Abstract
There is a large body of evidence suggesting that inhibitors of dipeptidyl peptidase-4, such as sitagliptin, may exhibit beneficial effects against different inflammatory disorders. This investigation was conducted to elucidate the potential ability of sitagliptin to counteract the injurious effects of doxorubicin in cardiac tissue. Male Wistar rats were pretreated with sitagliptin for 10 days then treated with a single dose of doxorubicin (20 mg/kg, i.p). Electrocardiography, biochemical estimation of serum and tissue markers, and histo- and immunopathological examinations were done. Results have shown that supplementation with sitagliptin resulted in significant improvement of cardiac function with contaminant decrease in serum markers of doxorubicin-induced cardiotoxicity. These results were supported by the histopathological results. Furthermore, a marked protection against oxidative stress was evident through reduction of lipid peroxidation and prevention of reduced glutathione content depletion and superoxide dismutase activity reduction in cardiac tissue of rats pretreated with sitagliptin in combination with doxorubicin. Moreover, sitagliptin ameliorated the activation of nuclear factor kappa-B and the release of inflammatory cytokines, tumour necrosis factor-alpha and nitric oxide. Finally, sitagliptin attenuated doxorubicin-induced increase in the expression of pro-apoptotic protein Bax and in the apoptotic marker, caspase-3. Collectively, these data indicate that sitagliptin pretreatment could alleviate doxorubicin-induced cardiotoxicity via reducing oxidative damage and its subsequent inflammation and apoptosis.
Keywords: Heart, doxorubicin, sitagliptin, oxidative stress, nuclear factor kappa-B, apoptosis
Introduction
Doxorubicin (DOX) is an anthracycline derivative that is potently used for treating a wide variety of human cancers. However, it has many adverse effects basically, serious dose-dependent cardiotoxicity and hepatotoxicity. This has resulted in essential careful monitoring of DOX during its therapeutical administration to decrease the cardiotoxicity. The mechanism underlying DOX-induced cardiotoxicity is still unclear. Accumulating evidence suggest the involvement of oxidative stress and lipid peroxidation in mediating DOX cardiotoxicity.1 However, some previous studies have showed failure of potent antioxidant such as vitamin E, N-acetylcysteine, and polyphenol to protect the heart against DOX toxicity.2,3 This has led to reconsider other molecular pathways involved in the pathogenesis of DOX-induced cardiotoxicity. Previous investigations have demonstrated the formation of an iron-anthracycline complex during DOX-induced cardiotoxicity which causes severe damage to the plasma membrane and interferes with the cytoskeletal structure leading to loss of myofibrils, dilation of the sarcoplasmic reticulum, and myocyte necrosis.4 DOX has been shown to induce the pro-apoptotic activation of nuclear factor-kappa B (NF-κB) in endothelial cells and cardiomyocytes.5–7 This leads to increase in the production of several proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and nitric oxide (NO).8 Administration of agents such as epigallocatechin-3-gallate that interferes with NF-κB signaling pathway has shown significant potential to attenuate DOX-induced cardiac damage.7 Recently, there is a growing evidence that cellular apoptosis (i.e. programmed cell death) may play a pivotal role in DOX-induced cardiotoxicity.9 As the use of DOX in chemotherapy is essential, the search for new therapeutic agents that can alleviate or reverse DOX-induced cardiotoxicity is mandatory.
Glucagon-like peptide-1 (GLP-1) is the most important incretin hormone that promotes insulin secretion in response to nutrient ingestion and it is secreted by the entero-endocrine L cells of the intestinal mucosa. However, GLP-1 has very short half-life (2 to 3 min) due to rapid degradation by dipeptidyl peptidase 4 (DPP4). DPP4 inhibitors lead to increased GLP-1 levels in the blood and extend the duration of GLP-1 action. In addition to the anti-diabetic effects of DPP4 inhibitors, accumulating evidence suggest that DPP-4 inhibitors have considerable therapeutic potential for different diseases such as tissue inflammation, diabetic nephropathy, and cardiovascular disorders.10–14 GLP-1 receptors are expressed in rodent and human heart and they improve energy metabolism via increasing glucose intake to myocytes.15,16 In isolated cardiomyocytes, GLP-1 enhances L-type Ca2+ current via activation of the cAMP-dependent protein kinase A.17 Many recent reports have focused on the protective effect of DPP4 inhibitors in various cardiovascular diseases such as hypertension, cardiac ischemia, and cardiomyopathy.18–22 The DPP-4 inhibitor, sitagliptin, has been shown to enhance the recovery of left ventricular developed pressure in a model of ischemia/reperfusion injury. Chronic treatment with sitagliptin increased survival in diabetic mice after left anterior descending coronary occlusion.23 Another study of Ye et al.18 showed the efficacy of sitagliptin in decreasing infarct size following induction of myocardial ischemia in non-diabetic rodents. Furthermore, DPP-4 inhibitors have also been demonstrated to improve left ventricular relaxation and decrease cardiomyocyte apoptosis and cardiac fibrosis in diabetic mice with cardiomyopathy.10 Interestingly, in most studies, the potential benefits of DPP-4 inhibitors were observed to be independent of blood glucose control. This suggests that DPP-4 inhibitors may improve heart function mainly through a GLP-1R-independent pathway.24 Up till now the possible protective role of DPP4 inhibition on DOX-induced cardiac toxicity has not been examined. Therefore, the aim of this study is to investigate the potential effects of sitagliptin on DOX-induced cardiotoxicity and explore the underlying mechanisms.
Materials and methods
Animals
The study was conducted on 32 male Wistar rats weighing 150–180 and of 9–10 week’s age. The animals were obtained from King Abdulaziz University, Jeddah, Saudi Arabia. The animals were maintained under standard conditions of hygiene, temperature, and a 12-h light/dark cycle with free access to standard rat chow and water. All the procedures concerning animal experiments were performed in accordance with the principles and guidelines for Care and Use of Laboratory Animals adopted by “The Research Ethics Committee”, Taibah University, Saudi Arabia which comply with “Principles of Laboratory Animals Care” (NIH publication No. 85-23, revised 1985).
Chemicals
The highly water soluble sitagliptin phosphate monohydrate was obtained as tablets “Januvia, Merck Sharp, UK” while DOX as ampoules “Adriblastina, Pharmacia, Italy” from the Medical Center of Taibah University, Al-Madinah Al-Munawwarah, Saudi Arabia. Other chemicals are of high analytical grade.
Experimental design
The rats were randomly assigned to four groups of eight animals each:
1. Control group, rats were administered physiological saline orally for 10 days.
2. DOX group, rats received physiological saline orally for 10 days and then DOX (20 mg/kg, i.p) on day 10.
3, 4. Sitagliptin + DOX groups, where rats were administered sitagliptin at two different doses (10, 20 mg/kg, orally) for 10 days before DOX administration, while DOX was given on the 10th day. Sitagliptin dosage regime was adapted based on the published studies in rodents reporting its antioxidant and anti-inflammatory activities.25–28
Forty-eight hours after DOX injection, rats were weighed, anesthetized using intraperitoneal injection of ketamine (75 mg/kg) then subjected to electrocardiography (ECG) recording. Following ECG, blood samples were collected and the serum was separated by centrifugation at 3000 r/min for 10 min then frozen at −20℃ for estimation of biochemical parameters. After that, rats were sacrificed and the hearts were harvested and washed with ice cold saline. Small piece of the hearts was cut, weighed, and then homogenized using normal saline. Another piece of the heart was fixed in 10% neutral buffered formalin for histopathological and immunohistochemical examination.
ECG
ECG was recorded at the beginning of the experiment to ensure the normal ECG pattern of the rats. ECG was recorded in ketamine anesthetized rats 48 h after DOX injection. Needle electrodes were inserted under the skin of the animals in lead II position. The ECG chart recording speed was 50 mm/s and the voltage was 1 mv/cm. Analysis of ECG waves was performed to calculate heart rate (beats/min), R-R interval (ms), QRS duration (ms), QT interval (ms) which was corrected for heart rate using the Bazett formula QTc = QT/(square root of RR interval),29 and R wave voltage (mv). For each parameter, measurements were done at three non-consecutive, randomly chosen points in every 5 min recording. The results are reported as mean of the three randomly selected segments.
Assessment of serum cardiotoxicity markers
Creatine kinase isoenzyme-MB (CK-MB) activity (a highly reliable biomarker of cardiac injury) was detected based on kinetic photometric method using available commercial kit (Elitech, France). The samples were measured at 340 nm and the increase in the absorbance at this wavelength is directly related to the activity of CK-MB and the results were expressed as U/L.
Lactate dehydrogenase (LDH) activity was estimated according to standard methods using available commercial kit (Human Gesllschaft fur Biochemica und Diagnostica, Germany).
Assessment of histopathological cardiac damage
Heart tissue samples fixed in 10% neutral buffered formalin were processed for paraffin sections of 4 µm thickness, stained with hematoxylin and eosin (H&E) and examined using a light microscope (Olympus BX-50 Olympus Corporation, Tokyo, Japan). The histopathological lesions in the hearts of rats of different groups were characterized. The structural alterations of tissue were assessed based on the degree of vascular congestion; hemorrhage; hemosidrosis; cloudy degeneration; fatty degeneration; muscle striation; apoptosis and necrosis. The severity of such pathological lesions was graded semiquantitatively depending on the degree and extent of the alteration as follows: score − was considered normal, score ± borderline, score + mild, score ++ moderate, and score +++ severe. Five high-power fields were observed from each animal.
Measurements of oxidative stress and antioxidant status
Estimation of malondialdehyde (MDA)
The MDA concentration was determined as an indicator of lipid peroxidation in heart tissue. The MDA content was measured using a commercial kit purchased from Bio-diagnostic Company (Giza, Egypt). Briefly, MDA content was determined by measuring the level of thiobarbituric acid-reactive substances spectrophotometrically at 534 nm. MDA level was expressed as nmol/g tissue.
Estimation of superoxide dismutase (SOD)
The SOD activity in the heart homogenate was determined using a commercial kit purchased from Bio-diagnostic Company (Giza, Egypt). Detection of SOD activity in heart homogenates relies on the ability of SOD to inhibit the reduction of nitro-blue tetrazolium dye. The SOD activity was expressed as units/g tissue.
Estimation of reduced glutathione (GSH)
The concentration of GSH in the heart homogenate was measured using a commercial kit purchased from Bio-diagnostic Company (Giza, Egypt). GSH content is proportional to the reduced yellow chromogen measured at 405 nm, that is produced by the reduction of Ellman’s reagent (5,5′ dithiobis-2-nitrobenzoic acid) (DTNB) with sulfhydryl (–SH) group of GSH. The GSH content was expressed as µmol/g tissue.
Immunohistochemical examinations
Mini tissue cores (5 mm) were extracted from each formalin fixed paraffin-embedded heart block of all animals and used to construct a tissue miniarray (TmA) as previously described.30 IHC staining of NF-κB, and Bax was carried out by using Ventana Bench Mark XT system (Ventana Medical Systems, Tucson, AZ). All procedures were performed automatically in the Bench Mark. IHC staining was performed on 4-µm slices of paraffin-embedded TmA tissue sections using primary antibodies against rabbit polyclonal anti NF-κBp65 antibody (Cat. No. RB-9034-R7, 1:100, Thermo Fisher Scientific, Fremont, CA 94538, USA) and rabbit polyclonal anti Bax antibody (Cat. No. orb4655, 1:100, Biorbyt, Cambridge, UK). The tissue sections were incubated with primary antibodies for 30 min at 42℃. The avidin-biotin technique was performed using diaminobenzidin (DAB) for visualization and sections were counterstained with hematoxylin. The slides were visualized under a light microscope. Immunohistochemical semi-quantitative analysis was carried out using image analysis software (ImageJ, 1.46a, NIH, USA). The immunohistochemical staining results in cardiac tissue were expressed as optical densities (OD) across 10 different fields for each TmA section.
Estimation of inflammatory cytokines
The levels of TNF-α and NO were determined in cardiac homogenates using commercially available kits according to the instructions of the manufacturer (R&D Systems, USA).
Estimation of apoptotic markers
The activity of myocardial caspase-3 was determined using a commercial colorimetric kit (Sigma Aldrich, USA) as per the standard methods provided. Caspase-3 activity was expressed in nmolpNA/min/ml.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey's Kramer Multiple Comparison Test. The values are means ± S.E.M for eight rats in each group. P value < 0.05 was considered as significant.
Results
Effect on ECG parameters in rats
As shown in Table 1, ECG monitoring was normal in control rats. DOX administration caused ECG abnormalities as presented by bradycardia, lengthen of R-R interval, increase of QRS duration, prolongation of QTc interval, and significant decrease in the R wave voltage as compared to the control group. Pretreatment with sitagliptin significantly attenuated DOX-induced abnormalities in ECG parameters in a dose-dependent manner as compared to DOX group.
Table 1.
Effects of doxorubicin with/without sitagliptin pretreatment on ECG parameters
| Groups | HR (bpm) | R-R interval (ms) | QRS duration (ms) | QTc interval (ms) | R wave voltage (mv) |
|---|---|---|---|---|---|
| Control | 363.3 ± 11.09 | 145 ± 8.5 | 47.5 ± 3.2 | 145 ± 8.6 | 0.69 ± 0.033 |
| DOX | 273 ± 6.03*** | 267.5 ± 6.3*** | 88.8 ± 8.4*** | 231.9 ± 9.3*** | 0.43 ± 0.017*** |
| Sitagliptin (10 mg/kg) + DOX | 322.9 ± 9.9*## | 235.6 ± 6.1***## | 63.1 ± 6.1# | 170.8 ± 3.5### | 0.5 ± 0.023*** |
| Sitagliptin (20 mg/kg) + DOX | 350 ± 10.6### | 190.7 ± 3.5***### | 56.9 ± 5.5## | 151.8 ± 9.7### | 0.62 ± 0.016### |
DOX: doxorubicin.
Note: Data are the mean ± SEM (n = 8).
P < 0.05, ***P < 0.001 significantly different as compared to the control.
P < 0.05, ##P < 0.01, ###P < 0.001 significantly different as compared to the DOX group (ANOVA followed by Tukey-Kramer multiple comparison as a post-hoc test).
Effect on cardiotoxicity markers in rats
CK-MB and LDH levels were determined as serum markers for myocardial injury. In DOX group, these markers were significantly elevated as compared to the control group. On the contrary, sitagliptin pretreated animals showed a significant decrease in LDH and CK-MB levels as compared to DOX group in a dose-dependent manner (Figure 1).
Figure 1.
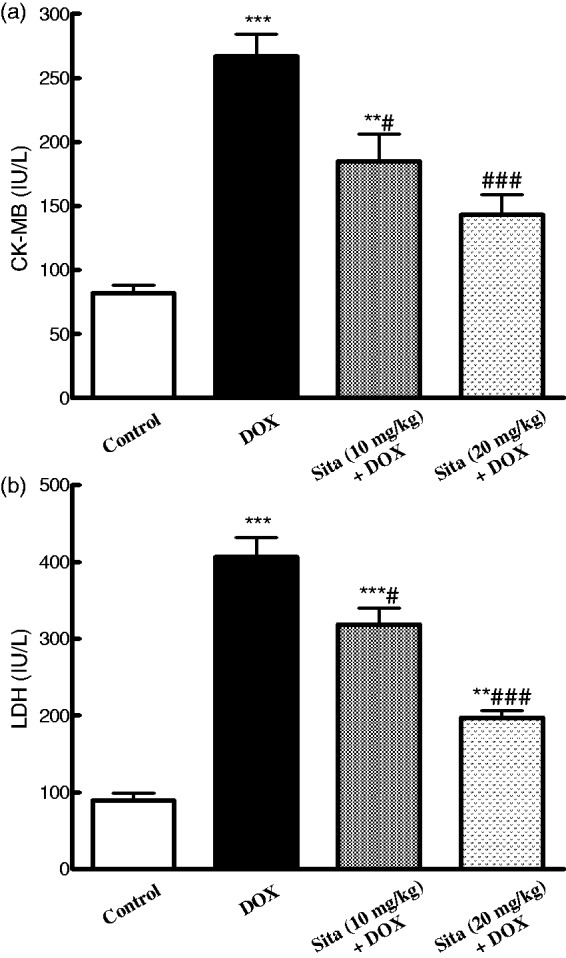
Effects of doxorubicin (DOX) with/without sitagliptin (Sita) pretreatment on serum cardiotoxicity markers. (a) CK-MB: creatine kinase-MB, (b) LDH: lactate dehydrogenase. Data are the mean ± SEM (n = 8).**P < 0.01, ***P < 0.001 significantly different as compared to the control. #P < 0.05, ###P < 0.001 significantly different as compared to the DOX group (ANOVA followed by Tukey-Kramer multiple comparison as a post-hoc test)
Histopathological examination
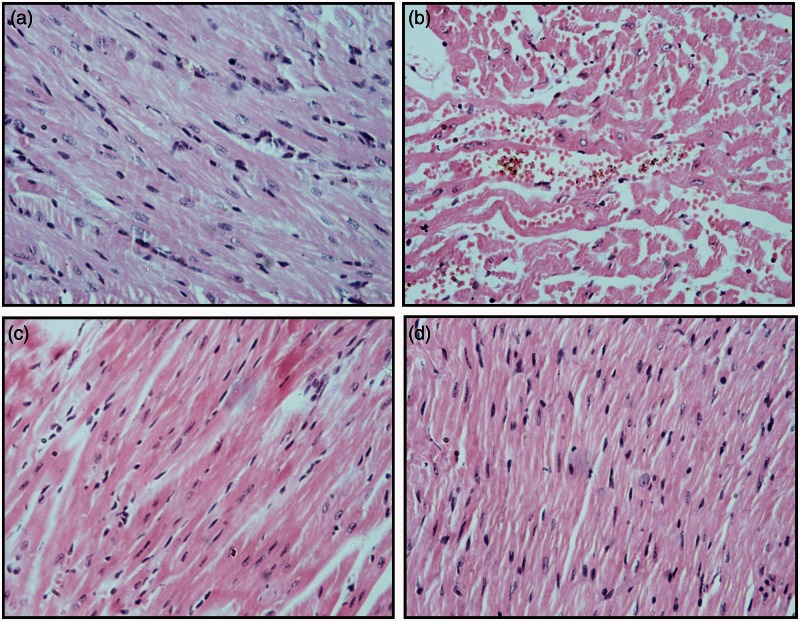
Rats of the control group showed normal appearance of cardiac muscles and blood vessels (Figure 2(a)). DOX intoxicated rats showed marked injury in the cardiac tissue as there was dispersion of the cardiac muscles architecture, loss of muscular striations, vascular congestion, and marked hydropic degeneration (Figure2(b)). Cardiac myocytes showed focal apoptosis and areas of coagulative necrosis with nuclear pyknosis, karyorrhexis, and karyolysis. Minimal to moderate inflammatory cellular infiltrate was detected. Heart specimen of sitagliptin pretreated rats at both doses showed attenuation of DOX-induced pathological changes (Figure 2(c) and (d)). Sitagliptin (20 mg/kg) significantly preserved almost normal heart cellular architecture from damaging effects of DOX. The semi-quantitative scoring of histological changes is presented in Table 2.
Figure 2.
Effects of doxorubicin (DOX) with/without sitagliptin pretreatment on heart histology of rats. (a) Control group: normal heart architecture was observed, (b) DOX group showed disruption of cardiac muscle architecture, loss of muscle striation, hydropic degeneration, apoptosis, and focal necrosis. Cardiac myocytes show focal apoptosis and signs of necrosis (pyknosis, kariorrhexis and karyolysis) with inflammatory cellular infiltrate (c, d) Sitagliptin + DOX groups; improvement of the cardiac lesions. Hematoxylin and eosin stain. Original magnification ×200. (A color version of this figure is available in the online journal.)
Table 2.
Histopathological semi-quantitative scoring showing the effects of doxorubicin (DOX) with/without sitagliptin (Sita) pretreatment on severity of histopathologic lesions in DOX-treated rats
| Histopathological changes | Control | DOX | Sitagliptin (10 mg/kg) + DOX | Sitagliptin (20 mg/kg) + DOX |
|---|---|---|---|---|
| Disruption of cardiac muscles architecture | − | + ++ | ++ | + |
| Loss of muscular striations | − | +++ | ++ | + |
| Vascular congestion | − | +++ | + | ± |
| Interstitial hemorrhage and hemosiderin deposition | − | +++ | ± | − |
| Cloudy swelling and hydropic degeneration | − | +++ | + | + |
| Fatty degeneration | − | + | − | − |
| Inflammatory cellular infiltrate | − | ++ | + | ± |
| Apoptosis | − | ++ | + | + |
| Necrosis (nuclear pyknosis, karyorrhexis, karyolysis) | − | +++ | + | − |
Note: Scores represent values obtained from tissue sections of six animals of each group, 5 fields/section (×40). Scores –, normal; ± borderline; + mild; ++, moderate; +++, severe levels.
Effect on oxidative stress and antioxidant status
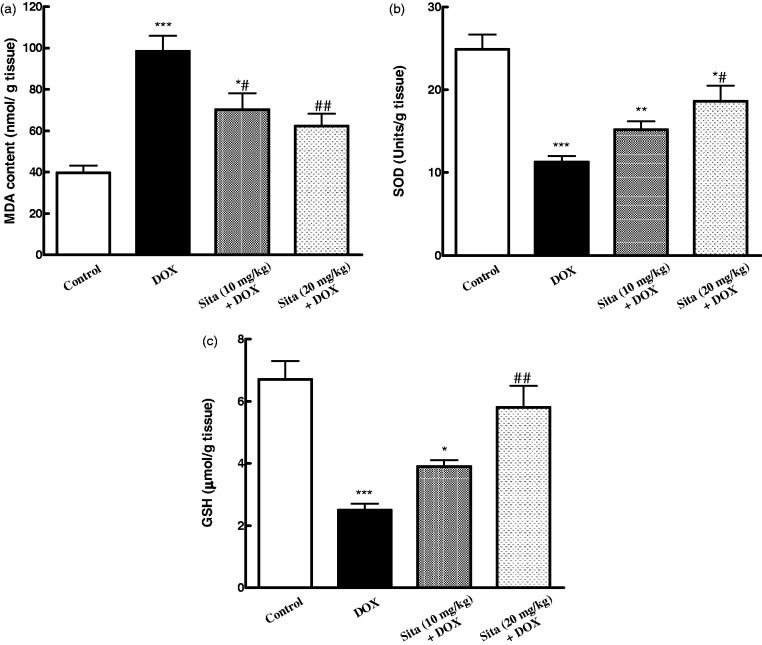
The level of lipid peroxidation in cardiac tissue was significantly increased with contaminant decrease in the antioxidant status in DOX-treated animals (Figure 3). Pretreatment with sitagliptin significantly attenuated the elevated MDA level and increased antioxidant markers, GSH content and SOD activity, as compared to DOX group.
Figure 3.
Effects of doxorubicin (DOX) with/without sitagliptin (Sita) pretreatment on cardiac oxidative stress markers. (a) MDA: malondialdehyde, (b) SOD: superoxide dismutase, (c) GSH: reduced glutathione. Data are the mean ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 significantly different as compared to the control. #P < 0.05, ##P < 0.01 significantly different as compared to the DOX group (ANOVA followed by Tukey-Kramer multiple comparison as a post-hoc test)
Effect on immunohistochemistry of NF-κB p65
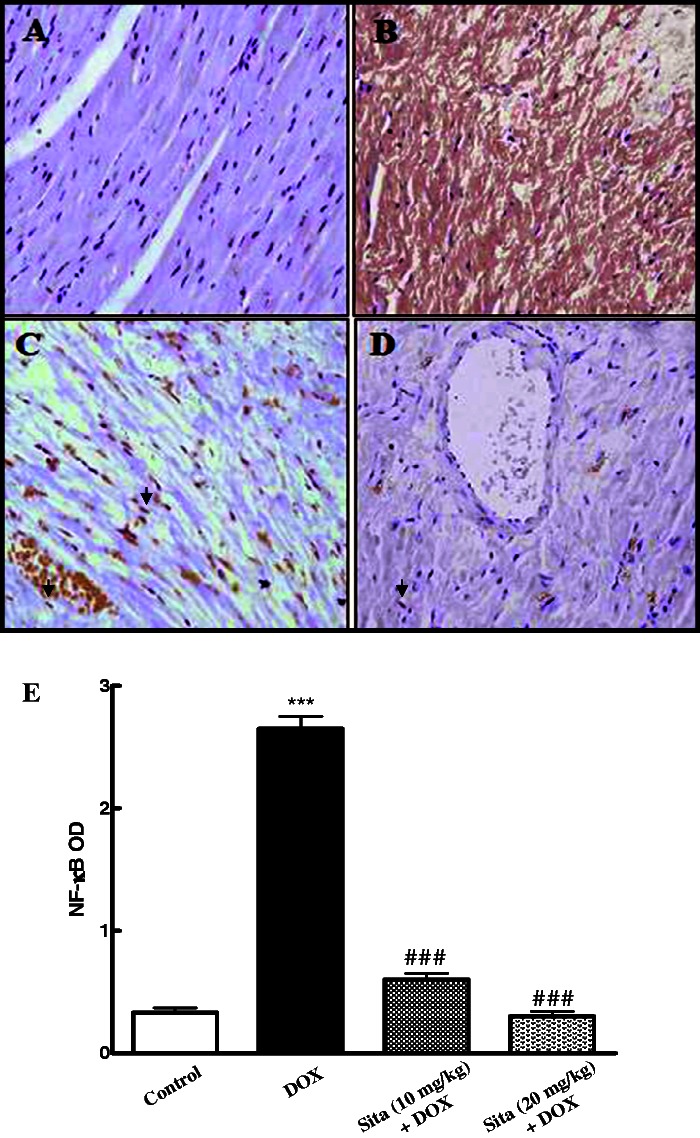
Regarding NF-κB immunoexpression and OD quantification, the control group showed minimal brown cytoplasmic immunostaining for NF-κB p65 subunit, while there was marked elevation in the level of p65 in case of DOX group as it was indicated by intense brown staining (Figure 4(a) and (b)). Sitagliptin pretreatment at both lower and higher doses induces a significant reduction in the expression of p65 (Figure 4(c) to (e)).
Figure 4.
Effects of doxorubicin (DOX) with/without sitagliptin (Sita) pretreatment on the expression of nuclear factor-kappa B (NF-κB) by immunohistochemical staining (200×). (a) Control group with mild light brown cytoplasmic immunostaining (b) DOX group, showing intense brown cytoplasmic immunostaining of NF-κB (c, d) Sitagliptin + DOX groups, showing a marked decrease in the cytoplasmic expression of NF-κB in a dose-dependent manner of sitagliptin and appearance of nuclear staining (arrow head) (e) Semiquantitative analysis of NF-κB immunohistochemical staining results in cardiac tissue expressed as optical densities (OD) across 10 different fields for each TmA section. Values are represented as means ± SEM. ***P < 0.001 significantly different as compared to the control. ###P < 0.001 significantly different as compared to the DOX group (ANOVA followed by Tukey-Kramer multiple comparison as a post-hoc test). (A color version of this figure is available in the online journal.)
Effect on inflammatory cytokines
As presented in Figure 5, DOX administration resulted in increase in the levels of inflammatory cytokines, TNF-α and NO, as compared to the control group, while sitagliptin pretreatment alleviated DOX-induced overproduction of these cytokines.
Figure 5.
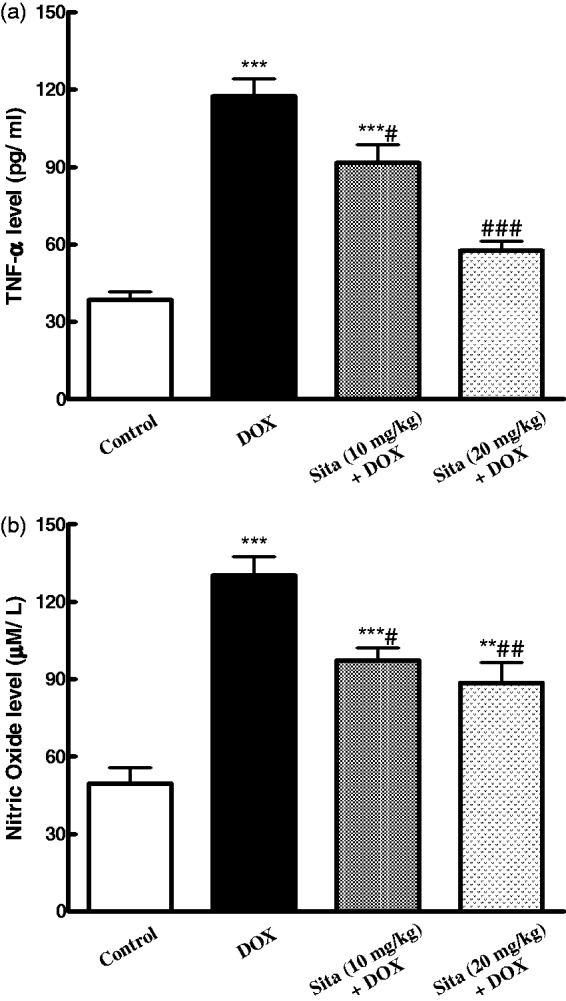
Effects of doxorubicin (DOX) with/without sitagliptin (Sita) pretreatment on inflammatory cytokines in cardiac tissue. (a) TNF-α (b) Nitric oxide. Data are the mean ± SEM (n = 8). **P < 0.01, ***P < 0.001 significantly different as compared to the control. #P < 0.05, ##P < 0.01, ###P < 0.001 significantly different as compared to the DOX group (ANOVA followed by Tukey-Kramer multiple comparison as a post-hoc test)
Effect on apoptotic markers
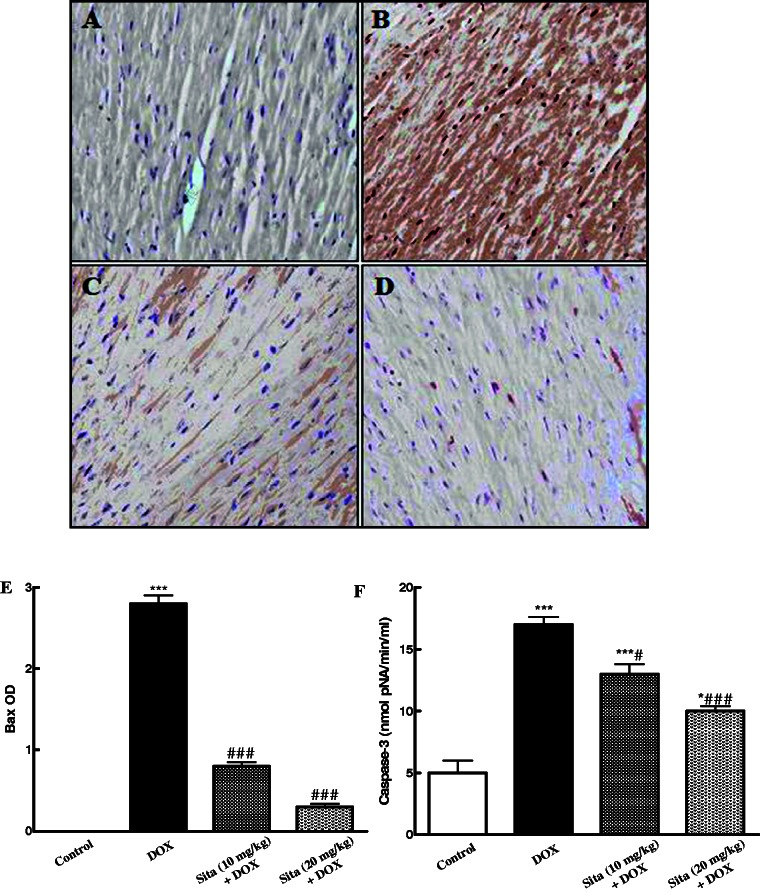
As shown in Figure 6, the immunoexpression and OD quantification of the pro-apoptotic marker Bax were markedly increased upon DOX administration as compared to the control group. However, sitagliptin pretreatment significantly attenuated Bax immunoexpression in a dose-dependent manner as compared to the DOX group (Figure 6(c) to (e)). Furthermore, the caspase-3 activity in the cardiac tissue was significantly elevated in DOX group as compared to the control one. Sitagliptin pretreatment ameliorated this increase and lowered the level of caspase-3 (Figure 6(f)).
Figure 6.
Effects of doxorubicin (DOX) with/without sitagliptin (Sita) pretreatment on the expression of Bax by immunohistochemical staining (200×) and caspase-3 activity in cardiac tissue. (a) Control group showing negative immunostaining, (b) DOX group, increased Bax expression as indicated by marked brown color (c, d) Sitagliptin + DOX groups, a marked decrease in the expression of Bax. (e) Semiquantitative analysis of Bax immunohistochemical staining results in cardiac tissue expressed as optical densities (OD) across 10 different fields for each TmA section. (f) Caspase-3 activity in cardiac tissue. Data are the mean ± SEM. *P < 0.05, ***P < 0.001 significantly different as compared to the control.
#P < 0.05, ###P < 0.001 significantly different as compared to the DOX group (ANOVA followed by Tukey-Kramer multiple comparison as a post-hoc test). (A color version of this figure is available in the online journal.)
Discussion
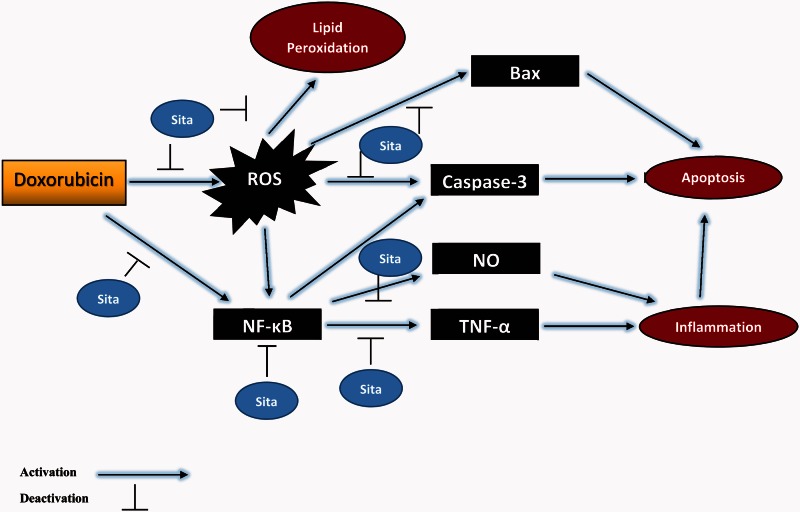
DOX is one of the most effective chemotherapeutic agents used in multiple cancer types but its clinical usefulness is seriously limited by the risk of developing cardiomyopathy and congestive heart failure. This study investigated the effects of sitagliptin on DOX-induced cardiotoxicity through estimation of various biochemical, electrophysiological, and histological parameters. The results showed that sitagliptin possesses cardioprotective effects against DOX cardiotoxicity which may be mediated through anti-oxidative, anti-inflammatory, and anti-apoptotic properties (Figure 7).
Figure 7.
Schematic diagram summarizing the possible mechanisms for sitagliptin (Sita) to counteract the oxidative and pro-apoptotic actions of doxorubicin in myocardial cells. (A color version of this figure is available in the online journal.)
The electrocardiographic results indicated that DOX cardiotoxicity is associated with ECG abnormalities as bradycardia, prolongation of R-R interval, QRS duration, QTc interval, and significant decrease in the R wave voltage. The prolongation of both QTc interval and R-R interval indicates delayed repolarization and a negative chronotropic effect in general.31 These results are in accordance with the previous investigations.32–34 Sitagliptin pretreatment counteracted these ECG abnormalities in a dose-dependent manner.
Furthermore, the development of cardiac damage induced by DOX administration was clearly evident through the significant elevation of serum CK-MB and LDH. Increased reactive oxygen species (ROS) generation following DOX administration causes membrane peroxidation and disruption of cardiomyocytes. This can lead to leakage of several biochemical markers in the serum or plasma, especially CK-MB, which is considered as standard diagnostic parameter for any form of myocardial damage.35,36 Pretreatment with sitagliptin attenuated the release of these cardiotoxicity markers into the serum in a dose-dependent manner. These biochemical results are strongly correlated with the above ECG observation and the histopathological examination of cardiac tissues. DOX administration induced degenerative changes in the cardiac tissue in the form of cloudy degeneration, muscle striation, hemorrhage, hemosidrosis, apoptosis, and necrosis. These pathological changes in cardiac tissue have been reported previously in acute DOX intoxication.34,37 On the contrary, marked attenuation of all DOX-induced histopathological lesions was observed in sitagliptin pretreated rats. Taking these results together, this study suggests that sitagliptin provokes protective effects against DOX-induced cardiotoxicity.
To further explore the molecular mechanisms that mediated the cardioprotective effects of sitagliptin, the effects of sitagliptin on oxidative stress, cytokine levels, NF-κB (p65) activity, and apoptosis were examined.
Multiple pathways are implicated in the pathogenesis of DOX-induced cardiotoxicity. Overproduction of ROS and decreased myocardial endogenous antioxidants have been considered as a corner stone in developing DOX-induced cardiotoxicity.38,39 ROS leads to lipid peroxidation and oxidative injury to cellular components which cause cardiomyocyte cell death. As documented, DOX has high affinity for the phospholipid component of mitochondrial membrane in cardiomyocyte, leading to accumulation of DOX in the heart tissue which in turn is characterized by high susceptibility to oxidative injury as compared to other organs due to low levels of free-radical detoxifying enzymes/molecules like SOD, GSH, and catalase.40,41 Results presented here demonstrated that DOX induced lipid peroxidation in cardiac tissue as it caused elevation in the cardiac content of MDA contaminant with significant decrease in GSH content and SOD activity. These findings confirm many previous reports that demonstrated increased cardiac lipid peroxidation accompanied by deteriorating antioxidant status in the DOX-treated animals.31,34,38,41 However, sitagliptin pretreatment attenuated DOX-induced oxidative damage and increased the antioxidant status in cardiac tissue. These results are in accordance with the previous studies which showed the ability of sitagliptin to reduce the oxidative burden in different experimental models of organs toxicities.22,28,42,43
In addition to its direct toxic effects, ROS can induce nuclear transcription factor-κB (NF-κB) activation which leads to its translocation into the nucleus where it binds to the promoter elements and activate the expression of inflammatory cytokine genes with subsequent proinflammatory mediators production such as TNF-α, COX-2, and NO.7,44 In the current study, DOX administration resulted in the increased expression of NF-κB as well as increased levels of TNF-α and NO. These results are in accordance with previous investigations that reported the ability of DOX to induce potent inflammatory response through activation of NF-κB and stimulating subsequent proinflammatory cytokine production.8 Interestingly, sitagliptin attenuated that inflammatory process. Sitagliptin pretreatment significantly suppressed the expression of NF-κB and reduced the production of TNF-α and NO in the cardiac tissue of rats exposed to DOX cardiotoxicity. These results confirm the anti-inflammatory action of sitagliptin that has been documented biochemically in animals and humans.11,14,23,42 Based on the presented results, it is acceptable to presume that the cardioprotective effects of sitagliptin against DOX cardiotoxicity are closely related to its ability to scavenge ROS and so blocking ROS induced activation of NF-κB and subsequent inflammatory mediator’s production, thus minimizing the tissue damage in response to oxidants.
Recently, increased apoptosis of cardiomyocytes has been implicated as one of DOX possible mechanisms of cardiotoxicity.34,45,46 However, increase in oxidative stress and depletion of endogenous antioxidants induces the intrinsic mitochondria-dependent apoptotic pathway in cardiomyocytes.47 Furthermore, NF-κB can potentiate DOX-induced apoptois in cardiomyocytes.5 Recently, Pointon et al.48 have suggested that the major mechanism of DOX cardiotoxicity is via damage or inhibition of electron transport chain (ETC) by involving alteration in the expression and translation of myocardial ETC genes, leading to ATP loss and caspase-3 activation that initiate the apoptotic degradation phase.
As reported before, the major regulators of apoptosis are members of the Bcl-2 family of proteins which include pro-apoptotic protein (as Bax) and anti-apoptotic proteins (as Bcl-2 and Bcl-xL).49 Increase of oxidative stress causes Bax translocation to the outer mitochondrial membrane resulting in increase in mitochondrial permeability and cytochrome c release into the cytosol which activate downstream effector caspases.50 In agreement with these early reports, DOX sharply increased Bax expression and caspase-3 activity with subsequent marked apoptosis of cardiomyocytes. Sitagliptin supplementation resulted in decreased in pro-apoptotic protein Bax expression and caspase-3 activity leading to attenuation of apoptosis. This finding is consistent with previous investigations that reported the antiapoptotic effects of sitagliptin.10,51,52
Finally, sitagliptin is a new anti-diabetic agent that has many advantages over other hypoglycemic agents as weight neutral and a low risk of hypoglycemia as well as lack of drug interactions. Until now no limitation has been reported concerning the use of sitagliptin. High cost and unknown long-term safety are the major disadvantages of sitagliptin.53 However, this study may add a new spectrum to the use of sitagliptin.
In conclusion, the current study demonstrates for the first time the cardioprotective effects of sitagliptin against DOX-induced cardiotoxicity. This effect may be mediated through potent antioxidative, anti-inflammatory, and anti-apoptotic activities of sitagliptin. Thus, it is reasonable to presume that sitagliptin may be beneficial in patient on DOX chemotherapy. Further clinical studies are needed to prove that effect.
Acknowledgments
The authors acknowledge Dr. Alaa O. Owaidah, Department of Biochemistry, College of Medicine, Taibah University, Al-Madinah Al-Munawwarah, Saudi Arabia for providing assistance. The authors thank Dr. Irfan Ansari for reviewing the language and grammar of the manuscript. This project was funded by the Deanship of Scientific Research (DSR), Taibah University, Al-Madinah Al-Munawwarah, Saudi Arabia, under grant no. 6242/1435. The authors are grateful for DSR financial support.
Authors’ contribution
DSE-A contributed to the protocol development and the concept of this study, ME-K performed histological and immunohistochemical analysis and HMA-H performed and analyzed the electrocardiography. All authors contributed to all other aspects of this study.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Segredo MP, Salvadori DM, Rocha NS, Moretto FC, Correa CR, Camargo EA, de Almeida DC, Reis RA1, Freire CM, Braz MG, Tang G, Matsubara LS, Matsubara BB, Yeum KJ, Ferreira AL. Oxidative stress on cardiotoxicity after treatment with single and multiple doses of doxorubicin. Hum Exp Toxicol 2014; 33: 748–60. [DOI] [PubMed] [Google Scholar]
- 2.Breed JG, Zimmerman AN, Dormans JA, Pinedo HM. Failure of the antioxidant vitamin E to protect against adriamycin-induced cardiotoxicity in the rabbit. Cancer Res 1980; 40: 2033–8. [PubMed] [Google Scholar]
- 3.Bruynzeel AM, Vormer-Bonne S, Bast A, Niessen HW, van der Vijgh WJ. Long-term effects of 7-mono hydroxyethylrutoside (monoHER) on DOX-induced cardiotoxicity in mice. Cancer Chemother Pharmacol 2007; 60: 509–14. [DOI] [PubMed] [Google Scholar]
- 4.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 2004; 56: 185–229. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B. Activation of nuclear factor-kappa B during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. Biochem J 2002; 367: 729–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou G, Dick R, Abrams GD, Brewer GJ. Tetrathiomolybdate protects against cardiac damage by doxorubicin in mice. J Lab Clin Med 2005; 146: 299–303. [DOI] [PubMed] [Google Scholar]
- 7.Saeed NM, El-Naga RN, El-Bakly WM, Abdel-Rahman HM, Salah ElDin RA, El-Demerdash E. Epigallocatechin-3-gallate pretreatment attenuates doxorubicin-induced cardiotoxicity in rats: a mechanistic study. Biochem Pharmacol 2015; 95: 145–55. [DOI] [PubMed] [Google Scholar]
- 8.Abd El-Aziz TA, Mohamed RH, Pasha HF, Abdel-Aziz HR. Catechin protects against oxidative stress and inflammatory-mediated cardiotoxicity in adriamycin-treated rats. Clin Exp Med 2012; 12: 233–40. [DOI] [PubMed] [Google Scholar]
- 9.Reeve JL, Szegezdi E, Logue SE, Chonghaile TN, O'Brien T, Ritter T, Samali A. Distinct mechanisms of cardiomyocyte apoptosis induced by doxorubicin and hypoxia converge on mitochondria and are inhibited by Bcl-xL. J Cell Mol Med 2007; 11: 509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenski M, Kazakov A, Marx N, Böhm M, Laufs U. Effects of DPP-4 inhibition on cardiac metabolism and function in mice. J Mol Cell Cardiol 2011; 51: 906–18. [DOI] [PubMed] [Google Scholar]
- 11.Makdissi A, Ghanim H, Vora M, Green K, Abuaysheh S, Chaudhuri A, Dhindsa S, Dandona P. Sitagliptin exerts an anti-inflammatory action. J Clin Endocrinol Metab 2012; 97: 3333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balakumar P, Dhanaraj SA. Cardiovascular pleiotropic actions of DPP-4 inhibitors: a step at the cutting edge in understanding their additional therapeutic potentials. Cell Signal 2013; 25: 1799–803. [DOI] [PubMed] [Google Scholar]
- 13.Panchapakesan U, Mather A, Pollock C. Role of GLP-1 and DPP-4 in diabetic nephropathy and cardiovascular disease. Clin Sci (Lond, England: 1979) 2013; 124: 17–26. [DOI] [PubMed] [Google Scholar]
- 14.Satoh-Asahara N, Sasaki Y, Wada H, Tochiya M, Iguchi A, Nakagawachi R, Odori S, Kono S, Hasegawa K, Shimatsu A. A dipeptidyl peptidase-4 inhibitor, sitagliptin, exerts anti-inflammatory effects in type 2 diabetic patients. Metabolism 2013; 62: 347–51. [DOI] [PubMed] [Google Scholar]
- 15.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008; 117: 2340–50. [DOI] [PubMed] [Google Scholar]
- 16.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation 2004; 110: 955–61. [DOI] [PubMed] [Google Scholar]
- 17.Xiao YF, Nikolskaya A, Jaye DA, Sigg DC. Glucagon-like peptide-1 enhances cardiac L-type Ca2+ currents via activation of the cAMP-dependent protein kinase A pathway. Cardiovasc Diabetol 2011; 10: 6–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye Y, Keyes KT, Zhang C, Perez-Polo JR, Lin Y, Birnbaum Y. The myocardial infarct size-limiting effect of sitagliptin is PKA-dependent, whereas the protective effect of pioglitazone is partially dependent on PKA. Am J Physiol Heart Circ Physiol 2010; 298: H1454–65. [DOI] [PubMed] [Google Scholar]
- 19.Chaykovska L, von Websky K, Rahnenfuhrer J, Alter M, Heiden S, Fuchs H, Runge F, Klein T, Hocher B. Effects of DPP-4 inhibitors on the heart in a rat model of uremic cardiomyopathy. PLoS One 2011; 6: e27861–e27861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pacheco BP, Crajoinas RO, Couto GK, Davel AP, Lessa LM, Rossoni LV, Girardi AC. Dipeptidyl peptidase IV inhibition attenuates blood pressure rising in young spontaneously hypertensive rats. J Hypertens 2011; 29: 520–8. [DOI] [PubMed] [Google Scholar]
- 21.Yin M, Sillje HH, Meissner M, van Gilst WH, de Boer RA. Early and late effects of the DPP-4 inhibitor vildagliptin in a rat model of postmyocardial infarction heart failure. Cardiovasc Diabetol 2011; 10: 85–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang G, Zhang P, Ye L, Lu K, Wang Y, Duan Q, Zheng A, Qin S, Zhang D. Protective effects of sitagliptin on myocardial injury and cardiac function in an ischemia/reperfusion rat model. Eur J Pharmacol 2013; 718: 105–13. [DOI] [PubMed] [Google Scholar]
- 23.Sauve M, Ban K, Momen MA, Zhou YQ, Henkelman RM, Husain M, Drucker DJ. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes 2010; 59: 1063–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadini GP, Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vascul Pharmacol 2011; 55: 10–16. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira L, Teixeira-de-Lemos E, Pinto F, Parada B, Mega C, Vala H, Pinto R, Garrido P, Sereno J, Fernandes R, Santos P, Velada I, Melo A, Nunes S, Teixeira F, Reis F. Effects of sitagliptin treatment on dysmetabolism, inflammation, and oxidative stress in an animal model of type 2 diabetes (ZDF rat). Mediators Inflamm 2010; 2010: 592760–592760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, Xu G, Pu Y, Zhu Z, Xu A, Lam KS, Chen ZY, Ng CF, Yao X, Huang Y. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension 2012; 60: 833–41. [DOI] [PubMed] [Google Scholar]
- 27.Lee TI, Kao YH, Chen YC, Huang JH, Hsu MI, Chen YJ. The dipeptidyl peptidase-4 inhibitor-sitagliptin modulates calcium dysregulation, inflammation, and PPARs in hypertensive cardiomyocytes. Int J Cardiol 2013; 168: 5390–5. [DOI] [PubMed] [Google Scholar]
- 28.Jung YA, Choi YK, Jung GS, Seo HY, Kim HS, Jang BK, Kim JG, Lee IK, Kim MK, Park KG. Sitagliptin attenuates methionine/choline-deficient diet-induced steatohepatitis. Diabetes Res Clin Pract 2014; 105: 47–57. [DOI] [PubMed] [Google Scholar]
- 29.Bazett HC. An analysis of time relations of electrocardiograms. Heart 1920; 7: 353–67. [Google Scholar]
- 30.Elkablawy MA, Albasri AM. High quality tissue miniarray technique using a conventional TV/radio telescopic antenna. Asian Pac J Cancer Prev 2015; 16: 1129–33. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien CE, Harik N, James LP, Seib PM, Stowe CD. Cesium-induced QT-interval prolongation in an adolescent. Pharmacotherapy 2008; 28: 1059–65. [DOI] [PubMed] [Google Scholar]
- 32.Jiang B, Zhang L, Li M, Wu W, Yang M, Wang J, Guo DA. Salvianolic acids prevent acute doxorubicin cardiotoxicity in mice through suppression of oxidative stress. Food Chem Toxicol 2008; 46: 1510–15. [DOI] [PubMed] [Google Scholar]
- 33.Elberry AA, Abdel-Naim AB, Abdel-Sattar EA, Nagy AA, Mosli HA, Mohamadin AM, Ashour OM. Cranberry (Vaccinium macrocarpon) protects against doxorubicin-induced cardiotoxicity in rats. Food Chem Toxicol 2010; 48: 1178–84. [DOI] [PubMed] [Google Scholar]
- 34.Mantawy EM, El-Bakly WM, Esmat A, Badr AM, El-Demerdash E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur J Pharmacol 2014; 728: 107–18. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Chen Z, Chua CC, Ma YS, Youngberg GA, Hamdy R, Chua BH. Melatonin as an effective protector against doxorubicin induced cardiotoxicity. Am J Physiol Heart Circ Physiol 2002; 283: H254–63. [DOI] [PubMed] [Google Scholar]
- 36.Zhu W, Soonpaa MH, Chen H, Shen W, Payne RM, Liechty EA, Caldwell RL, Shou W, Field LJ. Acute doxorubicin cardiotoxicity is associated with p53-induced inhibition of the mammalian target of rapamycin pathway. Circulation 2009; 119: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fouad AA, Yacoubi MT. Mechanisms underlying the protective effect of eugenol in rats with acute doxorubicin cardiotoxicity. Arch Pharm Res 2011; 34: 821–8. [DOI] [PubMed] [Google Scholar]
- 38.Oz E, Erbaş D, Sürücü HS, Düzgün E. Prevention of doxorubicin-induced cardiotoxicity by melatonin. Mol Cell Biochem 2006; 282: 31–7. [DOI] [PubMed] [Google Scholar]
- 39.Ichihara S, Yamada Y, Kawai Y, Osawa T, Furuhashi K, Duan Z, Ichihara G. Roles of oxidative stress and Akt signaling in doxorubicin cardiotoxicity. Biochem Biophys Res Commun 2007; 359: 27–33. [DOI] [PubMed] [Google Scholar]
- 40.Takacs IE, Matkovics B, Varga SI, Homolay P, Feer G, Seres T. Study of the myocardial antioxidant defense in various species. Pharmacol Res 1992; 25: 177–8. [Google Scholar]
- 41.Singh G, Singh AT, Abraham A, Bhat B, Mukherjee A, Verma R, Agarwal SK, Jha S, Mukherjee R, Burman AC. Protective effects of Terminalia arjuna against doxorubicin-induced cardiotoxicity. J Ethnopharmacol 2008; 117: 123–9. [DOI] [PubMed] [Google Scholar]
- 42.Vaghasiya J, Sheth N, Bhalodia Y, Manek R. Sitagliptin protects renal ischemia reperfusion induced renal damage in diabetes. Regul Pept 2011; 166: 48–54. [DOI] [PubMed] [Google Scholar]
- 43.Onoyama T, Koda M, Okamoto T, Kishina M, Matono T, Sugihara T, Murawaki Y. Therapeutic effects of the dipeptidyl peptidase-IV inhibitor, sitagliptin, on non-alcoholic steatohepatitis in FLS-ob/ob male mice. Mol Med Rep 2015; 12: 6895–902. [DOI] [PubMed] [Google Scholar]
- 44.Giri SN, Al-Bayati MA, Du X, Schelegle E, Mohr FC, Margolin SB. Amelioration of doxorubicin-induced cardiac and renal toxicity by pirfenidone in rats. Cancer Chemother Pharmacol 2004; 53: 141–50. [DOI] [PubMed] [Google Scholar]
- 45.Wu S, Ko YS, Teng MS, Ko YL, Hsu LA, Hsueh C, Chou YY, Liew CC, Lee YS. Adriamycin-induced cardiomyocyte and endothelial cell apoptosis: in vitro and in vivo studies. J Mol Cell Cardiol 2002; 34: 1595–607. [DOI] [PubMed] [Google Scholar]
- 46.Li S, E M, Yu B. Adriamycin induces myocardium apoptosis through activation of nuclear factor kappa-B in rat. Mol Biol Rep 2008; 35: 489–94. [DOI] [PubMed] [Google Scholar]
- 47.Li K, Sung RY, Huang WZ, Yang M, Pong NH, Lee SM, Chan WY, Zhao H, To MY, Fok TF, Li CK, Wong YO, Ng PC. Thrombopoietin protects against in vitro and in vivo cardiotoxicity induced by doxorubicin. Circulation 2006; 113: 2211–20. [DOI] [PubMed] [Google Scholar]
- 48.Pointon AV, Walker TM, Phillips KM, Luo J, Riley J, Zhang SD, Parry JD, Lyon JJ, Marczylo EL, Gant TW. Doxorubicin in vivo rapidly alters expression and translation of myocardial electron transport chain genes, leads to ATP loss and caspase 3 activation. PLoS ONE 2010; 5: e12733–e12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kunisada K, Tone E, Negoro S, Nakaoka Y, Oshima Y, Osugi T, Funamoto M, Izumi M, Fujio Y, Hirota H, Yamauchi-Takihara K. Bcl-xl reduces doxorubicin induced myocardial damage but fails to control cardiac gene downregulation. Cardiovasc Res 2002; 53: 936–43. [DOI] [PubMed] [Google Scholar]
- 50.Xin H, Liu XH, ZhuYZ Herba leonurine attenuates doxorubicin-induced apoptosis in H9c2 cardiac muscle cells. Eur J Pharmacol 2009; 612: 75–9. [DOI] [PubMed] [Google Scholar]
- 51.El-Sahar AE, Safar MM, Zaki HF, Attia AS, Ain-Shoka AA. Sitagliptin attenuates transient cerebral ischemia/reperfusion injury in diabetic rats: implication of the oxidative-inflammatory-apoptotic pathway. Life Sci 2015; 126: 81–6. [DOI] [PubMed] [Google Scholar]
- 52.Karabulut S, Coskun ZM, Bolkent S. Immunohistochemical, apoptotic and biochemical changes by dipeptidyl peptidase-4 inhibitor-sitagliptin in type-2 diabetic rats. Pharmacol Rep 2015; 67: 846–53. [DOI] [PubMed] [Google Scholar]
- 53.Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P. Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidas-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo controlled, parallel-group study. Clin Ther 2006; 28: 1556–68. [DOI] [PubMed] [Google Scholar]