Abstract
The traditional endpoint for assessing efficacy of chemotherapies for advanced/recurrent gastric cancer is overall survival (OS), but OS requires prolonged follow-up. We investigated whether progression-free survival (PFS) is a valid surrogate for OS. Using individual patient data from the GASTRIC meta-analysis, surrogacy of PFS was assessed through the correlation between the endpoints and through the correlation between the treatment effects on the endpoints. External validation of the prediction based on PFS was also evaluated. Individual data from 4069 patients in 20 randomized trials were analyzed. The rank correlation coefficient between PFS and OS was 0.853 (95% confidence interval [CI] = 0.852 to 0.854). The R 2 between treatment effects on PFS and on OS was 0.61 (95% CI = 0.04 to 1.00). Treatment effects on PFS and on OS were only moderately correlated, and we could not confirm the validity of PFS as a surrogate endpoint for OS in advanced/recurrent gastric cancer.
The prognosis of patients with advanced or recurrent gastric cancer (AGC) remains poor, with a 1-year median overall survival (OS) for commonly used chemotherapy regimens, consisting of fluoropyrimidine, platinum, taxane or anthracyclines agents (1). The most important issue in the development of agents for AGC is their ability to prolong OS with acceptable toxicity. Even though median postprogression survival ranges from 5 to 10 months, a validated shorter-term surrogate endpoint would likely reduce drug development costs, sample sizes, or the duration of trials aimed at establishing the benefit of new drugs. Progression-free survival (PFS) is commonly used in phase II and phase III trials. It has been evaluated as a surrogate endpoint for OS in several types of cancers (2–4). The ability to predict clinical benefits on OS from earlier benefits on PFS could be useful at all stages of clinical development. Here, we investigate the surrogacy of PFS for OS within the framework of the GASTRIC meta-analysis (5).
Trials were eligible if they were randomized, closed to accrual before the end of 2006, and collected individual patient data on PFS. To explore the correlation between the treatment effects at the trial level, we relied on the comparison between the experimental arms of the trials included in the meta-analysis with their corresponding control arms. We defined as experimental the treatment that contained the larger number of drugs (eg, triple combinations vs double combinations). In case of equal number of drugs, we defined as experimental the treatment that included the newer agent. When two experimental arms were tested in the same trial, we combined their data for the purposes of the analyses. All data were centrally checked for inconsistencies (6).
We used a meta-analytic validation approach (3,4,7). OS was defined as the time from randomization to death from any cause or to the last follow-up. PFS was the time to tumor progression or death from any cause or time to the last follow-up assessment. A detailed description of statistical methods used is provided in the Supplementary Material (available online). For external validation, we applied the identified relation to predict the hazard ratio (HR) for OS (HROS) from the hazard ratio for PFS (HRPFS) in randomized trials published since 2000 for which we had not obtained the individual patient data. We extracted the summary statistics for both endpoints (8) and compared the predicted value of HROS to the one reported in the articles. To determine whether surrogacy also applied to other classes of agents, we extended the validation to three published trials of targeted agents (9–11).
Individual data were obtained on 4069 patients from 20 eligible randomized trials (12–30). The characteristics of the trials have been described elsewhere (5). Thirteen trials defined the progression using radiological criteria, whereas seven used both clinical and radiological assessments. Overall and at the trial level, the treatment effect on PFS (HR = 0.79; 95% confidence interval [CI] = 0.74 to 0.85) tended to be larger than on OS (HR = 0.85; 95% CI = 0.79 to 0.92) as shown on the forest plot of Supplementary Figures 1 and 2 (available online).
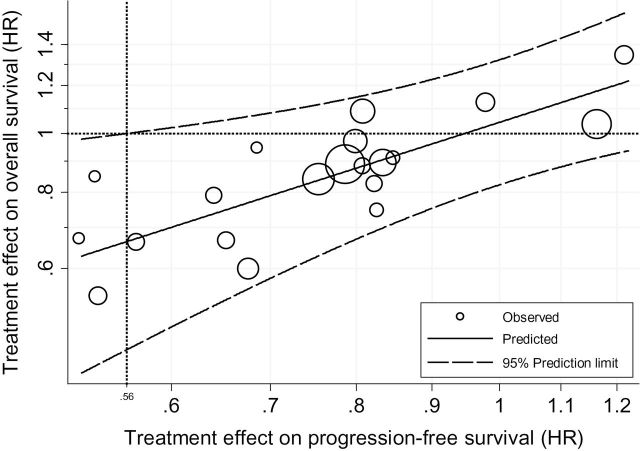
The individual-level association, as measured by the rank correlation coefficient, was 0.853 (95% CI = 0.852 to 0.854), indicating substantial correlation between PFS and OS for a given patient. The association at the trial level between log HROS and log HRPFS was only moderate, with a coefficient of determination, R 2, adjusted for the estimation errors (31), of 0.61 (95% CI = 0.04 to 1.00). The large confidence interval reflects the uncertainty around this estimate. The linear regression model that relates the treatment effect on PFS and on OS adjusted for estimations errors was
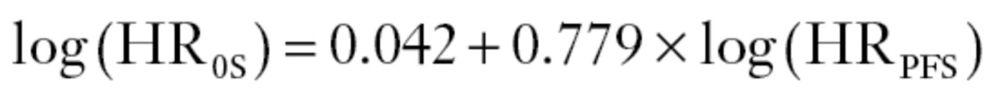 |
where the standard errors of the intercept and the slope were 0.79 and 0.295, respectively. This is shown as a straight line in Figure 1. The 95% prediction limits indicate the range of effect on OS that can be expected for a given effect on PFS. The moderate predictive accuracy at the trial level is reflected by the large interval width and a surrogate threshold effect of 0.56; hence, one should observe an HRPFS less than 0.56 to predict, with 95% probability, an HROS less than 1.
Figure 1.
Trial-level association between treatment effects. Log scale was used for the x and y axes; the horizontal line (circles) corresponds to the hazard ratio (HR) on overall survival of 1, which indicates the absence of effect on the overall survival. At the crossing point, the vertical line corresponds to the minimum amount of effect on PFS that will predict a hazard ratio on OS below 1 with 95% probability. This indicates the surrogate threshold effect.
Validation on independent literature data (9–11,32–39) is shown in Table 1 and Supplementary Figure 3 (available online). The larger the number of progressions, the more precise the prediction; however, precision is limited by the variability of the regression line. The observed HROS fell within the prediction interval in all trials, even in trials using humanized monoclonal antibodies [Trastuzumab (10), bevaxizumab (9), matuzumab (11)]. However, in the trial that concluded a statistically significant benefit of trastuzumab on OS (10), the effect on PFS was smaller than the surrogate threshold effect and therefore could not have been used to predict a statistically significant effect on OS.
Table 1.
Observed and predicted treatment effect on overall survival, based on the observed treatment effect on progression-free survival*
| Trial label | Trial | Observed HRPFS (95% CI) | Observed HROS (95% CI) | Predicted HROS (95% CI) |
|---|---|---|---|---|
| A | Jeung et al. (36) | 0.63 (0.38 to 1.05) | 0.56 (0.35 to 0.88) | 0.73 (0.46 to 1.04) |
| B | AIO (33) | 0.67 (0.43 to 1.04) | 0.82 (0.47,1.45) | 0.76 (0.53 to 1.07) |
| C | ToGA (10) | 0.71 (0.59 to 0.85) | 0.74 (0.60 to 0.91) | 0.80 (0.58 to 1.09) |
| D | AVAGAST (9) | 0.80 (0.68 to 0.93) | 0.87 (0.73 to 1.03) | 0.88 (0.76 to 1.14) |
| E | Kang et al. (35) | 0.80 (0.63 to 1.03) | 0.85 (0.64 to 1.13) | 0.88 (0.76 to 1.14) |
| F | Park et al. (38) | 0.86 (0.54 to 1.37) | 0.96 (0.60 to 1.52) | 0.93 (0.71 to 1.18) |
| G | REAL (a)† (34) | 0.92 (0.80 to 1.04) | 0.92 (0.80 to 1.10) | 0.98 (0.77 to 1.22) |
| H | REAL (b) (34) | 0.92 (0.81 to 1.05) | 0.86 (0.80 to 0.99) | 0.98 (0.77 to 1.22) |
| I | Ross et al. (39) | 0.95 (0.80 to 1.08) | 0.91 (0.76 to 1.04) | 1.00 (0.79 to 1.29) |
| J | FLAGS (32) | 0.99 (0.86 to 1.14) | 0.92 (0.80 to 1.05) | 1.03 (0.81 to 1.31) |
| K | Rao et al. (11) | 1.13 (0.63 to 2.01) | 1.02 (0.61 to 1.70) | 1.14 (0.89 to 1.46) |
| L | Moehler et al. (37) | 1.14 (0.59 to 2.21) | 0.77 (0.51 to 1.17) | 1.15 (0.90 to 1.48) |
* HR = hazard ratio; PFS = progression-free survival; CI = confidence interval; OS = overall survival.
† This trial was designed as a factorial 2×2 plan to test two comparisons: a platinum comparison (a) and a fluoropyrimidine comparison (b).
This is the first study based on individual patient data to evaluate whether PFS is a reasonable surrogate endpoint to use for randomized trials in AGC. Our results show a high correlation of PFS and OS in individual patients but only a modest correlation (R 2 = 0.61) between treatment effects on PFS and OS. It is lower than that found in trials of 5-fluorouracil–based therapies for advanced colorectal cancer (4). The correlation was also lower than in the adjuvant setting (40).
Possible limitations that may explain the moderate correlation observed in our analysis include the numerous processes involved in the progression of stomach cancer (eg, local or distant metastasis, peritoneum involvement), the use of clinical and radiological assessments for progression, and the impact of our definition of investigational treatment related to the heterogeneity in chemotherapies considered here; variability in the investigated treatments and in the effects of the treatments is a condition to generalize any results to future trials. Last, patients included in more recent trials received second-line treatments, including crossover (30), which may have diluted the effect of first-line treatment on OS (2). Because not all trials reported the same information at baseline, we could not assess the surrogacy in clinically relevant subset analyses.
All in all, we would not conclude that PFS is an adequate surrogate for OS in AGC. No precise prediction of the effect of a treatment on OS can be reliably drawn from the effect estimated on PFS.
Funding
This work was partially supported by the French Institut National du Cancer (grant PHRC GASTRIC); the Clinical Research Support Unit; and the Epidemiological and Clinical Research Information Network. A meeting was supported financially by unrestricted grants from GlaxoSmithKline. The funders were not present at the meeting, were not involved in the analyses of the data, and did not comment on the present paper.
Supplementary Material
X. Paoletti and K. Oba as well as T. Burzykovski and M. Buyse contributed equally to this work T. Burzykowski and M. Buyse contributed equally to this work. A first draft of this manuscript was developed at a meeting of investigators in Sapporo, Japan, September 24–28, 2010. The meeting was supported financially by unrestricted grants from GlaxoSmithKline.
The GASTRIC Investigators: Secretariat: Marc Buyse, Stefan Michiels, Kenichi Nakamura, Koji Oba, Xavier Paoletti, Philippe Rougier, and Seiichiro Yamamoto. Steering Committee: Yung-Jue Bang (Seoul National University College of Medicine, Seoul, Korea); Harry Bleiberg (Jules Bordet Hospital, Brussels, Belgium); Tomasz Burzykowski (Hasselt University, Diepenbeek, Belgium); Marc Buyse (International Drug Development Institute, Louvain-la-Neuve, Belgium);Catherine Delbaldo (Hôpital Louis Mourier, Colombes, France); Stefan Michiels (Institut Gustave Roussy, Université Paris XI, Villejuif, France); Satoshi Morita (Yokohama City University, Kanagawa, Japan); Koji Oba (Hokkaido University Hospital, Hokkaido, Japan); Yasuo Ohashi (University of Tokyo, Tokyo, Japan); Xavier Paoletti (Institut Curie, Paris, France); Jean-Pierre Pignon (Institut Gustave-Roussy, Villejuif, France); Philippe Rougier (University Hospital Europeen Georges Pompidou, Paris, France); Junichi Sakamoto (Tokai Central Hospital, Sohara, Japan); Daniel Sargent (Mayo Clinic, Rochester, MN); Mitsuru Sasako (Hyogo College of Medicine, Hyogo, Japan); and Eric Van Cutsem (Digestive Oncology Unit, University Hospital Gasthuisberf, Leuven, Belgium).Collaborators: J. Ajani, N. Boku, O. Bouche, J. Buckner, C. Coombes, S. Cullinan, M. Dank, N. Fuse, B. Glimelius, R. Hawkins, W. Koizumi, M. Moehler, Y. Nio, A. Ohtsu, A. Roth, K. Shitara, P. Thuss-Patience, A. Tsuburaya, E. Van Cutsem, U. Vanhoefer, J. Wils, and Y. Yamamura. Writing committee: Xavier Paoletti, Koji Oba, Tomasz Burzykowski, Yung-Jue Bang, Harry Bleiberg, Narikazu Boku, Olivier Bouché, Paul Catalano, Nozomu Fuse, Stefan Michiels, Markus Moehler, Satoshi Morita, Yasuo Ohashi, Atsushi Ohtsu, Arnaud Roth, Philippe Rougier, Junichi Sakamoto, Daniel Sargent, Mitsuru Sasako, Kohei Shitara, Peter Thuss-Patience, Eric van Cutsem, and Marc Buyse.
This project was initiated under the auspice of the French Institut National du Cancer, who served as a sponsor. The French Institut National du Cancer did not participate in the design of the study. It participated in the conduct of the study at an earlier stage by centralizing all the databases and by providing administrative and data management support. The sponsor had no role in the preparation, review, or approval of the manuscript.
The GASTRIC Group thanks all patients who took part in the trials and contributed to this research. The meta-analysis would not have been possible without their participation or without active participation of the collaborating institutions that provided their trial data (East Central Oncology Group; European Organization of Research and Treatment of Cancer; Fédération Francophone de Cancérologie Digestive; Italian Trials in Medical Oncology; Japanese Cooperative Oncology Group; North Central Cancer Treatment Group; South West Oncology Group; the V325 Study Group; the Swiss Group for Clinical Cancer Research; the Kyoto Research Group for Chemotherapy of Gastric Cancer, the Medizinische Klinik mit Schwerpunkt Haëmatologie und Onkologie, Charité, Universitatsmedizin Berlin; the Center for Caring Sciences, University of Uppsala Sweden. We thank Nicolas Thammavong for the data management.
References
- 1. Oba K, Paoletti X, Bang YJ, et al. Role of chemotherapy for advanced/recurrent gastric cancer: an individual-patient-data meta-analysis. Eur J Cancer. 2013;49(7):1565–1577. [DOI] [PubMed] [Google Scholar]
- 2. Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101(23):1642–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burzykowski T, Buyse M, Piccart-Gebhart MJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2008;26(12):1987–1992. [DOI] [PubMed] [Google Scholar]
- 4. Buyse M, Burzykowski T, Carroll K, et al. Progression-free survival is a surrogate for survival in advanced colorectal cancer. J Clin Oncol. 2007;25(33):5218–5224. [DOI] [PubMed] [Google Scholar]
- 5. GASTRIC. Role of chemotherapy for advanced/recurrent gastric cancer: an individual-patient-data meta-analysis. Eur J Cancer. 2013; 49(7):1565–1577 [DOI] [PubMed] [Google Scholar]
- 6. Stewart LA, Clarke MJ. Practical methodology of meta-analyses (overviews) using updated individual patient data. Cochrane Working Group. Stat Med. 1995;14(19):2057–2079. [DOI] [PubMed] [Google Scholar]
- 7. Burzykowski T, Molenberghs G, Buyse M, Geys H. Validation of surrogate endpoints in multiple randomized clinical trials with failure time endpoints. J R Stat Soc Series C Appl Stat. 2001; 50(4): 405–422. [Google Scholar]
- 8. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–2834. [DOI] [PubMed] [Google Scholar]
- 9. Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol.; 2011; 29(30):3968–3976. [DOI] [PubMed] [Google Scholar]
- 10. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. [DOI] [PubMed] [Google Scholar]
- 11. Rao S, Starling N, Cunningham D, et al. Matuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first-line treatment in patients with advanced oesophago-gastric cancer: a randomised, multicentre open-label phase II study. Ann Oncol.; 2010; 21(11):2213–2219. [DOI] [PubMed] [Google Scholar]
- 12. Douglass HO, Jr, Lavin PT, Goudsmit A, Klaassen DJ, Paul AR. An Eastern Cooperative Oncology Group evaluation of combinations of methyl-CCNU, mitomycin C, adriamycin, and 5-fluorouracil in advanced measurable gastric cancer (EST 2277). J Clin Oncol. 1984;2(12):1372–1381. [DOI] [PubMed] [Google Scholar]
- 13. Cullinan SA, Moertel CG, Fleming TR, et al. A comparison of three chemotherapeutic regimens in the treatment of advanced pancreatic and gastric carcinoma. Fluorouracil vs fluorouracil and doxorubicin vs fluorouracil, doxorubicin, and mitomycin. JAMA. 1985;253(14):2061–2067. [PubMed] [Google Scholar]
- 14. Wils JA, Klein HO, Wagener DJ, et al. Sequential high-dose methotrexate and fluorouracil combined with doxorubicin—a step ahead in the treatment of advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cooperative Group. J Clin Oncol. 1991;9(5):827–831. [DOI] [PubMed] [Google Scholar]
- 15. Kyoto Research Group for Chemotherapy of Gastric Aancer. A randomized, comparative study of combination chemotherapies in advanced gastric cancer: 5-fluorouracil and cisplatin (FP) versus 5-fluorouracil, cisplatin, and 4’-epirubicin (FPEPIR). Anticancer Res. 1992;12(6B):1983–1988. [PubMed] [Google Scholar]
- 16. Cullinan SA, Moertel CG, Wieand HS, et al. Controlled evaluation of three drug combination regimens versus fluorouracil alone for the therapy of advanced gastric cancer. North Central Cancer Treatment Group. J Clin Oncol. 1994;12(2):412–416. [DOI] [PubMed] [Google Scholar]
- 17. Glimelius B, Ekstrom K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8(2):163–168. [DOI] [PubMed] [Google Scholar]
- 18. Vanhoefer U, Rougier P, Wilke H, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000;18(14):2648–2657. [DOI] [PubMed] [Google Scholar]
- 19. Kim TW. A prospective randomized phase III trial of 5-fluorouracil and cusplatin (FP) versus epirubicin, cisplatin, and 5-FU (ECF) in the treatment of patients with previously untreated advanced gastric cancer (AGC). Eur J Cancer. 2001; 47(3)S314. [Google Scholar]
- 20. Ohtsu A, Shimada Y, Shirao K, et al. Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: the Japan Clinical Oncology Group Study (JCOG9205). J Clin Oncol. 2003;21(1):54–59. [DOI] [PubMed] [Google Scholar]
- 21. Koizumi W, Fukuyama Y, Fukuda T, et al. Randomized phase II study comparing mitomycin, cisplatin plus doxifluridine with cisplatin plus doxifluridine in advanced unresectable gastric cancer. Anticancer Res. 2004;24(4):2465–2470. [PubMed] [Google Scholar]
- 22. Pozzo C, Barone C, Szanto J, et al. Irinotecan in combination with 5-fluorouracil and folinic acid or with cisplatin in patients with advanced gastric or esophageal-gastric junction adenocarcinoma: results of a randomized phase II study. Ann Oncol. 2004;15(12):1773–1781. [DOI] [PubMed] [Google Scholar]
- 23. Bouche O, Ychou M, Burtin P, et al. Adjuvant chemotherapy with 5-fluorouracil and cisplatin compared with surgery alone for gastric cancer: 7-year results of the FFCD randomized phase III trial (8801). Ann Oncol. 2005;16(9):1488–1497. [DOI] [PubMed] [Google Scholar]
- 24. Ajani JA, Fodor MB, Tjulandin SA, et al. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol. 2005;23(24):5660–5667. [DOI] [PubMed] [Google Scholar]
- 25. Moehler M, Eimermacher A, Siebler J, et al. Randomised phase II evaluation of irinotecan plus high-dose 5-fluorouracil and leucovorin (ILF) vs 5-fluorouracil, leucovorin, and etoposide (ELF) in untreated metastatic gastric cancer. Br J Cancer. 2005;92(12):2122–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thuss-Patience PC, Kretzschmar A, Repp M, et al. Docetaxel and continuous-infusion fluorouracil versus epirubicin, cisplatin, and fluorouracil for advanced gastric adenocarcinoma: a randomized phase II study. J Clin Oncol. 2005;23(3):494–501. [DOI] [PubMed] [Google Scholar]
- 27. Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24(31):4991–4997. [DOI] [PubMed] [Google Scholar]
- 28. Roth AD, Fazio N, Stupp R, et al. Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2007;25(22):3217–3223. [DOI] [PubMed] [Google Scholar]
- 29. Dank M, Zaluski J, Barone C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19(8):1450–1457. [DOI] [PubMed] [Google Scholar]
- 30. Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10(11):1063–1069. [DOI] [PubMed] [Google Scholar]
- 31. Burzykowski T, Molenberghs G, Buyse M. The Evaluation of Surrogate Endpoints. New York: Springer; 2006. [Google Scholar]
- 32. Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28(9):1547–1553. [DOI] [PubMed] [Google Scholar]
- 33. Al-Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26(9):1435–1442. [DOI] [PubMed] [Google Scholar]
- 34. Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358(1):36–46. [DOI] [PubMed] [Google Scholar]
- 35. Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20(4):666–673. [DOI] [PubMed] [Google Scholar]
- 36. Jeung HC, Rha SY, Im CK, et al. A randomized phase 2 study of docetaxel and S-1 versus docetaxel and cisplatin in advanced gastric cancer with an evaluation of SPARC expression for personalized therapy. Cancer. 2011;117(10):2050–2057. [DOI] [PubMed] [Google Scholar]
- 37. Moehler MH, Siebler J, Hoehler T, Janssen J, Wein A, Menges M. CPT11/FA/5-FU versus ELF in chemonaive patients with advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction: a randomized phase II study. J Clin Oncol. 2004; 22(14S): 4064. [Google Scholar]
- 38. Park SH, Nam E, Park J, et al. Randomized phase II study of irinotecan, leucovorin and 5-fluorouracil (ILF) versus cisplatin plus ILF (PILF) combination chemotherapy for advanced gastric cancer. Ann Oncol. 2008;19(4):729–733. [DOI] [PubMed] [Google Scholar]
- 39. Ross P, Nicolson M, Cunningham D, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol. 2002;20(8):1996–2004. [DOI] [PubMed] [Google Scholar]
- 40. Burzykowski T, Bang Y. Disease-free survival as a surrogate endpoint for overall survival in an adjuvant trial of curatively resected stomach cancer using individual patient data meta-analysis. J Clin Oncol. 2009;27(15s):abstract 4517. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.