Abstract
1R, 1′S-isotetrandrine, a naturally occurring plant alkaloid found in Mahonia of Berberidaceae, possesses anti-inflammatory, antibacterial, and antiviral properties, but the antioxidative activity and mechanism action remain unclear. In this study, we demonstrated the antioxidative effect and mechanism of 1R, 1'S-isotetrandrine against tert-butyl hydroperoxide-induced oxidative damage in HepG2 cells. We found that 1R, 1′S-isotetrandrine suppressed cytotoxicity, reactive oxygen species generation, and glutathione depletion. Additionally, our study confirmed that 1R, 1′S-isotetrandrine significantly increased the antioxidant enzyme heme oxygenase-1 expression and nuclear translocation of factor-erythroid 2 p45-related factor 2 (Nrf2). Specifically, the nuclear translocation of Nrf2 induced by 1R, 1′S-isotetrandrine was associated with Nrf2 negative regulatory protein Keap1 inactivation and phosphorylation of both extracellular signal-regulated protein kinase and c-Jun NH2-terminal kinase. Preincubation with thiol-reducing agents reduced 1R, 1′S-isotetrandrine-induced heme oxygenase-1 expression, and treatment with either extracellular signal-regulated protein kinase or c-Jun NH2-terminal kinase inhibitors attenuated the levels of 1R, 1′S-isotetrandrine-induced Nrf2 activation and heme oxygenase-1 expression. Furthermore, the cytoprotective effect of 1R, 1′S-isotetrandrine was abolished by heme oxygenase-1, extracellular signal-regulated protein kinase, and c-Jun NH2-terminal kinase inhibitors. These results indicated that the 1R, 1′S-isotetrandrine ameliorated tert-butyl hydroperoxide-induced oxidative damage through upregulation of heme oxygenase-1 expression by the dissociation of Nrf2 from Nrf2-Keap1 complex via extracellular signal-regulated protein kinase and c-Jun NH2-terminal kinase activation and Keap1 inactivation.
Keywords: ITD, HO-1, Nrf2, oxidative stress
Introduction
Oxidative stress is widely involved in various diseases, such as chronic inflammation and a variety of cancers.1 In oxidative stress, reactive oxygen species (ROS) cause cytotoxic lipid peroxidation and serious damage to proteins and DNA.2 Under oxidative stress conditions, mammalian cells counteract ROS formation via the induction of cytoprotective enzymes such as heme oxygenase-1 (HO-1) and glutathione (GSH) synthetase.3,4
GSH is an intracellular antioxidant and free radical scavenger, which helps to maintain redox homeostasis.5 Some phytochemicals are known to exert antioxidant activity by reducing GSH depletion.6 HO-1, an important cytoprotective enzyme, catalyzes the degradation of heme into the precursor of bilirubin as a potent antioxidant.7 Increasing evidence indicates that HO-1 is involved in the mechanism of maintaining redox homeostasis and protecting against oxidative stress.8
Nrf2 is an oxidative stress-mediated nuclear transcription factor that regulates expression of antioxidative enzymes, including HO-1. In normal circumstances, Nrf2 is sequestered by binding Kelch-like ECH-associated protein 1 (Keap1), which keeps Nrf2 in ubiquitylated state, leading to Nrf2 degradation.9 The stimulus of adverse factors, including oxidative stress, can cause Nrf2 to dissociate from Keap1 and translocate into the nucleus, where it induces antioxidant gene expression.10 The mechanism of Nrf2 dissociation from the Keap1-Nrf2 complex remains to be established, but recent studies have suggested that the phosphorylation of Nrf2 by activating signal transduction pathways, such as the mitogen-activated protein kinases (MAPKs) and phosphoinositide 3-kinase (PI3K), can release Nrf2 from Keap1 and promote Nrf2 activation.11,12
Previous studies have demonstrated that natural compounds have the effect of inhibiting oxidative stress through inducing HO-1 expression in a variety of cells such as macrophage cells, retinal pigment epithelial cells, and liver cells.13–15 Upregulation of HO-1 expression by natural compounds in non-cytotoxic dose may represent a target for therapeutic intervention.
The Mahonia of Berberidaceae has the effect of antibacterial, anti-inflammatory and anticancer. 1R, 1′S-isotetrandrine (ITD) is a naturally occurring plant alkaloid that can be isolated from Mahonia. ITD has been reported to have numerous effects including anticancer, anti-inflammatory, and antibacterial activities, but there are no details yet on its possible antioxidant activity. Thus, we designed the current study to shed light on the possible antioxidative effect of ITD and its mechanisms of protection in a tert-butyl hydroperoxide (t-BHP) oxidative stress model in HepG2 cells.
Materials and methods
Reagents
ITD was purchased from Nanjing Zelang Medical Technology Co, Ltd (Nanjing, China). 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), t-BHP, SB203580 (p38 inhibitor), LY294002 (PI3K/Akt inhibitor), SP600125 (JNK) inhibitor), U0126 (extracellular signal regulated kinase (ERK) inhibitor), and SnPP (HO-1 inhibitor) were provided by Sigma Chemical Co (St Louis, MO). The reagents for cell culture such as streptomycin, penicillin, Dulbecco’s Modified Eagle’s Medium (DMEM), and fetal bovine serum (FBS) were obtained from Life Technologies Inc (Grand Island, NY). The kit for GSH determination was purchased from the Jiancheng Bioengineering Institute of Nanjing (Nanjing, China). Rabbit mAb Nrf2, HO-1, Keap1, Nrf2, Lamin B, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), phospho-extracellular signal regulated kinase (ERK), ERK, phospho-c-Jun NH2-terminal kinase (JNK), JNK, phospho-p38, and p38 were purchased from Cell Signaling (Boston, MA, USA). Horseradish-peroxidase-conjugated goat anti-rabbit IgG was purchased from Proteintech (Boston, MA, USA).
Cell culture and cell viability assay
The HepG2 human hepatoma cell line was obtained from the China Cell Line Bank (Beijing, China) and cultured in DMEM containing 10% FBS, 100 U/mL penicillin, 100 U/mL streptomycin and 3 mM glutamine at 37℃ with 95% air and 5% CO2. HepG2 cells, at a density of 2 × 104 cells/well, were transferred onto 96-well plates. After incubation 24 h, the cells were treated with ITD and various inhibitors in the presence or absence of t-BHP. Then, MTT solution (5 mg/mL) was added to each well. After 4 h of incubation, the supernatant was discarded, and DMSO was added to resolve the formazan. The absorbance was measured at 570 nm with a multidetection reader.
Isolation of cytosolic and nuclear proteins
Cytosolic and nuclear proteins were isolated by NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Pierce Biotechnology, Rockford, IL, USA), and all steps were on the basis of the manufacturer’s instructions.
Measurement of intracellular ROS
HepG2 cells were cultured in 24-well plates at the density of 1 × 105 cells/well. After 24 h, the cells were treated with various concentrations of ITD for 6 h. Subsequently, the cells were stained with 50 μM of DCFH-DA for 1 h and treated with t-BHP (10 mM) for 30 min in order to generate ROS. The fluorescence intensities were measured in a multidetection reader with excitation at 485 nm and emission at 535 nm.
Detection of intracellular GSH
HepG2 cells were grown at the concentration of 5 × 105 cells/well in 6-well plate 24 h before experiments. Then, cells were treated with ITD for 6 h and exposed to t-BHP (10 mM) for 3 h. The intracellular GSH levels were detected with GSH test kit (Nanjing JianCheng Bioengineering Institute, Nanjing, China).
Western blotting
For Western blot analysis, the prepared cells were harvested and washed twice with cold PBS. After lysing with radioimmunoprecipitation assay containing protease and phosphatase, the concentrations were measured by BCA protein assay kit (Beyotime, China). Equal amounts of protein (40 µg) were resolved by SDS-polyacrylamidegel, transferred to polyvinylidene difluoride membranes, and blocked with 5% non-fat dry milk in PBS for 2 h. Afterwards, the membrane was incubated with specific primary antibody at 4℃ overnight. After washing with PBS containing 0.05% Tween 20 three times, the membrane was incubated with peroxidase-conjugated secondary antibody for 1 h and detected using ECL detection kit. The variations of bands density were analyzed by ImageJ gel analysis software.
Transfection of small interfering RNA
To knockdown Nrf2, HepG2 cells were transfected with Nrf2-small interfering RNA (siRNA). Cells were grown in six-well plates (2 × 105 cells/well) 24 h before experiments. The siRNA for Nrf2 and for negative control was transfected into HepG2 cells using X-treme GENE siRNA Transfection Reagent purchased from Roche (Basel, Switzerland). After 24 h, ITD was added into the transfected cells and incubated for another 6 h.
Statistical analysis
Two-tailed Student’s t-test was performed using SPSS 18.0. The data were expressed as mean ± SEM. p value of < 0.05 or < 0.01 was thought as statistically significant.
Results
ITD protects HepG2 cells from t-BHP-induced cell death, ROS production and GSH depletion
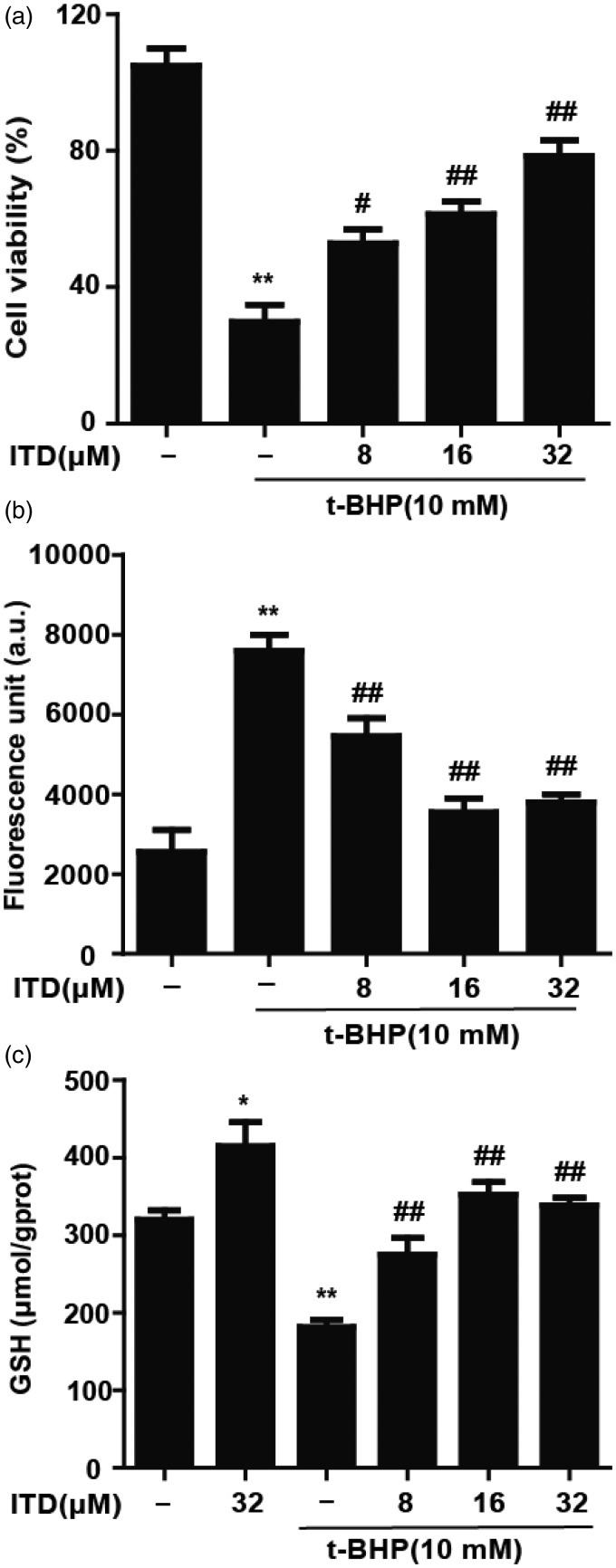
In biological systems, t-BHP treatment is commonly used to model oxidative stress. We examined the cytoprotection of ITD against t-BHP-induced cell death. HepG2 cells were pretreated with increasing concentrations of ITD (8, 16, and 32 μM) for 6 h and incubated with 10 mM t-BHP for 3 h. As shown in Figure 1(a), t-BHP-induced reduction in cell viability was inhibited by ITD in a dose-dependent manner. Furthermore, our results indicated that t-BHP could enhance intracellular ROS accumulation, which was reduced by treatment with ITD (Figure 1(b)). Our data demonstrated that t-BHP treatment significantly reduced the amount of GSH, but pretreatment with ITD significantly attenuated t-BHP-induced GSH depletion (Figure 1(c)).
Figure 1.
Effects of ITD on t-BHP-induced cell death, reactive oxygen species (ROS) generation and glutathione (GSH) depletion. (a) Cells were pro-treated with ITD (8, 16 and 32 μM) for 6 h and incubated with t-BHP for 3 h. MTT assay was used to determine the cell viability. (b) HepG2 cells were pro-incubated with various concentrations of ITD for 6 h and stained with DCFH-DA (50 µM) for 1 h, cells were exposed to t-BHP (10 mM) for additional 30 min. (c) HepG2 cells were treated with ITD (32 µM) for 6 h followed by t-BHP (10 µM) treatment for 3 h. A commercial GSH test kit was used to measure the level of GSH depletion. Each bar was expressed as the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01 versus control group, #P < 0.05, ##P < 0.01 versus t-BHP group.
ITD: isotetrandrine; t-BHP: tert-butyl hydroperoxide
ITD enhances the antioxidant protein levels of HO-1 in HepG2 cells
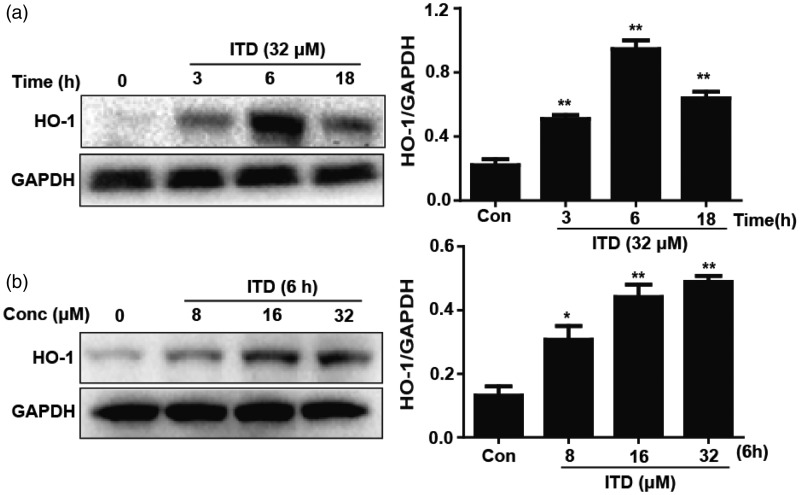
HO-1 is a cytoprotective enzyme, and previous studies have showed that it has antioxidant effects. The cells were treated with ITD (32 μM), Western blot analysis demonstrated that the most effective exposure period is 6 h for HO-1 activation (Figure 2(a)). Next, HepG2 cells were treated with ITD (8, 16, and 32 μM), the expression of HO-1 was significant increased in a dose-dependent manner (Figure 2(b)).
Figure 2.
Effects of ITD on the protein expression of HO-1 in HepG2 cells. (a) HepG2 cells were incubated with ITD (32 µM) for 3, 6 and 18 h. (b) HepG2 cells were treated with various concentrations of ITD (8, 16, and 32 µM) for 6 h. Each bar was expressed as the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01 versus control group.
ITD: isotetrandrine; HO-1: heme oxygenase-1
ITD induced the translocation of Nrf2 and attenuated the level of Keap1
Growing evidence suggested that the translocation of Nrf2 plays a crucial role in the expression of HO-1. HepG2 cells were pretreated with ITD, the level of total Nrf2 protein expression increased proportional to the decrease in levels of Keap1, which was the inhibitory protein of Nrf2 (Figure 3(a) and (b)). As compared with the control cells, preincubation with ITD (32 μM) resulted in an increase of the nuclear levels of Nrf2, which was directly proportional to decrease of the cytoplasmic levels in a time-dependently manner (Figure 3(c)).
Figure 3.
Effects of ITD on the translocation of Nrf2 and the inaction of Keap1. (a) HepG2 cells were incubated with ITD (32 µM) for 3 h, 6 h and 18 h. (b) HepG2 cells were treated with various concentrations of ITD (8, 16 and 32 µM) for 6 h, the levels of proteins were examined by Western blot analysis. (c) Cells were incubated with ITD (32 µM) for 1 h, 3 h and 6 h, and Western blot analysis were employed to analyze the nuclear and cytoplasmic levels of Nrf2. Each bar was expressed as the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01 versus control group.
ITD: isotetrandrine; Keap1: Kelch-like ECH-associated protein 1
ITD-induced HO-1 expression is mediated by Nrf2 in HepG2 cells
It is well known that Nrf2 is essential for expression of antioxidant genes, including HO-1. To examine the effect of Nrf2 in inducing HO-1 expression, siRNA transfection was used to develop a model of Nrf2 gene knockdown. After treatment with control siRNA or Nrf2 siRNA, we determined the levels of Nrf2 and HO-1 protein expression by Western blot analysis. Nrf2 siRNA suppressed the expression of HO-1 and Nrf2 (Figure 4). Therefore, ITD-induced HO-1 activation is mediated by Nrf2 in HepG2 cells.
Figure 4.
Effects of Nrf2-siRNA transfection on ITD-induced HO-1 protein expression. (a) Nrf2-siRNA or Nrf2-negative control siRNA was transfected into HepG2 cells for 24 h, the levels of proteins were examined by Western blot analysis. (b) Nrf2-siRNA was transfected into HepG2 cells for 24 h, cells were incubated with ITD for 6 h. Each bar was expressed as the mean ± SEM of three independent experiments. **P < 0.01 versus control group, ##P < 0.01 versus ITD group.
ITD: isotetrandrine; HO-1: heme oxygenase-1
ITD-induced Nrf2 translocation may be due to the cysteine residues of Keap1
It has been reported that some compounds and electrophilic agents interact with Keap1 by modifying thiol groups of Keap1, then release Nrf2. In order to demonstrate this possibility, HepG2 cells were incubated with ITD (32 μM) with or without DTT (100 μM) or β-mercaptoethanol (1.4 μM). DTT and β-mercaptoethanol abrogated the upregulation of HO-1 expression by ITD (Figure 5).
Figure 5.
Effect of thiol reducing agents on ITD-induced HO-1 expression. (a) HepG2 cells were incubated with DTT (100 μM) for 18 h followed by incubation with ITD (32 μM) for 6 h. (b) Cells were treated with β-ME (1.4 μM) for 18 h, and incubated with ITD (32 µM) for 6 h. Each bar was expressed as the mean ± SEM of three independent experiments. *P < 0.05, versus control group, #P < 0.05, ##P < 0.01 versus ITD group.
ITD: isotetrandrine; HO-1: heme oxygenase-1
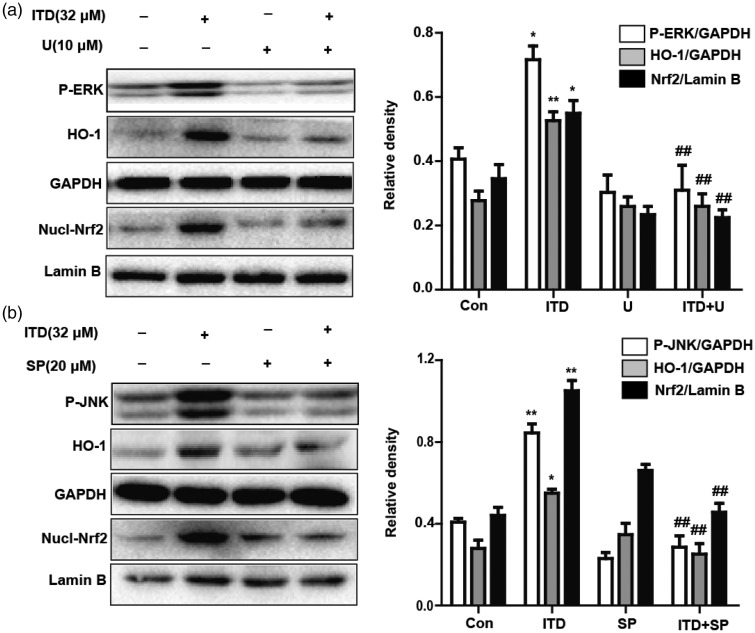
ITD-induced Nrf2 translocation and HO-1 expression via ERK and JNK activation
Previous studies have shown that the inducers of Nrf2 activation could modulate PI3K/Akt, MAPK activity.16 Firstly, we examined if ITD treatment could activate the PI3K/Akt, MAPK pathways. After HepG2 cells were incubated with ITD for 1, 2, 3, and 6 h, the levels of JNK and ERK phosphorylation increased, but not p38 and AKT (Figure 6). To further confirm JNK and ERK were upstream signaling events which were essential for Nrf2 translocation and HO-1 activation, HepG2 Cells were incubated with either SP600125 (JNK inhibitor, 20 μM), U0126 (ERK inhibitor, 10 μM) overnight before ITD treatment. These results indicated that the ability of ITD-induced Nrf2 and HO-1 expression was abolished when ERK or JNK was blocked by respectively inhibitor (Figure 7). Therefore, ERK and JNK activation are involved in Nrf2 translocation and HO-1 expression induced by ITD.
Figure 6.
Effect of ITD on the activation of the MAPK and PI3K/Akt pathways in HepG2 cells. (a, b, c, and d) Cells were treated with ITD (32 μM) for 6 h, Western blot analysis were used to explore the total and phosphorylated ERK, JNK, p38 and Akt protein expression. Each bar was expressed as the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01 versus control group.
ITD: isotetrandrine; ERK: extracellular signal-regulated protein kinase
Figure 7.
Effects of ERK and JNK activation on ITD-induced HO-1 expression and Nrf2 nuclear translocation. (a, b) HepG2 cells were incubated with SP600125 (JNK inhibitor, 20 μM) or U0126 (ERK inhibitor, 10 μM) followed by 6 h-incubation with ITD (32 μM). The whole cells lysates were examined with anti-HO-1 and anti-GAPDH antibodies by Western blot analysis, and nuclear extracts were subjected to Western blot analysis with anti-Nrf2 and anti-Lamin B antibodies. Each bar was expressed as the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01 versus control group, ##P < 0.01 versus ITD group.
ITD: isotetrandrine; ERK: extracellular signal-regulated protein kinase; HO-1: heme oxygenase-1
ITD alleviated cellular injury by upregulating Nrf2 and HO-1 via ERK and JNK activation
HepG2 cells were pretreated with SB203580 (p38 inhibitor, 10 μM), LY294002 (PI3K/Akt inhibitor, 20 μM), SP600125 (JNK inhibitor, 20 μM), U0126 (ERK inhibitor, 10 mM), or SnPP (HO-1 inhibitor, 40 μM) overnight, then cells were treated with ITD for 6 h and t-BHP (10 mM) for 3 h. We measured the cell viability by MTT colorimetric assay. We found that ITD could attenuate t-BHP-induced the reduction of cell viability. ERK, JNK and HO-1 inhibitors diminished the protective effects of ITD (Figure 8). These data demonstrated that ITD exerted cytoprotective effect by upregulating Nrf2 and HO-1 via activation of ERK and JNK signaling.
Figure 8.
Effects of ITD-induced ERK and JNK activation on t-BHP-induced cytotoxicity. Cells were preincubated with LY294002 (20 μM), U0126 (10 μM), SB203580 (10 μM), SP600125 (20 μM), and SnPP (40 μM) for 18 h and treated with ITD for 6 h followed by exposed to t-BHP (10 mM) for 3 h. Each bar was expressed as the mean ± SEM of three independent experiments. **P < 0.01 versus control group, #P < 0.05, ##P < 0.01 versus t-BHP group, ++P < 0.01 versus ITD (32 μM) group.
ITD: isotetrandrine; t-BHP: tert-butyl hydroperoxide
Discussion
Excessive exposure to ROS causes oxidative stress, which is associated with the mechanisms of several diseases.17 Therefore, one possible strategy of therapy for diseases is to regulate oxidative stress. Previous reports have suggested that various natural products could be considered as inhibitors of oxidative stress.18 In our study, we attempted to explore the antioxidant potential and mechanism of action of the natural plant alkaloid ITD in HepG2 cells, with a special focus on the cytoprotective enzyme HO-1, which has antioxidative function.
In the process of t-BHP metabolism by cytochrome P450, resultant lipid peroxidation leads to cell death. In this study, we examined whether ITD could protect against oxidative injury as modeled by t-BHP exposure in HepG2 cells. We found that ITD could alleviate the oxidative cytotoxicity in a dose-dependent manner (Figure 1(a)). Additionally, our data suggested that ITD suppresses ROS generation (Figure 1(b)). GSH, a non-enzymatic antioxidant in mammals, contributes to redox homeostasis by neutralizing ROS via its sulfhydryl group.19 We found that ITD could diminish t-BHP-induced GSH depletion (Figure 1(c)). These results clearly suggest that ITD possesses a significant protective effect against t-BHP-induced cell death and oxidative stress in HepG2 cells.
Increasing evidence suggests that the cytoprotection of antioxidants may be associated with expression of phase II enzymes like HO-1. HO-1 expression is induced in an adaptive response to resist a variety of abnormal stimulus conditions, including oxidative stress.20 In our study, we found that ITD increased the level of HO-1 expression in a time- and dose-dependent manner (Figure 2(a) and (b)), which suggested that the cytoprotection of ITD may account for its ability to induce antioxidant enzyme HO-1 expression.
Activation of nuclear transcription factor Nrf2 leads to expression of its target antioxidant genes, including HO-1. Under the condition of oxidative stress, Nrf2 disassociates from cytoplasmic Keap1 and transcribes into nuclear, then induces HO-1 expression. Our result suggested that ITD increased the level of total Nrf2 expression level and reduced Keap1 expression (Figure 3(a) and (b)). Furthermore, ITD increased the level of Nrf2 in the nucleus versus cytoplasmic Nrf2 (Figure 3(c)). In addition, ITD-induced HO-1 activation was abrogated by transfection with Nrf2 siRNA, which is demonstrated that Nrf2 is a regulator for ITD-induced HO-1 activation (Figure 4). One possible mechanism of Nrf2 activation is associated with Keap1 inactivation, which leads to the dissociation of Nrf2 from Nrf2-Keap1 complex.21 Keap1 contains six cysteine residues, some compounds and electrophilic agents can oxidize or covalently modify thiol groups of Keap1 leading to Keap1 inactivation.22 Dithiothreitol and β-mercaptoethanol, which are the thiol reducing agents, diminished ITD-induced HO-1 activation (Figure 5). It is more likely that ITD interacts with specific cysteine thiol of Keap1 directly, which allows Nrf2 to translocate into the nucleus and enhance the expression of HO-1.
The posttranscriptional modification of Nrf2 induced by kinases is another possible strategy of Nrf2 activation. Growing evidence shows that MAPK or PI3K are kinases upstream of Nrf2 transcription and upregulation of HO-1.23 These kinases promote the release of Nrf2 from Keap1 and its subsequent translocation by the phosphorylation of Nrf2 at serine and threonine residues. We found that preincubation with ITD enhanced the phosphorylation of ERK and JNK expression, but not p38 (Figure 6). Furthermore, the respective inhibitors of ERK and JNK abolished ITD-induced HO-1 upregulation (Figure 7). Therefore, ITD increased Nrf2-mediated HO-1 expression through activation of ERK and JNK in HepG2 cells. In addition, the treatment of ERK, JNK, or HO-1 inhibitor significantly decreased ITD-induced cytoprotection due to the abolished HO-1 expression (Figure 8). The results support that the cytoprotection of ITD is associated with Nrf2-mediated HO-1 expression through activation of ERK and JNK pathways.
In conclusion, ITD effectively alleviated t-BHP-induced oxidative damage via the suppression of cytotoxicity, ROS generation, and GSH depletion in HepG2 cells. Furthermore, our study demonstrated that ITD ameliorated oxidative stress through upregulation HO-1 expression by activation of upstream Nrf2. This may be due to ERK, JNK activation, and Keap1 inactivation. This study supports that ITD has therapeutic potency in the oxidative stress-induced diseases.
Acknowledgements
This work was supported by a grant from the Natural Science Foundation of Jilin (no. 20150520050JH).
Authors’ contributions
L-DW, X-XC contributed equally to this article. All authors participated in the completion of the manuscript. L-DW, X-XC and H-ML conducted most of the experiments and wrote the manuscript, X-SW, X-FQ and G-HC supplied critical reagents and contributed to the design and review of the study.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Breimer LH. Molecular mechanisms of oxygen radical carcinogenesis and mutagenesis: the role of DNA base damage. Mol Carcinog 1990; 3: 188–97. [DOI] [PubMed] [Google Scholar]
- 2.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg 2006; 391: 499–510. [DOI] [PubMed] [Google Scholar]
- 3.Chen XL, Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr Pharm Des 2004; 10: 879–91. [DOI] [PubMed] [Google Scholar]
- 4.Biljak VR, Rumora L, Cepelak I, Pancirov D, Popovic-Grle S, Soric J, Grubisic TZ. Glutathione cycle in stable chronic obstructive pulmonary disease. Cell Biochem Funct 2010; 28: 448–53. [DOI] [PubMed] [Google Scholar]
- 5.DeLeve LD, Kaplowitz N. Importance and regulation of hepatic glutathione. Semin Liver Dis 1990; 10: 251–66. [DOI] [PubMed] [Google Scholar]
- 6.Surh YJ, Kundu JK, Na HK, Lee JS. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutr 2005; 135: 2993S–3001S. [DOI] [PubMed] [Google Scholar]
- 7.Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Baranano DE, Dore S, Poss KD, Snyder SH. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol 1999; 1: 152–7. [DOI] [PubMed] [Google Scholar]
- 8.Shi X, Zhou B. The role of Nrf2 and MAPK pathways in PFOS-induced oxidative stress in zebrafish embryos. Toxicol Sci 2010; 115: 391–400. [DOI] [PubMed] [Google Scholar]
- 9.Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol 2011; 85: 241–72. [DOI] [PubMed] [Google Scholar]
- 10.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 2007; 47: 89–116. [DOI] [PubMed] [Google Scholar]
- 11.Kong AN, Owuor E, Yu R, Hebbar V, Chen C, Hu R, Mandlekar S. Induction of xenobiotic enzymes by the MAP kinase pathway and the antioxidant or electrophile response element (ARE/EpRE). Drug Metab Rev 2001; 33: 255–71. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul 2006; 46: 113–40. [DOI] [PubMed] [Google Scholar]
- 13.Kumar H, Kim IS, More SV, Kim BW, Choi DK. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat Prod Rep 2014; 31: 109–39. [DOI] [PubMed] [Google Scholar]
- 14.Farombi EO, Surh YJ. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J Biochem Mol Biol 2006; 39: 479–91. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Liu YY, Jiang Q, Li KR, Zhao YX, Cao C, Yao J. Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling. Free Radic Biol Med 2014; 69: 219–28. [DOI] [PubMed] [Google Scholar]
- 16.Weng CJ, Chen MJ, Yeh CT, Yen GC. Hepatoprotection of quercetin against oxidative stress by induction of metallothionein expression through activating MAPK and PI3K pathways and enhancing Nrf2 DNA-binding activity. N Biotechnol 2011; 28: 767–77. [DOI] [PubMed] [Google Scholar]
- 17.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 2003; 17: 1195–214. [DOI] [PubMed] [Google Scholar]
- 18.Vitaglione P, Morisco F, Caporaso N, Fogliano V. Dietary antioxidant compounds and liver health. Crit Rev Food Sci Nutr 2004; 44: 575–86. [DOI] [PubMed] [Google Scholar]
- 19.Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother 2003; 57: 145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 2010; 49: 1603–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JS, Surh YJ. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett 2005; 224: 171–84. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Liu J, Chen SY. Over-expression of Nrf2 diminishes ethanol-induced oxidative stress and apoptosis in neural crest cells by inducing an antioxidant response. Reprod Toxicol 2013; 42: 102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors 2003; 17: 287–96. [DOI] [PubMed] [Google Scholar]