Abstract
Autologous fibrin gel is commonly used as a scaffold for filling defects in articular cartilage. This biomaterial can also be used as a sealant to control small hemorrhages and is especially helpful in situations where tissue reparation capacity is limited. In particular, fibrin can act as a scaffold for various cell types because it can accommodate cell migration, differentiation, and proliferation. Despite knowledge of the advantages of this biomaterial and mastery of the techniques required for its application, the durability of several types of sealant at the site of injury remains questionable. Due to the importance of such data for evaluating the quality and efficiency of fibrin gel formulations on its use as a scaffold, this study sought to analyze the heterologous fibrin sealant developed from the venom of Crotalus durissus terrificus using studies in ovine experimental models. The fibrin gel developed from the venom of this snake was shown to act as a safe, stable, and durable scaffold for up to seven days, without causing adverse side effects. Fibrin gel produced from the venom of the Crotalus durissus terrificus snake possesses many clinical and surgical uses. It presents the potential to be used as a biomaterial to help repair skin lesions or control bleeding, and it may also be used as a scaffold when applied together with various cell types. The intralesional use of the fibrin gel from the venom of this snake may improve surgical and clinical treatments in addition to being inexpensive and adequately consistent, durable, and stable. The new heterologous fibrin sealant is a scaffold candidate to cartilage repair in this study.
Keywords: Fibrin gel, rattlesnake venom, scaffold, fibrin sealant
Introduction
Fibrin sealant (or fibrin gel) is a biomaterial that was initially developed as a hemostatic, surgical, and reparative agent.1,2 The advantages of this biological sealant include excellent tissue tolerance, complete reabsorption, and significant clinical improvement when combined with various treatments.3,4 Commonly used fibrin sealant is obtained by adding thrombin and calcium chloride to fibrinogen-rich plasma.1 This gel demonstrates hemostatic and reparative properties when used in a variety of clinical and surgical applications. These properties have also been employed successfully in scaffolds and adhesives to improve the repair and regeneration of fibrocartilage and elastic, craniofacial, and articular cartilages.5,6
Fibrin sealant has two main functions. The first is to serve as a carrier for the extracellular matrix and a scaffold for the administration of treatments, with the main advantage being its ability to preserve the biochemical and original properties of the implants.7–9 The second function of fibrin sealant is to speed up the repair of wounds and lesions, thereby lowering the probability of hemorrhage (hemostatic agent) and protecting the site against bacterial infection.10
This biomaterial can be prepared from fibrinogen-rich plasma, which contains many proteins including fibronectin and plasminogen and platelets. Fibrin sealant is also available in commercial formulas, which demonstrate strong tensile and adhesive strength, and gels with high fibrinogen concentrations generate a more viscous product.1,11 The fibrin gel obtained from the venom of the Crotalus durissus terrificus snake, a rattlesnake, contains serinoprotease, a protein in the venom that has been characterized as thrombin-like and acts in a similar manner as thrombin.12 In fact, this snake-derived fibrin gel has been used as a hemostatic agent to improve the healing of tissues,13–15 and this formulation seems to presents all of the beneficial characteristics that accompany the common fibrin sealant.
The use of this type of gel is required for cellular implants in articular cartilage lesions, for example, because it provides a 3-dimensional (3D) environment (as a scaffold for chondrocytes). Moreover, the new heterologous fibrin sealant derived from Crotalus durissus terrificus snake venom possesses the advantages of a thrombin-like protein with activities similar to other forms of thrombin but about 4000-fold more potent than human thrombin,. It is also inexpensive and easy to obtain and apply.13,15 Fibrin gel from this snake’s venom, as a commercial formulas for common use, is degraded by components of the extracellular matrix, such as macrophages and fibroblasts,16 resulting in the generation of nontoxic molecules to accelerate the healing process. However, the durability of this fibrin gel remains uncertain, and current formulations likely last one to three weeks in vivo.17,18 Moreover, its use may be restricted due to its lack of mechanical stability, depending on the species that it is used on and the repair protocol.18
Keeping in mind the importance of these data for the quality and efficiency of treatments using fibrin sealant, the current study sought to analyze the safety, durability (period that it remains intact in the implant site), and stability of fibrin gel developed from rattlesnake venom using both in vitro and in vivo ovine experimental models showing the results of preliminary studies.
Methods
This study was approved by the Ethics Committee on Animal Use on April 13, 2012, by the protocol: 46/2012—CEUA. Fibrin sealant developed from the venom of the rattlesnake was kindly provided by the Center for Studies of Venoms and Venomous Animals (Centro de Estudos de Venenos e Animais Peçonhentos—CEVAP) of São Paulo State University (UNESP—Univ Estadual Paulista) (Brazil). This gel is made of a thrombin-like compound derived from the venom of the snake and is cryoprecipitated in animal fibrinogen and a calcium chloride solution. This formulation is similar to the contents of commercial sealants but includes nonhuman products. Its composition is waiting for phase 2 clinical studies to be patented and registered by National Agency of Sanitary Surveillance (ANVISA, Brazil).
For implantation of the gel, chondral defects were experimentally induced by artrothomy in eight healthy ovines of the Texel-mix breed. The animals were between the ages of 18 and 36 months and had a mean weight of 30 kg. An area of approximately 14 mm2 in the access site to the patellofemoral joint underwent tricothomy, followed by antisepsis. With the animal placed in dorsal decubitus and duly anesthetized, the articular access was opened according to the technique described by Allen et al.19 An incision of the skin was performed in a semicircle, immediately medial to the patellar tendon. The adjacent tissue was separated until the entire articular capsule and patellar tendon were exposed. The articular capsule was penetrated without unnecessary damage to the patellofemoral ligaments.
The chondral defect was initiated on the articular surface of the medial femoral trochlea, using a shaver with a 3-mm circular drill and a diameter of approximately 0.07 mm per joint (animal). The hyaline cartilage and the calcified cartilage were removed via discrete drilling so that the gel could be adhered to the receiving site. The subchondral bone was not drilled to prevent the inflow of bone marrow cells.
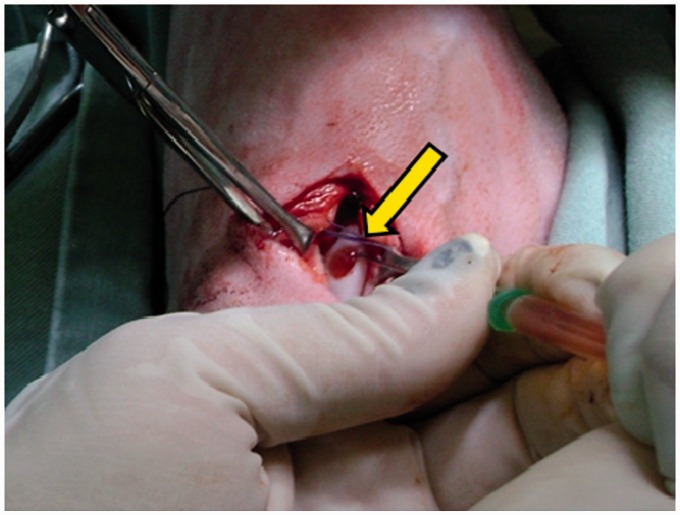
Immediately after performing the chondral defect, the gel compound was deposited strictly over the previously created defect, with the help of a syringe and a hypodermic needle. The gel was administered in its final stage of “gelation,” according to the recommendations by CEVAP, the developers of the gel, and formed an irregular-shaped 3D scaffold. The joint remained open until complete gelation, adaptation to the shape, and drying of the gel. All of the applications were photographed for posterior macroscopic comparison (Figure 1).
Figure 1.
Application of the gel made from the venom of the rattlesnake (arrow) on an experimentally induced chondral defect on the medial femoral trochlea. (A color version of this figure is available in the online journal.)
All planes were then sutured with separate stitches. After the surgeries, the animals received a regimen of antibiotics, tetanus serum, anti-inflammatory treatments, and daily wound cleaning for 10 days until the stitches were removed. All eight ovines were posteriorly divided in two groups. Group 1 (G1, 4 ovines) was euthanized 7 days after placement of the implant, and Group 2 (G2, 4 ovines) was euthanized 15 days after implanting the gel. This division was made because of the questionable durability of the gel, varying one to three weeks as reported in the literature.16–18 After euthanasia, both for G1 and G2, the medial trochlea of the femurs were removed so that the lesion site and subsequent implant site of the gel remained preserved. The same maneuver was performed on the contralateral member to obtain a control sample from each animal. These samples were macroscopically analyzed, photographed, and stored in formalin for subsequent microscopic and histopathological analysis at the Pathology Department of the School of Medicine of Botucatu (UNESP—Botucatu—FMB, SP, Brazil).
Magnetic resonance images were obtained from a healthy animal (without injuries) and another animal seven days after the lesion was induced (G1) and the gel was implanted, with the goal of analyzing the integration and permanence of the gel in the chondral defect site. Magnetic resonance imaging (MRI) was also performed on two other animals 15 days postoperatively (G2), with the goal of analyzing the behavior of the gel in the lesion during this period.
To complement the in vivo study, in vitro analyses were also performed using the same formulation of the rattlesnake venom fibrin gel. The contents of the gel were homogenized (mixed), similarly to the gel used in the in vivo implants, according to the recommendations of CEVAP. In the final gelation stage, the compound was poured onto a single spot in a Petri dish (Tissue Culture Petri Dishes—TPP, Switzerland), forming an irregular 3D scaffold. The plate was closed and maintained throughout the experiment in a 5% CO2 incubator at 37℃, with manipulations only performed in a laminar flow hood. After complete gelation, 3 mL of phosphate-buffered saline was added to each plate to maintain the moisture content. Five Petri dishes were studied, each containing the same amount of gel implanted in the in vivo study. The physical macroscopic characteristics of the “gels,” including consistency, durability, hydration, tridimensionality, and time to gelation, were then evaluated. The gels were photographed every 24 h for 15 days to record any alterations or problems in the gel characteristics.
Results
After the gel was applied, it occupied the whole injury site; slight leaking was observed to the area adjacent to the created circumference, but this did not interfere with the complete filling of the injury. The drying (or gelation) occurred within 1 min, on average, and the color changed from transparent to grey after drying. The gel was easily applied and handled; even without the use of commercial kits or special syringes, implantation was successful. There were no undesirable clinical reactions (such as exaggerated inflammation) over the 15 days of this experiment.
After performing euthanasia in G1, the gel could still be found at the injury site on the medial trochlea (7 days after implanting). The gel presented a stable macroscopic appearance and remained in its initial format, according to photographic comparison. There was no alteration in color, consistency, or shape of the gel, and it was perfectly adhered to the receiving site. However, there was significant synovial fluid present at the site of injury. After performing euthanasia in G2 (15 days after the implant), no vestige of the gel was observed upon exploration of the joint. In addition, there were no signs of any remaining implanted material, and the significant amount of synovial liquid detected in G1 was not observed.
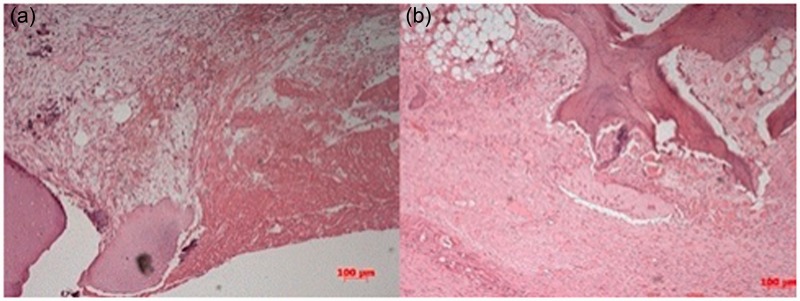
In the microscopic examination of the samples collected at day 7 (G1), the injury site was filled with an acellular structure, without any morphologic pattern, and we observed the presence of eosinophilic cells and interlaced fibrils, likely constituting the remaining gel (Figure 2(a)). The microscopic examination of the samples collected on day 15 (G2) revealed an interaction between the normal cartilage around the lesion and the expected neoformed fibrocartilage at the injury site, without modifications resulting from the implementation of gel (Figure 2(b)). There were no signs of the gel remaining in the injury site after 15 days.
Figure 2.
(a) G1 (7 days): The presence of red blood cells, an acellular structure without morphologic arrangement (arrow), and eosinophilic fibrils, which were likely the remains of the gel on the edges of the defect. (b) G2 (15 days): Normal neoformed fibrocartilage on the edge of the defect (arrow), after complete gel absorption. (A color version of this figure is available in the online journal.)
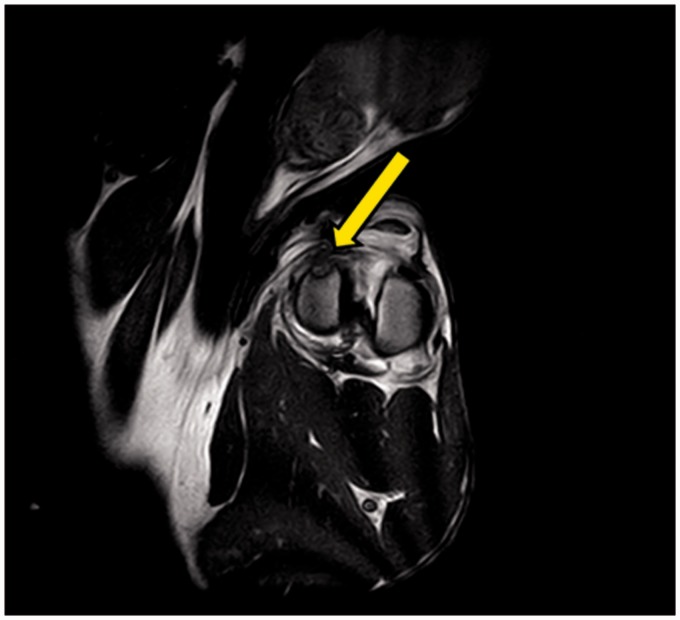
The images obtained from MRI analysis showed a clear difference between the gel and the cartilage. Specifically, at seven days after implantation, the gel was present at the injury site and stably attached to the surrounding healthy cartilage. The cartilage showed greater radiointensity than the gel, and a low-radiointensity halo could be observed between the gel and cartilage, which indicated the interaction of the gel with the receiving site (Figure 3). On the MRI examination, 15 days after the implant, only the injury site without the gel was observed.
Figure 3.
Magnetic resonance images showing the gel at the injury site seven days after implantation (arrow)
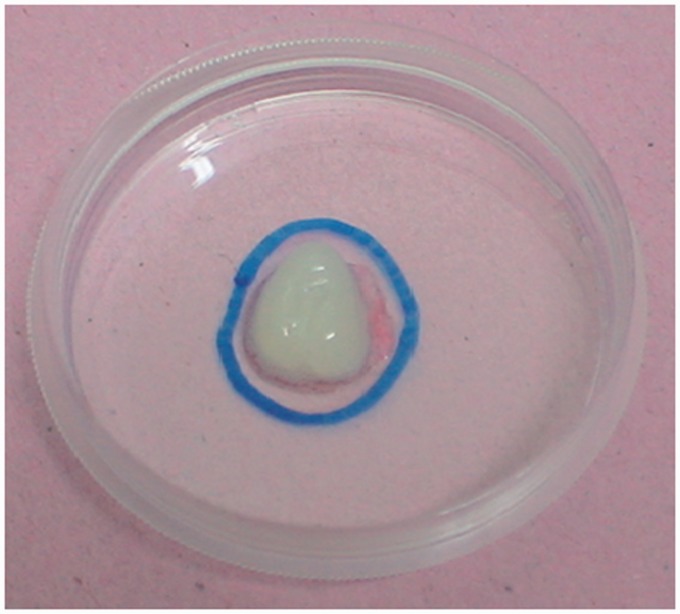
The macroscopic morphological characteristics of the gel in the complementary in vitro study were favorable. The gel maintained its consistency, moisture content, and tridimensionality. The time until complete gelation was 43 s, on average. All five gel plates demonstrated durability longer than 15 days (Figure 4).
Figure 4.
Image of the gel used in the in vitro study. (A color version of this figure is available in the online journal.)
Discussion
Fibrin gel (sealant) made from the venom of the Crotalus durissus terrificus snake contains serinoprotease, a thrombin-like protein with an activity similar to other forms of thrombin.12 In addition, the serinoprotease has a higher capacity to convert fibrinogen to fibrin than bovine thrombin,15 and this snake-derived fibrin may also be used with autologous, heterologous, or homologous fibrinogen.14 In this case, sealant with a better consistency (stronger) than those used with autologous thrombin or bovine lyophilized thrombin can be developed. Moreover, these new forms of fibrin gels can demonstrate better consistency than the commercial products (commercial kits or sealants and fibrin sealants), with the advantage of being far less expensive.11 Because it is a preliminary study, we did not aim to test the comparison with other commercial products, but only the applicability and safety of this sealant from venom of the Crotalus durissus terrificus. However, further studies should be conducted to compare commercial kits with sealant presented here.
The gel from the venom of the rattlesnake also possesses anti-inflammatory properties,14 and this material was previously used in other studies13 to evaluate the healing of hysterectomies performed in female dogs. Fibrin gel from snake venom was selected for this study because previous studies performed by CEVAP in Botucatu showed satisfactory use of this sealant with promising results; in particular, this gel performed in a similar way to previously developed fibrin sealant.20,21 Snake-derived fibrin gel also stimulates granulation tissue and does not trigger a strong inflammatory response.13 In our study, the rapid and normal generation of fibrocartilage, which completely filled the injury site in G2, showed the inert or beneficial effects of the gel implantation; however, detailed studies and quantitative analysis of innate and adaptive immunological response are still required, at different time points after implantation.
The anti-inflammatory effect of the gel in G2 may have also helped to prevent the increased production of synovial fluid observed on day 7 (G1), probably due to surgery. The synovitis caused by surgical manipulation was expected, and this condition typically results in the effusion of synovial liquid, protein and fibrinogen, and the influx of leukocytes.22 Alterations mainly involving the synovial membrane and the articular cartilage are a result of articular inflammation and the normal increase in synovial liquid.23,24 Therefore, the greater effusion observed on day 7 was not considered an adverse reaction to the “gel’s” implantation, and its remission, despite being natural and expected on day 15, was most likely improved by the presence of the gel. Similarly, previous studies have shown that treatment of chondral defects with gel also helps to reduce articular inflammation.25
Fibrin gel has been largely used as a tridimensional scaffold, mainly in the treatment of injuries of the articular cartilage.26 Its ease of use (in liquid form), polymerization, and high adherence to the injury site are some of the advantages of using this biomaterial. The scaffolds formed by the fibrin gel were effective in supporting cellular implants and promoting cell proliferation. In addition, fibrin clots have been shown to interact with and stimulate cell adhesion at the injury site.27,28 Therefore, according to the studies performed here, fibrin gel made from the venom of the rattlesnake showed satisfactory results of these preliminary measures, like adhesion and stability.
Fibrin is degraded by components of the extracellular matrix, resulting in the generation of nontoxic molecules that promote the healing process. According to the literature, the durability of fibrin sealant at the injury site is limited, ranging from one to three weeks and depending on the physiological time required to absorb a normal blood clot.11,17,18 In the present study, we observed that the absorption of the fibrin gel from snake venom persisted for longer than one week but less than 15 days. Thus, we concluded that absorption of the clot occurred near day 10, as the follow-up examinations did not detect any vestige of the gel on day 15. In contrast to reports in the literature that used common fibrin sealant, the gel tested here showed a slightly shorter reabsorption time, which represents one disadvantage of using this form of fibrin. This quick reabsorption may be explained by the heterologous components of the sealant, which could have stimulated a greater immune response than that observed in other experimental models.29 However, the use of this venom-derived fibrin gel did not show mechanical instability, according to what has been described in previous studies using common fibrin gels.18,30 Nevertheless, comparative controlled studies should be performed to put together commercial fibrin sealant kits and the fibrin sealant from snake venom as a development of this preliminary study.
In addition to several advantages observed in the use of this gel from rattlesnake venom presented in this study, it is well known that the commercial fibrin sealants using thrombin- and fibrinogen derived from human blood have expensive production and labor processes, plus pose the possibility of the transmission of infectious diseases.31–33 In contrast, the new heterologous fibrin sealant derived from Crotalus durissus terrificus snake venom contains a thrombin-like protein with an activity similar to other forms of thrombin but about 4000-fold more potent than human thrombin.34–35 Therefore, our fibrin sealant is produced from buffalo fibrinogen, a species able to donate a large amount of blood-diminishing product costs.36,37
Together, these findings led us to conclude that fibrin gel made from the venom of the rattlesnake seems to present adequate consistency and stability. It could be used as a scaffold, as well as being inexpensive and easy to obtain and apply. In particular, the durability of this gel was greater than one week but less than 15 days. Moreover, its applicability was excellent, and the gel did not trigger undesirable effects, such as inflammation, and allowed a normal repair process in this study. After performing additional and complementary studies, including the use of cell cultures and the in vivo comparison with other types of biological gels, this form of fibrin gel could be used as a scaffold or sealant, in several types of surgical treatment.
Acknowledgements
This study was financially supported by FAPESP (São Paulo Research Foundation) (2012/03600-6 and 2012/04516-9). Fibrin sealant derived from snake venom was kindly supplied by the Center for the Study of Venoms and Venomous Animals (CEVAP) of UNESP, its constituents and instructions for use are stated in its patents (registration numbers BR1020140114327 and BR1020140114360). At the time of use, the components were previously thawed, reconstituted, mixed, and applied according to the protocol of the project (Thomazini-Santos et al., 2001; Barros et al., 2010, 2011; Gasparotto et al. 2014; Ferreira Junior, 2014).
Author Contributions
ALGA, CNB, ALMY and JBS were responsible for the surgical and laboratory practical phases. RSFJ and BB were responsible for the gel development and CEVAP coordination. ALGA, CAH, MJW and CAR were responsible for the coordination of research and writing in final phase of article.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg 2001; 107: 229–37. [DOI] [PubMed] [Google Scholar]
- 2.Matras H. Fibrin seal: the state of the art. J Oral Max Surg 1985; 43: 605–11. [DOI] [PubMed] [Google Scholar]
- 3.Brennan M. Fibrin glue. Blood Rev 1991; 5: 240–4. [DOI] [PubMed] [Google Scholar]
- 4.Lupinetti FM, Stoney WS, Alford WC, Burrus GR, Glassford DM, Jr, Petracek MR, Thomas CS. Cryoprecipitate-topical thrombin glue. Initial experience in patients undergoing cardiac operations. J Thorac Cardiovasc Surg 1985; 90: 502–5. [PubMed] [Google Scholar]
- 5.Izuta Y, Ochi M, Adachi N, Deie M, Yamasaki T, Shinomiya R. Meniscal repair using bone marrow-derived mesenchymal stem cells: experimental study using green fluorescent protein transgenic rats. Knee 2005; 12: 217–23. [DOI] [PubMed] [Google Scholar]
- 6.Milano G, Sanna Passino E, Deriu L, Careddu G, Manunta L, Manunta A, Saccomanno MF, Fabbriciani C. The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects: an experimental study in a sheep model. Osteoarthritis Cartilage 2010; 18: 971–80. [DOI] [PubMed] [Google Scholar]
- 7.Peretti GM, Randolph MA, Zaporojan V, Bonassar LJ, Xu JW, Fellers JC, Yaremchuk MJ. A biomechanical analysis of an engineered cell-scaffold implant for cartilage repair. Ann Plast Surg 2001; 46: 533–7. [DOI] [PubMed] [Google Scholar]
- 8.Leo AJ, Grande DA. Mesenchymal stem cells in tissue engineering. Cells Tissues Organs 2006; 183: 112–22. [DOI] [PubMed] [Google Scholar]
- 9.Ringe J, Kaps C, Burmester GR, Sittinger M. Stem cells for regenerative medicine: advances in the engineering of tissues and organs. Naturwissenschaften 2002; 89: 338–51. [DOI] [PubMed] [Google Scholar]
- 10.Buchta C, Dettke M, Funovics PT, Höcker P, Knöbl P, Macher M, Quehenberger P, Treitl C, Worel N. Fibrin sealant produced by the CryoSeal FS System: product chemistry, material properties and possible preparation in the autologous preoperative setting. Vox Sang 2004; 86: 257–62. [DOI] [PubMed] [Google Scholar]
- 11.Brittberg M, Sjögren-Jansson E, Lindahl A, Peterson L. Influence of fibrin sealant (Tisseel) on osteochondral defect repair in the rabbit knee. Biomaterials 1997; 18: 235–42. [DOI] [PubMed] [Google Scholar]
- 12.Copley AL, Banerjee S, Devi A. Studies of snake venoms on blood coagulation I. The thromboserpentin (thrombin-like) enzyme in venoms. Thromb Res 1973; 2: 487–508. [Google Scholar]
- 13.Moraes JRE, Correia PHA, Camplesi AC, Moraes FR. Experimental use of fibrin glue derived from snake venom in non-pregnant canine uterus. J Venom Anim Toxins Incl Trop Dis 2004; 10: 133–43. [Google Scholar]
- 14.Sartori Filho RFC, Prestes NC, Thomazini IA, Mendes-Giannini MJ, Toscano E, Canavessi AMO, Barraviera B. Use of fibrin glue derived from snake venom in testicular biopsy in rams. J Venom Anim Toxins 1998; 4: 23–35. [Google Scholar]
- 15.Thomazini IAS. Avaliação do tempo de coagulação da trombina bovina, da reptilase e da fração do” tipo-trombina” de serpentes Crotalus durissus terrificus, empregando-se crioprecipitado de diferentes espécies animais [Evaluation of the coagulation time of bovine thrombin, reptilase and the “thrombin-like” fraction of Crotalus durissus terrificus serpents, using a cryoprecipitate from different animal species]. Thesis, Universidade Estadual Paulista, Faculdade de Medicina, Botucatu, 1996.
- 16.Pescatore P, Verbeke C, Harle M, Manefold BC. Fibrin sealing in peptic ulcer bleeding: the fate of the clot. Endoscopy 1998; 30: 519–23. [DOI] [PubMed] [Google Scholar]
- 17.Berrevoet F, Hemptinne B. Clinical application of topical sealants in liver surgery: does it work? Acta Chir Belg 2007; 107: 504–7. [DOI] [PubMed] [Google Scholar]
- 18.Yamada ALM. Efeito do implante autólogo de plasma rico em plaquetas (PRP) e células tronco mesenquimais na reparação de lesões condrais articulares induzidas experimentalmente em eqüinos [Effect of the autologous implant of plasma rich in platelets (PRP) and mesenchimal stem cells in reparing chondral articular injuries experimentally induced in equines]. Dissertation, Faculdade de Medicina Veterinária e Zootecnia, Universidade Estadual Paulista, Botucatu, 2011.
- 19.Allen MJ, Houlton JE, Adams SB, Rushton N. The surgical anatomy of the stifle joint in sheep. Vet Surg 1998; 27: 596–605. [DOI] [PubMed] [Google Scholar]
- 20.Ishimura M, Tamai S, Fujisawa Y. Arthroscopic meniscal repair with fibrin glue. Arthroscopy 1991; 7: 177–81. [DOI] [PubMed] [Google Scholar]
- 21.Laitakari K, Luotonen J. Autologous and homologous fibrinogen sealants: adhesive strength. Laryngoscope 1989; 99: 974–6. [DOI] [PubMed] [Google Scholar]
- 22.Stashak T. Diagnóstico de claudicação. In: Stashak TS. (ed). Claudicação em Eqüinos Segundo Adams [Lameness According to Adams], São Paulo: Editora Roca, 1994, pp. 99–159. [Google Scholar]
- 23.McIlwraith CW. From arthroscopy to gene therapy: 30 years of looking in joints. Am Assoc Equine Pract 2005; 51: 65–113. [Google Scholar]
- 24.Martins EAN, Silva LCLC, Baccarin RYA. Evaluation of the synovial fluid of the femuropatellar joint after experimental medial patellar desmotomy in horses. Ciênc Rural 2007; 37: 784–8. [Google Scholar]
- 25.Kon E, Mutini A, Arcangeli E, Delcogliano M, Filardo G, Nicoli Aldini N, Pressato D, Quarto R, Zaffagnini S, Marcacci M. Novel nanostructured scaffold for osteochondral regeneration: pilot study in horses. J Tissue Eng Regen Med 2010; 4: 300–8. [DOI] [PubMed] [Google Scholar]
- 26.Vinatier C, Mrugala D, Jorgensen C, Guicheux J, Noël D. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol 2009; 27: 307–14. [DOI] [PubMed] [Google Scholar]
- 27.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 2009; 37: 2259–72. [DOI] [PubMed] [Google Scholar]
- 28.Kretlow JD, Spicer PP, Jansen JA, Vacanti CA, Kasper FK, Mikos AG. Uncultured marrow mononuclear cells delivered within fibrin glue hydrogels to porous scaffolds enhance bone regeneration within critical-sized rat cranial defects. Tissue Eng Part A 2010; 16: 3555–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paletta GA, Arnoczky SP, Warren RF. The repair of osteochondral defects using an exogenous fibrin clot. An experimental study in dogs. Am J Sports Med 1992; 20: 725–31. [DOI] [PubMed] [Google Scholar]
- 30.Flanagan TC, Sachweh JS, Frese J, Schnöring H, Gronloh N, Koch S, Tolba RH, Schmitz-Rode T, Jockenhoevel S. In vivo remodeling and structural characterization of fibrin-based tissue-engineered heart valves in the adult sheep model. Tissue Eng Part A 2009; 15: 2965–76. [DOI] [PubMed] [Google Scholar]
- 31.Barbizan R, Castro MV, Ferreira RS, Barraviera B, Oliveira AL. Long-term spinal ventral root reimplantation, but not bone marrow mononuclear cell treatment, positively influences ultrastructural synapse recovery and motor axonal regrowth. Int J Mol Sci 2014; 15: 19535–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbizan R, Castro MV, Barraviera B, Ferreira RS, Jr, Oliveira AL. Influence of delivery method on neuroprotection by bone marrow mononuclear cell therapy following ventral root reimplantation with fibrin sealant. PloS One 2014; 9: e105712–e105712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbizan R, Castro MV, Rodrigues AC, Barraviera B, Ferreira RS, Oliveira AL. Motor recovery and synaptic preservation after ventral root avulsion and repair with a fibrin sealant derived from snake venom. PLoS One 2013; 8: e63260–e63260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barros LC, Ferreira RSJR, Barraviera SRCS, Stolf HO, Thomazini-Santos IA, Mendes-Giannini MJS, Toscano E, Barraviera B. A new fibrin sealant from Crotalus durissus terrificus venom: applications in Medicine. J Toxicol Environ Health B 2009; 12: 553–71. [DOI] [PubMed] [Google Scholar]
- 35.Barros LC, Soares AM, Costa FL, Rodrigues VM, Fuly AL, Giglio JR, Gallacci M, Thomazini-Santos IA, Barraviera SRCS, Barraviera B, Ferreira RS., Jr Biochemical and biological evaluation of gyroxin isolated from Crotalus durissus terrificus venom. J Venom Anim Toxins Incl Trop Dis 2011; 17: 23–33. [Google Scholar]
- 36.Benitez SU, Barbizan R, Spejo AB, Ferreira RS, Jr, Barraviera B, Góes AM, de Oliveira AL. Synaptic plasticity and sensory-motor improvement following fibrin sealant dorsal root reimplantation and mononuclear cell therapy. Front Neuroanatomy 2014; 8: 96–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gasparotto VPO, Landim-Alvarenga, Oliveira ALR, Simões GF, Lima-Neto JF, Barraviera B, Ferreira RS., Jr A new fibrin sealant as a three-dimensional scaffold candidate for mesenchymal stem cells. Stem Cell Res Ther 2014; 5: 78–78. [DOI] [PMC free article] [PubMed] [Google Scholar]