Abstract
Galectins are thought to be prognosticators for survival in renal cell cancer. However, the biological activity of galectin-3 (Gal-3) in renal carcinoma cells is still debated. In this study, immunohistochemical staining confirmed a high expression of Gal-3 in tumor tissue from renal cell carcinoma. Critically, Gal-3 expression was related to tumor cell differentiation. Consistent with Gal-3 expression in renal cell cancer, strong expression of Gal-3 was also observed in several renal tumor cell lines but not in normal renal cells. A Gal-3 high-expression cell line Caki-1 was chosen to study the biological activity of Gal-3. Using short hairpin RNA method, Gal-3 expression in Caki-1 cells was knocked down. We evidenced that Gal-3 knockdown inhibited cell proliferation and invasion, induced Caspase-3-dependent apoptosis and arrested cell cycle at G1 phase. Mechanically, Cyclin D1 expression decreased, but p27 increased after Gal-3 knockdown. Taken together, these results suggest that Gal-3 is related to the development of renal cell cancer and could serve as a target to therapy renal cell cancer.
Keywords: Galectin 3, renal carcinoma cells, cell cycle, p27
Introduction
Renal cell carcinoma (RCC) is the most common type of kidney cancer in adults. It occurs most often in men aging 50 to 70 years. Although the exact cause for carcinogenesis is unknown, many factors may increase the risk of kidney cancer, including dialysis treatment, family history of the disease, high blood pressure, horseshoe kidney, polycystic kidney disease, and smoking.1 The recent contribution to RCC is the finding of therapy target in the molecular level. Vascular endothelial growth factor signaling and the mammalian target of rapamycin are well-confirmed therapeutic targets in RCC,2 although its mechanisms of activation are not yet fully understood.
Galectins are a family of proteins defined by their binding specificity to β-galactoside sugars. This family of proteins is largely reported to regulate tumor proliferation, migration, and metastasis.3 As a member of galectins, Galectin-3 (Gal-3) is involved in multiple cellular processes including apoptosis, cell growth, cell adhesion, cell differentiation, and intracellular trafficking. Gal-3 was abnormally regulated in pathological conditions, such as inflammation and cardiovascular diseases.4,5 Moreover, expression and subcellular distribution of Gal-3 change with cellular differentiation, development, as well as carcinogenesis.6 Interestingly, Gal-3 expression was reported to increase in RCC tissue and was supposed to associate with prevalence of RCC.7–9 A mosaic pattern of Gal-3 expression was found in collecting ducts and distal tubules of normal kidney, however, was significantly increased in 79% of tumor samples as compared with normal tissues.10 By contrast, a loss of Gal-3 expression is also reported in renal carcinogenesis.11 Hence, Gal-3 might act as a pro or antitumor factor, depending on the cell type that expresses it.
Previously, we disclosed that Gal-3 inhibition sensitized human RCC cells to arsenic trioxide treatment.12 However, as debated, the function of Gal-3 itself in the carcinogenesis is not known in renal carcinoma. Whether Gal-3 provides a therapy target for renal cancer and potential mechanisms are not clear. In this study, Gal-3 expression was screened in RCC and a short hairpin RNA targeting Gal-3 was designed to evaluate the biological activity of Gal-3 in RCC. Our study provided novel data implicating Gal-3 as a potential therapy target in renal cancer.
Materials and methods
Clinical samples
Tumor samples were taken from 76 cases of RCC patients diagnosed in Cancer Hospital of Harbin Medical University and normal kidney tissues were obtained from patients with various benign kidney conditions. The criteria used to classify the degree of tumor differentiation were delineated in previous publications.13,14 Written informed consent was obtained from each patient, and the study was approved by the Clinical Ethics Committee of Cancer Hospital of Harbin Medical University.
Cell culture
Human RCC cell lines Caki-1 (ATCC, USA) and Caki-2 (ATCC, USA) were cultured in McCoy 5A (Sigma, USA) supplemented with 10% fetal bovine serum. Human renal cell adenocarcinoma cell lines 786-0 and ACHN (ATCC, USA) were grown in RPMI-1640 (NaHCO3 1.5 g/L, glucose 2.5 g/L, sodium pyruvate 0.11 g/L) and MEM (NaHCO3 1.5 g/L, Sodium Pyruvate 0.11 g/L [Gibco]), respectively, supplemented with 10% fetal bovine serum. All types of cells were cultured in a humidified incubator at 37℃ with 5% CO2.
Plasmid and short hairpin RNA transfections
Cells were transfected separately with 300 pmol of Gal-3 short hairpin RNA (shRNA; Addgene, Cambridge, MA, USA) or scrambled shRNA by using Lipofectamine 2000 (Invitrogen) in Caki-1 cells. Four candidate shRNAs were purchased from NeuronBioTech (Shanghai, China). The target sequences were GR311: CCCACGCTTC AATGAGAACA A, GR312: GCAAACAGAA TTGCTTTAG AT, GR313: GCCACTGATT GTGCCTTATA A, and GR314: GCTCCATGAT GCGTTATCTG G; 24 h after knockdown, experiments were carried out to detect cell growth, migration, apoptosis, and cell cycle distribution, as well as the protein expressions.
Cell proliferation
In vitro growth of renal carcinoma cells were determined using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, 3 × 103 cells were plated in 96-well plates and allowed to attach overnight. Five independent wells were repeated for each experiment. A total of 20 mL MTT (Sigma) solution (5 mg/mL in PBS) was added to each well and the plates were incubated for additional 4 h at 37℃; 150 μl dimethyl sulfoxide was added to each well before measurement at absorbance of 490 nm.
Transwell migration assay
Transwell migration assay was performed to test the migratory ability of RCC cells following Gal-3 knockdown. The same numbers of cells in serum-free Dulbecco's modified eagle medium in the upper chamber of 12-well plates were allowed to migrate for 8 h toward Dulbecco's modified eagle medium containing 10% FBS in the lower chamber. After migration, cells in the lower surface of the membrane were fixed and stained with Giemsa. Images were acquired with a bright field microscope at 20 × magnification. To quantify migratory cells, four independent fields were analyzed by using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The percentage of cells that migrated was normalized to control group.
Annexin V-Fluorescein isothiocyanate/propidium iodide
Apoptosis was determined using Annexin V-Fluorescein isothiocyanate/propidium iodide (PI) double staining. Briefly, 200 mL cells (1 × 106 cells per mL) were centrifuged at 1000 rpm for 5 min (4℃). Cells were washed with pre-cooled PBS and resuspended in 100 µL binding buffer with 2 µL Annexin V-Fluorescein isothiocyanate (20 µg/mL). The mixed samples were kept in dark for 15 min. A total of 400 mL PBS and 1 µL PI (50 µg/mL) were added to each sample before measurements. The value was read using flow cytometer (Beckman Coulter EPICS XL). Cells without staining were used as negative control.
PI staining
Twenty-four hours after shRNA transfection, cells were harvested and fixed in pre-colded ethanol overnight. PI (50 µg/mL) was added to each sample before measurements. Cell cycle distribution was analyzed using flow cytometer (Beckman Coulter EPICS XL).
Western blotting
Cells were grown to subconfluency on 6-well plates and exposed to Gal-3 knockdown. Twenty-four hours later, cells were collected, washed with PBS, and resuspended in hypotonic buffer (20 mM HEPES, 10 mM KCl, 1 mM MgCl2, 0.5 mM DTT, 0.1% Triton X-100, 20% glycerol, 5 µg leupeptin per mL, 10 µg aprotinin per mL, and 500 μM phenyl-methylsulfonyl fluoride). The cells were centrifuged at 850 g for 5 min. The supernatant was used as the cytoplasmic fraction. Protein concentrations were measured using BCA Protein Assay Kit (Beyotime Biotechnology, China). Total proteins were separated by SDS-PAGE (12.5% polyacrylamide) by the method of Laemmli and transferred to a PVDF membrane. The presence of proteins was detected using human anti-Gal-3 antibody H-160 (1:500) (Santa Cruz, USA), anti-Actin antibody (1:2000) (Sigma, USA), anti-p27 antibody (1:1000) (Santa Cruz, USA), anti-p21 (1:1000) (Santa Cruz, USA), and anti-Cyclin D1 antibody (1:1000) (Santa Cruz, USA). Expression levels of proteins were detected using the enhanced chemiluminescence system. For densitometric analysis of western blots, Image J software was used.
Quantification of Caspase-3 activity
Caspase-3 activity was determined using Caspase-3 assay kit (Beyotime Institute of Biotechnology, China) by detecting the color reporter molecule, p-nitroanilide (pNA), after cleavage from the substrate of Ac-DEVD-pNA.15 Cells were seeded at 4 × 105 cells per well in a 6-well plate and incubated overnight. Twenty-four hours after shRNA Gal-3 inhibition, cell lysates were assayed for Caspase-3 activity. Ten microliters of cell lysate was mixed with pre-cooled substrate solution Ac-DEVD-pNA (0.2 mM) and incubated at 37℃ for 60–120 min. The mixture without cell lysate was used as blank control. Relative fluorescent value was acquired at 405 nm wavelength. The activity of Caspase-3 in cell lysates was obtained by comparing with blank control and normalizing with the standard curve.
Immunohistochemistry
Tumor tissues and normal tissues were fixed in 4% paraformaldehyde, cryoprotected in 30% sucrose for 1 h at 4℃ and sectioned on a freezing microtome at 20 µm. After that, the sections were incubated with Gal-3 primary antibody (1:100) overnight at 4℃. The immunohistochemistry assay kit (Zhongshan Jianqiao Tech, Beijing, China) was applied to complete the staining. The images were captured using Olympus microscope (Japan).
Statistical analyzes
Data were expressed as mean ± standard deviation. Differences between multiple groups of data were analyzed by one-way ANOVA with Bonferroni correction (Graph Pad Prism 6.0, San Diego, CA, USA). Chi-square test was used to analyze the tumor cell differentiation in Gal-3 positive or negative cases. A p < 0.05 was considered statistically significant.
Results
Galectin 3 expression was elevated in RCC
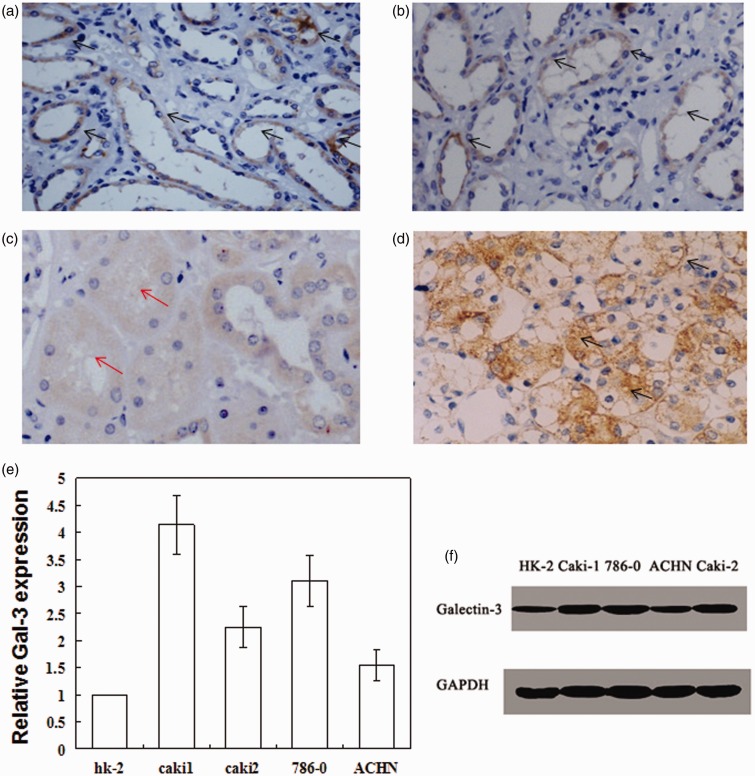
In the kidney tissue obtained from normal control, Gal-3 was mainly expressed in epithelial cells of the distal tubule and collecting duct (Figure 1(a, b)). Moreover, the expression was unequally and irregularly distributed in cytoplasm, while Gal-3 was not expressed in epithelial cells of proximal tubule (Figure 1(c)). By contrast, in tumor tissue from RCC patients, Gal-3 expression was completely different (Figure 1(d)). Our data showed that 53.9% of RCC tissues had a high expression of Gal-3. Importantly, Gal-3 was widely expressed on cell membrane, in cytoplasm, and nucleus. The association of Gal-3 expression with tumor differentiation was also analyzed (Table 1). The percentages of high differentiation, middle differentiation, and low differentiation in Gal-3 positive cases were 43.9%, 31.7%, and 24.4%, respectively. By contrast, the percentages of high differentiation, middle differentiation, and low differentiation in Gal-3 negative cases were 77.2%, 17.1%, and 5.7%, respectively. Through chi-square test, the p values in row and column comparison were 0.008 and 0.038, respectively. These data suggested that Gal-3 expression was related to the malignant degree of RCC and could be used as diagnostic or prognostic index for RCC.
Figure 1.
Gal-3 expression is up-regulated in renal cell carcinoma. Gal-3 was mainly expressed in epithelial cells of the collecting ducts (a) and distal tubules (b), but not in epithelial cells of proximal tubules (c). The black arrows indicate the expression of Gal-3; Red arrows indicate the low expression of Gal-3 in proximal tubules. (d) Gal-3 was highly expressed in RCC; (e) mRNA expression of Gal-3 in various renal tumor cells; (f) Representative blots of Gal-3 expression in renal tumor cells. (A color version of this figure is available in the online journal.)
Table 1.
The cases of high, middle and low differentiation of RCC in both of Gal-3+ and Gal-3− RCC patients
| High, Cases/% | Middle, Cases/% | Low, Cases/% | |
|---|---|---|---|
| Gal-3+ | 18 (43.9) | 13 (31.7) | 10 (24.4) |
| Gal-3− | 27 (77.2) | 6 (17.1) | 2 (5.7) |
We detected Gal-3 expression in renal tumor cell lines. As shown by Figure 1(e), Gal-3 mRNA level was highly expressed in renal tumor cell lines (Caki1, Caki2, 786-0, and ACHN cells). By contrast, Gal-3 was not expressed or expressed at a low level in normal renal cell line. Western blotting was further used to confirm the protein level of Gal-3 in those cell lines (Figure 1(f)). Consistent with mRNA expression, Gal-3 level was significantly higher in renal tumor cell lines than that in normal renal cells. Considering the high expression of Gal-3 in RCC or poor differentiation of RCC, we wondered whether Gal-3 was a potential therapeutic target for RCC. Thus, Caki1 cell line was chosen for the subsequent experiments.
Gal-3 knockdown inhibited cell proliferation and migration of renal carcinoma cells
We next designed different shRNAs to knock down Gal-3 expression. As shown in Figure 2(a, b), all of the three Gal-3 shRNA (Gal11 shRNA, Gal12 shRNA, and Gal13 shRNA) could significantly decrease Gal-3 expression at both of mRNA and protein levels. Moreover, Gal311 and Gal313 shRNA had the optimal knockdown effects, which were hence chosen to study their biological activity.
Figure 2.
Gal-3 knockdown inhibits cell proliferation of Caki-1 cells. (a) mRNA expression of Gal-3 after Gal-3 knockdown. (b) Protein expression of Gal-3 after Gal-3 knockdown. (c) Cell proliferation in different groups. **p < 0.01 compared with Caki-1 NC group
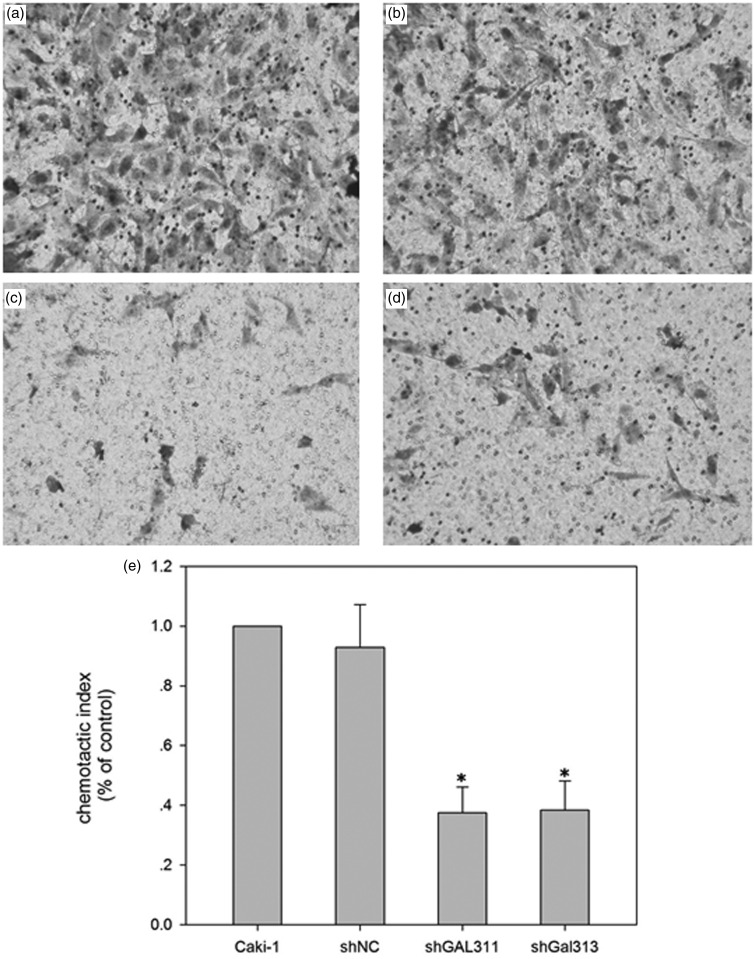
The proliferation of Caki-1 was measured after Gal-3 knockdown. As shown in Figure 2(c), the proliferation rate was significantly decreased by Gal311 and Gal313 shRNA. We also detected the cell migration after Gal-3 knockdown. As shown in Figure 3, Gal-3 knockdown by Gal311 and Gal313 significantly decreased cell migration compared with scrambled control.
Figure 3.
Gal-3 knockdown inhibits cell migration of Caki-1 cells. (a) Caki-1; (b) Caki-1 with NC shRNA; (c) Caki-1-GAL311; (d) Caki-1-GAL313; (e) Quantification of the migration. *p < 0.05 compared with Caki-1 NC group
Gal-3 knockdown-induced apoptosis of renal carcinoma cells
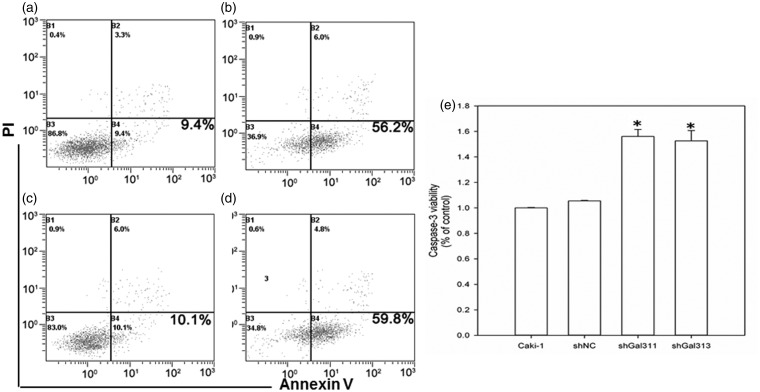
We also detected apoptosis after Gal-3 knockdown. As shown in Figure 4, scrambled shRNA did not affect apoptosis in Caki-1 cells. However, Gal-3 knockdown by Gal311 and Gal313 significantly increased apoptosis. The apoptotic rate in control, scrambled shRNA, Gal311, and Gal313 groups were 9.4%, 10.1%, 56.2%, and 59.8%, respectively. Moreover, we found that Caspase-3 activity was significantly up-regulated by Gal-3 knockdown (Figure 4). These data suggested that Gal-3 knockdown induced a Caspase-3 dependent apoptosis.
Figure 4.
Gal-3 knockdown elicits apoptosis of Caki-1 cells. (a) Caki-1; (b) Caki-1 with NC shRNA; (c) Caki-1-GAL311; (d) Caki-1-GAL313; (e) Caspase 3 activity after Gal-3 knockdown. **p < 0.01 compared with Caki-1 NC group
Gal-3 knockdown arrested cell cycle at G1 phase in renal carcinoma cells
We detected cell cycle distribution after Gal-3 knockdown. As shown in Figure 5, the G1 phase cells in control and scrambled groups were 42.5% and 48.6%, respectively. By contrast, G1 phase cells in Gal-3 knockdown groups were 55% (Gal311) and 65.8% (Gal313). These data suggested that Gal-3 knockdown could arrest the cells at G1 phase.
Figure 5.
Gal-3 knockdown arrests cell cycle at G1 phase. (a) Caki-1; (b) Caki-1 with NC shRNA; (c) Caki-1-GAL311; (d) Caki-1-GAL313; (e) Quantification data of cell numbers at G1 phase. *p < 0.05 compared with Caki-1 NC group
Gal-3 knockdown decreased Cyclin D1, but increased p27 expression
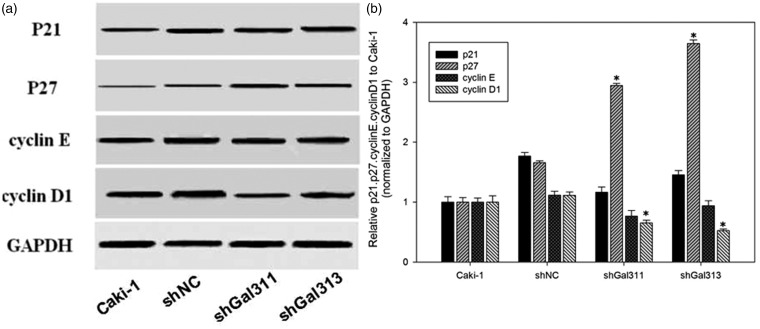
We identified the reasons for cell cycle arrest by Gal-3 knockdown. As shown in Figure 6, G1 phase regulation proteins p21, p27, cyclin D, and cyclin E were detected. p27 significantly increased, but Cyclin D1 significantly decreased after Gal-3 knockdown (p < 0.05 versus control). By contrast, p21 and Cyclin E were not affected by Gal-3 knockdown.
Figure 6.
Gal-3 knockdown promotes p27 expression, while suppresses Cyclin D1 expression. (a) Representative blots; (b) Quantification data of the protein expression. *p < 0.05 compared with Caki-1 NC group
Discussion
In this study, we depicted Gal-3 expression in RCC tissues using immunohistochemistry. Consistent with other studies,7,9,14 Gal-3 was up-regulated in RCC and tumor differentiation was negatively correlated with Gal-3 positive rate. Nevertheless, a decreased expression of Gal-3 was also found in RCC.11 This apparent discrepancy could be only caused by the methods used. In addition, Gal-3 positive expression was a critical index for evaluating the tumor cell differentiation. Moreover, we found high expression of Gal-3 in RCC cell lines at both of mRNA and protein levels.
Using the cell line model, we transferred Gal-3 shRNA to knockdown Gal-3 expression. In a previous study, Gal-3 expression was positively related to tumor cell proliferation and siRNA of Gal-3 could suppress the growth of human thyroid papillary carcinoma cells.16 Similar results were also observed in human breast cancer and human pituitary adenoma cells.17 T cells infected with human T-cell leukemia virus type I expressed high levels of Gal-3 and displayed higher growth rates than control transfectants.18 In our study, we used shRNA to knockdown Gal-3 expression to a level of 10% of normal group. Under this condition, it is beneficial to evaluate the biological activity of Gal-3 deficiency in RCC. With Gal-3 knockdown, cell proliferation was inhibited, cell migration was decreased, and apoptosis was increased. Significantly, cell cycle of RCC was also arrested at G1 phase.
Consistent with our study, Li et al.19 concluded that Gal-3 was an important mediator for conditioned media of adipose-derived MSCs stimulated the proliferation of human LoVo colorectal-cancer cells. One question is still left open that how Gal-3 facilitates tumor cell proliferation. In fact, a lot of studies verified carcinogenesis of Gal-3. For example, high expression of Gal-3 promoted cell growth by activating the mitogen-activated protein kinase pathway.19 Gal-3 can form multimers with other galectins in the extracellular milieu, which in turn cross-link glycoconjugates on the cell surface to generate galectin-glycan complexes that modulate intracellular signaling pathways and regulate cellular processes such as apoptosis, proliferation, migration, and angiogenesis.20 In addition, nucleocytoplasmic shuttling of Gal-3 also regulates tumor cell proliferation or apoptosis.21
Cyclin D1 is a critical inducer of cell cycle and supposed as a potential oncogene in human cancer.22 As a nucleocytoplasmic shuttle molecule, abnormal expression of Gal-3 could promote Cyclin D1 expression in RCC.23 By contrast, p27 is an inhibitor of cyclin-dependent kinase involved in the regulation of the cell cycle. Low expression of p27 implicates a poor prognosis of different cancer.24,25 In this study, we also disclosed that Gal-3 knockdown decreased Cyclin D1 and promoted p27 expressions.
Besides the function of Gal-3 in regulating cell proliferation, invasive ability was also regulated by Gal-3 expression. In squamous lung carcinoma cells, overexpression of Gal-3 increased cell migration, adhesion ability with cellular matrix and invasive ability.26 Down-regulation of Gal-3 expression decreased cell migration in rectal cancer cell line (LSLIM6 and HM7).27 In our study, Gal-3 knockdown is beneficial for decreased invasive ability. In the normal kidney tissue, Gal-3 was mainly expressed in cytoplasmic of epithelial cells of the distal tubule and collecting duct, but not in proximal tubule, which was consistent with previous publications.10,28 Although the reason for the expression discrepancy in proximal tubule with distal tubule is not known, there were some similar discussions in previous publication.9 As suggested, Gal-3 serves as a sorting receptor of endosomal organelles and recruits newly synthesized non-raft associated glycoproteins into transport vesicles destined for the apical cell surface. It is important for the maintenance of apical surface transport and epithelial cell polarity. In proximal tubules, Gal-3 would be replaced by other galectins.29 While in tumor tissue from RCC patients, Gal-3 expression was widely expressed, including membrane. As we known, Gal-3 has a high affinity with polysaccharide. The membrane distribution of Gal-3 would facilitate the binding of tumor cells to other normal cells with mucin, fibrin, and Mac-2 binding protein.30 The direct binding would facilitate the tumor migration or invasion. As reported previously, FAK/Src/Lyn activation and β-catenin expression stimulated by Gal-3 knockdown also contribute to osteosarcoma cell migration and invasion.31 RhoA and Myosin light-chain kinase activation were reported to regulate the effect of Gal-3 in hepatocellular carcinoma.32
Gal-3 also plays important roles in apoptosis.33 Anti-apoptotic and/or pro-apoptotic factor in various cell types were reported previously.34 However, as an inducing target for apoptosis in renal cells, Gal-3 still deserves deeply study. We used a genetic method to knockdown Gal-3 expression and displayed the antitumor effects of Gal-3 inhibition in RCC. Nevertheless, an effective compound which can directly inhibit Gal-3 is still lack. In the future study, the available inhibitor of galectin-3 will be screened and animal models with RCC will be also used to confirm the antitumor effect of Gal-3 inhibition.
Conclusion
Although Gal-3 expression was reported in RCC, the potential therapeutic target of Gal-3 was not verified. In this study, we not only confirmed the high expression of Gal-3 but also verified that Gal-3 knockdown inhibited cell growth, induced apoptosis, and suppressed invasive ability. Moreover, Gal-3 knockdown arrested cell cycle to G1 phase through promoting p27 and suppressing Cyclin D1 expressions. Gal-3 could serve as a potential therapeutic target for RCC.
Acknowledgements
This study was supported by grants from Education Department of Heilongjiang Province (12531367, 12511345). We are grateful to Prof. Xiaoming Jin (Department of Pathology, Harbin Medical University) for the kind help with the experiments.
Author contributions
YX, CL and XG conducted most of the experiments and analyzed the data; JS helped to collect the tumor sample; JL did some of the experiments; XG, WX designed the study and wrote the manuscript. YX and CL contributed equally to this study.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Tan HJ, Filson CP, Litwin MS. Contemporary, age-based trends in the incidence and management of patients with early-stage kidney cancer. Urol Oncol 2015; 33: 21.e19–e26. [DOI] [PubMed] [Google Scholar]
- 2.van der Veldt AA, Meijerink MR, van den Eertwegh AJ, Boven E. Targeted therapies in renal cell cancer: recent developments in imaging. Targeted Oncol 2010; 5: 95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thijssen VL, Heusschen R, Caers J, Griffioen AW. Galectin expression in cancer diagnosis and prognosis: a systematic review. Biochim Biophys Acta 2015; 1855: 235–247. [DOI] [PubMed] [Google Scholar]
- 4.French B, Wang L, Ky B, Brandimarto J, Basuray A, Fang JC, Sweitzer NK, Cappola TP. Prognostic value of galectin-3 for adverse outcomes in chronic heart failure. J Card Fail 2015. doi: 10.1016/j.cardfail.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Martinez E, Calvier L, Fernandez-Celis A, Rousseau E, Jurado-Lopez R, Rossoni LV, Jaisser F, Zannad F, Rossignol P, Cachofeiro V, López-Andrés N. Galectin-3 blockade inhibits cardiac inflammation and fibrosis in experimental hyperaldosteronism and hypertension. Hypertension 2015; 66: 767–775. [DOI] [PubMed] [Google Scholar]
- 6.Newlaczyl AU, Yu LG. Galectin-3—a jack-of-all-trades in cancer. Cancer Lett 2011; 313: 123–128. [DOI] [PubMed] [Google Scholar]
- 7.von Klot CA, Kramer MW, Peters I, Hennenlotter J, Abbas M, Scherer R, Herrmann TR, Stenzl A, Kuczyk MA, Serth J, Merseburger AS. Galectin-1 and Galectin-3 mRNA expression in renal cell carcinoma. BMC Clin Pathol 2014; 14: 15–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaneko N, Gotoh A, Okamura N, Matsuo E, Terao S, Watanabe M, Yamada Y, Hamami G, Nakamura T, Ikekita M, Okumura K, Nishimura O. Potential tumor markers of renal cell carcinoma: alpha-enolase for postoperative follow up, and galectin-1 and galectin-3 for primary detection. Int J Urol 2013; 20: 530–535. [DOI] [PubMed] [Google Scholar]
- 9.Straube T, Elli AF, Greb C, Hegele A, Elsässer HP, Delacour D, Jacob R. Changes in the expression and subcellular distribution of galectin-3 in clear cell renal cell carcinoma. J Exp Clin Cancer Res 2011; 30: 89–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.François C, van Velthoven R, De Lathouwer O, Moreno C, Peltier A, Kaltner H, Salmon I, Gabius HJ, Danguy A, Decaestecker C, Kiss R. Galectin-1 and galectin-3 binding pattern expression in renal cell carcinomas. Am J Clin Pathol 1999; 112: 194–203. [DOI] [PubMed] [Google Scholar]
- 11.Merseburger AS, Kramer MW, Hennenlotter J, Serth J, Kruck S, Gracia A, Stenzl A, Kuczyk MA. Loss of galectin-3 expression correlates with clear cell renal carcinoma progression and reduced survival. World J Urol 2008; 26: 637–642. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y, Gu X, Gong M, Guo G, Han K, An R. Galectin-3 inhibition sensitizes human renal cell carcinoma cells to arsenic trioxide treatment. Cancer Biol Ther 2013; 14: 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dancer JY, Truong LD, Zhai Q, Shen SS. Expression of Galectin-3 in renal neoplasms: a diagnostic, possible prognostic marker. Arch Pathol Lab Med 2010; 134: 90–94. [DOI] [PubMed] [Google Scholar]
- 14.Sakaki M, Fukumori T, Fukawa T, Elsamman E, Shiirevnyamba A, Nakatsuji H, Kanayama HO. Clinical significance of Galectin-3 in clear cell renal cell carcinoma. J Med Invest 2010; 57: 152–157. [DOI] [PubMed] [Google Scholar]
- 15.Zhu G, Wang X, Wu S, Li Q. Involvement of activation of PI3K/Akt pathway in the protective effects of puerarin against MPP+-induced human neuroblastoma SH-SY5Y cell death. Neurochem Int 2012; 60: 400–408. [DOI] [PubMed] [Google Scholar]
- 16.Yoshii T, Inohara H, Takenaka Y, Honjo Y, Akahani S, Nomura T, Raz A, Kubo T. Galectin-3 maintains the transformed phenotype of thyroid papillary carcinoma cells. Int J Oncol 2001; 18: 787–792. [DOI] [PubMed] [Google Scholar]
- 17.Huang CX, Zhao JN, Zou WH, Li JJ, Wang PC, Liu CH, Wang YB. Reduction of galectin-3 expression reduces pituitary tumor cell progression. Genet Mol Res 2014; 13: 6892–6898. [DOI] [PubMed] [Google Scholar]
- 18.Hsu DK, Hammes SR, Kuwabara I, Greene WC, Liu FT. Human T lymphotropic virus-I infection of human T lymphocytes induces expression of the beta-galactoside-binding lectin, galectin-3. Am J Pathol 1996; 148: 1661–1670. [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Xu X, Wang L, Liu G, Li Y, Wu X, Jing Y, Li H, Wang G. Senescent mesenchymal stem cells promote colorectal cancer cells growth via galectin-3 expression. Cell Biosci 2015; 5: 21–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elola MT, Blidner AG, Ferragut F, Bracalente C, Rabinovich GA. Assembly, organization and regulation of cell-surface receptors by lectin-glycan complexes. Biochem J 2015; 469: 1–16. [DOI] [PubMed] [Google Scholar]
- 21.Arnoys EJ, Ackerman CM, Wang JL. Nucleocytoplasmic shuttling of galectin-3. Methods Mol Biol 2015; 1207: 465–483. [DOI] [PubMed] [Google Scholar]
- 22.Shan J, Zhao W, Gu W. Suppression of cancer cell growth by promoting cyclin D1 degradation. Mol Cell 2009; 36: 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin HM, Pestell RG, Raz A, Kim HR. Galectin-3 enhances cyclin D(1) promoter activity through SP1 and a cAMP-responsive element in human breast epithelial cells. Oncogene 2002; 21: 8001–8010. [DOI] [PubMed] [Google Scholar]
- 24.Kirla RM, Haapasalo HK, Kalimo H, Salminen EK. Low expression of p27 indicates a poor prognosis in patients with high-grade astrocytomas. Cancer 2003; 97: 644–648. [DOI] [PubMed] [Google Scholar]
- 25.Moller MB. P27 in cell cycle control and cancer. Leuk Lymphoma 2000; 39: 19–27. [DOI] [PubMed] [Google Scholar]
- 26.O'Driscoll L, Linehan R, Liang YH, Joyce H, Oglesby I, Clynes M. Galectin-3 expression alters adhesion, motility and invasion in a lung cell line (DLKP), in vitro. Anticancer Res 2002; 22: 3117–3125. [PubMed] [Google Scholar]
- 27.Bresalier RS, Mazurek N, Sternberg LR, Byrd JC, Yunker CK, Nangia-Makker P, Raz A. Metastasis of human colon cancer is altered by modifying expression of the beta-galactoside-binding protein galectin 3. Gastroenterology 1998; 115: 287–296. [DOI] [PubMed] [Google Scholar]
- 28.Nishiyama J, Kobayashi S, Ishida A, Nakabayashi I, Tajima O, Miura S, Katayama M, Nogami H. Up-regulation of galectin-3 in acute renal failure of the rat. Am J Pathol 2000; 157: 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delacour D, Cramm-Behrens CI, Drobecq H, Le Bivic A, Naim HY, Jacob R. Requirement for galectin-3 in apical protein sorting. Curr Biol 2006; 16: 408–414. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Q, Duckworth CA, Wang W, Guo X, Barrow H, Pritchard DM, Rhodes JM, Yu LG. Peanut agglutinin appearance in the blood circulation after peanut ingestion mimics the action of endogenous galectin-3 to promote metastasis by interaction with cancer-associated MUC1. Carcinogenesis 2014; 35: 2815–2821. [DOI] [PubMed] [Google Scholar]
- 31.Park GB, Kim DJ, Kim YS, Lee HK, Kim CW, Hur DY. Silencing of galectin-3 represses osteosarcoma cell migration and invasion through inhibition of FAK/Src/Lyn activation and beta-catenin expression and increases susceptibility to chemotherapeutic agents. Int J Oncol 2015; 46: 185–194. [DOI] [PubMed] [Google Scholar]
- 32.Serizawa N, Tian J, Fukada H, Baghy K, Scott F, Chen X, Kiss Z, Olson K, Hsu D, Liu FT, Török NJ, Zhao B, Jiang JX. Galectin 3 regulates HCC cell invasion by RhoA and MLCK activation. Lab Invest 2015; 95: 1145–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakahara S, Oka N, Raz A. On the role of galectin-3 in cancer apoptosis. Apoptosis 2005; 10: 267–275. [DOI] [PubMed] [Google Scholar]
- 34.Harazono Y, Kho DH, Balan V, Nakajima K, Zhang T, Hogan V, Raz A. Galectin-3 leads to attenuation of apoptosis through Bax heterodimerization in human thyroid carcinoma cells. Oncotarget 2014; 5: 9992–10001. [DOI] [PMC free article] [PubMed] [Google Scholar]