21, Rb and FOXO3A, cell cycle regulators and apoptotic proteins.10,19,20 Expression of XPO1 is upregulated in both solid tumors and leukemias,21,22 and higher XPO1 levels correlate with a poor prognosis, suggesting the dependency of cancer cells on active XPO1-mediated nuclear export. Indeed, nuclear-cytoplasmic transport by XPO1 is required for the survival of several types of solid tumors and hematological malignancies.21–27 Interestingly, XPO1 blockade appears to be tolerated by non-neoplastic cells, including normal hematopoietic progenitor cells and proliferating cells of the gastrointestinal tract.28
Small-molecule inhibitors of XPO1, termed selective inhibitors of nuclear export (SINEs), were recently designed by exploiting an in silico molecular modeling strategy.29 The SINEs covalently bind to Cys528 in the nuclear export signal-binding groove of XPO1 to inhibit its nuclear export function.30 The orally bioavailable SINE compound selinexor (KPT-330) entered phase I clinical trials for solid tumors and hematological malignancies in July 2012 (NCT01607905 and NCT01607892), with AML patients first enrolled in the hematological malignancy study in July 2013. In 2014, selinexor entered phase I trial in children with relapsed or refractory AML or ALL (NCT02091245) and phase I and phase II trials to evaluate its activity in combination with chemotherapeutic drugs in patients with relapsed or refractory AML (NCT02249091, NCT02212561, NCT02088541, NCT02093403, NCT02299518). The preliminary results of the ongoing phase I study demonstrated clear activity of oral selinexor in inducing responses at tolerated doses, including complete remissions in a subset of relapsed/refractory AML patients.31 Previous studies by our group and others have shown that inhibition of XPO1 by SINEs induces apoptosis in AML cell lines with diverse genetic abnormalities and promotes apoptosis of AML cells in all cell cycle phases, including G0/G1.21,28,30,32 This finding supports the hypothesis that SINE-induced leukemia cell death does not depend on active proliferation. Moreover, xenograft studies have demonstrated that selinexor produces striking antileukemic activity against MV4–11 AML cells transplanted into immunodeficient mice, with minimal toxicity to normal hematopoietic cells.30,32 The antileukemic activity of selinexor, together with its lack of toxicity to normal hematopoietic cells, has also been shown in preclinical mouse models of several hematological malignancies, including T-cell acute lymphoblastic leukemia, chronic myeloid leukemia and multiple myeloma.22,26,33,34
The ability of the XPO1 inhibitor selinexor to induce apoptosis within the G0/G1 phase compartment of established AML cell lines suggested to us that it might also be active against slowly proliferating LIC populations in primary AML. To pursue this intriguing hypothesis, we tested selinexor in patient-derived xenografts, or PDX models, which have proven to be the most sensitive predictors of clinical responses in patients.35 Indeed, low-passage explants of AML cells that have not been cultured in tissue culture retain the hierarchical developmental structure of primary AML cell populations, including the retention of a small fraction of cells with the self-renewal properties of LIC. Here we demonstrate that selinexor is highly active against patient primary AML cells, including LIC, engrafted into immunodeficient mice and has little cytotoxicity against normal stem and progenitor cells. The xenografts used in our experiments were established from AML blasts of patients with a poor prognosis, including those with normal cytogenetics, FLT3-ITD+ AML and AML with complex karyotypes. Our findings indicate that inhibition of nuclear export by selinexor represents a novel approach to the eradication of LICs in patients with AML, thus directly addressing one of the major barriers to cure of this disease by combination chemotherapy.
MATERIALS AND METHODS
Patient samples
Primary AML cells, collected from bone marrow aspirates of three patients according to protocols approved by Dana-Farber Cancer Institute, represented two high-risk AML subtypes: cytogenetically normal with FLT3-ITD and complex karyotype disease (see Table 1). FLT3 status was determined by Sanger sequencing of genomic DNA.
Table 1.
Characteristics of AML specimens
| Patient | Specimen | Diagnosis | Specimen site | Time of collection | Cytogenetics | FLT3 status |
|---|---|---|---|---|---|---|
| 1 | AML-CK1 | AML | Bone marrow | Diagnosis | 46,XX,dup(2)(q21q33), t(8;16)(p11;p13), psu dic(22;1)(p11;p11)[10]/46,XX,dup(1)(q32q42), t(8;16),psu dic(9;1)(q34;p11) [4]/46,XX,t(8;16),psu dic(19;1) (p13;p11)[4]/46,XX[2] |
WT |
| 2 | AML-CK2 | AML | Bone marrow | Diagnosis | 46,XY,-2,der(5)t(2;5)(q3?1;q2?5), inv(11)(q21q23),add(15)(p11), del(20)(q12),+mar[19]/46, XY[1] |
WT |
| 3 | AML-CN | AML-M4 | Bone marrow | Relapse | 46, XX | FLT3-ITD |
Abbreviations: AML, acute myeloid leukemia; WT, wild type.
Patient-derived xenografts
All in vivo animal studies were performed according to a Dana-Farber Cancer Institute-approved protocol (DFCI no. 04–111). Bone marrow leukemic blasts from three AML patients were intravenously injected into NOD-SCID-IL2Rcγnull (NSG) mice (The Jackson Laboratory, Bar Harbor, ME, USA) for expansion. The NSG mice were monitored for leukemia development by flow cytometric analysis of peripheral blood for human CD45-positive (hCD45+) cells. To establish mouse models of patient primary AML, we injected the cryopreserved leukemic blasts into NSG mice via tail-vein injection and monitored the animals for leukemia progression, using flow cytometric analysis of peripheral blood for hCD45+ cells. hCD45+ cell counts in the bone marrow from the femurs and tibias or spine of euthanized animals were used to determine the extent of leukemia infiltration. The bone marrow cells were extracted from femurs, tibia or spine by crushing the bone in medium supplemented with 10% fetal bovine serum. To determine the efficacy of selinexor (KPT-330), mice were given orally either vehicle control (Pluronic F-68/PVP-K29/32) or selinexor (20 mg/kg) by oral gavage three times a week for 4 weeks. The drug response was determined by flow cytometric analysis for hCD45+ cells among the bone marrow leukocytes isolated from the femurs and tibias of mice in the vehicle- and selinexor-treated groups. Femurs, spines and spleen were fixed, sectioned and stained with hematoxylin and eosin as described before.30 For additional information, see Supplementary Materials and Methods.
Secondary transplantation assays
Bone marrow cells were isolated from the femurs and tibias of NSG mice transplanted with human primary AML cells that had been treated with either vehicle or selinexor at 20 mg/kg for 4 weeks. Serial dilutions of bone marrow leukocytes at doses from 106 to 102 hCD45+ cells were intravenously injected into untreated secondary recipients. After 19–22 weeks, mouse cohorts transplanted with serial dilutions of hCD45+ cells were screened for human engraftment. Infiltration of hCD45+ cells into the bone marrow of mouse femurs and tibias was used as an indicator of positive engraftment. For additional information, see Supplementary Materials and Methods.
Engraftment of normal human CD34+ cells into NSG mice
Human CD34+ cells were isolated from cord blood of consenting healthy donors according to procedures approved by the protocols of DFCI. hCD34+ cells (105) isolated from 10 donors were intravenously injected into sublethally (200 cGy) irradiated NSG mice, and peripheral blood was monitored for hCD45+ cells by flow cytometric analysis. Established human grafts were treated with vehicle or selinexor at 20 mg/kg three times a week for 4 weeks.
Dynamic BH3 profiling of AML cells
Bone marrow cells from AML xenografts were exposed ex vivo to 100 nM KPT-330 for 16 h. We then performed Dynamic BH3 profiling using several Bim peptide concentrations (0.03, 0.1, 0.3 and 1 µm) to determine the drug-induced increase in mitochondrial priming (Δ% priming) as described in our previous work.28,36,37
Statistical analysis
Percent engraftment and total hCD45+ cells in the bone marrow are reported as means ± s.e.m. Differences between treatment groups were analyzed with an unpaired Student’s t-test. LIC frequency was estimated from results of the serial transplantation assay using the Extreme Limiting Dilution software from the Walter and Eliza Hall Bioinformatics Institute of Medical Research (http://bioinf.wehi.edu.au/software/elda/).38
RESULTS
Selinexor is cytotoxic to primary AML cells engrafted into mice
To determine the antileukemic activity of selinexor in a clinically relevant setting, we established patient-derived xenografts, or PDX models, in which leukemic blast cells from the bone marrow of AML patients were transplanted intravenously into immunodeficient NSG mice. AML is very heterogeneous and includes high-risk subsets, including those with complex aberrant karyotypes and FLT3-activating mutations.1,2 The patients selected for this study were in high-risk subgroups: one had cytogenetically normal AML (AML-CN) in which the blast cells harbored an internal tandem duplication of FLT3 (FLT3-ITD), and two had complex karyotype AML (AML-CK1 and AML-CK2, the latter defined by a high-risk t(8;16) translocation;39 Table 1). Primary cells were injected intravenously into NSG mice, and when the mice were leukemic with primary human AML cells, as indicated by flow cytometric analysis of peripheral blood for human-specific CD45-positive (hCD45+) cells, the ‘first passage’ or ‘P0’ bone marrow AML cells were harvested and cryopreserved for these experiments.
Patient-derived xenograft AML-CK1
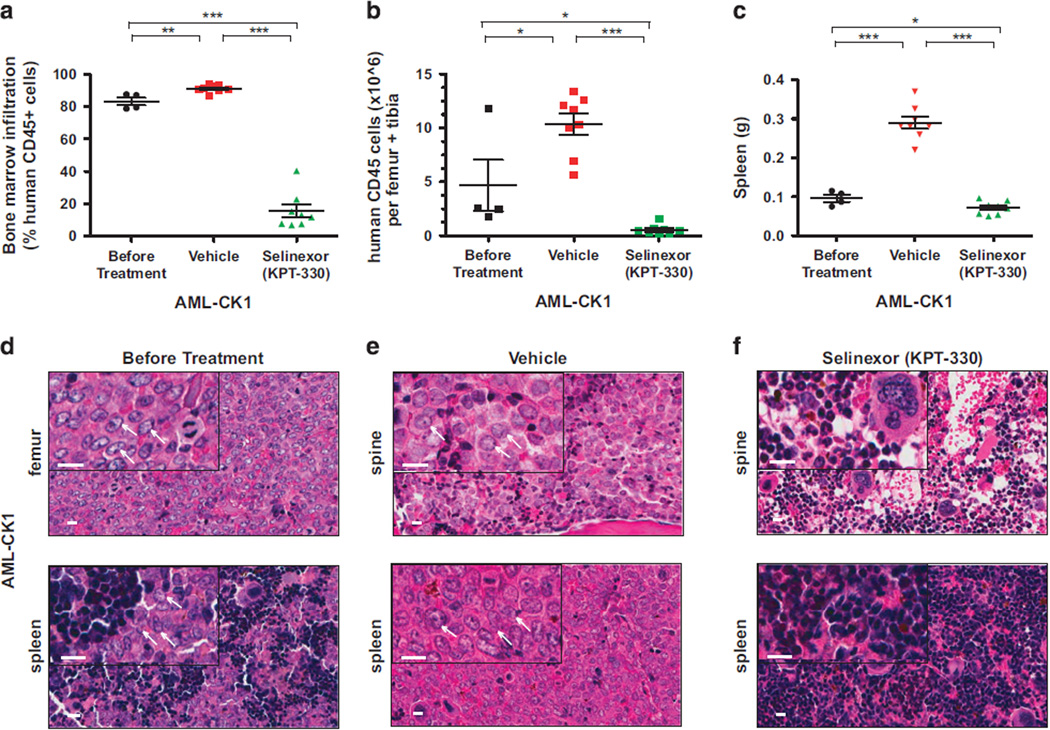
Twenty NSG mice each injected intravenously with 7.2 × 104 leukemic blasts from AML-CK1 were found to carry hCD45+ cells in their peripheral blood 3 months after tail-vein injection. Analysis of femurs extracted from four engrafted animals revealed that hCD45+ cells made up 83.2% ± 2.3% of total bone marrow leukocytes with 4.66 × 106 ± 2.4 × 106 hCD45+ cells per femur and tibia, documenting the human leukemia burden in these animals (Figures 2a and b; Supplementary Figure S1A). To determine the antileukemic activity of selinexor in xenograft AML-CK1, we divided the remaining mice into two groups and treated with either selinexor 20 mg/kg or vehicle control by oral gavage three times per week for 4 weeks (Figure 1). While on treatment, mice received high-calorie cherry or bacon supplemental diet to prevent weight loss, which is the dose-limiting toxicity of selinexor therapy in mice. In control mice, the bone marrow infiltration of hCD45+ cells increased to 91.0% ± 0.84% of bone marrow leukocytes with total hCD45+ cell counts rising to 10.4 × 106 ± 0.97 × 106 per femur and tibia (Figures 2a and b; Supplementary Figure S1B). By contrast, there was a remarkable decrease in human AML cell numbers in selinexor-treated mice, with hCD45+ cells accounting for only 15.6% ± 4.0% of bone marrow leukocytes and 0.54 × 106 ± 0.16 × 106 hCD45 cells per femur and tibia (~20-fold reduction in AML cell numbers; Figures 2a and b; Supplementary Figure S1C). The weight of spleens isolated from mice treated with vehicle decreased by approximately four-fold as compared with controls (Figure 2c). These results indicate that selinexor effectively kills the bulk human AML blast cells in the PDX model of AML-CK1.
Figure 2.
Selinexor (KPT-330) shows potent activity against primary AML-CK1 cells transplanted into NSG mice. (a) Percentage of human AML-CK1 cells in the bone marrow of NSG mice engrafted with AML-CN cells before and after treatment with vehicle or selinexor. (b) Counts of human CD45 cells per one femur and tibia in mice engrafted with AML-CK1 cells before treatment and following treatment with vehicle and selinexor. (c) Spleen weights of mice injected with human AML-CK1 cells before and after treatment with vehicle control and selinexor. (d–f) Histological analysis of the spine and femur bones and spleen from mice that had been engrafted with AML-CK1 cells isolated from killed animals before and after treatment with either vehicle control or selinexor. Human leukemia cell infiltration is apparent in the bone and spleen before treatment (d) and then after treatment with vehicle control (e), while normal hematopoiesis is seen after treatment with selinexor (f). Scale bar (white)=10 µm. White arrows point to the AML blast cells. In panels (a–c), each symbol denotes a single animal (n=4–8 per group). Error bars represent mean±s.e.m.; ***P < 0.0001 by unpaired t-test for comparisons between the indicated groups. *P < 0.05, **P < 0.001.
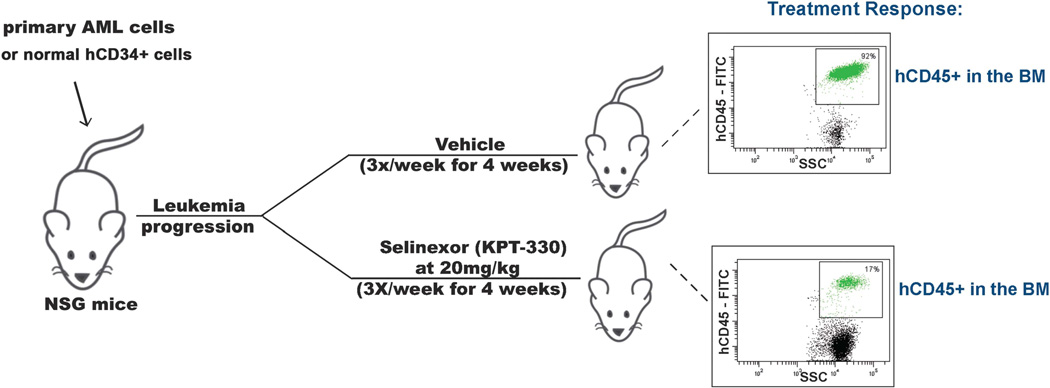
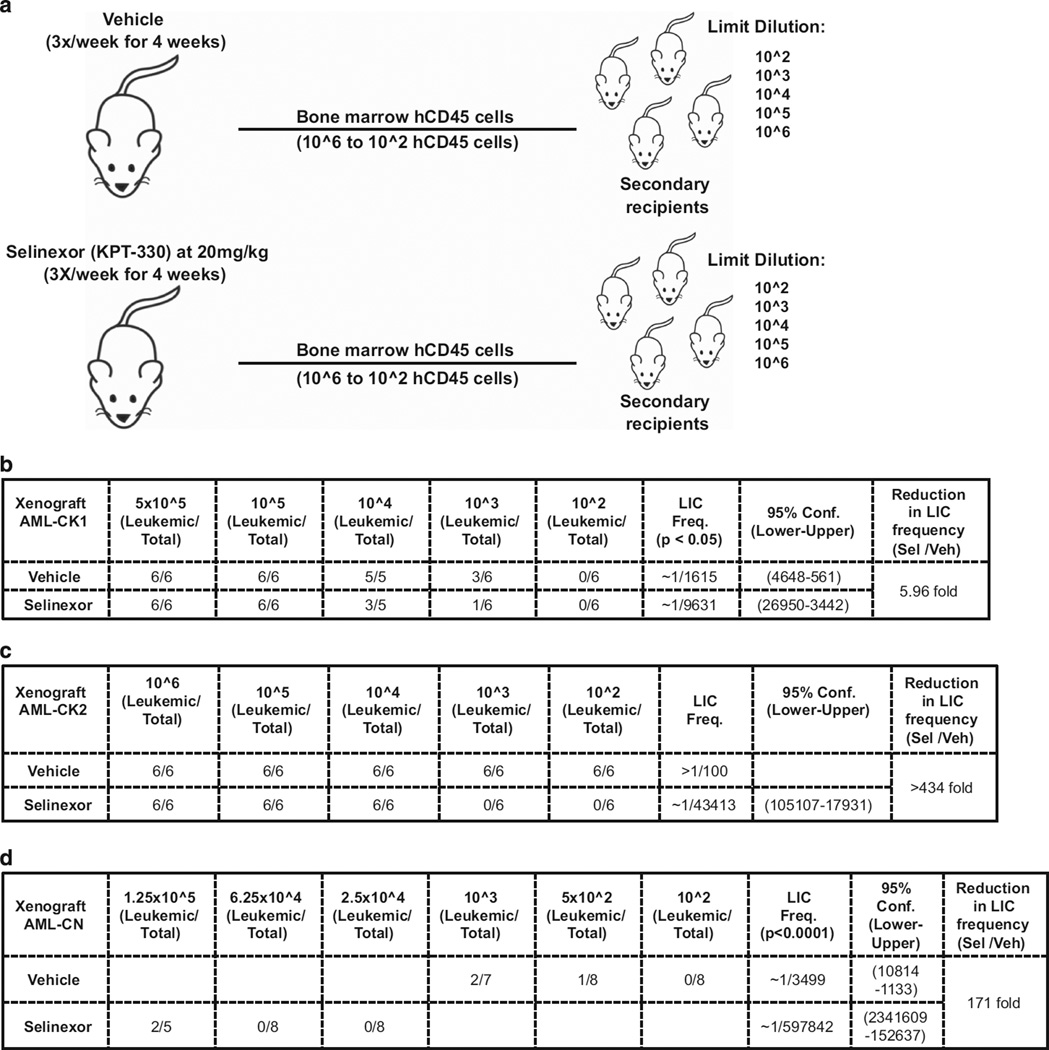
Figure 1.
Experimental scheme for study of the antileukemic activity of selinexor (KPT-330) in immunodeficient mice engrafted with primary human AML cells. Mouse xenograft models of human primary AML were established for use in therapeutic experiments to assess the efficacy of Selinexor against AML cells isolated from patients with AML-CK1, AML-CK2 and AML-CN (see Table 1). BM, bone marrow.
To define the toxicity of selinexor against normal hematopoietic cells, we examined histopathology on the bone marrow and spleens collected from mice bearing AML-CK1 before and after treatment with vehicle or selinexor. The hematoxylin and eosin-stained sections of AML-CK1 samples showed that leukemic cells had infiltrated the marrow and spleens of untreated and vehicle-treated mice; however, normal hematopoietic cell development in these organs was evident in the selinexor-treated mice (Figures 2d–f).
Patient-derived xenograft AML-CK2
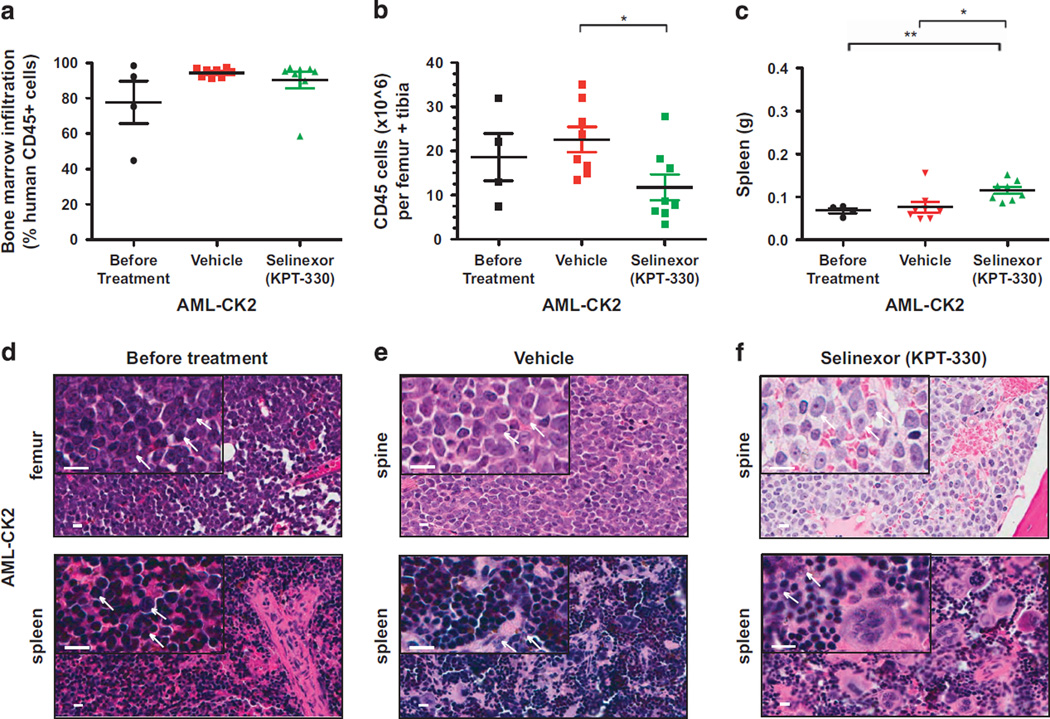
AML-CK2 cells caused leukemia in mice by 4 months after intravenous injection of 2.1 × 105 cells per animal. Infiltrating hCD45+ cells accounted for 77.7% ± 12% bone marrow leukocytes with 18.6 × 106 ± 5.4 × 106 hCD45+ cells per femur and tibia (Figures 3a and b; Supplementary Figure S2A). In mice with vehicle-treated AML-CK2 xenografts, the percentage of bone marrow infiltration by hCD45+ cells increased to 94.4% ± 0.81% leukocytes, with a total hCD45 count per femur and tibia increasing to 22.6 × 106 ± 2.9 × 106 cells. The AML-CK2 xenografts treated with selinexor showed hCD45+ infiltration of 90.3% ± 4.6% bone marrow leukocytes, with a slight decrease in total hCD45+ counts per femur and tibia to 11.8 × 106 ± 2.9 × 106 cells (Figures 3a and b; Supplementary Figure S2C). Thus, compared with the striking antileukemic activity in AML-CK1 xenografts, selinexor produced only moderate activity against the bulk leukemic population of AML-CK2 cells. The modest reduction of leukemic cell infiltrates in the bone marrow and spleen of selinexor-treated mice harboring AML-CK2 xenografts (Figures 3a–c) prevented the evaluation of toxicity in these animals (Figures 3d–f).
Figure 3.
Selinexor (KPT-330) induces only a moderate reduction of the leukemia burden in NSG mice engrafted with primary AML-CK2 cells. (a) Percentage engraftment of human AML-CK2 cells in the bone marrow of NSG mice before and after treatment with vehicle or selinexor. (b) Human CD45+ cells counts per femur and tibia in NSG mice transplanted with AML-CK2 cells before and after treatment with vehicle or selinexor. (c) Spleen weights of mice transplanted with human AML-CK2 cells before and after treatment with vehicle or selinexor. (d–f) Hematoxilin and eosin staining of the femur and spine bones and spleen of mice transplanted with AML-CK2 cells isolated from animals before treatment (d) and after treatment with either vehicle (e) or selinexor (f). Each symbol denotes an individual animal (n =4–8 per group). Scale bar (white) =10 µm. White arrows point to the AML blast cells. Error bars represent mean ±s.e.m. values; *P < 0.05 by unpaired t-test for comparisons between the indicated groups. **P < 0.001.
Patient-derived xenograft AML-CN
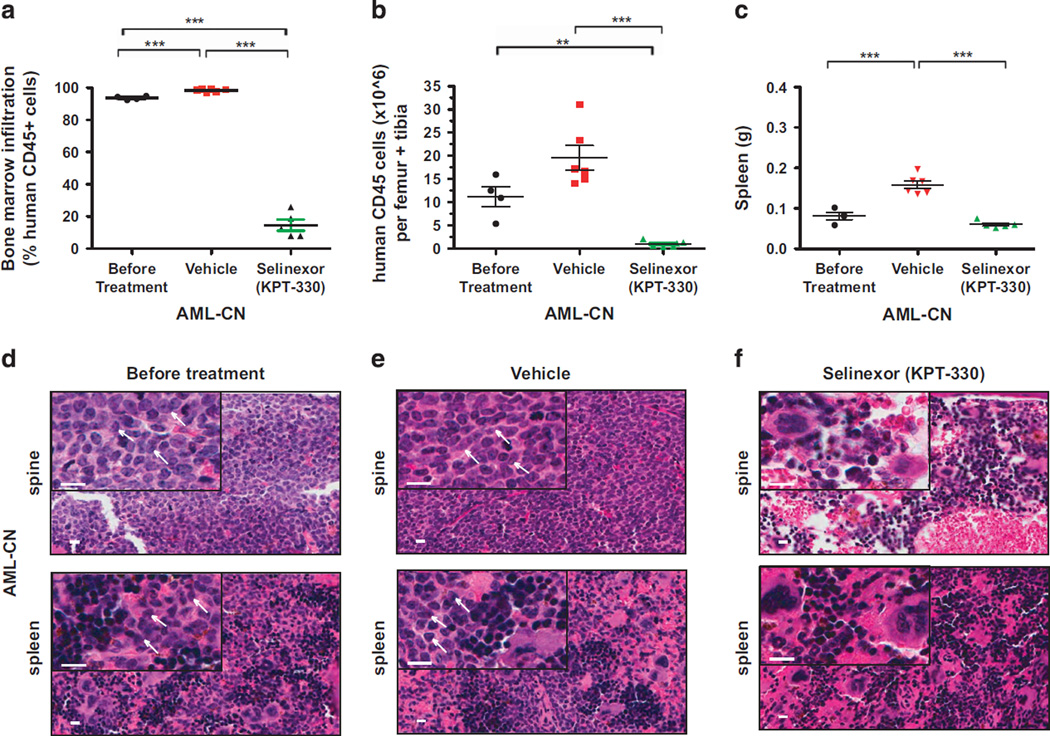
NSG mice injected with 1.74 × 105 leukemia cells from the patient with AML-CN developed leukemia 50 days after engraftment. hCD45+ cells accounted for 93.7% ± 0.57% of bone marrow leukocytes, and the AML blast count of 11.2 × 106 ± 2.2 × 106 hCD45+ cells per femur and tibia, confirming leukemia progression in these animals (Figures 4a and b; Supplementary Figure S3A). In vehicle-treated mice, hCD45+ cells made up 98.4% ± 0.38% of bone marrow leukocytes, with the total hCD45+ cell counts per femur and tibia increasing to 19.5 × 106 ± 2.6 × 106 cells. By contrast, the bone marrow infiltration by hCD45+ cells in selinexor-treated animals decreased to 14.5% ± 3.5% of bone marrow leukocytes, with hCD45+ cell counts per femur and tibia reduced by 11.2-fold to 1.00 × 106 ± 0.31 × 106 cells (Figures 4a and b; Supplementary Figure S3C). The weights of spleens isolated from mice treated with selinexor decreased by approximately threefold as compared with controls (Figure 4c). Hematoxylin and eosin-stained sections of the bone marrow and spleens from mice bearing AML-CN cells show reduction in leukemic infiltration and demonstrate a negligible toxic response to selinexor therapy (Figures 4d–f). These data demonstrate a dramatic reduction in the leukemia burden in selinexor-treated PDX model of AML-CN.
Figure 4.
Selinexor (KPT-330) demonstrates strong activity against primary AML-CN cells engrafted into NSG mice. (a) Infiltration of human leukemic cells in the bone marrow of NSG mice transplanted with AML-CN cells before and after treatment with vehicle or selinexor. Bone marrow infiltration is indicated by the percentage of human CD45+ cells in femurs and tibias. (b) Human CD45+ cells counts per femur and tibia in NSG mice transplanted with AML-CN cells, before and after treatment with either vehicle or selinexor. (c) Comparison of spleen weights of mice with human AML-CN leukemia before and after treatment with vehicle or selinexor. (d–f) Hematoxilin and eosin staining of spines of mice transplanted with AML-CN cells isolated from killed animals before and after treatment with either vehicle control (e) or selinexor (f). Extensive human leukemia cell infiltration is apparent in the spine bone and spleen before treatment (d) and in vehicle-treated mice (e). By contrast, normal hematopoietic cell differentiation and maturation can be seen in selinexor-treated mice (f). Each symbol denotes an individual animal (n=4–6 per group). Scale bar (white) indicator =10 µm. White arrow point to the AML blast cells. Error bars represent mean±s.e.m. values. ***P < 0.0001 by unpaired t-test for comparisons between the indicated groups. **P < 0.001.
To demonstrate the inhibitory effects of selinexor on XPO1 activity, we tested for the induction of XPO1 mRNA levels in AML-CN cells engrafted in PDX AML-CN in response to drug treatment. Upregulation of XPO1 mRNA levels likely occurs because of the compensatory effects in response to loss of XPO1 activity and has been previously established as a pharmacodynamic marker for selinexor response.34,40 As shown in Supplementary Figure 5, XPO1 mRNA levels increased by 1.7-fold in selinexor-treated AML-CN as compared with vehicle controls (Supplementary Figure 5).41 Moreover, mRNA levels of other known XPO1 response genes HSPA4L, ARRDC3, and NGFR are dramatically upregulated in selinexor-treated AML-CN blasts (Supplementary Figure S5). Furthermore, inhibition of XPO1 activity by selinexor induced nuclear retention of the XPO1 cargo FOXO3A (Supplementary Figure S6). Taken together, these data indicate the inhibitory effects of selinexor on XPO1 activity in primary human AML cells engrafted into mice.
Selinexor targets the LICs of primary AML
We next asked whether selinexor kills the LIC from patients AML-CK1, AML-CK2, or AML-CN. To determine the frequency of LICs before and after treatment with selinexor, we performed a limiting dilution transplantation assay. As shown in the experimental scheme in Figure 5a, the bone marrow cells were isolated from the femurs and tibias of mice engrafted with patient AML blasts after treatment with either vehicle or selinexor and retransplanted at serial dilutions (doses of 106–102 hCD45+ cells) by intravenous injection into new untreated recipient NSG mice (Figure 5a). After 19–22 weeks, the secondary recipients were analyzed for the development of leukemia as determined by the presence of hCD45+ cells in the mouse bone marrow. The number of leukemic mice in each group transplanted with specific numbers of hCD45+ cells was used to calculate the LIC frequency by linear regression analysis.38 Comparison of LIC frequency for patient xenografts treated with vehicle vs selinexor was then used to assess the antileukemic activity of selinexor against LICs in each of the three AML xenografts.
Figure 5.
Selinexor (KPT-330) targets the LICs of three primary AML samples engrafted into NSG mice. (a) Scheme for the limiting dilution transplantation assay. Bone marrow AML cells in xenografts treated with either vehicle or selinexor were re-transplanted at different serial dilutions into new recipient mice. The number of leukemic mice per total number of animals injected with AML cells in secondary recipients was used to determine the LIC frequency in vehicle- and selinexor-treated AML cell populations. LIC frequency of AML cells in AML-CK1 (b), AML-CK2 (c), and AML-CN (d) xenografts after treatment with either vehicle or selinexor. The table shows the number of leukemic mice in each secondary recipient group and reports the LIC frequencies with 95% confidence intervals for the three xenografts. *Pilot experiment for xenograft AML-CN (d), which demonstrated that 103 hCD45+ cells isolated from vehicle-treated mice were sufficient to initiate leukemia in secondary recipients, was used to guide the hCD45+ cell doses for determining the LIC frequencies in this xenograft.
For AML-CK1, results of the limiting dilution transplantation assay clearly illustrate a decrease in LIC frequency, from 1/1615 for vehicle-treated patient xenografts to 1/9631 for selinexor-treated xenografts, representing a 5.96-fold reduction in LIC number in response to therapy (Figure 5b). The most striking result was obtained for AML-CK2, where the LIC frequencies for vehicle and selinexor-treated xenografts were 1/100 and 1/43 413, respectively, representing a >434-fold reduction in the LIC frequency in response to treatment with selinexor (Figure 5c). The LIC frequency in the AML cells isolated from xenograft AML-CN was 1/3499, which decreased by ~ 171-fold to 1/597 842 after treatment with selinexor (Figure 5d). Taken together, these results demonstrate that selinexor is highly cytotoxic to the LIC isolated from patients with diverse forms of high-risk AML, including normal karyotype, FLT3-ITD AML and complex karyotype disease. Importantly, AML-CK2 xenografts showed only a modest response to selinexor treatment in primary mice (Figures 3a and b), demonstrating that assessment of reduction in bulk leukemic engraftment may underestimate the effects of this drug against LICs.
Selinexor has little cytotoxicity against normal CD34+ stem and progenitor cells
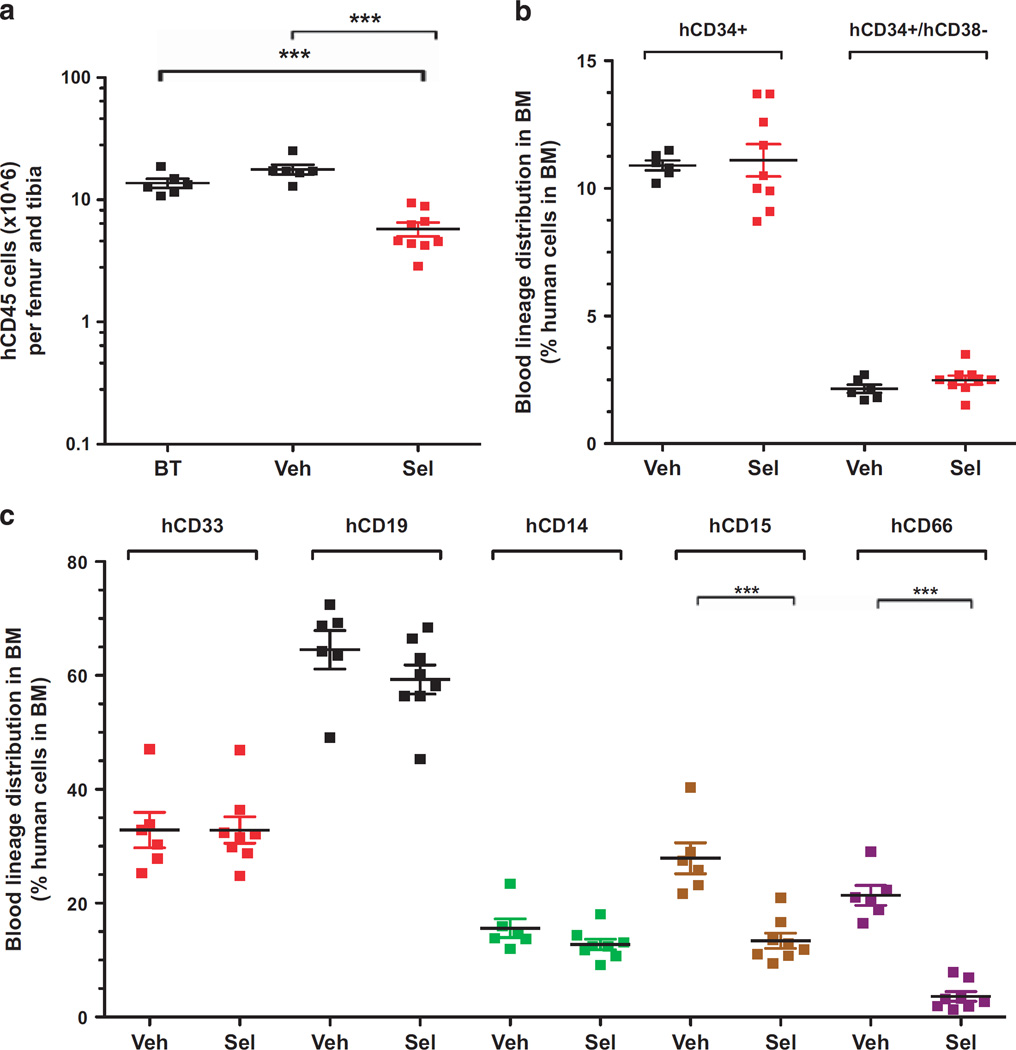
To determine the effects of KPT-330 on normal human hematopoietic cells, we established xenografts by injecting NSG mice with hCD34+ cells isolated from 10 pooled cord blood samples. Blood samples from engrafted mice were monitored at 9 weeks and then every 2 weeks thereafter for the appearance of hCD45+ cells. Eleven weeks postinjection, successful engraftment of human blood cells was indicated by the presence of 32.9% ± 1.9% of hCD45+ cells in the peripheral blood of mice (Supplementary Figure S4). Engrafted mice were split into two groups and treated orally with either vehicle or selinexor (20mg/kg) three times a week for 4 weeks. At the end of the treatment period, the bone marrows of mice treated with selinexor contained a slightly lower percentage of hCD45+ leukocytes compared with vehicle-treated controls: 70.5% ± 2.3% compared with 85.2% ± 1.5% (Supplementary Figure S4B). Total hCD45+ leukocyte counts per femur and tibia were reduced from 17.7 × 106 ± 1.6 × 106 cells in vehicle-treated mice to 5.8 × 106 ± 0.75 × 106 in selinexor- treated mice (Figure 6a). However, the percentage of hematopoietic stem and progenitor cells (hCD34+ and hCD34+CD38−) was not decreased in selinexor-treated compared with vehicle control mice, indicating that, by contrast with AML LIC, selinexor spares normal hematopoietic stem and progenitor cells (Figure 6b). Importantly, the fold decrease in absolute counts of normal hematopoietic stem and progenitor cells after selinexor therapy was much lower that the fold reduction in LIC frequency of the AML cells isolated from selinexor-treated xenografts, indicating that selinexor provides a therapeutic window for targeting LICs. Moreover, the myelo-lymphoid differentiation profile of the bone marrow cells of mice treated with selinexor demonstrated little effect of the drug on the distribution of myeloid (hCD33+), lymphoid (hCD19+) and monocytic (hCD14+) bone marrow progenitors (Figure 6c). However, the percentages of myelomonocytic (hCD15+) and granulocyte (hCD66+) progenitors were reduced by ~ 2.1- and ~ 6.2-fold, respectively, in response to treatment with selinexor compared with controls (Figure 6c). Taken together, these results suggest that selinexor has selective activity against LICs, with relative sparing of normal hematopoietic stem and progenitor cells.
Figure 6.
Selinexor (KPT-330) spares normal human hematopoietic cells engrafted into NSG mice. (a) Total counts of hCD45+ cells per femur and tibia in mice engrafted with cord blood hCD34+ cells before and after treatment with either vehicle or selinexor at 20 mg/kg for 4 weeks. (b) Percentage of hCD34+ and hCD34+/CD38 − cells in the bone marrow (BM) of established grafts treated with either vehicle or selinexor. (c) Distribution of human blood lineages in the BM of grafts treated with either vehicle or selinexor. Sel, Selinexor, BT, before treatment. n=6–9 mice per group; Error bars represent mean±s.e.m.; ***P < 0.0001 by Student’s t-test for comparison between the indicated groups.
Selinexor rapidly induced death signaling in AML cells treated ex vivo
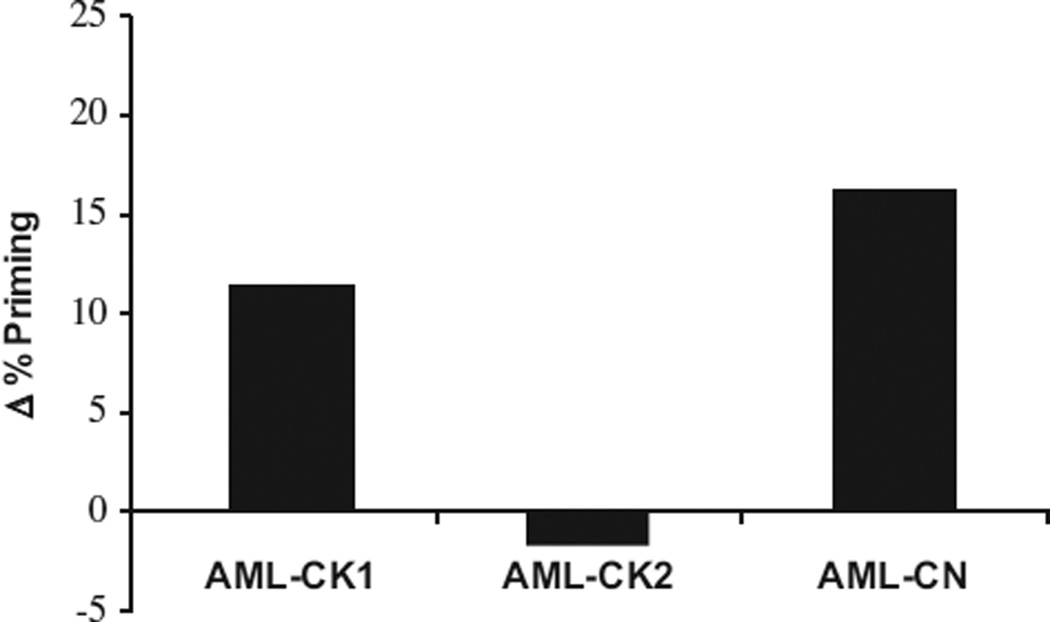
Our previous work demonstrated involvement of intrinsic apoptosis and an increase in mitochondrial priming in triggering cell death in response to selinexor.28,30 In this study, we determined the increase in apoptotic priming in response to selinexor of the three xenografts by a dynamic BH3 profiling strategy.36,37,41,42 This technique measures drug-induced increase in mitochondrial apoptotic priming after a 16-h incubation to predict if tumor cells will respond to therapy. As shown in Figure 5, PDXs AML-CK1 and AML-CN, but not AML-CK2, were primed for apoptosis upon 16 h of ex vivo selinexor treatment (Figure 7), correlating with the observed in vivo response (Figures 2–4). These data indicate that selinexor promotes mitochondrial apoptosis to kill primary AML cells. It also suggests that dynamic BH3 profiling should be prospectively evaluated as a predictive biomarker for selinexor response in upcoming clinical trials.
Figure 7.
Selinexor induces death signaling in primary AML cells. Bone marrow cells from AML xenografts were exposed ex vivo to 100 nM KPT-330 for 16 h and then Dynamic BH3 profiling was performed. Selinexor induced an increase in mitochondrial priming (Δ% priming) in AML-CN and AML-CK1 but not AML-CK2.
DISCUSSION
Our findings establish the antileukemic activity of the nuclear export inhibitor selinexor, an oral clinical stage SINE class of XPO1 antagonists, in human primary AML cells engrafted into immunodeficient NSG mice. Results of the therapeutic experiments in mice bearing AML xenografts demonstrate that selinexor is highly active against blast cells from two of the three patients with poor-prognosis disease. Importantly, serial dilution transplantation assays reveal that selinexor therapy greatly reduced the frequency of LICs in xenografts derived from all three patients, indicating that this agent not only targets the bulk population of leukemic cells but eliminates LICs as well. Moreover, in mice engrafted with AML cells derived from one of the patients with complex karyotype AML (AML-CK2), selinexor significantly decreased LIC frequency despite exerting only modest antileukemic activity against bulk disease. These results underscore the importance of using stem cell assays when evaluating the effects of new cancer drugs, as measurements of reduction in bulk populations may underestimate potency against the disease-sustaining stem cells.
The capability of selinexor to target the AML LICs in xenografts established from human AML samples has important therapeutic implications. Current regimens of chemotherapy for AML often lead to initial disease remissions, but these are typically followed by a high relapse rate, regardless of the intensity of postinduction chemotherapy. Because AML likely relapses due to resistance of the LIC subpopulation to conventional therapies, agents that target both LICs and the bulk leukemia cell population are needed to achieve long-term remissions with curative potential. Thus the ability of selinexor to eliminate the LICs that give rise to the rapidly proliferating myeloid blasts and ultimately lead to disease recurrence portends the clinical utility of this new, first-in-class agent in the treatment of AML. Because AML is a genetically heterogeneous clonal disorder, future work is needed to identify biomarkers to predict the responsiveness of LICs to selinexor, as well as optimal schedules for the integration of selinexor with standard chemotherapeutic agents to eradicate the LIC subpopulation.
Most drugs used in the treatment of AML also kill normal hematopoietic progenitor cells, often leading to bone marrow hypoplasia with pancytopenia as the dose-limiting toxicity. Our findings show that selinexor causes minimal toxicity to normal hematopoietic cells, as demonstrated by the normal differentiation and maturation of hematopoietic cells in the bone marrows and spleens of mice after 4 weeks of treatment with selinexor (Figures 2, 3, 4 and 6 and our previous work28,30). Notably, normal bone marrow cellularity and function were restored in the mice while they are receiving selinexor. In mice engrafted with normal cord blood human CD34+ cells, selinexor reduced bone marrow hCD45+ cell numbers, but the impact on normal hematopoietic stem and progenitor cells was modest compared with the decreases seen in LIC frequency after selinexor treatment, supporting the existence of a therapeutic window for targeting LICs.
Nuclear export by XPO1 maintains the cellular distribution of a variety of protein and RNA molecules that function as tumor suppressors and are involved in apoptotic signaling and cell cycle regulation. Selinexor spares normal hematopoietic cells, raising the possibility that normal cells are less reliant on continuous high levels of nuclear export for survival. Although the exact molecular mechanisms underlying the selective antileukemic activity of selinexor remain to be elucidated, it is likely that nuclear retention of XPO1-regulated tumor-suppressor proteins, such as p53, p21, FOXO, PP2A, BRCA1 and survivin, leads to apoptotic signaling in neoplastic cells. We suggest that requirement for nuclear-cytoplasmic export of key proteins in normal cells is not as stringent as that in malignant cells that undergo apoptosis when treated with selinexor.
These combinatorial changes in protein or RNA distribution may also contribute to the high degree of differential sensitivity of the AML blasts and LICs observed in mice engrafted with AML cells derived from one of the patients with complex karyotype AML (AML-CK2). We hypothesize that the differential sensitivity of LICs and the bulk AML cells in response to selinexor observed in patient AML-CK2 could be due to the effects of the drug on self-renewal pathways that are aberrantly activated in LICs but are not active in the bulk leukemia cell population. Because they represent a minor cell population, the responsiveness of LICs cannot be inferred from studies of the bulk AML cell population and thus have to be assayed independently through serial dilution retransplantation studies, such as those we have performed here.
From previous studies, it is clear that LICs are often not as responsive as the bulk AML cells to cytarabine, presumably because the LIC are relatively quiescent. We showed in AML cell lines that selinexor-induced cytotoxicity is not cell-cycle dependent, suggesting the importance of PDX models to assay the responsiveness of LICs through retransplantation. Because of the unique activity of selinexor in inhibiting XPO1, we postulate that it is more active against quiescent LICs than the bulk leukemia cells, because one of its mechanisms of action is to interfere with pathways that are needed for the re-entry of resting or G0 cells into the cell cycle. This particular aspect of selinexor activity would be expected to focus on the LIC and not the regular growth of the ‘transit-amplifying’ cells that comprise the bulk AML cell population.
Selinexor entered clinical Phase I trial in patients with hematological malignancies (NCT01607892) in 2012 and is currently being tested in patients with relapsed or refractory AML (NCT02249091, NCT02088541, NCT02093403, NCT02299518). The preliminary results of this trial are encouraging, as they have demonstrated that selinexor is active in patients with relapsed or refractory AML at well-tolerated dosages, in that 4 of the 14 heavily pretreated patients enrolled have achieved complete response or complete response with incomplete hematological recovery.31 In summary, our results demonstrate that selinexor potently targets self-renewing LIC populations in AML, making it one of the very few drugs that are in clinical trials in patients that kill self-renewing AML cells. Given that LICs are thought to be responsible for causing relapse after successful initial induction of remission in patients with AML, our findings strongly support further clinical testing of selinexor to clarify its activity in combination regimens for the treatment of patients with both newly diagnosed and advanced AML.
Supplementary Material
Acknowledgments
We thank the patients for their cooperation in providing bone marrow samples for this study; Ilene Galinsky for providing the patient samples used to generate mouse PDX models of human AML; Nancy Kohl, Paul Kirschmeier and Prafulla Gokhale for assistance in guiding in vivo studies at the Lurie Family Imaging Center; John Gilbert for his editorial review and critical reading of the manuscript; and John Daley and the flow cytometry team for their valuable advice. This research was supported by William Lawrence and Blanche Hughes Foundation (to ATL), Leukemia and Lymphoma Society’s Translational research grant (to ATL), Alex’s Lemonade Stand Foundation’s Young Research Investigator grant (to JE), Luck2Tuck Foundation (to JE), Claudia Adams Barr Innovative Basic Science Research grant (to MRM) and Lady Tata Memorial Trust (to CELN).
Footnotes
CONFLICT OF INTEREST
BK, SS, MK and YL are employees of Karyopharm Therapeutics Incorporated and receive compensation and hold equity in the company. The other authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
JE designed and performed experiments, analyzed data and wrote the manuscript. BTL and AB performed experiments and analyzed data. ALC, CR and AB helped design and perform in vivo mouse studies. AK, MRM, CELN and SA participated in experimental design and data analysis. SS and MK designed the KPT-330 compound and participated in the design of in vivo mouse studies. IAG and RMS provided AML patient samples and helped to analyze the data. SJR interpreted the hematopathology. BK, YL, WCC, JCYW and ALK designed experiments and analyzed the data. JM and AL designed and performed BH3 profiling experiments and analyzed data. ATL guided the research, analyzed the results and wrote the paper.
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
REFERENCES
- 1.Estey EH. Acute myeloid leukemia: 2014 update on risk-stratification and management. Am J Hematol. 2014;89:1063–1081. doi: 10.1002/ajh.23834. [DOI] [PubMed] [Google Scholar]
- 2.Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–1089. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 4.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5:738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 5.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 6.Gilliland DG, Jordan CT, Felix CA. The molecular basis of leukemia. Hematology Am Soc Hematol Educ Program. 2004:80–97. doi: 10.1182/asheducation-2004.1.80. [DOI] [PubMed] [Google Scholar]
- 7.Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M. CRM1 is responsible for intracellular transport meditted by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 9.Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- 10.Turner JG, Dawson J, Sullivan DM. Nuclear export of proteins and drug resistance in cancer. Biochem Pharmacol. 2012;83:1021–1032. doi: 10.1016/j.bcp.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stade K, Ford CS, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- 12.Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL. eIF4E is a central node of an RNA regulon that governs cellular proliferation. J Cell Biol. 2006;175:415–426. doi: 10.1083/jcb.200607020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu D, Farmer A, Chook YM. Recognition of nuclear targeting signals by Karyopherin-beta proteins. Curr Opin Struct Biol. 2010;20:782–790. doi: 10.1016/j.sbi.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong X, Biswas A, Chook YM. Structural basis of assembly and disassembly of the CRM1 nuclear export complex. Nat Struct Mol Biol. 2009;16:558–560. doi: 10.1038/nsmb.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong X, Biswas A, Suel KE, Jackson LK, Martinez R, Gu H, et al. Structural basis for leucine-rich nuclear export signal recognition by CRM1. Nature. 2009;458:1136–1141. doi: 10.1038/nature07975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guttler T, Gorlich D. Ran-dependent nuclear export mediators: a structural perspective. EMBO J. 2011;30:3457–3474. doi: 10.1038/emboj.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutten S, Kehlenbach RH. CRM1-mediated nuclear export: to the pore and beyond. Trends Cell Biol. 2007;17:193–201. doi: 10.1016/j.tcb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Koyama M, Matsuura Y. An allosteric mechanism to displace nuclear export cargo from CRM1 and RanGTP by RanBP1. EMBO J. 2010;29:2002–2013. doi: 10.1038/emboj.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu D, Farmer A, Collett G, Grishin NV, Chook YM. Sequence and structural analyses of nuclear export signals in the NESdb database. Mol Biol Cell. 2012;23:3677–3693. doi: 10.1091/mbc.E12-01-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu D, Grishin NV, Chook YM. NESdb: a database of NES-containing CRM1 cargos. Mol Biol Cell. 2012;23:3673–3676. doi: 10.1091/mbc.E12-01-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima K, Kornblau SM, Ruvolo V, Dilip A, Duvvuri S, Davis RE, et al. Prognostic impact and targeting of CRM1 in acute myeloid leukemia. Blood. 2013;121:4166–4174. doi: 10.1182/blood-2012-08-447581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lapalombella R, Sun Q, Williams K, Tangeman L, Jha S, Zhong Y, et al. Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia. Blood. 2012;120:4621–4634. doi: 10.1182/blood-2012-05-429506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang WY, Yue L, Qiu WS, Wang LW, Zhou XH, Sun YJ. Prognostic value of CRM1 in pancreas cancer. Clin Invest Med. 2009;32:E315. [PubMed] [Google Scholar]
- 24.Inoue H, Kauffman M, Shacham S, Landesman Y, Yang J, Evans CP, et al. CRM1 blockade by selective inhibitors of nuclear export attenuates kidney cancer growth. J Urol. 2013;189:2317–2326. doi: 10.1016/j.juro.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen A, Wang Y, Zhao Y, Zou L, Sun L, Cheng C. Expression of CRM1 in human gliomas and its significance in p27 expression and clinical prognosis. Neurosurgery. 2009;65:153–159. doi: 10.1227/01.NEU.0000348550.47441.4B. discussion 159–160. [DOI] [PubMed] [Google Scholar]
- 26.Walker CJ, Oaks JJ, Santhanam R, Neviani P, Harb JG, Ferenchak G, et al. Preclinical and clinical efficacy of XPO1/CRM1 inhibition by the karyopherin inhibitor KPT-330 in Ph+ leukemias. Blood. 2013;122:3034–3044. doi: 10.1182/blood-2013-04-495374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao Y, Dong Y, Lin F, Zhao H, Shen Z, Chen P, et al. The expression of CRM1 is associated with prognosis in human osteosarcoma. Oncol Rep. 2009;21:229–235. [PubMed] [Google Scholar]
- 28.Etchin J, Sanda T, Mansour MR, Kentsis A, Montero J, Le BT, et al. KPT-330 inhibitor of CRM1 (XPO1)-mediated nuclear export has selective anti-leukaemic activity in preclinical models of T-cell acute lymphoblastic leukaemia and acute myeloid leukaemia. Br J Haematol. 2013;161:117–127. doi: 10.1111/bjh.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalid O, Toledo Warshaviak D, Shechter S, Sherman W, Shacham S. Consensus Induced Fit Docking (cIFD): methodology, validation, and application to the discovery of novel Crm1 inhibitors. J Comput Aided Mol Des. 2012;26:1217–1228. doi: 10.1007/s10822-012-9611-9. [DOI] [PubMed] [Google Scholar]
- 30.Etchin J, Sun Q, Kentsis A, Farmer A, Zhang ZC, Sanda T, et al. Antileukemic activity of nuclear export inhibitors that spare normal hematopoietic cells. Leukemia. 2013;27:66–74. doi: 10.1038/leu.2012.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savona M, Garzon R, de Nully Brown P, Yee K, Lancet JE, Gutierrez M, et al. Phase I trial of selinexor (KPT-330), a first-in-class oral selective inhibitor of nuclear export (SINE) in patients (pts) with advanced acute myelogenous leukemia (AML) Blood. 2013;122 [Google Scholar]
- 32.Ranganathan P, Yu X, Na C, Santhanam R, Shacham S, Kauffman M, et al. Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood. 2012;120:1765–1773. doi: 10.1182/blood-2012-04-423160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt J, Braggio E, Kortuem KM, Egan JB, Zhu YX, Xin CS, et al. Genome-wide studies in multiple myeloma identify XPO1/CRM1 as a critical target validated using the selective nuclear export inhibitor KPT-276. Leukemia. 2013;27:2357–2365. doi: 10.1038/leu.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tai YT, Landesman Y, Acharya C, Calle Y, Zhong MY, Cea M, et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia. 2014;28:155–165. doi: 10.1038/leu.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarry JE, Murphy K, Perry R, Sanchez PV, Secreto A, Keefer C, et al. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rgammac-deficient mice. J Clin Invest. 2011;121:384–395. doi: 10.1172/JCI41495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan J, Letai A. BH3 profiling in whole cells by fluorimeter or FACS. Methods. 2013;61:156–164. doi: 10.1016/j.ymeth.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan JA, Brunelle JK, Letai A. Heightened mitochondrial priming is the basis for apoptotic hypersensitivity of CD4+ CD8+ thymocytes. Proc Natl Acad Sci USA. 2010;107:12895–12900. doi: 10.1073/pnas.0914878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Borrow J, Stanton VP, Jr, Andresen JM, Becher R, Behm FG, Chaganti RS, et al. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14:33–41. doi: 10.1038/ng0996-33. [DOI] [PubMed] [Google Scholar]
- 40.Yosef Landesman WS, Jean-Richard S-M, Trinayan K, Louis P, Vincent S, Sharon S, et al. Abstract 3775: Pharmacokinetic (PK)/pharmacodynamic (PD) and efficacy relationship of selective inhibitors of nuclear export (KPT-SINE) Cancer Res. 2012;72:3775. [Google Scholar]
- 41.Del Gaizo Moore V, Letai A. BH3 profiling--measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer Lett. 2013;332:202–205. doi: 10.1016/j.canlet.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montero J, Sarosiek KA, DeAngelo JD, Maertens O, Ryan J, Ercan D, et al. Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell. 2015;160:977–989. doi: 10.1016/j.cell.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.