Abstract
Tobacco-sourced carcinogen including benzopyrene (B[a]P) in lung cancer metastasis has not been fully reported. In this study, lung carcinoma A549 cell line was used to investigate the potential roles of tobacco-sourced B[a]P on cell metastasis and invasion and to assess its underlying mechanism. Effects of tobacco-sourced carcinogen on A549 cell proliferation, metastasis, and invasion were analyzed using MTT assay, Transwell assay, and scratch method, respectively. The effects of tobacco-sourced carcinogen on cytokines and chemokines secretion were detected using enzyme-linked immunosorbent assay. Moreover, correlation between inflammatory factor expression and cancer cell migration and invasion was assessed using siRNA-mediated gene silencing. Data showed that both B[a]P and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone either at high or low dose performed no significant difference on A549 cell proliferation with time increasing. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone performed no significant difference on A549 cell migration and invasion while B[a]P significantly increased A549 cell migration and invasion compared to the control group (P < 0.05). Consequently, except for IL-6, IL-8, CCL-2, and CCL-3, secretions were significantly increased by B[a]P treatment compared to the control (P < 0.05). Furthermore, when CCL-2 and CCL-3 were silenced, the migrated and invasive A549 cells were significantly decreased compared to the control, respectively (P < 0.05), while silenced IL-8 drastically decreased the migrated and invasive cells compared to the control (P < 0.01). Taken together, this study illustrated that there may be significant correlation between smoking and lung cancer metastasis. B[a]P maybe an excellent contributor for lung cancer metastasis through up-regulating IL-8, CCL-2, and CCL-3 expression.
Keywords: Lung cancer, cell migration, cell invasion, chemokine CCL-2 and CCL-3, cytokine IL-8
Introduction
Lung cancer remains to be one of the most common malignancies, which is well known for the easy metastasis to variety kinds of organs including clavicle lymph node, bone, liver and pleura, and other sites.1–4 Statistics show that morbidity and mortality for lung cancer are drastically increasing in recent years, especially among male persons.5 Varieties of treatment methods including genetic therapy, surgery, and chemotherapy for lung cancer have been discussed a lot in previous papers.6,7 However, there is no radical cure method for lung cancer due to its complicate pathogen.
Increasing evidence presented that smoking is closely correlated to lung cancer development and reported that the long-term smokers who get lung cancer are far more than the non-smokers.8,9 More than 2500 kinds of harmful substances have been researched in tobacco, including the strong carcinogens of benzopyrene (B[a]P) or nitrosamine (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, NNK).10,11 The carcinogenesis for lung cancer by tobacco smoke derived significant attentions on B[a]P and NNK in lung cancer development and progression, inducing the mutations of Ras genes or P53 via different signal pathways.12,13 For example, NNK-induced Kras mutation from GGT to GAT at site 12 in human lung cancer tissue,14 and B[a]P resulted in methylation of CpG on p16 and then enhanced P53 mutation in lung cancer progression.15 However, few papers have reported the correlation between B[a]P in lung cancer metastasis.
Chemokines are some gene-induced peptides which play crucial roles in tumor development, which are correlated to lung cancer cell growth, angiogenesis, and even metastasis.16,17 In addition to the genetic factors and environmental factors, literature refers that immunologic imbalance may also play pivotal roles in lung carcinoma.18 Interleukin (IL)-8 is a multi-sourced cytokine which has been reported to be highly secreted in serum from lung cancer patients, as well as in para-carcinoma tissue.19 Besides, CCL-2 and CCL-3 have been reported in several kinds of cancer’s tumorigenesis and metastasis, including CCL2 is progressively up-regulated in prostate cancer and CCL3 promotes murine lung metastasis process through accumulating leukocytes and fibroblasts.20,21 Nonetheless, the effects of B[a]P in lung cancer metastasis and its mechanism still remain unknown.
In this study, we used human lung adenocarcinoma A549 cell line to investigate the potential effects of B[a]P and NNK on cell migration and invasion. Varieties of experimental methods were used to assess the effects of B[a]P and NNK on cytokines and chemokines secretion during cell migration and invasion. Additionally, the possible role of B[a]P in lung cancer metastasis was further verified in another lung cancer H1299 cell line. This study was aimed to investigate the potential roles of tobacco-sourced carcinogens B[a]P on lung cancer metastasis and its possible mechanism.
Materials and methods
Cell culture and cell treatment
Human lung adenocarcinoma A549 and H1299 cell lines (purchased from Shanghai Bioleaf Bio Co., Ltd, Shanghai, China) were cultured in RPMI-1640 medium (Invitrogen, USA) containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 µg/mL streptomycin, and 1% L-glutamine (Sigma, USA) in an atmosphere of 5% CO2 at 37℃.
Cells were plated onto the six-well plates in 2 mL of fresh RPMI-1640 medium to adjust the cell density of 1 × 105 cells/well. After 24 h of cultivation, cells were mixed with benzopyrene or nitrosamine (C20H12; purity, > 96%; Sigma) for 24, 48, and 72 h. Each experiment was conducted three times independently.
MTT assay
A549 cells were plated onto the 96-well plates at a cell density of 5 × 105 cells/well in 1 mL of fresh RPMI-1640 medium containing 10% FBS. After 24 h of incubation, benzopyrene or nitrosamine was added into each well and incubated for 48 h. Consequently, 20 µL of MTT was added into each well and then the cells were incubated for 4 h at 37℃. After that, 150 µL of dimethylsulfoxide was supplemented into each well to mix with cells for 10 min. Absorbance of cells in each well was observed at 570 nm under an absorption spectrophotometer (Olympus, Japan). All experiments were conducted independently for three times.
Enzyme-linked immunosorbent assay
After 24 or 48 h treatment of drugs, conditioned medium was collected for enzyme-linked immunosorbent assay (ELISA). Standard ELISA methods as described by the manufacturers were used to measure IL-6, IL-8, CCL-2, and CCL-3 protein concentrations.
Cell migration and invasion assay
Cell migration and invasion were conducted using Transwell migration chambers (8 µm pore size; USA); in addition, the membranes for the invasion assay were coated with a diluted extracellular matrix (ECM) (namely the Matrigel, which consists of the laminin and type IV collagen) solution (Sigma). Briefly, A549 (5 × 104 cells/well) cells were seeded in the upper portion of a chamber with serum-free medium after transfection. Medium contained 10% FBS with or without B[a]p (or NNK), served as a chemoattractant in the lower chamber. After 24 or 48 h incubation at 37℃, non-invaded cells on the top of the membrane were scraped and removed by cotton swabs, and the invaded cells were fixed, stained with Diff-Quik staining, and then counted using light microscopy. The migration and invasion assay was repeated three times.
siRNA transfection
siRNA-mediated gene silencing was used to investigate the effects of IL-8, CCL-2, and CCL-3 expression on A549 cell migration and invasion. In brief, three kinds of vectors (purchased from SantaCruz, siRNA specific for IL-8 (sc-39631), CCL-2 (sc-43913), and CCL-3 (sc-43933)) were transfected into the A549 cells or H1299 cells using Lipofectamine® RNAiMAX Transfection Reagent based on the manufacturers’ protocol (Life Technologies, USA). Cells transfected with the siRNA without the target gene sequence were considered as controls. After 48 h of transfection, cells were prepared for further analysis.
Western blotting
Cells treated with drugs treatment in each group were lysed with radioimmunoprecipitation assay (Sangon Biotech) containing phenylmethanesufonyl fluoride and then were centrifuged at 12,000 rpm at 4℃ for 5 min.22 Supernatant was collected to determine the concentration of lysed proteins using bicinchoninic acid protein assay kit (Pierce, Rochford, IL). For Western blotting,23 a total of 50 µg protein per lane was subjected onto a 10% sodium dodecylsulfate-polyacrylamide gel electrophoresis and then transferred onto a polyvinylidencefluoride membrane (Mippore). Membranes were blocked in tris-buffered saline tween supplemented with 5% non-fat milk for 1 h and subsequently incubated with primary antibodies (1:1000 for IL-6, IL-8, CCL-2, CCL-3, and 1:5000 for GAPDH) overnight at 4℃, followed by incubation with horseradish peroxidase-labeled goat anti-rat secondary antibody. The immunoreactive protein bands were developed by enhanced chemiluminescence, and the immunoreactive bands were analyzed by a densitometer. Phosphoglyceraldehyde dehydrogenase (GAPDH, Sigma) was considered as the internal control.
Statistical analysis
The results of multiple experiments are presented as the mean ± SD. Statistical analyses were performed using graph prism 5.0 statistical software (GraphPad Prism, San Diego, CA). The P-values were calculated using a one-way analysis of variance with the post-hoc test. P < 0.05 was considered as statistically significant.
Results
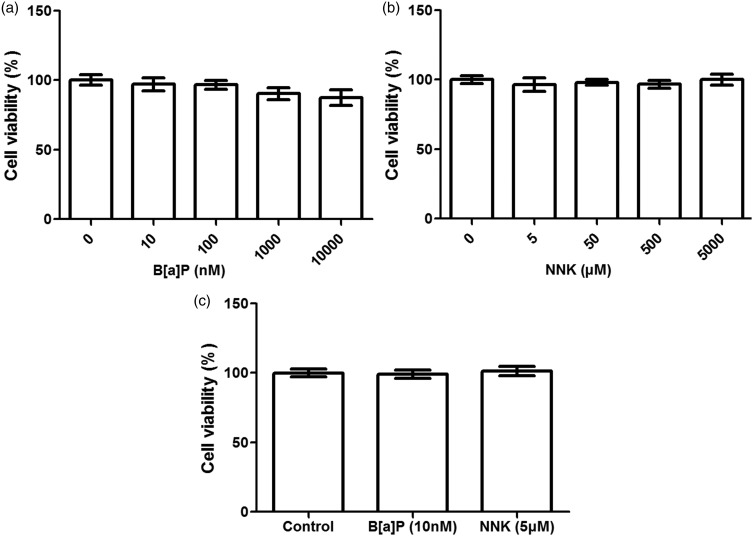
Effects of B[a]P or NNK on A549 cell viability
The effects of B[a]P or NNK on A549 cell proliferation were assessed using MTT assay (Figure 1). The results showed that there were no significant differences among groups when cells were treated with B[a]P at different concentrations (from 0 nM to 10,000 nM) at 48 h (Figure 1(a)). Similarly, no significant difference between the NNK at different concentration (from 0 to 5000 μM) and the A549 cell viability at 48 h was observed (Figure 1(b)), indicating that both B[a]P and NNK performed no significant effects on A549 cell viability at the early culture stage. Therefore, low dose of B[a]P and NNK on A549 cell viability with long time incubation were assessed to confirm whether the two kinds of drugs can influence A549 cell proliferation or not (Figure 1(c)). Interestingly, there were no significant effects of low dose B[a]P or NNK on A549 cell viability at five days’ incubation, indicating that both B[a]P and NNK would perform no significant effects on A549 cell viability.
Figure 1.
Effects of benzopyrene (B[a]P) or nitrosamine (NNK) treatment on A549 cell viability. (a, b): B[a]P or NNK with different concentration performed no significant influence on A549 cell viability after 48 h incubation, respectively; (c): B[a]P or NNK with low dose performed no significant differences on A549 cell viability after five days incubation
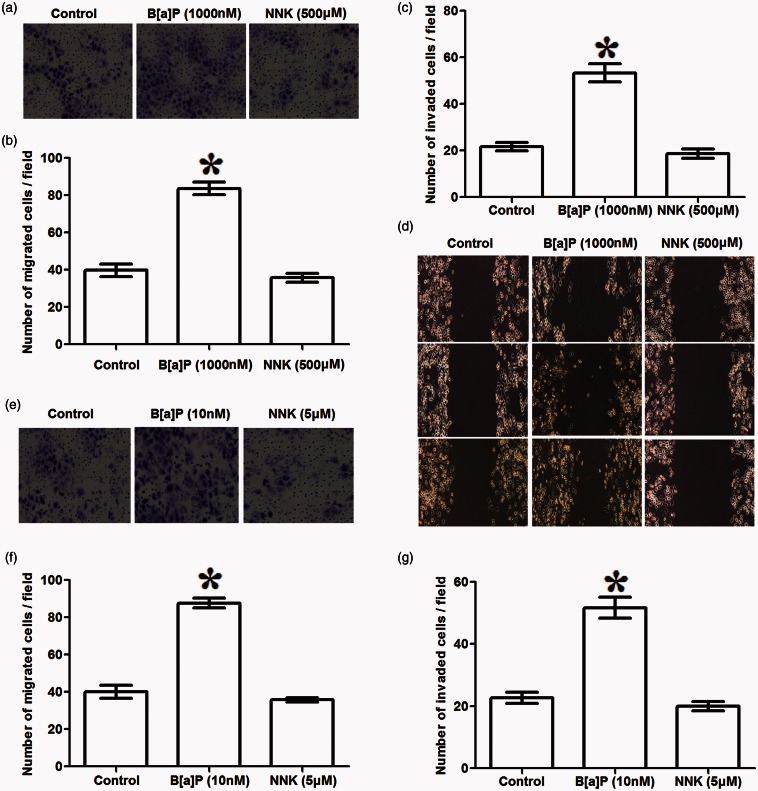
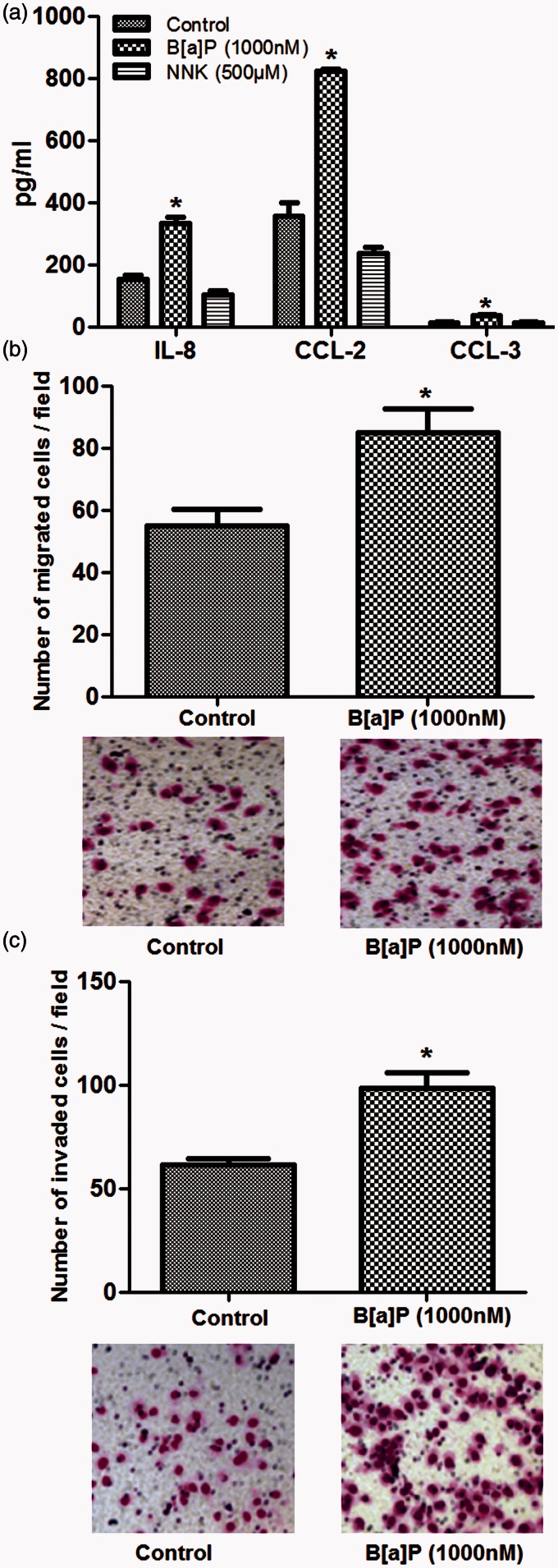
Effects of B[a]P or NNK on A549 cell migration and invasion
Transwell and in vitro scratch assay were conducted to assess whether B[a]P or NNK could influence A549 cell migration and invasive or not (Figure 2). Transwell assay showed that when A549 cells were treated with high dose of B[a]P in long time incubation (1000 nM, 48 h), numbers of the migrated cells and the invaded cells were significantly increased compared to the control (P < 0.05, Figure 2(a) to (c)); besides, the same tendency was observed in scratch assay (P < 0.05, Figure 2(d)). However, the results presented that NKK treatment with high dose in long time incubation (500 μM, 48 h) could not significantly influence A549 cell migration and invasion. On the other hand, effects of B[a]P or NNK with low dose on A549 cell migration and invasion were also assessed. When cells were treated with B[a]P (10 nM, 5 day), the number of migrated and invasive cells were statistically increased compared to the control (P < 0.05, Figure 2(e) to (g)). However, no significant difference was found between NNK treatment and migrated or invasive A549 cells. These data indicate that B[a]P could significantly increase A549 cell migration and invasion, but NNK could not.
Figure 2.
Effects of benzopyrene (B[a]P) or nitrosamine (NNK) treatment on A549 cell migration and invasion. (a–d): transwell assay and scratch assay showed that high dose of B[a]P (1000 nM, 48 h) significantly increased the migrated (a, b) and invasive (c, d) numbers of A549 cells, but high dose of NNK (500 μM, 48 h) performed no significant influence on A549 cell migration and invasion; (e–g): low dose of B[a]P (10 nM, 5 day) significantly increased migrated (e, f) and invasive (g) A549 cells, but NNK showed no statistical effects on A549 cell migration and invasion. *P < 0.05 compared to the control
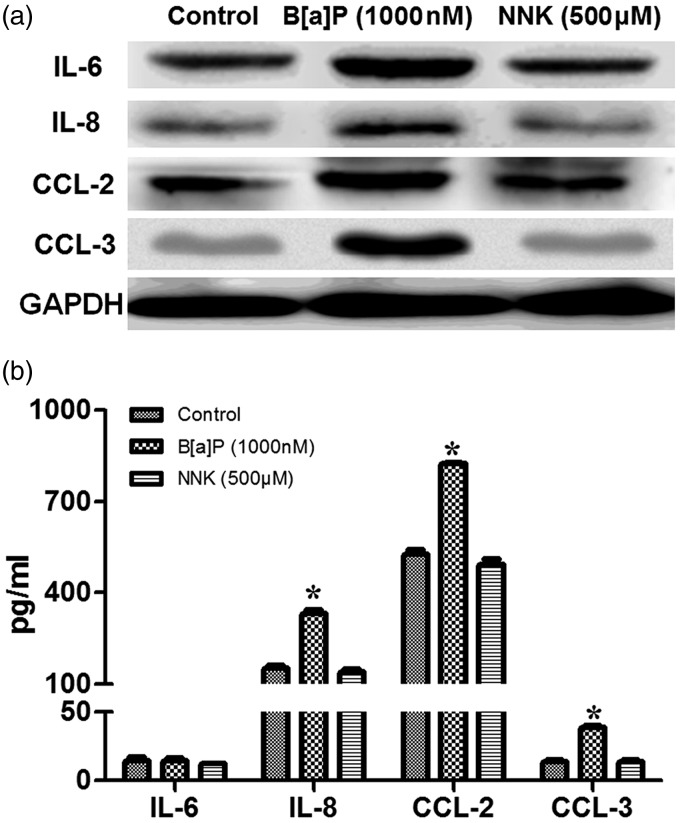
Effects of drug treatment on cytokine and chemokine secretion
Cytokines and chemokines secretion in A549 cells which were treated with B[a]P or NNK at low dose were analyzed using the Western blot and ELISA, respectively (Figure 3). The results showed that secretions of IL-8, CCL-2, and CCL-3 in B[a]P-treated A549 cells were drastically increased compared to that in controls (P < 0.05, Figure 3(a) and (b)) but no effects for B[a]P on IL-6 was found. In contrast to the influence of B[a]P on protein secretion in A549 cells, no significant difference between NNK treatment and proteins secretion was observed (Figure 3(a) and (b)). These results suggested that there may be correlation between B[a]P treatment and cytokines and chemokines secretion in A549 cells except for NNK.
Figure 3.
Influence of drug treatment on cytokines and chemokines secretion in A549 cells. (a): except IL-6, expressions of IL-8, CCL-2, and CCL-3 were all increased in cells treated with B[a]P (10 nM); (b): ELISA showed that B[a]P (1000 nM) treatment significantly increased IL-8, CCL-2, and CCL-3 secretion in cells but showed no effect on IL-6 secretion. However, no significant difference of IL-8, CCL-2, and CCL-3 secretion among groups was observed when cells treated with NNK. *P < 0.05, compared to the control
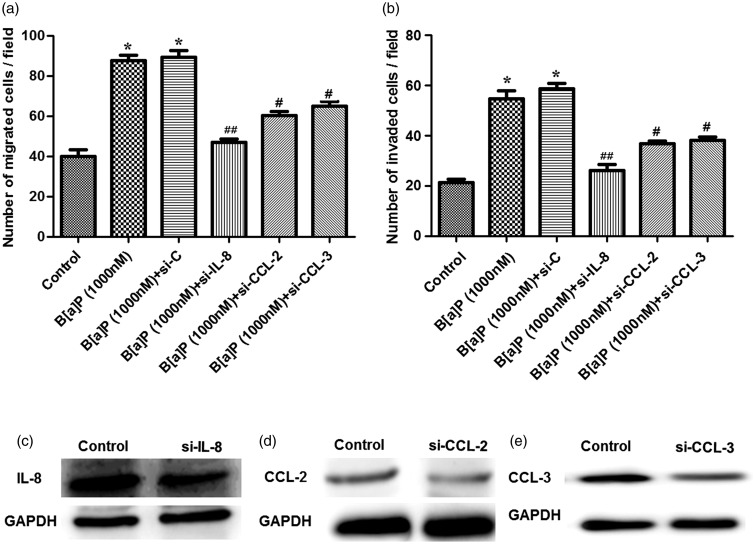
Effects of cytokine and chemokine silenced on A549 cell migration and invasion
In order to further verify whether the cytokine and chemokine levels were correlated with A549 cell migration and invasion, IL-8, CCL-2, and CCL-3 were silenced in B[a]P-treated A549 cells using gene silencing (Figure 4). When cells were treated with B[a]P, the migrated and invasive cells were both significantly increased compared to the control, as well as the B[a]P-treated cells transfected with siRNA-control (P < 0.05, Figure 4(a) and (b)). However, when cells were transfected with si-CLL-2 or si-CCL-3, cell migration and invasion ability were both statistically decreased compared to the B[a]P + si-C group (P < 0.05, Figure 4(a), (b), (d), and (e)). Besides, silenced IL-8 drastically decreased the migrated and invasive cells compared to the B[a]P + si-C group (P < 0.01, Figure 4(a) to (c)).
Figure 4.
Influence of the silenced cytokine or chemokine on B[a]P-treated A549 cell migration and invasion. (a): the silenced CCL-2 and CCL-3 both significantly declined the number of migrated A549 cells, while the silenced IL-8 only drastically decreased the migrated cells; (b): the silenced CCL-2 and CCL-3 both significantly declined the number of invasive A549 cells, while silenced IL-8 drastically decreased the invasive cells; (c–e): Western blot showed that the expressions of IL-8, CCL-2, and CCL-3 in B[a]P-treated A549 cells were all down-regulated. *P < 0.05 compared to the control, #P < 0.05, and ##P < 0.01, compared to the si-C group. Si-C stands for cells transfected with the siRNA without any target gene sequence; si-CCL-2, siCCL-3, or si-IL-8 stands for the cells transfected with the siRNA with target gene CCL-2, CCL-3, or IL-8 sequence
Influence of B[a]P on H1299 cell migration and invasion and the inflammatory factor secretion
In order to further verify the effects of B[a]P on lung cancer cell metastasis and invasion, another lung cancer H1299 cell was used to assess the effects of B[a]P on cell metastasis and inflammatory factors secretion (Figure 5). The levels for IL-8, CCL-2, and CCL-3 in the B[a]P-treated H1299 cells were significantly increased compared to that in the control cells (P < 0.05, Figure 5(a)). However, no significant difference was observed for the influence of NNK on the three kinds of cytokines secretion. Besides, the migrated and invaded H1299 cells were also increased by the B[a]P treatment (Figure 5(b) and (c)), suggesting the promoting role of B[a]P on H1299 metastasis.
Figure 5.
Influence of the silenced cytokine or chemokine on B[a]P-treated H1299 cell migration and invasion. (a): the protein levels for IL-8, CCL-2, and CCL-3 were increased by the B[a]P treatment than the control but no significant effect for NNK on the inflammatory secretion was found; (b): B[a]P treatment resulted in the increasing number of migrated H1299 cells; (c): B[a]P treatment resulted in the increasing number of invaded H1299 cells. *P < 0.05 compared to the control (cells without B[a]P treatment)
Discussion
It has been demonstrated that inflammatory factors such as cytokines and chemokines play crucial roles in lung cancer tumorigenesis.16,19 There are vast evidence about the harmful effects of tobacco-derived carcinogen in a variety of tumor developments, especially in lung cancer.14,15 However, little is known about the roles of tobacco-derived carcinogen on lung cancer metastasis and invasion. In this present study, we assessed the effects of tobacco-sourced B[a]P and NNK on human lung carcinoma A549 cell proliferation, metastasis, and invasion and on cytokines and chemokines secretion. Our data presented that both B[a]P and NNK had no significant effect on lung cancer cell growth, but B[a]P could significantly enhance cell migration and invasion, as well as increase IL-8, CCL2, and CCL3 secretion in A549 cells, suggesting that smoking may be a significant contributor in lung cancer.
Cancer metastasis and invasion is a result of variety of complicate factor interactions including inflammatory factors, enzymes and adhesion proteins, and other molecules.24,25 B[a]P is a ubiquitous polycyclic aromatic hydrocarbon and potent carcinogen which exists in water, smoke, and air.26 Increasing evidence have demonstrated the pivotal roles of B[a]P in lung tumorigenesis including induced p53 or Ras genes mutation by damaging DNA or changing amino code.27,28 However, little has been known about B[a]P in lung cancer metastasis and invasion. Ueng et al.29 proved that the induction for B[a]P on lung tumor invasion and metastasis was suppressed by fibroblast growth factor-9. In this study, B[a]P at any concentration showed the approximate similar influence on A549 cell proliferation ability (Figure 1), suggesting its high carcinogenesis in lung cancer. Therefore, we further analyzed the roles of B[a]P application on lung metastasis and invasion. Our results showed that B[a]P application significantly increased migrated and invasive A549 cells compared to the control (Figure 2), indicating that B[a]P may be correlated to lung cancer metastasis.
On the other side, immune factors such as chemokines and cytokine have been illustrated in variety of diseases including cancers carcinogenesis.16 For instance, CCL-9 and CCL-21 are the therapeutic targets for lung cancer because they are contributors in lung carcinogenesis and migration.30,31 CCL-2 and CCL-3 in lung metastasis have not been fully discussed. However, a recent study which was conducted by Kitamura et al.32 demonstrated that CCL-2 had a therapeutic impact in breast cancer metastasis through enhancing several macrophages retention. Meanwhile, abundant evidence have referred the pivotal carcinogenesis of IL-8 in lung cancer. Podechard et al.33 demonstrated that IL-8 secretion induced by the air-sourced B[a]P would lead to lung inflammation. The data in this study revealed that B[a]P application would significantly increase the expression levels of CCL-2, CCL-3, and IL-8 in A549 cells (Figure 3), suggesting that B[a]P may contribute the inflammatory factors secretion in lung cancer metastasis. In order to further verify this result, we silenced the three factors of CCL-2, CCL-3, and IL-8 to assess the number of migrated and invasive A549 cells. Interestingly, the results tell us that the migrated and invasive A549 cells were less when CCL-2 and CCL-3 were silenced, and when IL-8 was silenced, the migrated cells were drastically decreased (Figure 4), implying that the three kinds of inflammatory factors may involve in lung cancer metastasis and invasion. From another point of view, we also assessed the influences of B[a]P on lung cancer metastasis in another lung cancer cell line of H1299. Similar to that in A549 cells, the inflammatory factors secretion including CCL-2, CCL-3, and IL-8 was increased by B[a]P in H1299 cells, as well as the increasing impact for B[a]P on H1299 migration and invasion (Figure 5), indicating that except for A549 cells, B[a]P treatment could also affect the migration and invasion by affecting the inflammatory cytokines secretion. These data revealed that the influence of B[a]P on lung cancer metastasis was not a specific action.
In conclusion, this study suggests that the tobacco-sourced carcinogen B[a]P may play crucial roles in accelerating lung cancer metastasis through up-regulating the cytokine IL-8 and chemokine CCL-2 and CCL-3 expression. Our study may provide a basis for illustrating the correlation between smoking and lung cancer metastasis and may explain the therapeutic impact of cytokines and chemokines in metastasis of lung cancer. Further studies are still needed to confirm the deep regulating mechanism of cytokine and chemokine secretion on the metastasis of lung cancer at the transcriptional level.
Acknowledgements
This research was supported by the grants from National Natural Science Foundation of China (Nos. 30960439, 81560488 and 81101693), National Key Clinical Specialty (Oncology) Fund, and Key Project of Department of Education of Yunnan Province (2015Z090).
Authors' contributions
JZ and LC contributed equally to this work. JZ and LC carried out the experiments, performed the statistical analysis, and drafted the manuscript. HJ carried out the experiments and collected the data. WL and HC designed the study, collected the funding, and helped to draft the manuscript. All authors read and approved the final manuscript.
Declaration Of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Krüger S, Buck AK, Mottaghy FM, Hasenkamp E, Pauls S, Schumann C, Wibmer T, Merk T, Hombach V, Reske SN. Detection of bone metastases in patients with lung cancer: 99mTc-MDP planar bone scintigraphy, 18F-fluoride PET or 18F-FDG PET/CT. Eur J Nucl Med Mol Imag 2009; 36: 1807–12. [DOI] [PubMed] [Google Scholar]
- 2.Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P. Members of IASLC Staging Committee. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009; 4: 568–77. [DOI] [PubMed] [Google Scholar]
- 3.She J, Yang P, Hong Q, Bai C. Lung cancer in China: challenges and interventions. Chest J 2013; 143: 1117–26. [DOI] [PubMed] [Google Scholar]
- 4.Gutschner T, Hämmerle M, Eißmann M, Hsu J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Groß M. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res 2013; 73: 1180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, Abdel-Rahman M, Wang X, Levine AD, Rho JK. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet 2012; 44: 852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, Zhou C, Su W-C, Wang M, Sun Y. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012; 13: 528–38. [DOI] [PubMed] [Google Scholar]
- 8.Bittoni MA, Harris RE, Clinton SK, Focht BC. The relationships between c-reactive protein, smoking, and lung cancer mortality in the Third National Health and Nutrition Examination Survey. Cancer Res 2013; 73: 3632–3632. [Google Scholar]
- 9.Moolgavkar SH, Holford TR, Levy DT, Kong CY, Foy M, Clarke L, Jeon J, Hazelton WD, Meza R, Schultz F. Impact of reduced tobacco smoking on lung cancer mortality in the United States during 1975 C2000. J Natl Cancer Inst 2012; 104: 541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Dai Y, Feng G, He R, Yang W, Li D, Zhou X, Zhu L, Tan L. Addition of porphyrins to cigarette filters to reduce the levels of benzo [a] pyrene (B [a] P) and tobacco-specific N-nitrosamines (TSNAs) in mainstream cigarette smoke. J Agr Food Chem 2011; 59: 7172–7. [DOI] [PubMed] [Google Scholar]
- 11.WHO. Standard operating procedure for determination of tobacco-specific nitrosamines in mainstream cigarette smoke under ISO and intense smoking conditions, Geneva: WHO, 2014. [Google Scholar]
- 12.Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res 2012; 72: 2457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer 2012; 131: 2724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Calvez F, Mukeria A, Hunt JD, Kelm O, Hung RJ, Tanière P, Brennan P, Boffetta P, Zaridze DG, Hainaut P. TP53 and KRAS mutation load and types in lung cancers in relation to tobacco smoke: distinct patterns in never, former, and current smokers. Cancer Res 2005; 65: 5076–83. [DOI] [PubMed] [Google Scholar]
- 15.Hussain SP, Amstad P, Raja K, Sawyer M, Hofseth L, Shields PG, Hewer A, Phillips DH, Ryberg D, Haugen A. Mutability of p53 hotspot codons to benzo (a) pyrene diol epoxide (BPDE) and the frequency of p53 mutations in nontumorous human lung. Cancer Res 2001; 61: 6350–5. [PubMed] [Google Scholar]
- 16.Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett 2007; 256: 137–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levina V, Marrangoni AM, DeMarco R, Gorelik E, Lokshin AE. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PloS One 2008; 3: e3077–e3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, Geng Y, Gray JW, Moses HL, Yang L. Gr-1+ CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res 2010; 70: 6139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpagnano GE, Palladino GP, Lacedonia D, Koutelou A, Orlando S, Foschino-Barbaro MP. Neutrophilic airways inflammation in lung cancer: the role of exhaled LTB-4 and IL-8. BMC Cancer 2011; 11: 226–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Patel L, Pienta KJ. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev 2010; 21: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Li YY, Matsushima K, Baba T, Mukaida N. CCL3-CCR5 axis regulates intratumoral accumulation of leukocytes and fibroblasts and promotes angiogenesis in murine lung metastasis process. J Immunol 2008; 181: 6384–93. [DOI] [PubMed] [Google Scholar]
- 22.Bae S, Jung Y, Choi YM, Li S. Effects of Er-Miao-San extracts on TNF-alpha-induced MMP-1 expression in human dermal fibroblasts. Biol Res 2015; 48: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jo DH, Kim JH, Cho CS, Cho YL, Jun HO, Yu YS, Min JK, Kim JH. STAT3 inhibition suppresses proliferation of retinoblastoma through down-regulation of positive feedback loop of STAT3/miR-17-92 clusters. Oncotarget 2014; 5: 11513–11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett 2008; 269: 199–225. [DOI] [PubMed] [Google Scholar]
- 25.Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol 2001; 11: S37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szmigielski S, Szudzinski A, Pietraszek A, Bielec M, Janiak M, Wrembel JK. Accelerated development of spontaneous and benzopyrene-induced skin cancer in mice exposed to 2450-MHz microwave radiation. Bioelectromagnetics 1982; 3: 179–91. [DOI] [PubMed] [Google Scholar]
- 27.Hecht SS, Carmella SG, Villalta PW, Hochalter JB. Analysis of phenanthrene and benzo [a] pyrene tetraol enantiomers in human urine: relevance to the bay region diol epoxide hypothesis of benzo [a] pyrene carcinogenesis and to biomarker studies. Chem Res Toxicol 2010; 23: 900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamaraj S, Ramakrishnan G, Anandakumar P, Jagan S, Devaki T. Antioxidant and anticancer efficacy of hesperidin in benzo (a) pyrene induced lung carcinogenesis in mice. Investig New Drugs 2009; 27: 214–22. [DOI] [PubMed] [Google Scholar]
- 29.Ueng TH, Chang YL, Tsai Y-Y, Su JL, Chan P-K, Shih JY, Lee YC, Ma YC, Kuo ML. Potential roles of fibroblast growth factor-9 in the benzo (a) pyrene-induced invasion in vitro and the metastasis of human lung adenocarcinoma. Arch Toxicol 2010; 84: 651–60. [DOI] [PubMed] [Google Scholar]
- 30.Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol 2009; 9: 377–84. [DOI] [PubMed] [Google Scholar]
- 31.Koizumi K, Kozawa Y, Ohashi Y, Nakamura ES, Aozuka Y, Sakurai H, Ichiki K, Doki Y, Misaki T, Saiki I. CCL21 promotes the migration and adhesion of highly lymph node metastatic human non-small cell lung cancer Lu-99 in vitro. Oncol Rep 2007; 17: 1511–6. [PubMed] [Google Scholar]
- 32.Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, Kato Y, Li J, Pollard JW. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med 2015; 212: 1043–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Podechard N, Lecureur V, Le Ferrec E, Guenon I, Sparfel L, Gilot D, Gordon JR, Lagente V, Fardel O. Interleukin-8 induction by the environmental contaminant benzo (a) pyrene is aryl hydrocarbon receptor-dependent and leads to lung inflammation. Toxicol Lett 2008; 177: 130–7. [DOI] [PubMed] [Google Scholar]