Abstract
Toll-like receptors play essential roles in the modulation of melanogenesis, which has been implicated in the pathogenesis of hyper- or hypopigmentation-related diseases. However, little is currently known regarding the role of TLR9 in human melanocytes. TLR9 recognizes unmethylated cytosine-phosphate-guanine motif-containing oligodeoxynucleotides, and cytosine-phosphate-guanine ODN2006 acts as an hTLR9 agonist. The aim of the present study was to investigate the effect of cytosine-phosphate-guanine ODN2006 on melanogenesis in the human melanocyte cells. MTT assay and enzyme-linked immunosorbent assay indicated that ODN2006 stimulation (0, 1, 5, 10 µM) dose-dependently reduced cell viability and promoted the production of TNF-α, IL-6, and IL-8 in PIG1 melanocytes. The mRNA and protein levels of PMEL and TYRosinase were elevated at 6 h, and then decreased 24 h later, but were significantly augmented 72 h later following ODN2006 stimulation; whereas, TLR9 expressions were time-dependently increased in PIG1 melanocytes. Moreover, ultraviolet B irradiation combined with ODN2006 stimulation induced much more significant enhancement of PMEL, TYRosinase, and TLR9 mRNA and protein after three days in PIG1 melanocytes, and the similar results were obtained using the primary human melanocytes. The expression of TLR9 protein was down-regulated by TLR9 siRNA transfection. ODN2006 had an additive effect on ultraviolet B-induced melanogenesis and PMEL expression, as well as NF-κB activation, which could be blocked by TLR9 knockdown, the NF-κB specific inhibitor PDTC, or the TBK1 inhibitor BX795. Collectively, we concluded that TLR9 regulates melanogenesis through NF-κB activation, suggesting that TLR9 may play a role in microbial-induced melanogenesis.
Keywords: Toll-like receptor 9, melanogenesis, PMEL, ODN2006, NF-κB
Introduction
Chronic dermatological conditions often give rise to abnormal skin pigmentation. Skin pigment-related diseases, including melanoma, vitiligo, and seborrheic keratosis (SK) are often associated with excessive or reduced production of melanin.1 Epidermal melanocytes synthesize melanin pigments from TYRosine (TYR) and accumulate melanin in specialized cellular organelles called melanosomes, which are transported from melanocytes to adjacent keratinocytes.2,3 Melanogenesis is the process of producing the melanin pigment, and involves a series of chemical and enzymatic pathways.4 Hence, modulation of this process may become an important approach in the treatment of hyper- or hypopigmentation-related diseases.
Human melanocytes are not simply professional pigment-producing cells, but also have the phagocytic capacity and produce pro-inflammatory mediators. Toll-like receptors (TLRs) have been implicated in both innate host defense against pathogens and inflammatory response. Research has also shown that TLRs activation in melanocytes may play a role in the modulation of melanogenesis.5 However, the mechanisms of recognition of microbes by TLRs in melanocytes have not yet been fully explored.
Among the TLR family members, TLR9 is primarily expressed on antigen-presenting cells and is one of a group of intracellular receptors located in endosomal compartments that are responsible for the recognition of nucleic acids derived from viruses, bacteria, and the host. TLR9 recognized non-methylated cytosine-phosphate-guanine (CpG) motifs in bacterial or viral DNA as foreign, thus playing an essential role in the specific cellular response to CpG DNA.6 Un-methylated CpG motifs are considered to be pathogen-associated molecular patterns (PAMPs) due to their abundance in microbial genomes but scarcity in vertebrate genomes.7 The activation of TLR9 by microbial DNA or synthetic oligonucleotides is based on these motifs, leading to the induction of innate immune responses. In the specific cellular response to CpG DNA, TLR9−/− mice are completely unresponsive to CpG DNA, whereas TLR9+/+, TLR2β/−, and TLR4−/− mice respond normally to CpG DNA.8,9 Additionally, TLR9 has been indicated to play an important role in detecting and combatting viral infections.10 Accordingly, we speculated that TLR9 signaling may participate in the process of melanogenesis in human melanocytes.
Melanocytes can produce substances with a range of biological functions including antimicrobial defense. Meanwhile, TLRs play an important role in the cellular response through the recognition of pathogens. Currently, unmethylated CpG motif-containing oligodeoxynucleotides (ODNs) have been well characterized as agonists of TLR9. The sequence of ODN2006 is a CpG ODN that acts as an hTLR9 agonist and is used as the basic CpG ODN material. Here, we evaluated the stimulatory effects of the TLR9 agonist ODN2006 on melanogenesis in human melanocyte cell line PIG1, as a possible therapeutic agent to address hypopigmentation disorders.
Materials and methods
Cell culture and cell treatment
The immortalized human melanocyte cell line PIG1 was a gift from Professor Caroline Le Poole11 from the Department of Dermatology, University of Cincinnati, USA. Normal primary human melanocytes were isolated from teenaged epidermal foreskin and cultured according to the normal method.11 Cells were cultured in 254 medium supplemented with 5% fetal calf serum (FCS) and human melanocyte growth supplement (S-002-5) at 37℃ in a humidified atmosphere with 5% CO2. The 254 medium, FCS, and S-002-5 were all purchased from Gibco BRL (Gaithersburg, MD, USA). In the following assays, after serum starvation for 24 h, cells were preconditioned with 25 µM pyrrolidine dithiocarbamate (PDTC) or 5 µM BX795 (both from Sigma, St. Louis, MO, USA) for 1 h prior to ODN2006 stimulation (Invitrogen, Carlsbad, CA, USA) and ultraviolet B (UVB) irradiation as indicated for three days.
Immunofluorescence microscopy
Cultured PIG1 melanocytes (3 × 105) were plated onto a glass coverslip. After overnight incubation, cells were washed twice with PBS and fixed with methanol at −20℃ for 15 min, followed by two further washes with PBS. Coverslips were incubated in blocking buffer containing the appropriate primary antibodies at 4℃ for 1 h, and then washed for four times with PBS. The primary antibodies were rabbit anti-premelanosome protein (PMEL) polyclonal Ab, rabbit anti-TYRosinase (TYR) polyclonal Ab, and mouse anti-TLR9 monoclonal Ab (Abcam, Cambridge, MA, USA). Fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (IgG) (Molecular Probes, Eugene, OR) and FITC-conjugated rabbit anti-mouse IgG (Invitrogen, Carlsbad, CA, USA) were used as secondary antibodies. At the final step, the cells were incubated with 1 mg/mL fluorescent dye DAPI (Sigma, Saint Louis, MO, USA) for 30 min to evaluate the position of nucleus. Images of treated cells were captured using an Olympus FluoView FV1000 confocal laser scanning microscope (Olympus, Tokyo, Japan) and analyzed with Olympus FV1000 software FV10-ASW version 2.1 b.
UVB irradiation
UVB radiation was performed using a modification of a previously published protocol.12 In brief, PIG1 melanocytes were cultured in six-well plates and washed once with PBS. Cells were then covered with 1 mL PBS and exposed to UVB (wavelength 296–298 nm) at 20 mJ/cm2 for three days. The UVB was emitted by SS-01B-2 UV phototherapy equipment (Shanghai Sigma High Technology Co., LTD, Shanghai, China) with a light intensity of 11.2 mW/cm2. Immediately after radiation, the PBS was replaced with 2 mL fresh complete medium, and cells were further cultured for a period before being harvested. Cells were divided into four groups to examine the effects of ODN2006 and UVB: control group, ODN2006-treated group (5 µM for three days), UVB-irradiated group (20 mJ/cm2 once daily for three days), and ODN2006 + UVB-treated group [treated with UVB (20 mJ/cm2 once daily for 3 days) following pretreatment with ODN2006].
MTT assay
PIG1 melanocytes (5 × 103) were plated into 96-well plates (Corning Inc., Corning, NY, USA) and incubated overnight. After serum starvation for 24 h, cells were stimulated with ODN2006 (1, 5, 10 µM; Invitrogen, Carlsbad, CA, USA) at 37℃ in 5% of CO2. After 72 h, 20 µL of modified tetrazolium salt 3 -(4,5-dimethyl-2-thiazolyl)-2,5-dipheny-2H-tetrazolium-bromide (MTT, 5 mg/mL; Sigma, Saint Louis, MO, USA) was added to each well and samples were incubated at 37℃ for 4 h. Then, the supernatant was carefully removed and 100 µL of DMSO (Sigma, Saint Louis, MO, USA) was added to lyse the cells. Once the dark-blue MTT crystals had dissolved, the absorbance was measured at 490 nm using a Benchmark microplate reader (Bio-Rad, Hercules, CA, USA).
Enzyme-linked immunosorbent assay PIG1 melanocytes (4 × 105) were plated into six-well plates (Corning Inc.) and incubated overnight. After serum starvation for 24 h, cells were cultured in the presence of different concentrations of ODN2006 (1, 5, 10 µM) at 37℃ in 5% of CO2. After stimulation for 72 h, culture supernatants were harvested. Cytokines, including TNF-α, IL-6, and IL-8, contained in the conditioned medium were analyzed in triplicate using sandwich-type ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. The absorbance at 450 nm was determined using a microplate reader.
Real-time reverse transcription-polymerase chain reaction (RT-PCR)
PIG1 melanocytes (4 × 105) were plated into six-well plates and cultured overnight. After serum starvation for 24 h, cells were treated as indicated. The total RNA was purified using the RNeasy Mini kit (Qiagen, German) according to the manufacturer’s instructions. For real-time reverse transcription-polymerase chain reaction (RT-PCR) analyses, 1 µg of DNase-treated total RNA was reverse transcribed. The amplification of the cDNA was accomplished using the ABI Prism 7900HT sequence detection system (Applied Biosystems) in the presence of the commercially available SYBR Green PCR Master Mix (Takara) in a 40-cycle PCR. The denaturing, annealing, and extension conditions of each PCR cycle were 95℃ for 5 s, 60℃ for 20 s and 72℃ for 34 s, respectively. The relative expression was calculated using the 2−ΔΔCt method. The mRNA expression levels were normalized to the levels of β-actin and represented as fold induction. The primer sequences of PMEL, TYR, TLR9, and β-actin used in this RT-PCR analysis are shown in Table 1 and were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China).
Table 1.
The sequence of primers used for real-time RT-PCR
| Gene | Sequence |
|---|---|
| Pmel17 | 5′-CCC CAG GAA ACT GAC GAT GC-3′ |
| 5′-AGC CAC AGG AGG TGA GAG GAA T-3′ | |
| Tyr | 5′-GGC CTC AAT TTC CCT TCA CA-3′ |
| 5′-CAG AGC ACT GGC AGG TCC TAT-3′ | |
| TLR9 | 5′-GTG CCC CAC TTC TCC ATG-3′ |
| 5′-GGC ACA GTC ATG ATG TTG TTG-3′ | |
| β-actin | 5′-CTG GAA CGG TGA AGG TGA CA-3′ |
| 5′-AAG GGA CTT CCT GTA ACA ATG CA-3′ |
RT-PCR: reverse transcription-polymerase chain reaction.
Melanin content determination
After serum starvation for 24 h, cells were treated as indicated. The total amount of melanin was measured using the sodium hydroxide solubilization method.13 Briefly, the harvested cells were dissolved in 1 mL of 1 N NaOH at 100℃ for 30 min and centrifuged for 20 min at 16,000 g. The optical densities of the supernatants were measured at 400 nm using an ELISA reader. The absorbance was compared with a standard curve of synthetic melanin (Sigma, St. Louis, MO, USA).
siRNA transfection
For siRNA transfection, PIG1 melanocytes (4 × 105) were seeded in six-well plates, grown overnight, and transfected on the following day with TLR9-siRNA (50 nM; GenePharma, Shanghai, China) or the non-silencing control siRNA (si-NS, 50 nM; GenePharma, Shanghai, China), as indicated using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. The knockdown efficiency was verified 48 to 72 h after transfection.
Western blot analysis
Cell lysates were collected and protein concentrations were determined using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA). Equal amounts of protein were processed for Western blotting following the standard protocols. The primary antibodies used were rabbit anti-PMEL monoclonal Ab, rabbit anti-TYR polyclonal Ab, mouse anti-TLR9 monoclonal Ab, and rabbit anti-β-actin polyclonal Ab (dilution, 1:1000; Abcam, Cambridge, MA, USA), and rabbit anti-NF-κB p65 polyclonal Ab (dilution, 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After incubation with proper secondary antibodies linked to horseradish peroxidase (HRP), the resultant protein bands were visualized by ECL (Beyotime, Shanghai, China). The absorbance values of the target proteins were calculated using Gel-Pro Analyzer version 4.0 software (Media Cybernetics, Silver Spring, MD, USA) and normalized to β-actin for quantification analysis.
Statistical analysis
Data are expressed as mean ± standard deviation of results derived from three individual experiments performed in triplicate. All analyses were conducted using SPSS software (SPSS Inc., Chicago, IL, USA). Statistical analysis was performed using Student's t-test and analysis of variance. *P < 0.05 was considered to indicate a statistically significant difference.
Results
ODN2006 reduced cell viability and promoted pro-inflammatory cytokine production in PIG1 melanocytes
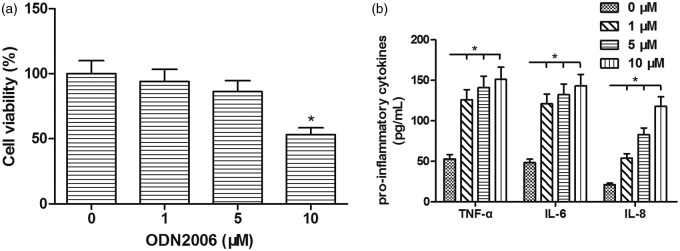
Using the MTT assay, we found that ODN2006 stimulation (1, 5, 10 µM) reduced the cell viability of PIG1 melanocytes in a dose-dependent manner. After 72 h incubation, the viability of cells exposed to 1 µM ODN2006 showed little change. A slight decrease of 14% was induced by 5 µM ODN2006, while 10 µM ODN2006 led to a notable 47% reduction in cell viability in PIG1 melanocytes (Figure 1(a)). In addition, ELISA demonstrated that ODN2006 incubation (1, 5, 10 µM) enhanced the levels of TNF-α, IL-6, and IL-8 in a dose-dependent manner in PIG1 melanocytes (Figure 1(b)). Thus, 5 µM was chosen as the optimal ODN2006 dose for subsequent assays.
Figure 1.
Effect of ODN2006 stimulation on cell viability and pro-inflammatory cytokines production in PIG1 melanocytes. After serum starvation for 24 h, PIG1 melanocytes were treated with ODN2006 at different concentrations (1, 5, 10 µM) for 72 h, and then subjected to respective detection. (a) The cell viability of PIG1 melanocytes was assessed using MTT assay. (b) The levels of TNF-α, IL-6, and IL-8 in PIG1 melanocytes were assessed using commercial ELISA kits
ODN2006 regulated the expression of PMEL, TYR, and TLR9 in PIG1 melanocytes
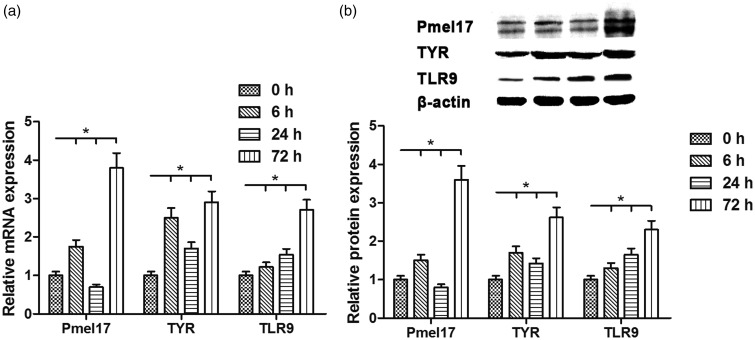
The results indicated that ODN2006 stimulation clearly regulated the expression of PMEL, TYR, and TLR9 to different degrees as indicated by real-time RT-PCR and Western blotting in PIG1 melanocytes. The mRNA and protein levels of PMEL and TYR were increased after 6 h, and then decreased 24 h later, but significantly augmented 72 h later following ODN2006 stimulation; in contrast, the expression of TLR9 was enhanced at the mRNA and protein levels in a time-dependent manner (Figure 2(a) and (b)).
Figure 2.
Effects of ODN2006 stimulation on the mRNA and protein expression levels of PMEL, TYR, and TLR9 in PIG1 melanocytes. After serum starvation for 24 h, PIG1 melanocytes were treated with 5 µM ODN2006 for 0, 6, 24, and 72 h, and then subjected to respective detection. (a) The mRNA levels of PMEL, TYR, and TLR9 were evaluated using real-time RT-PCR. (b) The protein expression levels of PMEL, TYR, and TLR9 were evaluated using Western blotting
ODN2006 had an additive effect on UVB-induced expression of PMEL, TYR, and TLR9 in human melanocytes
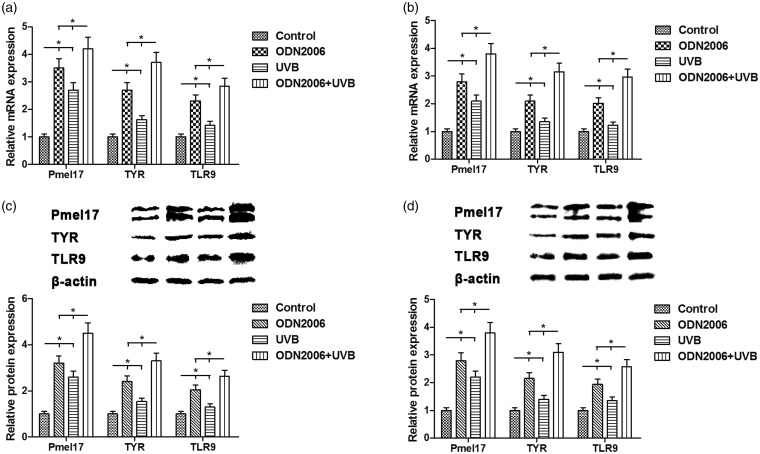
Real-time RT-PCR and Western blotting analyses showed that the expression levels of PMEL, TYR, and TLR9 mRNA and protein were also obviously elevated after UVB irradiation for three days, and this was reinforced by ODN2006 stimulation in PIG1 melanocytes (Figure 3(a) and (c)). The similar results were obtained using the primary human melanocytes in this study (Figure 3(b) and (d)). Hence, we concluded that ODN2006 stimulation could regulate the expression of PMEL, TYR, and TLR9 by strengthening the action of UVB irradiation in human melanocytes.
Figure 3.
Effects of ODN2006 stimulation and UVB irradiation on the mRNA and protein expression levels of PMEL, TYR, and TLR9 in human melanocytes. (a, c) After serum starvation for 24 h, PIG1 melanocytes were treated with UVB (20 mJ/cm2 once daily) following pretreatment with ODN2006 for three days, and then subjected to respective detection. The mRNA and protein expression levels of PMEL, TYR, and TLR9 were evaluated using real-time RT-PCR and Western blotting, respectively. (b, d) After serum starvation for 24 h, the primary human melanocytes were treated with UVB (20 mJ/cm2 once daily) following pretreatment with ODN2006 for three days, and then subjected to respective detection. The mRNA and protein expression levels of PMEL, TYR, and TLR9 were evaluated using real-time RT-PCR and Western blotting, respectively
ODN2006 stimulation enhanced melanogenesis via TLR9/NF-κB signaling in PIG1 melanocytes
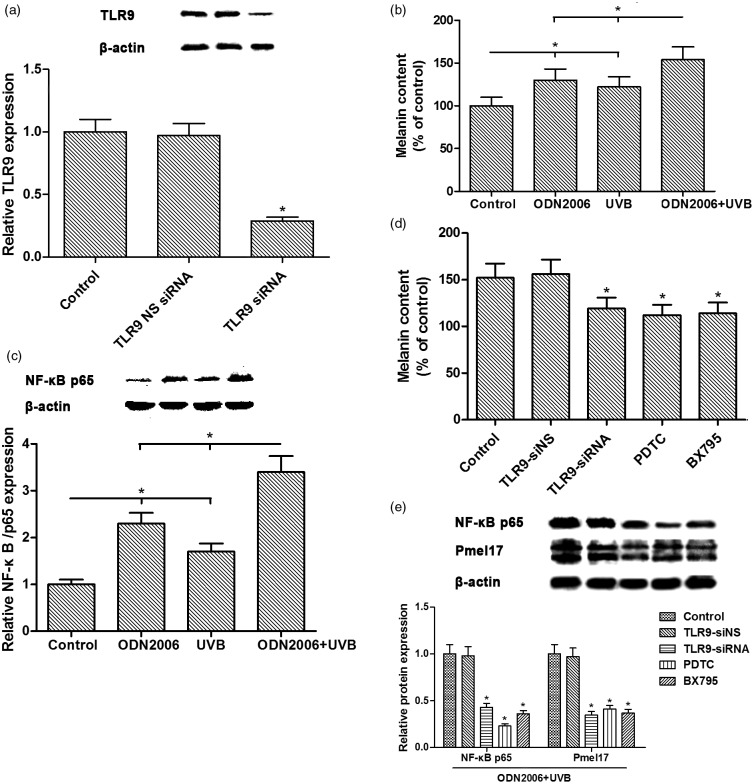
Compared with the control group, ODN2006 stimulation and UVB irradiation induced a significant increase in melanin content. Compared with the TLR9 NS siRNA group, the expression of TLR9 protein was down-regulated by TLR9 siRNA transfection (Figure 4(a)). Compared with the UVB-irradiated group, the ODN2006 + UVB-treated group showed a much more significant increase in melanin content, suggesting that ODN2006 treatment had an additive effect on UVB-induced melanin synthesis (Figure 4(b)). To elucidate the intracellular signaling pathways involved in ODN2006- and UVB-induced melanogenesis, we analyzed NF-κB signaling in human melanocytes treated with ODN2006 and UVB irradiation. Western blot analysis revealed that the expression of NF-κB p65 was significantly up-regulated in the ODN2006-treated group and UVB-treated group, and was even higher in the ODN2006 + UVB-treated group (Figure 4(c)). The increase in melanin content and the expression of NF-κB p65 and PMEL caused by ODN2006 stimulation and UVB irradiation were alleviated by TLR9 siRNA transfection and NF-κB-specific inhibitor PDTC, as well as TANK-binding kinase 1 (TBK1) inhibitor BX795 pretreatment, respectively (Figure 4(d) and (e)).
Figure 4.
Effects of ODN2006 stimulation and UVB irradiation on melanin synthesis and TLR9/NF-κB signaling pathway in PIG1 melanocytes. After serum starvation for 24 h, PIG1 melanocytes transfected with or without TLR9-siRNA/TLR9-siNS were preconditioned with 25 µM PDTC or 5 µM BX795 for 1 h, and then treated with ODN2006 followed by UVB treatment (20 mJ/cm2 once daily) for three days, and then subjected to respective detection. (a, c, e) The expression of TLR9, NF-κB p65, PMEL, and β-actin proteins was evaluated using Western blotting. (b, d) Melanin content was determined by measuring the absorbance at 400 nm. The sodium hydroxide solubilization method was used to determine the melanin content
Discussion
Several studies suggest that TLRs play essential roles in the pathogenesis of melanogenesis. Among them, TLR9 is specific for unmethylated CpG ODNs that are present in bacterial DNA, and the short synthetic single-stranded CpG ODNs can induce the Th1-type immune response through interaction with TLR9. But whether TLR9 is involved in the pathogenic process of melanogenesis and further, the related cellular mechanisms are still not well understood. In this study, we treated the human melanocytes PIG1 with ODN2006 and UVB to evaluate the role of TLR9 signaling in melanin biosynthesis.
The involvement of TLRs in inflammatory disorders has been related to numerous cutaneous infections.14,15 Environmental factors such as UV irradiation and dermatological conditions such as atopic dermatitis, psoriasis, and other autoimmune disorders can affect cytokine levels in human skin tissues.16,17 Cytokines and related inflammatory mediators directly or indirectly regulate the proliferation and rate of melanogenesis in human epidermal melanocytes.18 TLR9 activation induces the production of IL-6 and TNFα in response to ODN2006.19,20 In addition, CpG-ODN 2006 elevates the production of chemokines and inflammatory cytokines in a dose-dependent manner,21 and CpG-ODNs can suppress the proliferation of A549 lung adenocarcinoma cells via TLR9 signaling.22 Consistently, our results showed that ODN2006 enhanced the levels of TNF-α, IL-6, and IL-8 in PIG1 melanocytes and also led to a notable reduction in cell viability. Therefore, abnormal inflammatory responses and loss of cell viability may underlie the pathological process of melanogenesis in response to ODN2006.
Skin pigment plays a critical role in protecting the organism. It is well known that synthesis and transfer of melanin are pivotal in the study of pigmentary skin diseases. UV irradiation can directly induce melanogenesis in several types of cultured melanocytes and promote skin pigmentation in mouse.23,24 Melanogenic enzyme TYR regulates melanin synthesis and catalyzes the rate-limiting step of melanogenesis.25 In addition, the melanosome is a specialized membrane-surrounded organelle that is involved in the synthesis, storage, and transport of melanin. PMEL (also called Pmel17, gp100, and silver) in humans is a 668-amino acid type I transmembrane melanosome-specific structural glycoprotein,26 which initiates pre-melanosome morphogenesis within multivesicular bodies and thus directly involves in the biogenesis of premelanosomes.27 Real-time RT-PCR and Western blot analyses showed that the expression of PMEL and TYR was elevated at 6 h, and then decreased 24 h later, but was significantly augmented 72 h later following ODN2006 stimulation. This variation in the expression of PMEL was, to some extent, consistent with that of TYR, which is known to be regulated by UVB.24,28 Research has shown that inhibition of UVB-induced melanogenesis and TYR expression was associated with enhanced levels of ubiquitination-dependent proteolysis.29 UVB-induced TYR-related protein 2 (TRP-2) expression plays an important role along with TYR in melanogenesis, and TRP-2 down-regulation could be inhibited by ubiquitination inhibitor MG-132 through proteasomal degradation.30 Therefore, we speculated that the down-regulation of PMEL and TYR proteins 24 h after UVB was involved in ubiquitination-dependent proteolysis.
As the first line of the innate immune system, the skin plays a crucial role in defense against cutaneous microbial infection. Despite evidence for the existence of TLRs in melanocytes, little is currently known regarding the role of TLR9 in human melanocytes. TLRs recognize specific PAMPs and subsequently trigger innate immunity. CpG motifs, immunostimulatory components of bacterial DNA, can activate innate immunity through TLR9,31 and are thought to have potential for various immune therapies such as for cancer, asthma, pollinosis, and infectious diseases. CpG ODN2006 stimulation directly activates TLR9 in human tonsillar B cells.20 In our study, ODN2006 stimulation enhanced TLR9 expression in a time-dependent manner. Compared with the UVB-induced increase in PMEL/TYR/melanin, much more significant increase in PMEL/TYR/melanin associated with prominent NF-κB activation was shown in the UVB + ODN2006-treated group. This finding demonstrated that ODN2006 has an additive effect on UVB-induced melanogenesis via PMEL and TYR elevation, as well as significant NF-κB activation.
Moreover, CpG ODN 2006 promotes the activation of p65-NF-κB and MAPK signaling pathways in L02 hepatocytes in a dose-dependent manner.21 The NF-kB-specific inhibitor PDTC reduces the effects induced by ODN2006 in a study on hemocytic immune responses.32 TBK1, a member of IκB Kinase (IKK)-related kinases in the activation of NF-κB pathway, plays a role in regulating innate immunity and inflammation. BX795 inhibits NF-κB signaling and arrests cell proliferation in oral cancer cells.33 We found that the increase in PMEL/melanin and NF-κB p65 activation was induced by ODN2006 stimulation and UVB irradiation, and these effects were distinctly reduced by TLR9 knockdown, PDTC pretreatment, or TBK1 inhibitor BX795 pretreatment, respectively. These results support the notion that the impairment of physiological TLR9/NF-κB signaling may be able to neutralize or negate the enhancing effect of ODN2006 on PMEL/melanin expression.
Collectively, we demonstrated that the expression of PMEL, TYR, and TLR9 could be regulated by ODN2006 stimulation. ODN2006 stimulated inflammatory responses and reduced cell viability, and further, had an additive effect on UVB-induced melanogenesis associated with NF-κB activation. These results suggest that TLR9 may play a role in microbial-induced melanogenesis through NF-κB signaling.
Acknowledgments
We thank Professor Caroline Le Poole for kindly providing the melanocyte cell line PIG1.This research was supported by Basic Research Project of Natural Science in Shaanxi Province (No. 2015JM8416).
Authors’ contributions
LS and SP contributed equally to this work. XX and JH conceived and designed the experiments. LS, SP, and YY performed the experiments. JS analyzed the data. DL and XW contributed reagents/materials/analysis tools. LS, XX and JH wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
References
- 1.Petersson F, Ivan D, Kazakov DV, Michal M, Prieto VG. Pigmented Paget disease – a diagnostic pitfall mimicking melanoma. Am J Dermatopathol 2009; 31: 223–6. [DOI] [PubMed] [Google Scholar]
- 2.Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J 2007; 21: 976–94. [DOI] [PubMed] [Google Scholar]
- 3.Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature 2007; 445: 843–50. [DOI] [PubMed] [Google Scholar]
- 4.Pillaiyar T, Manickam M, Jung SH. Inhibitors of melanogenesis: a patent review (2009 - 2014). Expert Opin Ther Pat 2015; 25: 775–88. [DOI] [PubMed] [Google Scholar]
- 5.Jin SH, Kang HY. Activation of Toll-like receptors 1, 2, 4, 5, and 7 on human melanocytes modulate pigmentation. Ann Dermatol 2010; 22: 486–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011; 34: 637–50. [DOI] [PubMed] [Google Scholar]
- 7.Weiner GJ, Liu HM, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci U S A 1997; 94: 10833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature 2000; 408: 740–5. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Serrano ME, Estrada-Garcia I, Mogica-Martinez D, Gonzalez-Garay A, Lopez-Herrera G, Berron-Ruiz L, Espinosa-Padilla SE, Yamazaki-Nakashimada MA, Vargas-Hernandez A, Santos-Argumedo L, Estrada-Parra SA, Espinosa-Rosales FJ. Increased pro-inflammatory cytokine production after lipopolysaccharide stimulation in patients with X-linked agammaglobulinemia. J Clin Immunol 2012; 32: 967–74. [DOI] [PubMed] [Google Scholar]
- 10.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett 2003; 85: 85–95. [DOI] [PubMed] [Google Scholar]
- 11.Le Poole IC, van den Berg FM, van den Wijngaard RM, Galloway DA, van Amstel PJ, Buffing AA, Smits HL, Westerhof W, Das PK. Generation of a human melanocyte cell line by introduction of HPV16 E6 and E7 genes. In Vitro Cell Dev Biol Anim 1997; 33: 42–9. [DOI] [PubMed] [Google Scholar]
- 12.Magina S, Esteves-Pinto C, Moura E, Serrao MP, Moura D, Petrosino S, Di Marzo V, Vieira-Coelho MA. Inhibition of basal and ultraviolet B-induced melanogenesis by cannabinoid CB(1) receptors: a keratinocyte-dependent effect. Arch Dermatol Res 2011; 303: 201–10. [DOI] [PubMed] [Google Scholar]
- 13.Tsuboi T, Kondoh H, Hiratsuka J, Mishima Y. Enhanced melanogenesis induced by TYRosinase gene-transfer increases boron-uptake and killing effect of boron neutron capture therapy for amelanotic melanoma. Pigment Cell Res 1998; 11: 275–82. [DOI] [PubMed] [Google Scholar]
- 14.Esteve LO, Saz SV, Hosein S, Solano-Gallego L. Histopathological findings and detection of Toll-like receptor 2 in cutaneous lesions of canine leishmaniosis. Vet Parasitol 2015; 209: 157–63. [DOI] [PubMed] [Google Scholar]
- 15.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001; 413: 732–8. [DOI] [PubMed] [Google Scholar]
- 16.Dessinioti C, Stratigos AJ, Rigopoulos D, Katsambas AD. A review of genetic disorders of hypopigmentation: lessons learned from the biology of melanocytes. Exp Dermatol 2009; 18: 741–9. [DOI] [PubMed] [Google Scholar]
- 17.Niwano T, Terazawa S, Nakajima H, Wakabayashi Y, Imokawa G. Astaxanthin and withaferin A block paracrine cytokine interactions between UVB-exposed human keratinocytes and human melanocytes via the attenuation of endothelin-1 secretion and its downstream intracellular signaling. Cytokine 2015; 73: 184–97. [DOI] [PubMed] [Google Scholar]
- 18.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 2004; 84: 1155–228. [DOI] [PubMed] [Google Scholar]
- 19.Rozkova D, Novotna L, Pytlik R, Hochova I, Kozak T, Bartunkova J, Spisek R. Toll-like receptors on B-CLL cells: expression and functional consequences of their stimulation. Int J Cancer 2010; 126: 1132–43. [DOI] [PubMed] [Google Scholar]
- 20.Mansson A, Adner M, Hockerfelt U, Cardell LO. A distinct Toll-like receptor repertoire in human tonsillar B cells, directly activated by PamCSK, R-837 and CpG-2006 stimulation. Immunology 2006; 118: 539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lou XE, Xu N, Yao HP, Chen Z. Bicyclol attenuates pro-inflammatory cytokine and chemokine productions in CpG-DNA-stimulated L02 hepatocytes by inhibiting p65-NF-kappaB and p38-MAPK activation. Pharmazie 2010; 65: 206–12. [PubMed] [Google Scholar]
- 22.Barnie PA, Zhang P, Lu P, Chen X, Su Z, Wang S, Xu H. CpG-oligodeoxynucleotides suppress the proliferation of A549 lung adenocarcinoma cells via toll-like receptor 9 signaling and upregulation of Runt-related transcription factor 3 expression. Biomed Rep 2014; 2: 374–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizutani Y, Hayashi N, Kawashima M, Imokawa G. A single UVB exposure increases the expression of functional KIT in human melanocytes by up-regulating MITF expression through the phosphorylation of p38/CREB. Arch Dermatol Res 2010; 302: 283–94. [DOI] [PubMed] [Google Scholar]
- 24.Shimoda H, Shan SJ, Tanaka J, Maoka T. beta-Cryptoxanthin suppresses UVB-induced melanogenesis in mouse: involvement of the inhibition of prostaglandin E2 and melanocyte-stimulating hormone pathways. J Pharm Pharmacol 2012; 64: 1165–76. [DOI] [PubMed] [Google Scholar]
- 25.Hearing VJ, Jimenez M. Mammalian TYRosinase – the critical regulatory control point in melanocyte pigmentation. Int J Biochem 1987; 19: 1141–7. [DOI] [PubMed] [Google Scholar]
- 26.Watt B, van Niel G, Raposo G, Marks MS. PMEL: a pigment cell-specific model for functional amyloid formation. Pigment Cell Melanoma Res 2013; 26: 300–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berson JF, Harper DC, Tenza D, Raposo G, Marks MS. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol Biol Cell 2001; 12: 3451–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang P, Liu W, Zhu C, Yuan X, Li D, Gu W, Ma H, Xie X, Gao T. Silencing of GPNMB by siRNA inhibits the formation of melanosomes in melanocytes in a MITF-independent fashion. PLoS One 2012; 7: e42955–e42955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yun CY, You ST, Kim JH, Chung JH, Han SB, Shin EY, Kim EG. p21-activated kinase 4 critically regulates melanogenesis via activation of the CREB/MITF and beta-catenin/MITF pathways. J Invest Dermatol 2015; 135: 1385–94. [DOI] [PubMed] [Google Scholar]
- 30.Lee EJ, Lee YS, Hwang S, Kim S, Hwang JS, Kim TY. N-(3,5-dimethylphenyl)-3-methoxybenzamide (A(3)B(5)) targets TRP-2 and inhibits melanogenesis and melanoma growth. J Invest Dermatol 2011; 131: 1701–9. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann C, Dunger N, Doser K, Lippert E, Siller S, Edinger M, Falk W, Obermeier F. Physiologic TLR9-CpG-DNA interaction is essential for the homeostasis of the intestinal immune system. Inflamm Bowel Dis 2014; 20: 136–43. [DOI] [PubMed] [Google Scholar]
- 32.Sung HH, Yang CW, Lin YH, Chang PT. The effect of two CpG oligodeoxynucleotides with different sequences on haemocytic immune responses of giant freshwater prawn, Macrobrachium rosenbergii. Fish Shellfish Immunol 2009; 26: 256–63. [DOI] [PubMed] [Google Scholar]
- 33.Bai LY, Chiu CF, Kapuriya NP, Shieh TM, Tsai YC, Wu CY, Sargeant AM, Weng JR. BX795, a TBK1 inhibitor, exhibits antitumor activity in human oral squamous cell carcinoma through apoptosis induction and mitotic phase arrest. Eur J Pharmacol 2015; 769: 287–96. [DOI] [PubMed] [Google Scholar]