Abstract
Myocardial infarction (MI) is associated with a high mortality rate, which is attributed to the effects of myocyte loss that occurs as a result of ischemia-induced cell death. Very few therapies can effectively prevent or delay the effects of ischemia. Polyamines (PAs) are polycations required for cell growth and division, and their use may prevent cell loss. The aim of this study was to investigate the relationship between hypoxia/ischemia (H/I)-induced cell apoptosis and PA metabolism and to investigate the ability of spermine to limit H/I injury in cardiomyocytes by blocking the mitochondrial apoptotic pathway. Neonatal rat cardiomyocytes were placed under hypoxic conditions for 24 h after being subjected to 5 μM of spermine as a pretreatment therapy. H/I induced PA catabolism, which was indicated by a 1.3-fold up-regulation of spermidine/spermine N1-acetyltransferase expression. Exogenous spermine significantly reduced H/I-induced cell death rate (60 ± 2 to 36 ± 2%) and apoptosis rate (42 ± 2 to 21 ± 2%); it also attenuated lactate dehyodrogenase and creatine kinase leakage (440 ± 13 and 336 ± 16 U/L to 275 ± 15 and 235 ± 13 U/L). Furthermore, it decreases calcium overload (3.8 ± 0.2 to 2.2 ± 0.1 a.u.). Moreover, spermine pretreatment remarkably decreased cytochrome c release from the mitochondria to the cytosol, lowering the expression of cleaved caspase-3 and -9. With spermine pretreatment, there was an increase in Bcl-2 levels and phosphorylation of ERK1/2, phosphoinositide 3-kinase, Akt, and GSK-3β, preserving mitochondrial membrane potential and inducing an mitochondrial permeability transition pore opening. In conclusion, H/I decreased endogenous spermine concentrations in cardiomyocytes, which ultimately induced apoptosis. The addition of exogenous spermine effectively prevented myocyte cell death.
Keywords: Spermine, hypoxia/ischemia, cardiomyocytes, myocardial infarction, mitochondrial permeability transition pore
Introduction
Myocardial infarction (MI) is caused by an acute and persistent ischemia, which occurs due to coronary artery occlusion. Furthermore, MI is frequently associated with serious complications, including arrhythmias, shock, or heart failure. Cardiomyocyte cell death is induced when the mitochondrial apoptotic pathway is activated; this phenomenon is widely accepted as the cellular mechanism through which an ischemic injury is caused in the myocardium.1,2 Mitochondrial-mediated apoptosis is characterized by mitochondrial dysfunction. In this apoptosis, caspase activators are released through an opening of the mitochondrial permeability transition pore (mPTP). There is loss of mitochondrial transmembrane potential (ΔΨm), release of cytochrome c (Cyt c), and participation of pro- and antiapoptotic Bcl-2 family proteins. Subsequently, caspase-3 and -9 are activated in this process.3 Mitochondria provides a critical and viable intervention target to protect cardiomyocytes.4,5
Polyamines (PAs) are composed of putrescine (PU), spermidine (Spd), and spermine (Sp). They are non-protein compounds in which the small molecules carry polyvalent positive charges and nitrogen. They and their metabolic enzymes are classically known to be important mediators of cell growth and division.6,7 Furthermore, spermine exerts the strongest biological activity including antioxidant effect which indicates a protection in cardiovascular care like the carotenoids.8 PAs play pivotal roles in the development of cardiac diseases,9,10 while the prosurvival kinases ERK1/2 and phosphoinositide 3-kinase (PI3K)-Akt are involved in regulating PA synthesis.11 However, scientists still need to unravel the specific mechanisms through which spermine provides cardioprotection in a hypoxic/ischemic environment by regulating various proteins.
Materials and methods
Isolation of neonatal cardiomyocytes and hypoxia/ischemia (H/I) treatment
Newborn Wistar rats, aged 1–3 days and weighing 5–8 g, were used in this study. Primary cultures of neonatal cardiomyocytes were prepared, as described in a previous study.12 All the experiments were approved by the Animal Care Committee for the Use of Experimental Animals at Harbin Medical University, Heilongjiang, China. The cardiomyocytes were plated in 6- or 96-well plates coated with collagen. Thereafter, they were maintained at 37℃ in a 5% CO2 incubator that was humidified using Dulbecco’s Modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 1% penicillin, and streptomycin. In this experiment, we used cardiomyocytes that were spontaneously beating 72 h after plating. For the hypoxic/ischemic protocol, hypoxic condition was produced using Hank’s buffered saline solution that was saturated with 95% N2 and 5% CO2. To mimic ischemic solution, the pH was maintained at 6.8 using lactate. Cells were placed in a hypoxic incubator, which was equilibrated with 1% O2/5% CO2/94% N2 for 24 h.
Experimental protocols
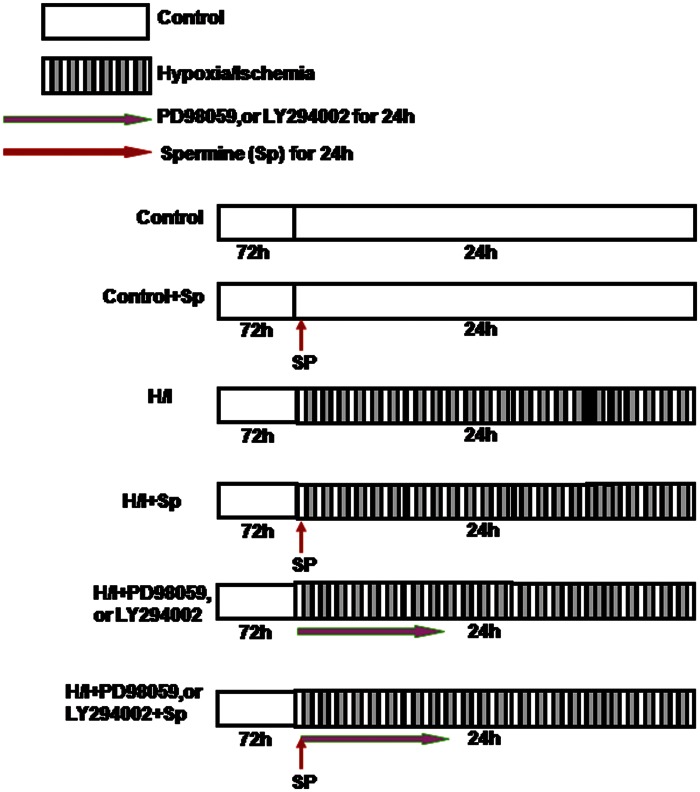
At the end of the initial culture period, cells were randomly divided into the following six groups (n = 8 independent experiments, Figure 1): (1) Control group (Control): cardiomyocytes were cultured with DMEM containing 10% FBS for 24 h; (2) Control + spermine group (Control + Sp): cardiomyocytes were cultured for 24 h in a media using 5 μM/L spermine; (3) H/I group: cardiomyocytes were exposed to H/I condition for 24 h; (4) H/I + spermine group (H/I + Sp): 5 μM/L spermine was added to an ischemic medium showing signs of hypoxia, and the cells were subsequently incubated for 24 h; (5) H/I + PD98059 (or LY294002) group (H/I + PD98059/LY294002): 10 mM of PD98059 (or 10 mM LY294002) was added to the medium 30 min before the development of hypoxia; the resulting medium was incubated for 24 h; (6) H/I + PD98059 (or LY294002) + spermine group (H/I + PD98059/LY294002 + Sp): 10 mM PD98059 (or 10 mM LY294002) was added to the medium 30 min before the commencement of hypoxia and the medium was incubated for 24 h.
Figure 1.
Summary of experimental treatment protocol. At the end of 72 h of culturing with DMEM +10% fetal bovine serum, cardiomyocytes were exposed to a hypoxic culture medium for 24 h with or without including the different experimental groups, as illustrated. (A color version of this figure is available in the online journal.)
Determination of PAs by RP-HPLC
Precolumn derivatization was carried out using RP-HPLC, which was equipped with ODS-C18 column (COSMOSIL, Japan).13–15 In this process, PAs (putrescine, spermidine, and spermine, Sigma, St. Louis, MO) were used as standards, while benzoyl chloride was used as the derivative reagent. Thus, in the control and H/I groups, we compared the content of PAs. Derivatization was carried out at 50℃ for 8 h under the following conditions: methanol–water (45:55) was used as the mobile phase; the flow rate was kept fixed at 1.0 mL/min. The wavelength of detection was also fixed at 234 nm. Thus, each peak of the derivatives was separated clearly in the chromatogram obtained by HPLC instrument. Using the size of peak area, we determined the difference in the content present in the two groups.
Cell viability assay
Cell viability was measured by Cell Counting Kit-8 (CCK-8) (Dojindo, WTS, Japan). Cells were seeded in 96-well plates at a concentration of 3 × 103 cells/well. After 24 h of each treatment, 10 µL was added to each well of CCK-8 immediately. Subsequently, they were incubated for 2 h at 37℃. Using a microplate spectrophotometer, the plates were read at 570 nm (A570) to determine their optical density.
Assay of lactate dehydrogensase (LDH) and creatine kinase (CK) activity
The extent of cellular injury was determined by measuring the amount of LDH and CK released from cardiomyocytes. The culture media was collected, while LDH and CK activity was measured using commercial kits (Jian Cheng Bioengineering Institute, Nanjing, China); the plates were read using a microplate spectrophotometer.
Apoptosis assay by Annexin V/PI staining
The apoptotic rate was detected by flow cytometry using FITC Annexin V Apoptosis Detection Kit I (BD Pharmingen, USA). Cells were washed using cold PBS, and then they were suspended in a binding buffer at a concentration of 1 × 106 cells/mL. The solution (100 µL) was transferred into a 5 mL culture tube; thereafter, we added 5 µL of FITC-Annexin V and 5 µL propidium iodide. The cells were gently vortexed and incubated in the dark for 15 min at room temperature (RT). Thereafter, the fluorescence was analyzed by flow cytometry. The percentage of apoptotic cells was determined using the Mod Fit LT software (Verity Software House Inc., Topsham, ME, USA).
Morphological analysis of apoptotic nuclei
Cells apoptosis was determined by visualizing the morphology of nuclei using the fluorescent DNA-binding dye, Hoechst 33342 (Santa Cruz, Bergheimer, Germany). Cells were rinsed with PBS and incubated for 10 min using 5 mg/mL Hoechst 33342. Nuclei were visualized under a fluorescent microscope at 400 × magnification (Nikon Corporation, Tokyo, Japan); the excitation wavelength was maintained in the range of 330–380 nm. Apoptotic nuclei of cells were assessed by counting the number of cells that displayed morphological changes in the nuclei, such as chromatin condensation and fragmentation.
Measurement of [Ca2+]i
The [Ca2+]i was determined by molecular probes according to manufacturer’s instructions. After treatment, cardiomyocytes (1 × 106 cells/sample) were either cultured on a 12-well plate or slides were washed using a buffer of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid to maintain the pH at 7.4. Then, the washed cells were stained using 5 μM fluo-8 AM (Santa Cruz, Bergheimer, Germany) for 30 min at RT. The fluorescence of Ca2+ was measured using a laser confocal scanning microscope (Olympus, LSM, Japan); the excitation wavelength was kept at 485 nm, while the emission wavelength was set at 530 nm. The fluorescent intensity was reflected by [Ca2+]i.
Determination of mitochondrial membrane potential (Δψm)
ΔΨm was estimated using mitochondrial membrane potential assay kit, equipped with JC-1(Santa Cruz, Bergheimer, Germany). Briefly, cells were collected and incubated at 37℃ for 20 min using JC-1 (1×). Then, they were washed twice using phosphate buffered saline. Using a fluorescent microscope, observations were conducted at 400× magnification (Nikon Corporation, Tokyo, Japan). Furthermore, the fluorescence of JC-1 monomer (green) was observed at excitation wavelength of 514 nm; the emission wavelength was maintained at 529 nm. Thereafter, the fluorescence of JC-1 aggregate (red) was observed at an emission wavelength of 585 nm/590 nm. At least 100 areas were randomly selected from each image, and the average intensity of each region was quantified. We calculated the ratio of JC-1 aggregate to monomer (red/green) intensity in each region. A decrease in this ratio was interpreted as a decrease of Δψm, whereas an increase in this ratio was interpreted as a gain of Δψm.
Assay of mPTP opening
The changes of mPTP opening were measured by co-incubating calcein AM (Santa Cruz, Bergheimer, Germany) and cobalt chloride (Sigma Chemical Co., St. Louis, MO, USA). Cardiomyocytes were plated in 24-well plates (5 × 105 cells/well). After stimulating with different treatments, we loaded the cells with 2 μM of calcein AM in the presence of 5 mM of cobalt chloride solution; the reaction mixture was kept in the dark for 15 min, and the temperature of the reaction mixture was maintained at 37℃. Using a laser confocal scanning microscope (Nikon, Japan), we measured fluorescence at an excitation wavelength of 488 nm and an emission wavelength of 505 nm. The fluorescence intensity of individual cells was measured using the Sigma Scan Pro 5 software (SPSS Inc., Chicago). In the control group, the fluorescence intensity was set at 100% viable.
Detection of Cyt c released from mitochondria
Cyt c released from mitochondria was measured by immunoblotting. Mitochondrial and cytosolic protein fractions were prepared using a Cell Mitochondria Isolation Kit (Beyotime, Jiangsu, China). Briefly, cells were collected and suspended in a mitochondrial isolation reagent A, containing phenyl methyl sulfonyl fluoride and other protease inhibitors. Then, the cell culture was vortexed for 5 s and incubated on ice for 15 min. Mitochondrial isolation reagent B was added, vortexed, and centrifuged at 14,000 rpm for 5 min; the temperature was maintained at 4℃ during this process. The supernatant was transferred to a fresh 1.5 mL microcentrifuge tube and was termed as the cytosolic fraction. Lactate dehydrogenase (LDH) served as a cytosolic marker. The expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was considered as the loading control.
Immunoblotting
Cells were harvested and lysed. Protein concentration was measured using a BCA Protein Assay Kit (Beyotime, Jiangsu, China). We boiled equal quantities of proteins, and then we separated them by performing sodium dodecyl sulfate polyacrylamide gel electrophoresis. After separating these proteins, we electrophoretically transferred them into a nitrocellulose membrane. Membranes were blocked at RT for 1 h using tris-buffered saline containing 5% non-fat milk. Thereafter, they were incubated overnight at 4℃ along with specific primary antibodies (ornithine decarboxylase [ODC], spermidine/spermine N1-acetyltransferase (SSAT), anticleaved caspase-3 and -9, Bcl-2, Cyt c, and GAPDH were purchased from Santa Cruz and anti-ERK1/2and PI3K–Akt–GSK-3β antibodies were purchased from Cell Signaling Technology, Danvers, MA, USA). The membrane was washed thrice with 1 × Tris-buffer saline-Tween 20 (TBST) buffer. Subsequently, the membrane was incubated in TBST solution along with alkaline phosphatase-labeled secondary antibody; the incubated mixture was kept in a shaker for 2 h at RT. Finally, the membrane was washed thrice with TBST solution. Antibody–antigen complexes were detected using Western Blue® Stabilized Substrate for Alkaline Phosphatase (Promega, USA). The intensities of protein bands were quantified by a Bio-Rad ChemiDoc™ EQ densitometer and Bio-Rad Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
The data were expressed as mean ± SE (n = 8 for each group). Each measurement was obtained by performing at least three independent experiments. Statistical comparisons were made using paired or unpaired t-tests or one-way ANOVA followed by Bonferroni post-test. Significance level was set at p < 0.05.
Results
Changes of PAs metabolism under H/I condition
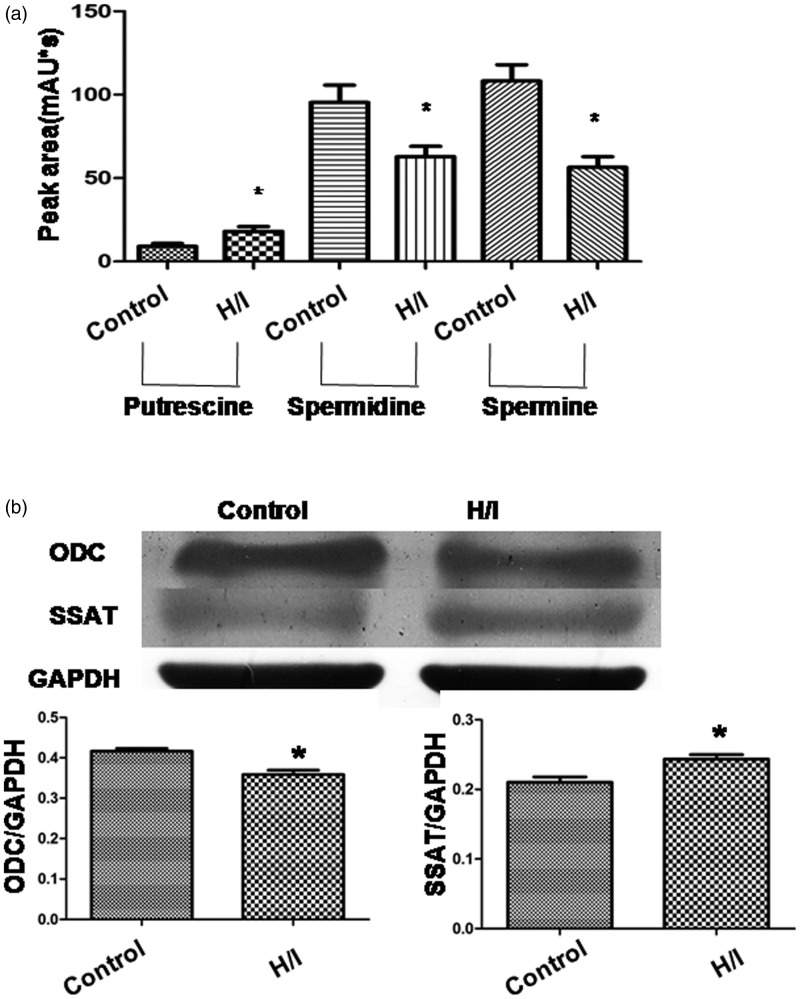
In this experiment, HPLC chromatogram was used to determine the content of PAs. Standard solutions were used as positive control. The peaks of the three derivatives were obtained at about 8, 17, and 37 min, respectively. Compared with the control group, the peak area of putrescine was greater, while that of spermidine and spermine was lower in the H/I group (p < 0.05 for all 3; Figure 2(a)). Furthermore, ODC is an enzyme that performs the first step of PA biosynthesis; SSAT is a key regulatory enzyme catabolizing PA. Compared with the control group, the expression of ODC was lower while that of SSAT was higher in the H/I group (both p < 0.05; Figure 2(b)). These results indicate that H/I inhibited the production of endogenous spermine.
Figure 2.
Polyamines content remarkably decreases with hypoxia/ischemia (H/I). (a) HPLC chromatogram of polyamines after derivatization, and the peak areas of putrescine (PU), spermidine (Spd), and spermine (Sp). (b) Protein expression of ornithine decarboxylase (ODC) and spermidine/spermine N1-acetyltransferase (SSAT). The intensity of each band was quantified by densitometry, and the data were normalized to GAPDH. All the data were in the range of n = 4–8 for independent experiments. *p < 0.05 versus control
The effects of spermine on cell viability and apoptosis
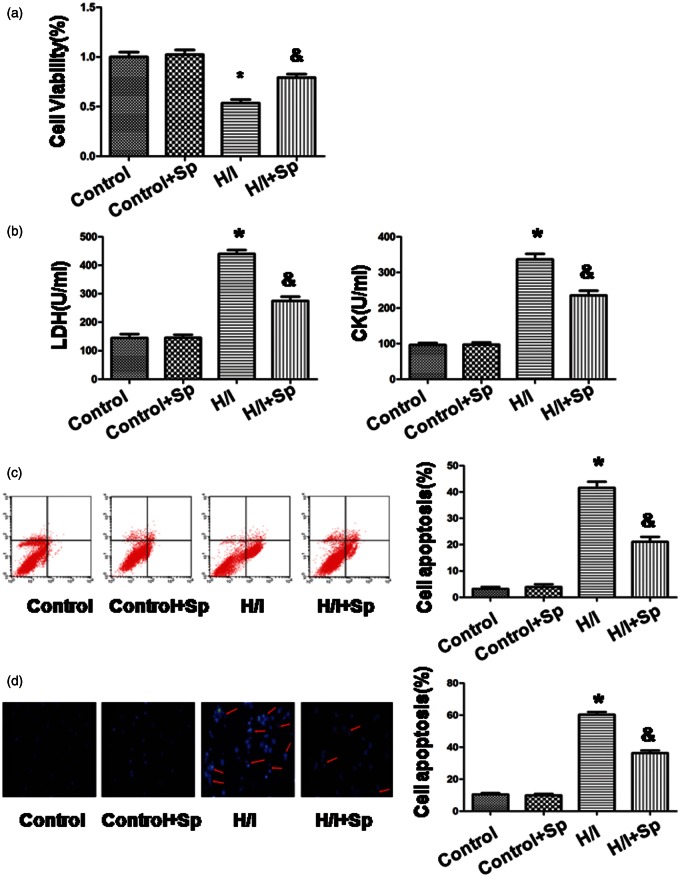
Compared with the control group, cell viability was lower in the H/I group at 54 ± 3% (100%, p < 0.05; Figure 3(a)). In contrast, in the presence of spermine, cell viability increased to 79 ± 4% versus H/I group (p < 0.05). Compared with the control group, there was no significant difference in the control + Sp group.
Figure 3.
Spermine inhibited the effects of hypoxia/ischemia on cardiomyocytes apoptosis. (a) Cell viability was measured by CCK-8 assay. The cells that were incubated with the control medium were considered to be 100% viable. (b) The extent of cellular injury was detected by LDH and CK activity. (c) Apoptosis was analyzed by flow cytometry. (d) Detection of nuclear morphology in apoptotic cells using Hoechst 33342 nuclear staining. Apoptotic cells were marked as cells with condensed, disrupted nuclei (arrow, × 400). Apoptotic cells were counted in at least 10 random fields. All the data are mean ± SEM for n = 8 independent experiments. *p < 0.05 versus control group; &p < 0.05 versus H/I group. (A color version of this figure is available in the online journal.)
The extent of cellular injury was estimated from the leakage of LDH and CK (Figure 3(b)). With H/I treatment, extracellular LDH increased to 440 ± 13 U/L, while CK increased to 336 ± 16 U/L. In the control group, the corresponding values of LDH and CK were 145 ± 14 and 96 ± 6 U/L, respectively (p < 0.05). With spermine incubation, we significantly reduced the leakage of LDH and CK to 275 ± 15 and 235 ± 13 U/L, respectively. There was no significant difference between control + Sp group and the control group.
Flow cytometry was used to examine cardiomyocyte apoptosis (Figure 3(c)). Thus, we detect whether spermine altered cardiac apoptosis that was induced by H/I. The control group exhibited a low apoptotic rate of 3 ± 1%, while H/I significantly increased the rate to 42 ± 2% (p < 0.05). Compared with the H/I group, the apoptotic rate was reduced to 21 ± 2% in the presence of spermine (p < 0.05). In the absence of H/I, spermine did not influence the apoptotic rate.
We further confirmed the morphological changes in the nuclei by Hoechst 33342 staining (Figure 3(d)). In the H/I group, the number of apoptotic nuclei with typical features of fragmentation and condensation was significantly increased to (60 ± 2%) as compared to that in the control group (10 ± 1%, p < 0.05). In the H/I + Sp group, the percentage of apoptotic nuclei was lower as compared to that in the H/I group (36 ± 2%, p < 0.05). Compared to the control, there was no significant difference in the number of apoptotic nuclei of control + Sp group.
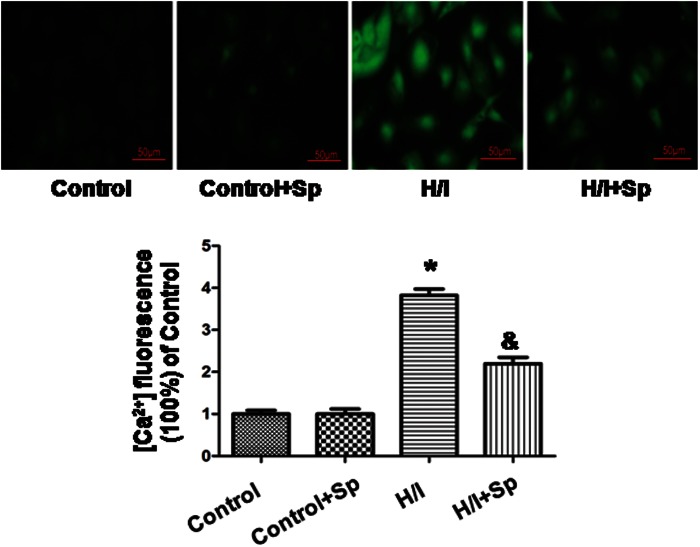
Spermine decreases calcium overload
Calcium overload occurred in the early stage of H/I, and we detected [Ca2+]i by fluo-8 AM staining 24 h after H/I (Figure 4). Since the fluorescence intensity in the H/I group was 3.8 ± 0.2 a.u., it was significantly higher than that of control group (1.0 ± 0.1 a.u., p < 0.05). After administering spermine, the [Ca2+]i was much lower (2.2 ± 0.1 a.u.) than H/I (p < 0.05). Spermine had no effect in the absence of H/I.
Figure 4.
Spermine decreases calcium overload caused by hypoxia/ischemia. An intracellular Ca2+ concentration was determined by fluo-8 AM staining. An intracellular calcium ([Ca2+]i) was detected after 24 h of hypoxia/ischemia. Fluorescence intensities of [Ca2+]i were recorded by a laser scanning confocal microscope. All data were from n = 4 in independent experiments. *p < 0.05 versus control; &p < 0.05 versus H/I. (A color version of this figure is available in the online journal.)
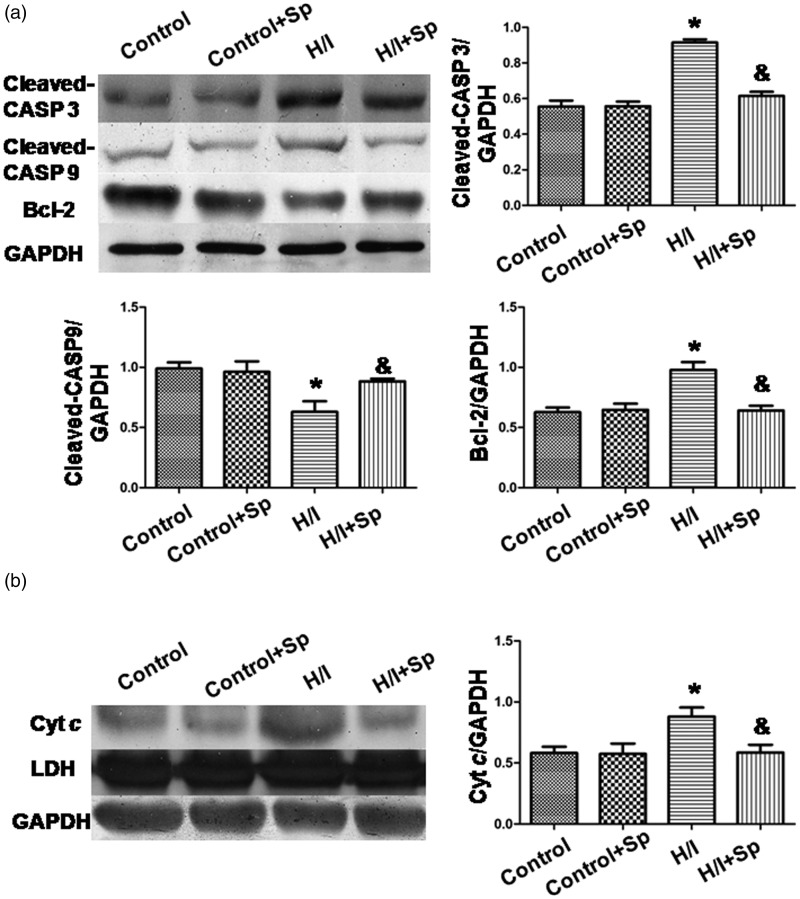
Spermine increased Bcl-2 expression, decreased the expressions of cleaved caspase-3 and -9 expressions, and suppressed the release of Cyt c
The expression of Bcl-2 and cleaved caspase-3 and -9 decreased, while Cyt c release increased in the H/I group (p < 0.05 versus control; Figure 5). By spermine treatment, the effect of H/I was reversed (p < 0.05); however, the use of spermine had no effect in the absence of H/I.
Figure 5.
Protein expression of cleaved caspase-3 and-9, Bcl-2, and cytosolic cytochrome c. (a) Cleaved caspase-3 and -9 and Bcl-2 expression. (b) Cytochrome c release. The intensity of each band was quantified by densitometry, and the data were normalized to GAPDH. Lactate dehydrogenase (LDH) served as a cytosolic marker. All the data were from n = 4 independent experiments. *p < 0.05 versus control; &p < 0.05 versus H/I
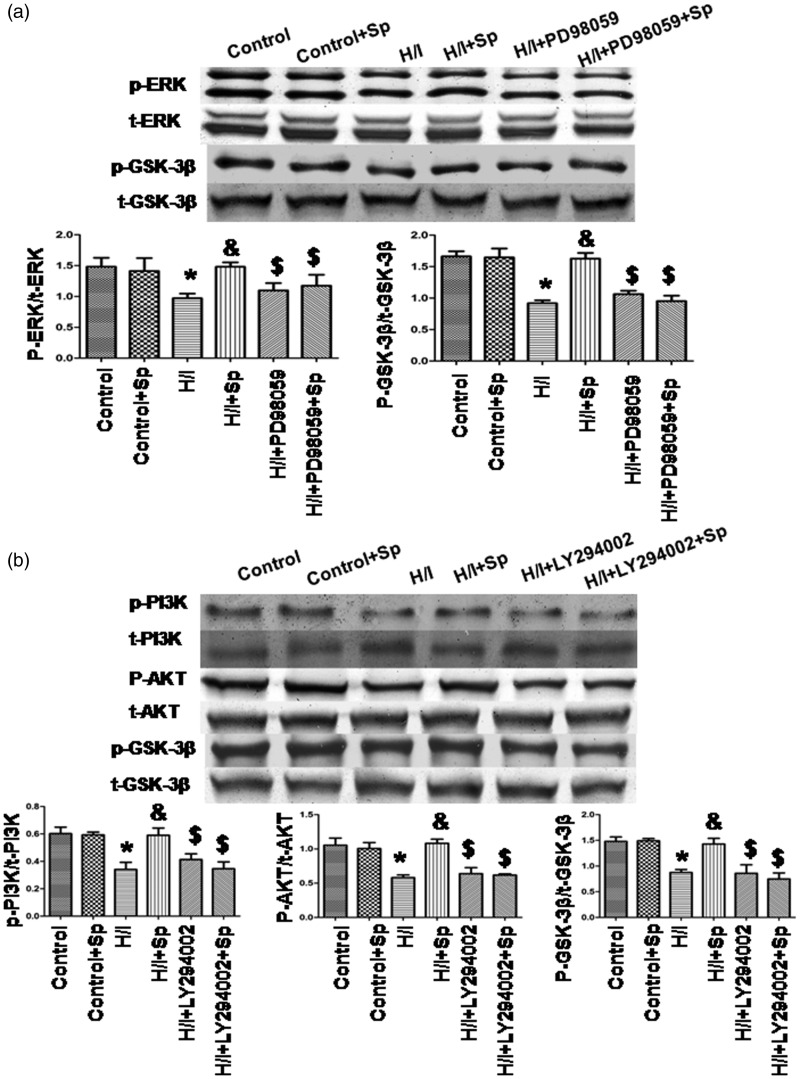
Spermine activates ERK1/2 and PI3K–Akt–GSK-3β pathway
To determine whether ERK1/2 and PI3K–Akt–GSK-3β pathways were activated by spermine in the presence of H/I, we cultured cardiomyocytes using spermine and a media conditioned with hypoxia (Figure 6). Compared with the control group, the levels of phosphorylated ERK1/2, PI3K, Akt, and GSK-3β were lower in the H/I group (p < 0.05). Spermine prevented H/I-induced changes of ERK1/2 and PI3K–Akt–GSK-3β pathways (p < 0.05); however, spermine had no effect in the absence of H/I. The total amount of ERK1/2, PI3K, Akt, and GSK-3β protein remained unchanged in different conditions.
Figure 6.
Spermine induced the increasing activity of ERK1/2, PI3K, Akt, and GSK-3β. (a) ERK1/2 and GSK-3β expression. (b) PI3K, Akt, and GSK-3β. Cells were collected and cell lysates were analyzed by immunoblotting. The graphs in (a) and (b) represented the optical density of the band of phosphorylated ERK1/2, PI3K, Akt, and GSK-3β, which were normalized with that of total ERK1/2, PI3K, Akt, and GSK-3β, respectively. All the data were from n = 4 for independent experiments. *p < 0.05 versus control; &p < 0.05 versus H/I; $ p < 0.05 versus H/I+Sp
To determine whether ERK1/2 and PI3K–Akt–GSK-3β pathways were involved in spermine-inhibited apoptosis induced by H/I, we used PD98059 (an ERK1/2 inhibitor) and LY294002 (a PI3K inhibitor) to inhibit the signaling pathways (Figure 6). We found that the inhibitors of ERK1/2 and PI3K significantly suppressed phosphorylation of ERK1/2, PI3K, Akt, and GSK-3β, which was previously induced by spermine treatment.
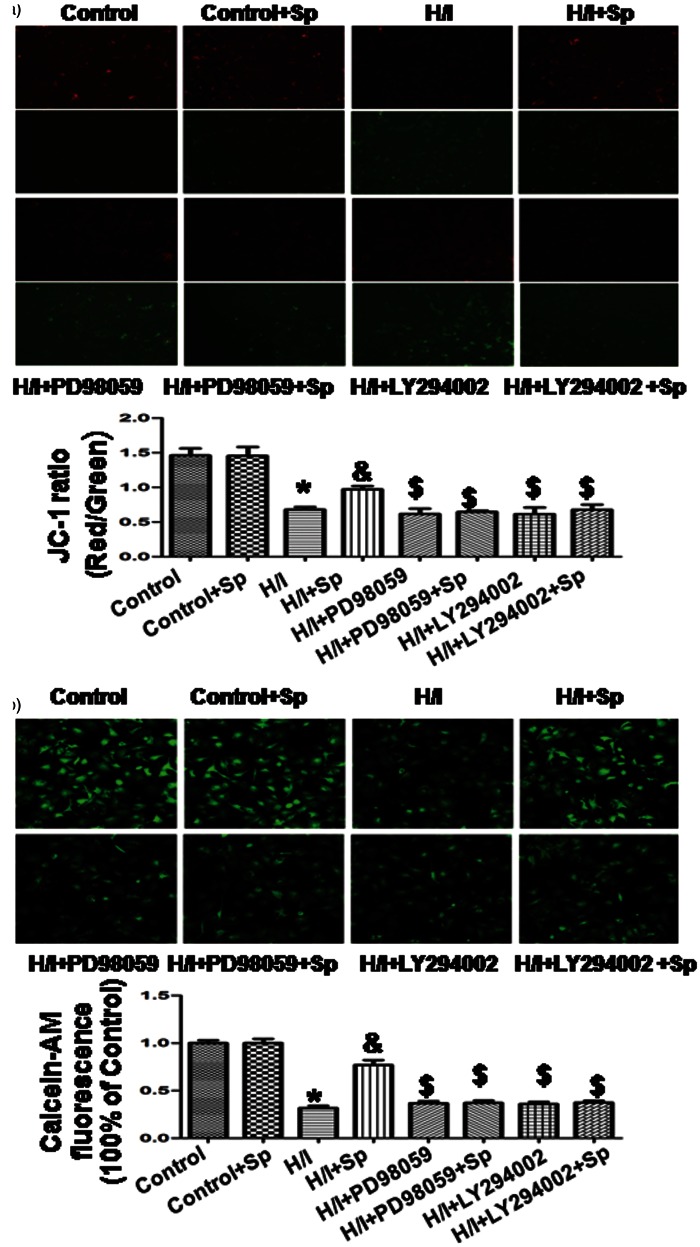
Effects of spermine on ΔΨm and mPTP opening
As shown in Figure 7(a), cardiomyocytes exposed to H/I exhibited disruption of ΔΨm, a phenomenon that was not observed in the control group; this effect was detected in the cardiomyocytes with an increase in green fluorescence. However, a co-treatment with spermine served as a protection against this effect. The ratio of red and green fluorescence was used to determine the ΔΨm change induced by H/I treatment and the protective effect offered by Sp. In the control group, JC-1 aggregated in mitochondria at a ratio of 1.5 ± 0.1, while the H/I group was 0.7 ± 0.1 (p < 0.05 versus control group). The ratio was increased to 1.0 ± 0.1 following co-treatment with spermine (p < 0.05 versus H/I group), while PD98059 and LY294002 reversed the effect of spermine. These results indicate that the mitochondria-mediated pathway was involved in the cardioprotective effect of spermine.
Figure 7.
Measurement of mitochondrial membrane potential (Δψm) and mitochondrial pore (mPTP) opening. (a) Δψm was measured by JC-1 imaging. Cells of different groups were stained using the JC-1 probe and the images were obtained by fluorescent microscopy. JC-1 spontaneously forms J-aggregate and exhibits red fluorescence under a high potential. The JC-1 monomeric form shows green fluorescence under a low potential. The individual red and green average fluorescence intensities are expressed as the ratio of red to green fluorescence. (b) Calcein AM was used to measure the changes of mPTP opening. The fluorescence intensity in the control group was considered to be 100% viable. The changes in fluorescence intensity are inversely proportional with the degree of mPTP opening. All the data were from four independent experiments. *p < 0.05 versus control; &p < 0.05 versus H/I; $ p < 0.05 versus H/I + Sp. (A color version of this figure is available in the online journal.)
In different groups, there were also changes in the rates of mPTP opening in response to calcein AM. The fluorescence intensity in the control group was set at 100%. The level of fluorescence intensity was inversely proportional to the degree of mPTP opening. As shown in Figure 7(b), fluorescence intensity was reduced from 100 ± 3 to 32 ± 2% in the H/I group (p < 0.05 versus control). With the use of spermine, the fluorescence intensity increased to 77 ± 5% versus the H/I group (p < 0.05); however, spermine did not have any effect in the absence of H/I. Both PD98059 and LY294002 individually reversed an increase in fluorescence intensity that was observed with spermine treatment.
Discussion
In the present study, we have investigated the effect of H/I on the concentrations of endogenous spermine in cardiomyocytes. We also investigated whether an exogenous administration of spermine could offer protective effects. The most significant findings of this study are as follows: (1) spermine decreased in cardiomyocytes that were subjected to hypoxia; (2) the loss of spermine was associated with induced apoptosis; and (3) the addition of spermine attenuated apoptosis in cardiomyocytes by inhibiting the opening of mPTP and by activating ERK1/2, PI3K–Akt–GSK-3β pathways. These findings provide a novel target to prevent the myocardial damage induced by MI.
PA levels are tightly regulated by key enzymes that control the biosynthesis and interconversion of PAs; ODC and SSAT are the most prominent enzymes in this context.6,16 SSAT is the central molecular regulator of PA metabolism, and it mediates catabolism associated with drug response, apoptosis, and stress responses.17,18 On the other hand, RP-HPLC has been recognized as an effective method to monitor PA cellular content.13–15 Hypoxia increased putrescine content and SSAT expression, but it also simultaneously reduced spermidine/spermine content and ODC expression. An increase in SSAT expression indicated an enhanced PA catabolism, which was responsible for the reduced levels of spermidine and spermine. Spermine offers protection against ischemic injury in some cell types,19,20 and the current study indicates that this protection extends to cardiomyocytes.
H/I induces cardiomyocyte apoptosis by activating death receptor and mitochondrial pathways. During an mPTP opening, the mitochondrial pathway is a major apoptosis-inducing pathway that is initiated by stress signals associated with the release of Cyt c from the mitochondrial intermembrane space. Cyt c along with apoptosis-activating factor-1 forms a complex called the apoptosome, which recruits and activates the death effector caspase-9; this caspase-9 subsequently activates caspase-3 that in turn activates the apoptosis process at an early stage.21–23 The activation of caspase-3 and caspase-9 is a key irreversible point in mitochondria-mediated apoptosis. In addition, an increased level of intracellular calcium can change the permeability of outer mitochondrial membrane, which causes the release of Cyt c and apoptotic-induced factor; it can also cause an irreversible injury to cardiomyocytes, including reduced cell-to-cell coupling, intracardiac reentry, and an abnormal rhythmic activity.24 The mitochondrial permeability and the release of apoptosome can be regulated by Bcl-2 proteins.25 The antiapoptotic protein Bcl-2 resides in the mitochondrial outer membrane. Thus, it can stabilize the function of mitochondrial membrane and inhibit Caspase or Cyt c release.26,27 In our study, we observed that spermine significantly affected the following changes: (i) increase in cell viability, (ii) decrease in LDH and CK leakage, (iii) increase in apoptosis of cardiomyocytes, (iv) increase in intracellular calcium concentration and Cyt c release, (v) increase in Bcl-2 expression, and (vi) reduction in caspase-3 and -9 expression.
To further explore the mechanisms of spermine protection against H/I-induced apoptosis, we detected the changes of Δψm, mPTP opening, and related signaling pathways. Mitochondrial membrane potential (Δψm) and membrane permeability play a pivotal role in the apoptosis of cardiomyocytes. A loss of Δψm was mainly attributed to an irreversible opening of the mPTP, leading to an arrest of mitochondrial ATP synthesis, mitochondrial swelling, and outer membrane permeabilization.28,29 As one cascade of mitogen-activated protein kinase (MAPK) family, ERK regulates cell differentiation and growth; moreover, phosphorylation of ERK can protect cardiomyocytes from apoptosis.30 Akt signaling, a central molecule of cellular survival mechanisms, is activated by PI3K. Subsequently, Akt signaling phosphorylates many downstream targets, including glycogen synthase kinase 3β (GSK-3β) and Bcl-2 family.31,32 In addition, GSK-3β can be inactivated by MAPK upon phosphorylation, because it mediates the convergence of myocyte protection signaling by inhibiting an mPTP opening.33,34 Presently, several research studies have shown that PI3K/Akt/GSK-3β and ERK1/2 signaling pathways are involved in regulating the survival of cardiomyocytes.29,35,36 Spermine treatment restored H/I-induced loss of Δψm through an abnormal mPTP opening, promoting the expressions of pho-ERK1/2 and pho-PI3K/Akt/GSK-3β. Furthermore, PD98059 (an inhibitor of ERK1/2 signaling) and LY 294002 (a PI3K inhibitor) reversed the effects of spermine.
In conclusion, the current study revealed that H/I impaired an endogenous metabolism of PAs in cardiomyocytes by decreasing spermine content. An addition of exogenous spermine mitigated the apoptosis of cardiomyocytes by preventing the activation of mitochondrial pathway. Spermine may be an effective preventive medication in the treatment of MI and other ischemic heart diseases; it may be used during cardiac surgery to limit ischemic injury.
Highlights
Spermine decreased in cardiomyocytes that were subjected to hypoxia.
The loss of spermine was associated with induced apoptosis.
Addition of spermine attenuated apoptosis in cardiomyocytes by inhibition of mPTP opening and activation of ERK1/2 and PI3K–Akt–GSK-3β pathway.
Acknowledgements
This research study is supported by the National Natural Science Foundation of China (No. 81270311, 81070123).
Authors’ contribution
CW and CQX conceived and designed the research, and drafted the manuscript; CW, HZL, YHW and HJS completed the experiment and data analysis; XP, HXL and SZB revised the paper and gave some suggestions. All authors read and approved the final version of the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Kaitsis RN, Mann DL. Apoptosis and the heart: a decade of progress. J Mol Cell Cardiol 2005; 38: 1–2. [DOI] [PubMed] [Google Scholar]
- 2.Van Empel VP, Hofstral BAT, Crijins HJ. Myocyte apoptosis in heart failure. Cardiovasc Res 2005; 67: 21–29. [DOI] [PubMed] [Google Scholar]
- 3.Gustafsson AB, Gittlieb RA. Mechanisms of apoptosis in the heart. J Clin Immunol 2003; 23: 447–59. [DOI] [PubMed] [Google Scholar]
- 4.Huang H, Wu K, You Q. Naringin inhibits high glucose-induced cardiomyocyte apoptosis by attenuating mitochondrial dysfunction and modulating the activation of the p38 signaling pathway. Int J Mol Med 2013; 32: 396–402. [DOI] [PubMed] [Google Scholar]
- 5.Li Q, Zhou L, Gao G. Mitochondrial network in the heart. Protein Cell 2012; 3: 410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Persson L. Polyamine homeostasis. Essays Biochem 2009; 4: 11–24. [DOI] [PubMed] [Google Scholar]
- 7.Wallace HM, Ftaser AV, Hughes A. A perspective of polyamine metabolism. Biochem J 2003; 376: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciccone MM, Cortese F, Gesualdo M. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediators Inflamm 2013; 2013: 782137–782137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao YJ, Xu CQ. Role of polyamines in myocardial ischemia/reperfusion injury and their interactions with nitric oxide. Eur J Pharmacol 2007; 562: 236–46. [DOI] [PubMed] [Google Scholar]
- 10.Tantini B, Fiumana E, Cetrullo S. Involvement of polyamines in apoptosis of cardiac myoblasts in a model of simulated ischemia. J Mol Cell Cardiol 2006; 40: 775–82. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Xue G, Zhang W, Wang L. Akt and Erk1/2 activate the ornithine decarboxylase/polyamine system in cardioprotective ischemic preconditioning in rats: the role of mitochondrial permeability transition pores. Mol Cell Biochem 2014; 390: 133–42. [DOI] [PubMed] [Google Scholar]
- 12.Yan X, Tian J, Wu H. Ginsenoside Rb1 protects neonatal rat cardiomyocytes from hypoxia/ischemia induced apoptosis and inhibits activation of the mitochondrial apoptotic pathway. Evid Based Complement Alternat Med 2014; 2014: 149195–149195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carl FV, Johan CR, Fritz KS. Quantitation of polyamines in cultured cells and tissue homogenates by reversed-phase high-performance liquid chromatography of their benzoyl derivatives. J Chromatogr 1988; 426: 41–45. [DOI] [PubMed] [Google Scholar]
- 14.Zhao YJ, Xu CQ, Li QF, Ma LY. Analysis of polyamines in tissue from perfusion of isolated rat heart by RP-HPLC. Chin J Pathophysiol 2005; 21: 412–3. [Google Scholar]
- 15.Sui JJ. Determination of polyamines in rat intestinal epithelial cell line IEC-6 by RP-HPLC. Chin Pharmacol Bull 2011; 27: 1309–12. [Google Scholar]
- 16.Hasegawa S, Nakano M, Hamana K. Decrease myocardial polyamine concentration in rats with myocardial infarction. Life Sci 1997; 19: 1643–50. [DOI] [PubMed] [Google Scholar]
- 17.Matsui-Yuasa I, Otani S, Yukioka K. Two mechanisms of spermidine/spermineN1-acetyltransferase-induction. Arch Biochem Biophys 1989; 268: 209–14. [DOI] [PubMed] [Google Scholar]
- 18.Casero RA, Pegg AE. Polyamine catabolism and disease. Biochem J 2009; 421: 323–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Wang S. Spermine attenuates the preconditioning of diazoxide against transient focal cerebral ischemia in rats. Neurol Res 2014; 36: 666–72. [DOI] [PubMed] [Google Scholar]
- 20.Han LP, Yuan LB, Shentu YP. Spermine reduced no-reflow size induced by ischemia-reperfusion through regulating autophagy. Int J Cardiol 2013; 168: 3145–7. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Nijhawan D, Budihardjo I. Cytochrome c and dATP- dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997; 91: 479–89. [DOI] [PubMed] [Google Scholar]
- 22.Li HZ, Han LP, Xu CQ. Effect of dopamine receptor 1 on apoptosis of cultured neonatal rat cardiomyocytes in simulated ischaemia/reperfusion. Basic Clin Pharmacol Toxicol 2008; 102: 329–36. [DOI] [PubMed] [Google Scholar]
- 23.Honda HM, Ping P. Mitochondrial permeability transition in cardiac cell injury and death. Cardiovasc Drugs Therapy 2006; 20: 425–32. [DOI] [PubMed] [Google Scholar]
- 24.Itoh K, Yoshizumi M, Kitagawa T. Extracellularly-administered lysophosphatidylcholine causes Ca2+ efflux from freshly isolated adult rat cardiomyocytes. Basic Res Cardiol 1998; 93: 23–29. [DOI] [PubMed] [Google Scholar]
- 25.Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood 1996; 88: 386–401. [PubMed] [Google Scholar]
- 26.Huang PR, Hung SC, Pao CC. N-(1-pyrenyl) maleimide induces bak oligomerization and mitochondrial dysfunction in jurkat cells. Biomed Res Int 2015; 2015: 798489–798489. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Estaquier J, Vallette F, Vayssiere JL, Mignotte B. The mitochondrial pathways of apoptosis. Adv Exp Med Biol 2012; 942: 157–83. [DOI] [PubMed] [Google Scholar]
- 28.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev 2007; 87: 99–163. [DOI] [PubMed] [Google Scholar]
- 29.Duchen MR. Contributions of mitochondria to animal physiology from homeostatic sensor to calcium signaling and cell death. J Physiol 1999; 516: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin F, Shite J, Liang CS. Antioxidants attenuate myocyte apoptosis and improve cardiac function in CHF: association with changes in MAPK pathways. Am J Physiol Heart Circ Physiol 2003; 285: 822–32. [DOI] [PubMed] [Google Scholar]
- 31.Morello F, Perino A, Hirsch E. Phosphoinositide 3-kinase signalling in the vascular system. Cardiovasc Res 2009; 82: 261–71. [DOI] [PubMed] [Google Scholar]
- 32.Hers I, Vincent EE, Tavaré JM. Akt signalling in health and disease. Cell Signal 2011; 23: 1515–27. [DOI] [PubMed] [Google Scholar]
- 33.Zhou BP, Deng J, Xia W. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 2004; 6: 931–40. [DOI] [PubMed] [Google Scholar]
- 34.Sunaga D, Tanno M, Kuno A. Accelerated recovery of mitochondrial membrane potential by GSK-3β inactivation affords cardiomyocytes protection from oxidant-induced necrosis. PLoS One 2014; 9: e112529–e112529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umoh NA, Walker RK, Millis RM. Calcitonin gene-related peptide regulates cardiomyocyte survival through regulation of oxidative stress by PI3K/Akt and MAPK signaling pathways. Ann Clin Exp Hypertens 2014; 2: 1007–1007. [PMC free article] [PubMed] [Google Scholar]
- 36.Kim DE, Kim B, Shin HS. The protective effect of hispidin against hydrogen peroxide-induced apoptosis in H9c2 cardiomyoblast cells through Akt/GSK-3β and ERK1/2 signaling pathway. Exp Cell Res 2014; 327: 264–75. [DOI] [PubMed] [Google Scholar]