Abstract
Depression is associated with an altered immune response, which could be normalized by antidepressant drugs. However, little is known about the influence of antidepressants on the peripheral immune response and function of macrophages in individuals not suffering from depression. Our studies were aimed at determining the influence of antidepressant drugs on the humoral and cellular immune response in mice. Mice were treated intraperitoneally with imipramine, fluoxetine, venlafaxine, or moclobemide and contact immunized with trinitrophenyl hapten followed by elicitation and measurement of contact sensitivity by ear swelling response. Peritoneal macrophages from drug-treated mice were either pulsed with sheep erythrocytes or conjugated with trinitrophenyl and transferred into naive recipients to induce humoral or contact sensitivity response, respectively. Secretion of reactive oxygen intermediates, nitric oxide, and cytokines by macrophages from drug-treated mice was assessed, respectively, in chemiluminometry, Griess-based colorimetry and enzyme-linked immunosorbent assay, and the expression of macrophage surface markers was analyzed cytometrically. Treatment of mice with fluoxetine, venlafaxine, and moclobemide results in suppression of humoral and cell-mediated immunity with a reduction of the release of macrophage proinflammatory mediators and the expression of antigen-presentation markers. In contrast, treatment with imipramine enhanced the humoral immune response and macrophage secretory activity but slightly suppressed active contact sensitivity. Our studies demonstrated that systemically delivered antidepressant drugs modulate the peripheral humoral and cell-mediated immune responses, mostly through their action on macrophages. Imipramine was rather proinflammatory, whereas other tested drugs expressed immunosuppressive potential. Current observations may be applied to new therapeutic strategies dedicated to various disorders associated with excessive inflammation.
Keywords: Immune regulation, immune suppression, fluoxetine, venlafaxine, moclobemide, imipramine
Introduction
Depression is one of the most common diseases resulting from complex interactions of genetic, immunological, and environmental factors. However, the etiology of depressive disorder is not yet completely understood. The previously hypothesized inflammatory basis of depression1 was raised by Smith2 as the “macrophage theory of depression” after the observation that administration of monokines to healthy volunteers may lead to the development of symptoms of a major depressive episode. Furthermore, the higher incidence of depression in women and in patients with autoimmune disorders suggests an important role of activation of macrophages (Mfs) by estrogens and mediators of excessive inflammation in the pathogenesis of depression.2 Finally, the higher serum levels of proinflammatory cytokines and activation of circulating monocytes were also detected in patients with depression in comparison to healthy individuals.3 In addition, treatment of co-existing inflammatory disease with inhibitors of cytokines in depressed patients also ameliorated the symptoms of depression,4 and possibly could improve the response to treatment with conventional antidepressant drugs.5 Intriguingly, a clinical trial of the clinical applicability of tumor necrosis factor antagonist, infliximab, in treatment-resistant depression gave promising positive results.6 Altogether, these observations strongly supported the inflammatory and Mf theories of depression. It is worth noting that proinflammatory factors, such as cytokines secreted by peripheral Mfs, are able to cross the blood–brain barrier through various postulated mechanisms, which then activate local inflammation in the central nervous system (CNS).7,8 Additionally, microglia activated by systemic or local stimuli play an important role in the inflammatory response in the CNS, which could result in the alteration of the synthesis and metabolism of neurotransmitters, for instance by increasing monoamine re-uptake.9 During depression microglia were shown to activate the 2,3-dioxygenase-dependent metabolism of tryptophan, which inhibits serotonin synthesis.10 Furthermore, inflammation associated with depression may increase the risk of cardiovascular disorders11 and cancer development12 in depressed patients.
Resident Mfs of the phagocyte system are involved in various innate immune mechanisms, especially in antimicrobial and antitumor defense, and cellular debris clearance. Moreover, tissue Mfs, especially those of monocytic origin, play an important role in the induction and orchestration of adaptive humoral and cellular immune response through their ability to phagocytose, process and present antigens and to release various pro and antiinflammatory factors. Apart from antigen-presenting activity, Mfs act as effector cells in delayed-type hypersensitivity, including the cutaneous contact sensitivity (CS) reaction.13 As such they are a critical lynchpin in the overall immune response. Due to their expression of various cell-membrane-associated and intracellular receptors, Mfs can react to numerous signaling molecules, including cytokines, hormones, neurotransmitters, and bioactive compounds of different medications.14 Additionally, Mfs express a high plasticity of their current phenotype, which contemporarily is classified as classically or alternatively activated.13 The population of Mfs in the CNS mainly consists of microglia as well as perivascular, meningeal, and choroid Mfs.15 Resident Mfs, including microglia, colonize the brain tissue prenatally to support neurogenesis, mainly through secretion of neurotrophic factors, and to provide the phagocytic and other recently reviewed functions in the CNS.16 Interestingly, microglia can sense and respond to the alterations of neurotransmission in the CNS16 and peripheral Mfs are likely to possess the same ability.
Pharmacotherapy of depression includes several groups of drugs that differ greatly in their chemical and pharmacological properties.17 Since disturbances of neurotransmission are a leading abnormality in the pathogenesis of depression, drugs ameliorating this process are clinically important. These are represented by tricyclic antidepressant drugs (TCA), such as imipramine (IMI); selective serotonin re-uptake inhibitors (SSRI), such as fluoxetine (FLUO); serotonin-norepinephrine re-uptake inhibitors (SNRI), such as venlafaxine (VENLA); and reversible monoamine oxidase (MAO)-A inhibitors like moclobemide (MOCLO). The most important clinical effect of antidepressants’ action in the CNS results from an increase of the level of neurotransmitters, especially serotonin, in the synapses. It should be stressed that different cells of the immune system, especially Mfs, can sense the alterations of neurotransmitters concentrations and their relation due to the expression of serotonin18 and norepinephrine19 receptors.
Therapy with antidepressant drugs seems to impact the dysregulated immune response mechanisms in patients with depression,20 mainly through the amelioration of the cytokine profile in serum.21 Nevertheless, their possible effect on systemic immunity is not well described, especially in individuals not suffering from depression. Therefore, our present studies aimed to investigate the influence of treatment with the aforementioned antidepressant drugs on the immune activity of peripheral Mfs in mice.
Materials and methods
Mice
In all experiments, 10–12-week-old male mice (24 ± 2 g) of the inbred CBA/J strain from the breeding unit of the Department of Immunology, Jagiellonian University Medical College, Krakow, Poland were used according to the guidelines of the 1st Local Ethics Committee (approval no. 152/2012). Mice were fed autoclaved food and water ad libitum. The general scheme of experiments is shown in the Figure 1(a).
Figure 1.
Experimental design. (a) The general scheme of successive experiments performed. In each experiment, mice were treated for seven days with each respective antidepressant drug. To assess humoral immune response, thioglycollate-induced peritoneal macrophages (TMfs) collected from these mice were pulsed with SRBCs and transferred intraperitoneally into drug-untreated recipients. For evaluation of active contact sensitivity (CS) reaction, drug-treated mice were contact sensitized, then challenged with PCL and elicited CS was measured as ear swelling response. Oil-induced peritoneal macrophages (OIL Mfs) from drug-treated mice were tested either for expression of surface markers in cytometry, generation of ROIs in a chemiluminescence assay, or for secretion of cytokines and nitric oxide during cell culture. Finally, drug-treated mice were the source of OIL Mfs, which, after conjugation with trinitrophenyl (TNP) hapten (TNP-Mfs) were transferred intravenously into mice that were then epicutaneously (e.c.) sensitized and subsequently challenged with PCL to estimate CS reaction measured as ear swelling response in TNP-Mf recipients. (b) Scheme of experiment testing for early 2-h and late 24-h phases of active CS reaction in antidepressant drug-treated, e.c. sensitized mice. (c) Presentation of the model of intravenous adoptive transfer of TNP-Mfs from antidepressant drug-treated donors into naive recipients one day before PCL e.c. sensitization for testing of drug-influenced TNP-Mf impact on early and late phases of CS reaction measured as ear swelling response after challenge with PCL. (A color version of this figure is available in the online journal.)
Antidepressant drug administration
Fluoxetine hydrochloride (FLUO, cat. no. F132) in a daily dose of 10 mg/kg per mouse, imipramine hydrochloride (IMI, cat. no. I7379) in a daily dose of 20 mg/kg per mouse, MOCLO (cat. no. M3071) in a daily dose of 5 mg/kg per mouse and venlafaxine hydrochloride (VENLA, cat. no. V7264) in a daily dose of 5 mg/kg per mouse (Sigma, St. Louis, MO, USA) were used as sterile phosphate-buffered saline (PBS) solutions for intraperitoneal injections. Both, donors of Mfs as well as mice actively sensitized with hapten, were treated with the proper drug for seven days.
Reagents
Several reagents were used, including acetone, ethanol (P.O.Ch. S.A., Gliwice, Poland), Dulbecco’s phosphate-buffered saline (DPBS), fetal calf serum (FCS), PBS, RPMI1640, thioglycollate medium (Gibco Life Technologies, Grand Island, NY, USA), heparin sodium salt, lucigenin (bis-N-methylacridinum nitrate), luminol (3-aminophthalic hydrazide), 2-mercaptoethanol (2-ME), mineral oil (heavy fraction), zymosan (Sigma, St. Louis, MO, USA), extra virgin olive oil (Basso Fedelee Figli s.r.l., San Michele di Serino, Italy), 2,4,6-trinitrobenzene sulfonic acid (TNBSA) (Eastman Kodak, Rochester, NY, USA), twice recrystallized 2,4,6-trinitrophenyl chloride (picryl chloride, PCL) (Chemica Alta, Edmonton, Alberta, Canada), sheep red blood cells (SRBC) (Graso Biotech, Starogard Gdanski, Poland).
Enzyme-linked immunosorbent assay kits
The following enzyme-linked immunosorbent assay (ELISA) kits were used: Mouse tumor necrosis factor (TNF) alpha ELISA Ready-SET-Go!® (sensitivity 8 pg/ml, cat. no. 88-7324-22), Mouse IL-6 ELISA Ready-SET-Go!® (sensitivity 4 pg/ml, cat. no. 88-7064-22), Mouse IL-10 Platinum ELISA Test (sensitivity 5 pg/ml, cat. no. BMS614/2), Mouse transforming growth factor (TGF)-beta1 Platinum ELISA Test (sensitivity 7.8 pg/ml, cat. no. BMS608/4), (eBioscience Inc., San Diego, CA, USA); Mouse IL-12p40 BD OptEIA™ Set (sensitivity 15.6 pg/ml, cat. no. 555165; BD Biosciences, San Diego, CA, USA).
Antibodies
In cytometric analysis, fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse Mac-3 monoclonal antibody (mAb), phycoerythrin (PE)-conjugated rat anti-mouse I-Ak, CD80, CD86, CD40, CD11b, CD14, CD16/32 mAb (BD Pharmingen, San Diego, CA, USA) were used.
Harvest of oil-induced or thioglycollate-induced peritoneal Mfs
Peritoneal exudates were induced by intraperitoneal injection (on the second day of drug treatment) of either 1 ml of mineral oil or 2 ml of thioglycollate medium (in the case of Mfs tested in humoral response assays). Five days later, peritoneal exudate cells (containing over 95% of non-specific esterase-positive cells)22 were harvested by washing the peritoneal cavity with 5 ml of ice-cold DPBS containing heparin (5 U/ml) and, after washing, were used in the assays as peritoneal Mfs collected from either drug-treated donors or untreated control mice.
Chemiluminescence assay
The generation of reactive oxygen intermediates (ROIs) by control Mfs from naive mice and by Mfs from donors treated with drugs was assessed in a luminol-dependent and a lucigenin-dependent chemiluminescence assay. Mfs were placed in duplicates on 96-well black plates (Nunc, Roskilde, Denmark) at a concentration of 1 × 106 cells per well in 200 µl of RPMI1640 with 10% FCS. After a 15-min incubation at 37℃ of Mfs with chemiluminescence probes, Mf oxidative burst was stimulated in selected wells with mouse serum-opsonized zymosan (10 particles per Mf) just before measurement of chemiluminescence with a Lucy 1 luminometer (Anthos, Salzburg, Austria), which lasted for 75 min. The averaged results of the kinetics of ROIs generation were expressed in relative units of luminescence emission per second.
Measurement of cytokines and nitric oxide concentration
Mfs from control or drug-treated mice were partially stimulated with lipopolysaccharide (LPS) (200 ng) and cultured in standard conditions (37℃ and 5% CO2) at a concentration of 2 × 106 cells per well in 2 ml of RPMI1640 with 5% FCS. Culture supernatants were collected for evaluation of nitric oxide (NO), IL-6, IL-12p40, and TNFα concentrations after 24 h and for IL-10 and TGF-β1 measurement after 48 h of culture. Nitric oxide concentration was measured in freshly collected supernatants in a method based on a modified Griess reaction.23 Cytokine concentrations were measured after storage of supernatants at −80℃ in ELISA according to manufacturer procedures.
Flow cytometry
Apart from testing CD16/32 expression, peritoneal Mfs from naive or drug-treated mice were incubated with mAb (clone 2.4G2) to block Fc receptors. Then cells were incubated with FITC-conjugated anti-mouse Mac-3 mAb and PE-conjugated mAb against either I-Ak (MHC class II), CD80, CD86, CD40, CD11b, CD14, or CD16/32 surface markers. After washing, Mfs were analyzed by flow cytometry (FACSCalibur, BD Bioscience, San Jose, CA, USA) for expression of selected markers. In each case, 5 × 104 cells were analyzed for data acquisition.
Hemolytic plaque forming and direct hemagglutination assays24
Thioglycollate-induced Mfs harvested from control untreated or drug-treated mice were incubated with SRBC for 30 min at 37℃ in ratio 1:10. After lysis of non-phagocytosed SRBC by osmotic shock, SRBC-pulsed Mfs were transferred intraperitoneally into naive recipients (4 × 106 Mf per mouse). Seven days later blood, sera and spleens were collected individually with a measurement of spleen weight for each. Serum titers of total anti-SRBC antibodies (in collected sera) as well as IgG antibodies (in sera pre-incubated with 0.15 M 2-ME) were measured in direct hemagglutination (HA) and expressed as a log2 of titers. Titers of specific IgM antibodies were calculated by subtraction of IgG titers from total antibody serum titers. Briefly, two-fold dilutions of sera in DPBS were incubated with 1% SRBC suspension for 90 min at 37℃ to show agglutination. Further, single cell suspensions of each spleen in RPMI1640 were incubated with 1% SRBC in the presence of guinea pig complement for 90 min at 37℃ to enumerate the number of plaque forming cells (PFC) in a plaque forming assay (PFA) performed in triplicates by a slide technique. The averaged results were expressed as the number of PFC per spleen (PFC/spl).
Active sensitization and assessment of CS reaction (Figure 1(b))
Control naive mice or mice treated with drugs were actively sensitized (on the second day of the treatment) by application of 150 µl of a 5% PCL solution in acetone:ethanol (1:3 v/v) on the shaved abdomen of the animals. Five days later, mice were challenged by topical application of 10 µl of a 0.4% PCL solution in acetone:olive oil (1:1 v/v) on both sides of both ears. Two and 24 h later, ear swelling was measured with an engineer’s micrometer (Mitutoyo, Tokyo, Japan) and, after subtraction of ear thickness increase in non-sensitized but challenged littermate animals, were expressed as delta ± standard error (SE) representing the average net ear swelling reaction in each group, consisting of five mice.
Transfer of trinitrophenyl-conjugated Mfs (Figure 1(c))
Peritoneal Mfs from control or drug-treated mice were conjugated with trinitrophenyl (TNP) hapten by incubation with TNBSA solution in DPBS (2 mg TNBSA per 1 × 108 cells) for 10 min in darkness at room temperature. Washed TNP-conjugated Mfs (TNP-Mfs) were then transferred intravenously into naive recipients that on the next day were sensitized with a 5% PCL solution (see supra). Five days later, actively sensitized recipients of TNP-Mfs were challenged with a 0.4% PCL, and subsequent ear swelling was measured as described above.
Statistical analysis
All experiments were performed two to five times, and representative results are shown in the figures. Analysis of variance (ANOVA) with a post hoc RIR Tukey test was used to estimate the statistical significance of differences observed between all, control and experimental groups; and p < 0.05 was considered as a minimum level of significance.
Results
Antidepressant drugs influence the induction of humoral immunity by antigen-pulsed Mfs
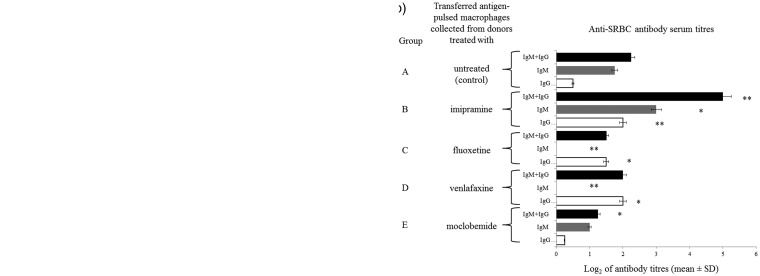
Mfs as efficient phagocytes and antigen-presenting cells are involved in the induction of the humoral immune response to corpuscular antigens. Thus, Mfs harvested from mice treated with each antidepressant drug were pulsed with SRBC (as a cellular antigen) and then transferred intraperitoneally into naive mice, from which seven days later, sera and spleens were collected for assessment of the parameters of humoral immunity, i.e. serum antibody titers in HA and number of antibody-producing cells in PFA. A significantly decreased number of antibody-producing cells was observed in spleens of recipients of SRBC-pulsed Mfs from donors treated with MOCLO and VENLA (Figure 2(a), groups E and D, respectively). Similar tendency was observed after treatment of Mf donors with FLUO (Figure 2(a), group C). In contrast, an increased number of antibody-producing cells were determined in spleens of recipients of SRBC-pulsed Mfs from donors treated with IMI (Figure 2(a), group B). Furthermore, approximately half-reduced titers of the total SRBC-specific antibodies were measured in sera of recipients of SRBC-pulsed Mfs from MOCLO-, VENLA- and FLUO-treated donors (Figure 2(b), groups E, D and C, respectively), while recipients of SRBC-pulsed Mfs from IMI-treated donors expressed enhanced serum titer of SRBC-specific antibodies (Figure 2(b), group B). Interestingly, SRBC-specific antibodies of IgM class were not detected in sera of recipients of SRBC-pulsed Mfs from donors treated with FLUO and VENLA (Figure 2(b), groups C and D).
Figure 2.
Antidepressant drugs impact humoral immune response. Mouse donors of macrophages were treated with imipramine (20 mg/kg/day), fluoxetine (10 mg/kg/day), venlafaxine (5 mg/kg/day), or moclobemide (5 mg/kg/day) for seven days. Then, isolated thioglycollate-induced peritoneal macrophages, after pulsing with sheep red blood cells (SRBC), had been injected intraperitoneally into recipients, from which sera and spleens were collected individually seven days later. (a) Number of plaque-forming cells (PFC) expressing specific antibody-producing B cells in spleens of macrophage recipients was estimated through a plaque-forming assay and indicated as a mean number of PFC per spleen. (b) SRBC-specific antibody titers in sera of donor mice were assessed through a direct hemagglutination assay and expressed as a mean value of log2 of antibody titer. N = 5; *P < 0.05; **P < 0.01; compared to respective control
Treatment with antidepressant drugs affects CS response
To determine the influence of antidepressant therapy on cellular allergic immune response, on the second day, mice treated with the studied drugs were contact immunized with PCL hapten, and five days later a CS reaction was elicited by ear challenge with PCL and then measured as an ear swelling response. A significantly suppressed 2-h CS reaction, reflecting early B1 B cell and mast cell-dependent phase of cutaneous allergy, was observed in mice treated with FLUO and VENLA and to a lesser extent with IMI (Figure 3(a), groups C, D and B, respectively). Similarly, suppression of the CS late phase mediated by effector T cells and Mfs was demonstrated in mice treated with IMI, FLUO, and VENLA (Figure 3(b), groups B, C and D, respectively), whereas MOCLO insignificantly affected both CS phases (Figure 3(a) and (b), group E).
Figure 3.
Repeated administration of antidepressant drugs affects active contact sensitivity in mice. On the second day of treatment with each respective antidepressant drug mice were contact sensitized with picryl chloride (PCL), and five days later were challenged by topical application of PCL on the skin of both ears. Elicited contact sensitivity reaction was measured (a) 2 h and (b) 24 h later as ear swelling response and, after subtraction of ear thickness increase in non-sensitized but challenged littermate animals, expressed as delta ± standard error (SE). N = 5; *P < 0.05; **P < 0.01; ***P < 0.005
Hapten-conjugated Mfs, when transferred intravenously into naive mice, were shown to be tolerogenic rather than immunogenic and to very weakly induce CS response.25 Our current studies determined whether antidepressant drugs influence this phenomenon by the transfer of TNP-Mfs from drug-treated donors into recipients that two days later were contact immunized with PCL followed by ear challenge to elicit CS reaction. Both the early and late phases of CS were strongly suppressed in immunized recipients of TNP-Mfs from donors treated with FLUO (Figure 4(a) and (b), group C), and, to a lesser extent, with VENLA and MOCLO (Figure 4(a) and (b), groups D and E), while transferred TNP-Mfs from IMI-treated donors enhanced both, the early (Figure 4(a), group B) and late (Figure 4(b), group B), phases of CS reaction.
Figure 4.
Hapten-conjugated macrophages from antidepressant drug-treated donors influence the contact sensitivity reaction in sensitized recipients. Oil-induced peritoneal macrophages from mice treated for seven days with respective antidepressant drug were conjugated with trinitrophenyl (TNP) hapten and then transferred intravenously into naive recipients that on the next day were sensitized with picryl chloride (PCL). Five days later, recipients of TNP-conjugated macrophages were challenged with PCL on the skin of both ears. Contact sensitivity reaction was measured (a) 2 h and (b) 24 h later as ear swelling response and, after subtraction of ear thickness increase in non-sensitized but challenged littermate animals, expressed as delta ± standard error (SE). N = 5; *P < 0.05; **P < 0.01
Expression of surface markers of Mfs from mice treated with antidepressant drugs
Cytofluorometrically analyzed expression of CD11b, CD14, and CD16/32 phagocytosis markers was reduced on the surface of peritoneal Mfs harvested from donors treated with FLUO, VENLA, and MOCLO, whereas a slight increase of the expression of these markers was demonstrated in Mfs from IMI-treated donors (Table 1). Further, analysis of MHC class II, CD80, CD86, and CD40 markers of antigen presentation showed a similar trend, i.e. their expression was reduced in Mfs from donors treated with FLUO, VENLA, and MOCLO and increased in Mfs from donors treated with IMI (Table 1). It is worth noting that the strongest reduction of MHC class II expression was observed in Mfs from FLUO-treated mice, while the lowest expression of CD11b and CD16/32 receptors was detected in the case of Mfs from MOCLO-treated donors.
Table 1.
Antidepressant drug treatment alters the expression of macrophage surface markers of antigen phagocytosis and presentation
| (% of cells) | CD14 |
CD11b |
CD16/32 |
MHC II |
CD80 |
CD86 |
CD40 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Mac3+ | Total | Mac3+ | Total | Mac3+ | Total | Mac3+ | Total | Mac3+ | Total | Mac3+ | Total | Mac3+ | |
| Mf ctrl | 34.5 | 31.6 | 87.6 | 34.7 | 80.2 | 36.2 | 42.0 | 23.0 | 70.9 | 34.4 | 50.3 | 33.1 | 49.4 | 31.0 |
| Mf IMI | 37.6 | 34.9 | 80.8 | 39.7 | 78.7 | 38.9 | 43.2 | 18.7 | 68.4 | 37.7 | 53.6 | 37.3 | 52.7 | 34.8 |
| Mf FLUO | 28.4 | 26.8 | 85.9 | 30.5 | 72.8 | 29.2 | 34.8 | 16.5 | 66.9 | 30.5 | 44.1 | 29.6 | 41.4 | 26.7 |
| Mf VENLA | 28.9 | 26.6 | 84.6 | 29.0 | 86.6 | 30.7 | 49.5 | 18.1 | 74.9 | 29.1 | 51.4 | 30.7 | 54.7 | 28.0 |
| Mf MOCLO | 33.7 | 31.4 | 65.9 | 19.2 | 60.8 | 27.5 | 28.7 | 22.0 | 65.7 | 29.9 | 34.6 | 28.0 | 20.4 | 14.5 |
Note: The level of expression of markers of phagocytosis (CD14, CD11b, CD16/32) and antigen presentation (MHC class II, CD80, CD86, CD40) on the surface of oil-induced peritoneal macrophages from mice treated for seven days with each respective antidepressant drug was analyzed cytometrically. Results were shown as a percentage of macrophages expressing a particular marker within either the total population of analyzed macrophages or the Mac3+ subpopulation of macrophages. N = 4; statistically non-significant.
Mf: macrophages; Mf ctrl: control macrophages; Mf IMI: macrophages from imipramine-treated mice; Mf FLUO: macrophages from fluoxetine-treated mice; Mf VENLA: macrophages from venlafaxine-treated mice; Mf MOCLO: macrophages from moclobemide-treated mice.
Treatment of mice with antidepressant drugs affects the secretion of cytokines, NO, and ROIs by Mfs
The plasticity of the phenotype of Mfs orchestrating the immune response, apart from the profile of expressed surface molecules, also depends on the ability to release various soluble factors, including cytokines, NO and ROIs, which secretion was assessed for Mfs from mice treated with assayed drugs.
Unstimulated Mfs from donors treated with FLUO, VENLA, and MOCLO expressed a decreased release of proinflammatory IL-6, TNFα, and IL-12p40, while unstimulated Mfs from mice treated with IMI showed an enhanced release of IL-6 (Figure 5(a)). On the other hand, the treatment of mice with all tested drugs resulted in a reduction of the secretion of IL-6, TNFα, and IL-12p40 by Mfs stimulated with LPS (Figure 5(a)). Furthermore, the secretion of IL-10 was enhanced in the case of unstimulated Mfs from mice treated with FLUO and MOCLO as well as LPS-stimulated Mfs from MOCLO-treated mice (Figure 5(b)). In addition, the release of TGF-β1 was increased in the case of unstimulated Mfs from donors treated with IMI and VENLA as well as LPS-stimulated Mfs from mice treated with IMI and MOCLO (Figure 5(b)).
Figure 5.
Secretion of cytokines by macrophages is affected by repeated administration of antidepressant drugs. Oil-induced peritoneal macrophages from mice treated with each respective antidepressant drug were cultured in standard conditions, in some cases after stimulation with LPS (200 ng), at concentration of 2 × 106 cells per well in 2 ml of culture medium. Enzyme-linked immunosorbent assay (ELISA) was used to measure (a) concentration of IL-6, TNFα, and IL-12p40 in supernatants collected after 24 h of culture and (b) concentration of IL-10 and TGF-β1 in supernatants collected after 48 h of culture. N = 5; *P < 0.05; **P < 0.01; compared to respective control
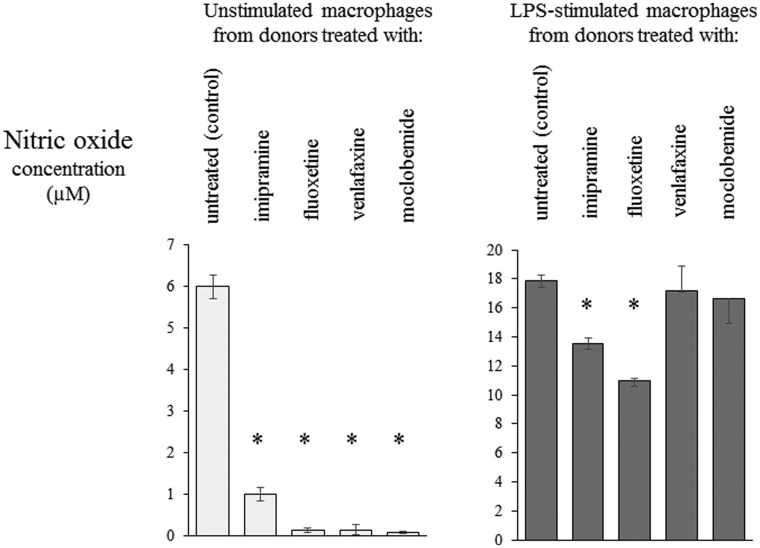
Furthermore, the treatment of mice with all tested drugs resulted in inhibition of NO production by standard-cultured unstimulated Mfs (Figure 6). LPS-stimulated secretion of NO was also inhibited in the case of Mfs from donors treated with IMI and FLUO, while treatment of animals with VENLA and MOCLO only moderately affected Mf production of NO after stimulation with LPS (Figure 6).
Figure 6.
Nitric oxide release by macrophages is altered by treatment with antidepressant drugs. Oil-induced peritoneal macrophages from mice treated with each respective antidepressant drug were cultured in standard conditions, in some cases after stimulation with LPS (200 ng), at concentration of 2 × 106 cells per well in 2 ml of culture medium. Nitric oxide concentration in the 24-h-culture supernatant was measured in method based on modified Griess reaction. N = 5; *P < 0.05; compared to respective control
Luminol- and lucigenin-dependent chemiluminescence analysis demonstrated inhibited generation of ROIs by zymosan-stimulated Mfs from mice treated with VENLA and, to a lesser extent, with FLUO and MOCLO (Figure 7(a) and (b)). In contrast, zymosan-induced generation of ROIs by Mfs from IMI-treated mice was significantly increased (Figure 7(a) and (b)).
Figure 7.
Repeated administration of antidepressant drugs influence the generation of reactive oxygen intermediates (ROIs) by macrophages. Oil-induced peritoneal macrophages from mice treated for seven days with each respective antidepressant drug were incubated on 96-well black plates at concentration of 1 × 106 cells per well with chemiluminescence probes, i.e. (a) luminol or (b) lucigenin, and then stimulated with zymosan. Kinetics of ROIs generation was then measured as chemiluminescence emission in luminometer. The averaged results were expressed in relative units of luminescence emission (RULE) per second
Mf ctrl: control macrophages; Mf IMI: macrophages from imipramine-treated mice; Mf FLUO: macrophages from fluoxetine-treated mice; Mf VENLA: macrophages from venlafaxine-treated mice; Mf MOCLO: macrophages from moclobemide-treated mice N = 4
Discussion
The antiinflammatory and immunosuppressive activities of various antidepressant drugs were frequently shown in patients with major depression mainly as a normalization of the previously increased level of proinflammatory cytokines in blood plasma during therapy.26,27 However, the influence of antidepressant drugs on the immune response of healthy individuals was poorly characterized in the past. Therefore, our studies aimed to investigate whether antidepressant drugs can modulate humoral and cellular immune responses with a special emphasis on the role of Mfs in observed drug-mediated effects. Our studies demonstrated that treatment of mice with FLUO, VENLA, and MOCLO generally leads to suppression of humoral and cell-mediated immunity with a reduction of Mf secretory activity, while treatment of mice with IMI enhanced humoral immune response and Mf secretory activity, but slightly suppressed active CS. Furthermore, it was confirmed that Mf immune activity was significantly affected by drug treatment. Thus, it proved that Mfs can perceive and respond to drug-derived modulatory signals as well as transmit them further, even into untreated recipient animals.
Previously, it was reported that orally administered paroxetine, another representative of SSRIs, did not affect the humoral immune response to SRBC evaluated in PFA nor altered Mf phagocytosis and engulfment of SRBC,28 which contrasts with our current results (Figure 2). Thus, to our knowledge, the modulation of humoral immunity by the assayed antidepressant drugs through the direct impact on Mfs was shown here for the first time. Interestingly, B lymphocytes from recipients of Mfs from FLUO- and VENLA-treated donors seemed to undergo an accelerated immunoglobulin class switching, since specific IgM antibodies were not detected in the sera of these mice.
In line with current results is former observation that treatment of mice with FLUO strongly suppresses CS reaction induced by PCL29 as well as 2,4-dinitrofluorobenzene (DNFB) haptens.30 Immunization of mice with DNFB hapten activates effector T CD8+ cytotoxic lymphocytes, in contrast to PCL hapten, which activates the effector response of T CD4+ lymphocytes and Mfs.
Previous reports hypothesized that the antiinflammatory effect of antidepressant therapy in humans results from the ability of prescribed drugs to modulate the cytokine-dependent signaling pathways, possibly through impact on cytokine release.31 This is in accordance with our observations and several other studies,27 which also revealed that the addition of MOCLO to the cultures of healthy volunteer whole blood cells increased the LPS-stimulated release of IL-10 and significantly decreased the unstimulated release of TNFα.32 Recently, it was also shown that repeated administration of FLUO into hapten-immunized mice also influenced cytokine secretion by splenocytes with the enhancement of IL-10 and the reduction of TNFα release.29,30 The influence of antidepressant drugs on the production of cytokines by human cells was previously reviewed.33 Among other things, it was determined that FLUO, VENLA, and IMI addition to the culture of LPS-stimulated blood cells of patients with depression increased the release of IL-6 and did not affect TNFα secretion.34 In contrast, other in vitro studies demonstrated the inhibition of LPS-stimulated release of IL-6 and TNFα by human blood monocytes cultured in the presence of IMI.35 This is consistent with our observations that IMI treatment downregulated LPS-stimulated secretion of IL-6 and TNFα. Paroxetine, the aforementioned representative of SSRIs, has recently been shown to reduce the secretion of IL-6 by LPS-stimulated mouse Mfs through inhibition of intracellular IκBα phosphorylation. Interestingly, these results were duplicated with fluoxetine,36 which is in line with our observations.
Apart from cytokine secretion, Mfs also orchestrate the immune response through the generation of ROIs and NO,13 which, as currently shown, can be modulated by antidepressant drugs. Previously, the reduction of basal NO release was observed in peritoneal Mfs from rats that received FLUO thrice and subsequently were subjected to a forced swimming test.16,37 On the contrary to our studies, the moderate inhibitory effect of IMI added to Mf culture on the generation of ROIs was previously mentioned.38 Further, no significant effect of single administration of FLUO on ROIs generation was recently reported in mice.39 However, the direct impact of repeated administration of antidepressant drugs on the generation of ROIs and NO by Mfs harvested from drug-treated mice, to our knowledge, was investigated here for the first time.
The aforementioned results imply that repeated administration of antidepressant drugs, especially FLUO, VENLA, and MOCLO, leads to a reduction of Mf capability to phagocytose, process, and present antigens followed by suppression of humoral and cellular immune response. This effect is also associated with a reduction of the expression of Mf surface markers of antigen phagocytosis and presentation. Thus, as a result of the repeated administration of antidepressant drug into healthy (not suffering from depression) individuals, the suppression of antibody-mediated humoral as well as allergic cellular immunity is observed. These clinically important effects may promote the inclusion of antidepressant drugs into the therapy of other diseases, including those of allergic, autoimmune, and inflammatory origin. A formerly suggested40 possibility of a future application of antidepressant drugs to therapy of cutaneous allergic contact dermatitis was recently reviewed.41 FLUO was also suggested to be a promising alternate therapeutic agent in drug-resistant Burkitt’s lymphoma.42 The therapeutic effect of chosen antidepressant drugs used as an adjuvant analgesics43,44 may also be augmented by their antiinflammatory potential. Furthermore, excessive inflammation is observed in various metabolic, neuroendocrine, and cardiovascular disorders. Therefore, the application of antidepressant drugs in the complex therapy of these syndromes may have beneficial effects, primarily by enhancing the mood of the patient and secondarily by suppressing chronic inflammation. On the other hand, the potentially adverse suppressive effect of chronic antidepressant therapy in patients with depression should be considered, because it may impair antimicrobial or antitumor immunity. This assumption seems to be supported by the observation that, although FLUO administered in an acute manner into healthy animals greatly enhances leukocyte rolling and adhesion on the endothelium through the increase of serotonin plasma concentrations, chronic treatment with FLUO significantly disturbs the early phase of inflammation through the severe impairment of leukocyte recruitment.45
Our research findings indicate that antidepressant drugs may have diverse effects, conceivably dependent on the cellular target. For instance, treatment of mice with IMI leads to proinflammatory activation of Mfs, expressed among other things as an enhancement of CS in sensitized recipients of hapten-conjugated Mfs, while repeated administration of IMI into actively sensitized animals resulted in slight suppression of CS reaction. Currently, various mechanisms are considered to be responsible for the antiinflammatory action of antidepressant drugs.46 It is most likely that the observed antiinflammatory effects are a result of both, the direct impact of the drug’s bioactive compound on Mfs and other immune cells as well as changes in the concentration and relation of neurotransmitters sensed by these cells, especially Mfs.47 The antiinflammatory effect of FLUO was shown to result from the inhibition of the expression of inducible NO synthase and cyclooxygenase-2 in an LPS-stimulated RAW cell line in a glycogen synthase kinase-3beta-dependent manner, suggesting a significant role of this kinase in immunomodulation by FLUO.39 Additionally, under the action of FLUO, the reduced expression of cyclooxygenase-2 in a rat ligature-induced periodontitis model48 and the suppressed production of prostaglandin E2 by LPS-stimulated human synovial cells49 were also detected. Other studies revealed the ability of FLUO to alleviate symptoms of experimental mouse colitis by inhibition of proinflammatory signaling dependent on nuclear factor κB (NFκB) in epithelial cells.50 Targeting of NFκB was also responsible for the aforementioned inhibition of cytokine release by mouse Mfs treated with paroxetine and FLUO.36 Furthermore, the inhibition of secretion of proinflammatory cytokines under the influence of antidepressant drugs was assumed to be a result of an increased intracellular concentration of cyclic adenosine monophosphate due to the stimulation of G-protein coupled receptors, and antidepressant drugs are suspected to be able to bind directly to these receptors.27 However, since serotonin receptors are members of the family of G-protein coupled receptors, and their agonist was recently shown to inhibit systemic inflammation induced by TNFα,51 it is also possible that antidepressant drug-mediated enhancement of serotonin level may be responsible for the observed antiinflammatory effect. Thus, the interaction of a particular antidepressant drug with cells of the immune system seems to be a complex phenomenon,52 which requires further investigation.
In conclusion, our studies strongly suggest that antidepressant drugs repeatedly administered to individuals not suffering from depression modulate the humoral and cell-mediated immune response, mostly through action on Mfs. Additionally, it was shown previously that experimental administration of either FLUO or a TCA representative alleviated the clinical symptoms of rheumatoid arthritis53,54 or inflammatory bowel disease.55 A similar beneficial effect was obtained by administration of VENLA into mice with experimental autoimmune encephalomyelitis.56 In addition, it was reported that IMI inhibited in vitro differentiation of human monocytes into macrophage-like cells.57 Our results showed that, apart from the regulation of cytokine production, antidepressant drugs modulate other immune functions of Mfs. Hence, currently observed effects of treatment with different antidepressant drugs possess significant translational potential and indicate that these drugs could be considered for application in complex therapeutic strategies dedicated to various inflammatory diseases.
Acknowledgements
The authors express their gratitude to Prof. Krzysztof Bryniarski (Department of Immunology, Jagiellonian University Medical College) for his precious help, consultation of experimental protocols and revision of the manuscript, and to Dr. Bernadeta Nowak (Department of Immunology, Jagiellonian University Medical College) for her precious help with flow cytometry analysis. This study was supported by grant of Polish Ministry of Science and Higher Education No K/DSC/003095 to K.N. The authors would like to express their gratitude to the Leading National Research Centre (KNOW) 2012-2017 for their financial support of the process of printing the article.
Authors’ contributions
KN contributed to study conception and design, performed the experiments, analyzed the data and drafted the manuscript. MK performed the experiments, analyzed the data and prepared the linguistic correction of the manuscript. PB performed the experiments and analyzed the data. SS performed the experiments and prepared the linguistic correction of the manuscript. AB, MM, AT, PK and JN performed the experiments. IF-B contributed to study design and data analysis, and revised the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Hickie I, Lloyd A. Are cytokines associated with neuropsychiatric syndromes in humans? Int J Immunopharmacol 1995; 17: 677–83. [DOI] [PubMed] [Google Scholar]
- 2.Smith RS. The macrophage theory of depression. Med Hypotheses 1991; 35: 298–306. [DOI] [PubMed] [Google Scholar]
- 3.Beumer W, Gibney SM, Drexhage RC, Pont-Lezica L, Doorduin J, Klein HC, Steiner J, Connor TJ, Harkin A, Versnel MA, Drexhage HA. The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. J Leukoc Biol 2012; 92: 959–75. [DOI] [PubMed] [Google Scholar]
- 4.Kulmatycki KM, Jamali F. Drug disease interactions: role of inflammatory mediators in depression and variability in antidepressant drug response. J Pharm Pharm Sci 2006; 9: 292–306. [PubMed] [Google Scholar]
- 5.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009; 65: 732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 2013; 70: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther 2011; 130: 226–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon ML, McNeil LK, Freund GG. Macrophages make me sick: how macrophage activation states influence sickness behavior. Psychoneuroendocrinology 2011; 36: 1431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leonard BE. The concept of depression as a dysfunction of the immune system. Curr Immunol Rev 2010; 6: 205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 2012; 37: 137–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinan TG. Inflammatory markers in depression. Curr Opin Psychiatry 2009; 22: 32–6. [DOI] [PubMed] [Google Scholar]
- 12.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol 2004; 5: 617–25. [DOI] [PubMed] [Google Scholar]
- 13.Nazimek K, Bryniarski K. The biological activity of macrophages in health and disease. Postepy Hig Med Dosw 2012; 66: 507–20. [DOI] [PubMed] [Google Scholar]
- 14.Nazimek K, Filipczak-Bryniarska I, Bryniarski K. The role of medicaments, exosomes and miRNA molecules in modulation of macrophage immune activity. Postepy Hig Med Dosw 2015; 69: 1114–29. [PubMed] [Google Scholar]
- 15.Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci 2011; 14: 1227–35. [DOI] [PubMed] [Google Scholar]
- 16.Roman A, Kreiner G, Nalepa I. Macrophages and depression – a misalliance or well-arranged marriage? Pharmacol Rep 2013; 65: 1663–72. [DOI] [PubMed] [Google Scholar]
- 17.Haenisch B, Bönisch H. Depression and antidepressants: insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacol Ther 2011; 129: 352–68. [DOI] [PubMed] [Google Scholar]
- 18.Shajib MS, Khan WI. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol (Oxf) 2015; 213: 561–74. [DOI] [PubMed] [Google Scholar]
- 19.Slota C, Shi A, Chen G, Bevans M, Weng NP. Norepinephrine preferentially modulates memory CD8 T cell function inducing inflammatory cytokine production and reducing proliferation in response to activation. Brain Behav Immun 2015; 46: 168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee KM, Kim YK. The role of IL-12 and TGF-beta1 in the pathophysiology of major depressive disorder. Int Immunopharmacol 2006; 6: 1298–304. [DOI] [PubMed] [Google Scholar]
- 21.Hannestad J, Dellagioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology 2011; 36: 2452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czajkowska B, Ptak M, Bobek M, Bryniarski K, Szczepanik M. Different isoenzyme patterns of nonspecific esterases and the level of IL-6 production as markers of macrophage functions. Folia Histochem Cytobiol 1995; 33: 111–5. [PubMed] [Google Scholar]
- 23.Marzinzig M, Nussler AK, Stadler J, Marzinzig E, Barthlen W, Nussler NC, Beger HG, Morris SM, Jr, Bruckner UB. Improved methods to measure end products of nitric oxide in biological fluids: nitrite, nitrate, and S-nitrosothiols. Nitric Oxide 1997; 1: 177–89. [DOI] [PubMed] [Google Scholar]
- 24.Filipczak-Bryniarska I, Nowak B, Sikora E, Nazimek K, Woron J, Wordliczek J, Bryniarski K. The influence of opioids on the humoral and cell-mediated immune responses in mice. The role of macrophages. Pharmacol Rep 2012; 64: 1200–15. [DOI] [PubMed] [Google Scholar]
- 25.Ptak W, Bereta M, Ptak M, Iverson GM, Green DR. Suppression and contrasuppression in the induction of contact sensitivity by the administration of cell bound antigen-antibody complexes. J Immunol 1985; 135: 2312–8. [PubMed] [Google Scholar]
- 26.Janssen DG, Caniato RN, Verster JC, Baune BT. A psychoneuroimmunological review on cytokines involved in antidepressant treatment response. Hum Psychopharmacol 2010; 25: 201–15. [DOI] [PubMed] [Google Scholar]
- 27.Kenis G, Maes M. Effects of antidepressants on production of cytokines. Int J Neuropharmacol 2002; 5: 401–12. [DOI] [PubMed] [Google Scholar]
- 28.Henderson DC, Edwards RG, Weston BJ, Dewdney JM. Immunological studies on paroxetine, a novel anti-depressant drug. Int J Immunopharmacol 1988; 10: 361–7. [DOI] [PubMed] [Google Scholar]
- 29.Kubera M, Curzytek K, Majewska-Szczepanik M, Szczepanik M, Marcinska K, Ptak W, Leskiewicz M, Maes M, Basta-Kaim A, Budziszewska B, Detka J, Duda W, Lason W. Inhibitory effect of antidepressant drugs on contact hypersensitivity reaction. Pharmacol Rep 2012; 64: 714–22. [DOI] [PubMed] [Google Scholar]
- 30.Curzytek K, Kubera M, Majewska-Szczepanik M, Szczepanik M, Marcinska K, Ptak W, Duda W, Leskiewicz M, Basta-Kaim A, Budziszewska B, Lason W, Maes M. Inhibition of 2,4-dinitrofluorobenzene-induced contact hypersensitivity reaction by antidepressant drugs. Pharmacol Rep 2013; 65: 1237–46. [DOI] [PubMed] [Google Scholar]
- 31.Kubera M, Lin AH, Kenis G, Bosmans E, van Bockstaele D, Maes M. Anti-inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J Clin Psychopharmacol 2001; 21: 199–206. [DOI] [PubMed] [Google Scholar]
- 32.Lin A, Song C, Kenis G, Bosmans E, De Jongh R, Scharpe S, Maes M. The in vitro immunosuppressive effects of moclobemide in healthy volunteers. J Affect Disord 2000; 58: 69–74. [DOI] [PubMed] [Google Scholar]
- 33.Maes M. The immunomodulatory effects of antidepressants. Hum Psychopharmacol 2001; 16: 95–103. [DOI] [PubMed] [Google Scholar]
- 34.Kubera M, Kenis G, Bosmans E, Kajta M, Basta-Kaim A, Scharpe S, Budziszewska B, Maes M. Stimulatory effect of antidepressants on the production of IL-6. Int Immunopharmacol 2004; 4: 185–92. [DOI] [PubMed] [Google Scholar]
- 35.Xia Z, DePierre JW, Nassberger L. Tricyclic antidepressants inhibit IL-6, IL-1 beta and TNF-alpha release in human blood monocytes and IL-2 and interferon-gamma in T cells. Immunopharmacology 1996; 34: 27–37. [DOI] [PubMed] [Google Scholar]
- 36.Durairaj H, Steury MD, Parameswaran N. Paroxetine differentially modulates LPS-induced TNFa and IL-6 production in mouse macrophages. Int Immunopharmacol 2015; 25: 485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roman A, Kusmierczyk J, Klimek E, Rogoz Z, Nalepa I. Effects of co-administration of fluoxetine and risperidone on properties of peritoneal and pleural macrophages in rats subjected to the forced swimming test. Pharmacol Rep 2012; 64: 1368–80. [DOI] [PubMed] [Google Scholar]
- 38.Hadjimitova V, Traykov T, Goliyski P, Ribarov S. Effects of some psychotropic drugs on the activated macrophage-induced luminol-dependent chemiluminescence. Pharmacol Toxicol 1999; 84: 170–3. [DOI] [PubMed] [Google Scholar]
- 39.Su HC, Ma CT, Yu BC, Chien YC, Tsai CC, Huang WC, Lin CF, Chuang YH, Young KC, Wang JN, Tsao CW. Glycogen synthase kinase-3β regulates anti-inflammatory property of fluoxetine. Int Immunopharmacol 2012; 14: 150–6. [DOI] [PubMed] [Google Scholar]
- 40.El-Nour H, Lundeberg L, Abdel-Magid N, Lonne-Rahm SB, Azmitia EC, Nordlind K. Serotonergic mechanisms in human allergic contact dermatitis. Acta Derm Venereol 2007; 87: 390–6. [DOI] [PubMed] [Google Scholar]
- 41.Curzytek K, Kubera M, Szczepanik M, Basta-Kaim A, Leskiewicz M, Budziszewska B, Lason W, Maes M. Crosstalk between contact hypersensitivity reaction and antidepressant drugs. Pharmacol Rep 2013; 65: 1673–80. [DOI] [PubMed] [Google Scholar]
- 42.Cloonan SM, Williams DC. The antidepressants maprotiline and fluoxetine induce type II autophagic cell death in drug-resistant Burkitt’s lymphoma. Int J Cancer 2011; 128: 1712–23. [DOI] [PubMed] [Google Scholar]
- 43.Dharmshaktu P, Tayal V, Kalra BS. Efficacy of antidepressants as analgesics: a review. J Clin Pharmacol 2012; 52: 6–17. [DOI] [PubMed] [Google Scholar]
- 44.Marchettini P, Formaglio F, Lancerenza M. Neuropathic pain. In: Bruera E, Higginson I, von Gunten CF, Morita T. (eds). Textbook of palliative medicine and supportive care, Boca Raton: CRC Press and Taylor & Francis Group, 2015, pp. 481–92. [Google Scholar]
- 45.Herr N, Mauler M, Witsch T, Stallmann D, Schmitt S, Mezger J, Bode C, Duerschmied D. Acute fluoxetine treatment induces slow rolling of leukocytes on endothelium in mice. PLoS One 2014; 9: e88316–e88316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castanon N, Leonard BE, Neveu PJ, Yirmiya R. Effects of antidepressants on cytokine production and actions. Brain Behav Immun 2002; 16: 569–74. [DOI] [PubMed] [Google Scholar]
- 47.de Las Casas-Engel M, Corbi AL. Serotonin modulation of macrophage polarization: inflammation and beyond. Adv Exp Med Biol 2014; 824: 89–115. [DOI] [PubMed] [Google Scholar]
- 48.Branco-de-Almeida LS, Franco GCN, Castro ML, dos Santos JG, Anbinder AL, Cortelli SC, Kajiya M, Kawai T, Rosalen PL. Fluoxetine inhibits inflammatory response and bone loss in a rat model of ligature-induced periodontitis. J Periodontol 2012; 83: 664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yaron I, Shirazi I, Judovich R, Levartovsky D, Caspi D, Yaron M. Fluoxetine and amitriptyline inhibit nitric oxide, prostaglandin E2, and hyaluronic acid production in human synovial cells and synovial tissue cultures. Arthritis Rheum 1999; 42: 2561–8. [DOI] [PubMed] [Google Scholar]
- 50.Koh S, Kim JM, Kim I, Kim N, Jung HC, Song IS, Kim JS. Fluoxetine inhibits NF-κB signaling in intestinal epithelial cells and ameliorates experimental colitis and colitis-associated colon cancer in mice. Am J Physiol Gastrointest Liver Physiol 2011; 301: G9–19. [DOI] [PubMed] [Google Scholar]
- 51.Nau F, Jr, Yu B, Martin D, Nichols CD. Serotonin 5-HT2A receptor activation blocks TNF-α mediated inflammation in vivo. PLoS One 2013; 8: e75426–e75426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leonard BE. The immune system, depression and the action of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry 2001; 25: 767–80. [DOI] [PubMed] [Google Scholar]
- 53.Krishnadas R, Krishnadas R, Cavanagh J. Sustained remission of rheumatoid arthritis with a specific serotonin reuptake inhibitor antidepressant: a case report and review of the literature. J Med Case Rep 2011; 5: 112–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sacre S, Medghalchi M, Gregory B, Brennan F, Williams R. Fluoxetine and citalopram exhibit potent antiinflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors. Arthritis Rheum 2010; 62: 683–93. [DOI] [PubMed] [Google Scholar]
- 55.Iskandar HN, Cassell B, Kanuri N, Gyawali CP, Gutierrez A, Dassopoulos T, Ciorba MA, Sayuk GS. Tricyclic antidepressants for management of residual symptoms in inflammatory bowel disease. J Clin Gastroenterol 2014; 48: 423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vollmar P, Nessler S, Kalluri SR, Hartung HP, Hemmer B. The antidepressant venlafaxine ameliorates murine experimental autoimmune encephalomyelitis by suppression of pro-inflammatory cytokines. Int J Neuropsychopharmacol 2009; 12: 525–36. [DOI] [PubMed] [Google Scholar]
- 57.Ying G, Karlsson H, DePierre JW, Nassberger L. Tricyclic antidepressants prevent the differentiation of monocytes into macrophage-like cells in vitro. Cell Biol Toxicol 2002; 18: 425–37. [DOI] [PubMed] [Google Scholar]