Abstract
Salmonella enterica infections continue to be a significant burden on public health worldwide. The ability of S. enterica to produce hydrogen sulfide (H2S) is an important phenotypic characteristic used to screen and identify Salmonella with selective medium; however, H2S-negative Salmonella have recently emerged. In this study, the H2S phenotype of Salmonella isolates was confirmed, and the selected isolates were subjected to antimicrobial susceptibility testing and molecular identification by multilocus sequence typing, pulsed-field gel electrophoresis, and clustered regularly interspaced short palindromic repeat (CRISPR) analysis. The phs genetic operon was also analyzed. A total of 160 S. enterica serovar Aberdeen isolates were detected between 2005 and 2013 in China. Of them, seven non-H2S-producing isolates were detected. Notably, four samples yielded four pairs of isolates with different H2S phenotypes, simultaneously. The data demonstrated that H2S-negative isolates were genetically closely related to H2S-positive isolates. Three new spacers (Abe1, Abe2, and Abe3) were identified in CRISPR locus 1 in four pairs of isolates with different H2S phenotypes from the same samples. Sequence analysis revealed a new nonsense mutation at position 208 in the phsA gene of all non-H2S-producing isolates. Additionally, we describe a new screening procedure to avoid H2S-negative Salmonella, which would normally be overlooked during laboratory and hospital screening. The prevalence of this pathogen may be underestimated; therefore, it is important to focus on improving surveillance of this organism to control its spread.
Introduction
Salmonella enterica is a common food-borne zoonotic pathogen found worldwide [1]. All non-typhoidal Salmonella (over 2,700 serovars) are considered human pathogens, with an estimated 93.8 million cases of infection, and up to 150,000 deaths a year annually in humans [2]. A substantial number of human diseases related to non-typhoidal Salmonella occur in developed countries [3, 4]. In China, an estimated 9.03 million cases of S. enterica infection are reported annually, and outbreaks are common [5]. In recent years, S. enterica serotype Aberdeen (S. Aberdeen) has been detected in Shanghai and Nanjing using our Salmonella surveillance system. This pathogen has a wide host range, infecting cattle and swine, however, only a few cases have been reported over the last 50 years [6–8]. S. Aberdeen virulence is inferior to that of other serotypes, which often cause sporadic disease [9]. S. Aberdeen mainly infects individuals with weakened immune systems, such as infants and young children, and causes abdominal pain and diarrhea as the primary clinical manifestations.
The ability to produce hydrogen sulfide (H2S) is an important phenotypic characteristic used for the screening and identification of Salmonella with selective medium, including deoxycholate hydrogen sulfide lactose (DHL), Salmonella-Shigella (SS), and triple sugar iron (TSI) agar [10]. However, a range of H2S-negative Salmonella serotypes, including S. Typhimurium, S. Infantis, S. Kentucky, S. Senftenberg, S. Enteritidis, S. Heidelberg, and S. Derby, have emerged recently in Japan, Kuwait, China, and Hong Kong [10–13]. Using our laboratory Salmonella surveillance system, 160 (7.3%) S. Aberdeen isolates were detected among 2,179 Salmonella isolates between 2005 and 2013 in China. Among these 160 isolates, seven (4.4%) were identified as non-H2S-producing S. Aberdeen. To the best of our knowledge, this is the first report of the identification of H2S-negative S. Aberdeen.
Therefore, in this report, we investigated genetic variations among non-H2S-producing and selected H2S-producing S. Aberdeen isolates by multilocus sequence typing (MLST), pulsed-field gel electrophoresis (PFGE), and clustered regularly interspaced short palindromic repeat (CRISPR) analysis [14–18], with the aim to clarify the molecular basis of the inability of these isolates to produce H2S.
Materials and Methods
Ethics statement
During our routine surveillance of Salmonella, fecal samples from individual outpatients with diarrhea were collected and screened in sentinel hospitals based on a national pathogen monitoring system. The study was approved and authorized by the institutional ethics committees of the Academy of Military Medical Sciences of the Chinese People’s Liberation Army (Beijing, China). The institutional review board of the Academy of Military Medical Sciences waived the requirement for written informed consent from the participants.
Isolates
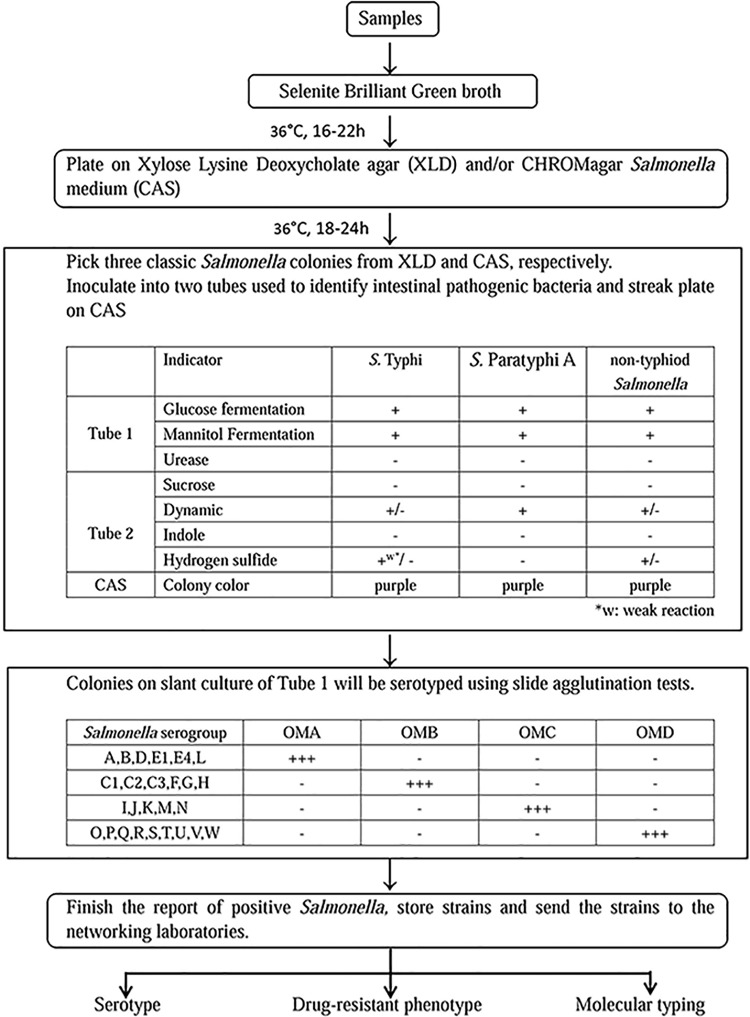
A surveillance system of infectious diseases has been established in our laboratory. As the center monitoring laboratory in this system, we are responsible for collecting samples from network laboratories and sentinel hospitals. In our study, the vegetable, aquatic product, and swine manure were collected from markets of agricultural products from different districts in Shanghai, and water samples were collected from the Huangpu River. The samples were analyzed as described below. Our Salmonella detection procedure involved several processes: enrichment, isolation, species identification, and sero-typing. The overall methodology is presented as a flow diagram in Fig 1. Samples were added to Selenite Brilliant Green broth (CHROMagar, Shanghai, China) and incubated at 36°C for 16–22 h to enrich bacteria. The pre-enriched samples were plated on xylose lysine deoxycholate agar (XLD; CHROMagar) and CHROMagar Salmonella medium (CAS; CHROMagar) simultaneously, followed by incubation at 36°C for 18–24 h. Three classic Salmonella colonies were picked from XLD and CAS, inoculated into two tubes (Tube 1 and Tube 2; see Fig 1), used to identify intestinal pathogenic bacteria, and plated on CAS to confirm the H2S phenotype. The samples were then incubated at 36°C for 18–24 h. Colonies on Tube 1 slant cultures were serotyped using slide agglutination tests (SSI Diagnostica, Hillerød, Denmark). API 20E test strips (bioMérieux SA, Marcy l’Etoile, France) were used to confirm agglutinative and incomplete agglutinative bacterial colonies and to test for the H2S phenotype.
Fig 1. The methodology of Salmonella detection procedure.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was carried out using automated broth microdilution (Sensititre; Thermo Fisher Scientific, USA). Twenty-one antibiotics, including amikacin, ampicillin, aztreonam, cefazolin, cefepime, cefoperazone, cefoxitin, ceftazidime, ceftriaxone, chloramphenicol, gentamicin, piperacillin, tetracycline, thienamycin, ticarcillin, ticarcillin/clavulanic acid, tobramycin, trimethoprim/sulfamethoxazole, levofloxacin, nitrofurantoin and norfloxacin, were used to test antimicrobial susceptibility. According to the breakpoints outlined by the Clinical and Laboratory Standards Institute (CLSI), each isolate was recorded as resistant or susceptible for each antimicrobial. The CLSI-specified susceptible control strain was Escherichia coli ATCC 25922.
MLST analysis
MLST analysis was conducted according to the protocols described on the MLST website (http://mlst.ucc.ie/mlst/dbs/Senterica/documents/primersEnterica_html). Briefly, the S. Aberdeen isolates were subcultured into Luria broth (LB) at 37°C for 18 h; then, total DNA was extracted using a TIANamp bacteria DNA kit (Tiangen Biotech, Beijing, China) and stored at –20°C prior to use. Seven housekeeping genes (thrA, purE, sucA, hisD, aroC, hemD, and dnaN) were amplified by polymerase chain reaction (PCR) using primer sequences downloaded from the MLST database. PCR amplified products were sequenced by the BGI. The resulting sequence data were imported into the MLST database (http://mlst.warwick.ac.uk/mlst/mlst/dbs/Senterica) and information of the sequence type (ST) was obtained.
PFGE analysis
PFGE was performed as previously described [19]. The purified total DNA was digested with XbaI (TaKaRa, Dalian, China) at 37°C for 3 h. DNA macrorestriction fragments were resolved over 20 h on 1% SeaKem gold agarose (Lonza, Rockland, ME, USA), in 0.5M Tris-borate-EDTA buffer, using a CHEF Mapper PFGE system (Bio-Rad, Hercules, CA, USA). The electrophoresis conditions had an initial switch time of 2.16 s, with final switch times of 63.8 s. S. enterica serotype Braenderup H9812 was used as the molecular size standard [20]. The gel images were digitally captured for analysis using BioNumerics software version 6.0 (Applied Maths, Belgium). The genetic similarity coefficients were calculated, and dendrograms were constructed by the unweighted pair group method of arithmetic average (UPGMA). The analysis parameters used in this study were based on 1.2% tolerance values.
CRISPR analysis
Two CRISPR loci (CRISPR 1 and CRISPR 2) are reported to be present in all Salmonella genomes [21], and molecular epidemiological investigations have strongly correlated CRISPR polymorphisms with serotype [16]. CRISPR typing was performed between four paired isolates from the same samples with different H2S phenotypes. Primer pairs were selected based on a previous study [15]. PCR conditions were as follows: 95°C for 5 min; 30 cycles of 95°C for 30 s, 63°C for 40 s, and 72°C for 45 s; and 72°C for 7 min, using Ex Taq DNA polymerase (TaKaRa/Clontech, Beijing, China). PCR-amplified products were sequenced by the BGI. The resulting sequence data were imported into the CRISPRfinder Website (http://crispr.u-psud.fr/Server/) to obtain spacer and direct repeat information [21]. The name of each spacer was obtained using the Institute Pasteur CRISPR database for Salmonella (http://www.pasteur.fr/recherche/genopole/PF8/crispr/CRISPRDB.html) [13]. In cases where the spacer or direct repeat was unknown in the CRISPR dictionary, a new spacer name was assigned according to recognized spacer nomenclature [15].
Sequence analysis of the phs operon
The phs operon is critical for the reduction of thiosulfate to hydrogen sulfide and contains three genes (phsA, phsB, and phsC) encoding thiosulfate reductases [22, 23]. To determine the molecular basis of the inability of some strains to produce hydrogen sulfide, the phs operon was amplified, and the resulting sequences were analyzed. PCR conditions were as follows: 95°C for 5 min; 30 cycles of 95°C for 30 s, 57°C for 40 s, and 72°C for 45 s; and 72°C for 7 min, using Ex Taq DNA polymerase (TaKaRa/Clontech). Primer pairs [13] are presented in Table 1. The sequence data obtained were imported into DNAman 6.0, and genetic differences were detected using Mega 6.0. The genome sequence of strain Salmonella Typhimurium LT2 (NC_003197.1) was used as a reference.
Table 1. Primers for PCR amplification of the phsABC genes.
| Gene | Primer | Products length |
|---|---|---|
| phsA1 | F 5'-CGTTGGATGCCTGTTCAG-3' | 938 |
| R 5'-AGGTCGTAGAGCCGATTG-3' | ||
| phsA2 | F 5'-CGCCGTTCAACTGATAGA-3' | 959 |
| R 5'-AATGGTGAGCTTCGATCC-3' | ||
| phsA3 | F 5'-CATCGTAGAGCTGTTCATCA-3' | 975 |
| R 5'-CATGTGCGTGTTCAGGAA-3' | ||
| phsB | F 5'-CAAGCATGAGCAGCACCAC-3' | 687 |
| R 5'-ATGAGGGAGGAGGGAACCAT-3' | ||
| phsC | F 5'-GATGGTCTCTATTTGCCGTTCT-3' | 803 |
| R 5'-GGTGCTGCTCATGCTTGTT-3' |
Nucleotide sequence accession numbers
The nucleotide sequences obtained in this study have been deposited in the NCBI under GenBank accession numbers KU143714–KU143732.
Results
Identification of S. Aberdeen isolates
In total, 160 S. Aberdeen isolates were detected in our laboratory from 2005 to 2013. Among these isolates, 58.75% were isolated from human cases, 30.63% were isolated from aquaculture products, 3.12% were isolated from vegetable, 3.12% were isolated from surface water, 2.51% were isolated from poultry, and 1.87% were isolated from swine manure. Additionally, 39 (24.37%) of 49 aquaculture products isolates were detected from Ballamya quadrata (spiral shell). We identified seven (4.4%) non-H2S-producing S. Aberdeen isolates. Notably, two stool samples yielded two pairs of isolates with different H2S phenotypes simultaneously (SH06084+ and SH06084–, SH11G1146+ and SH11G1146–; where + indicates an H2S-producing isolate and–indicates a non-H2S-producing isolate). Similar findings were obtained from one surface water sample (SH11SF112+ and SH11SF112–) and one vegetable sample (SH06SF12+ and SH06SF12–). According to molecular typing results, isolation origin, source, H2S phenotype, and isolation year, a total of 45 isolates, including 39 isolates from Shanghai and six isolates from Nanjing, were selected for further analysis, with seven non-H2S-producing isolates and 38 H2S-producing isolates (used as reference strains).
Antimicrobial susceptibility testing
All 45 S. Aberdeen isolates tested were susceptible to all 21 antibiotics.
MLST analysis
All 45 S. Aberdeen isolates belonged to the same sequence type, ST426. Previously, two S. Aberdeen isolates belonging to ST426 were reported in the MLST database; one was isolated from the UK (1934) and the other was isolated from Australia (2001). A strain in the MLST database isolated from China in 2008 (serotype unknown), belonging to ST653, was also identified as a single-locus variant of ST426.
PFGE analysis
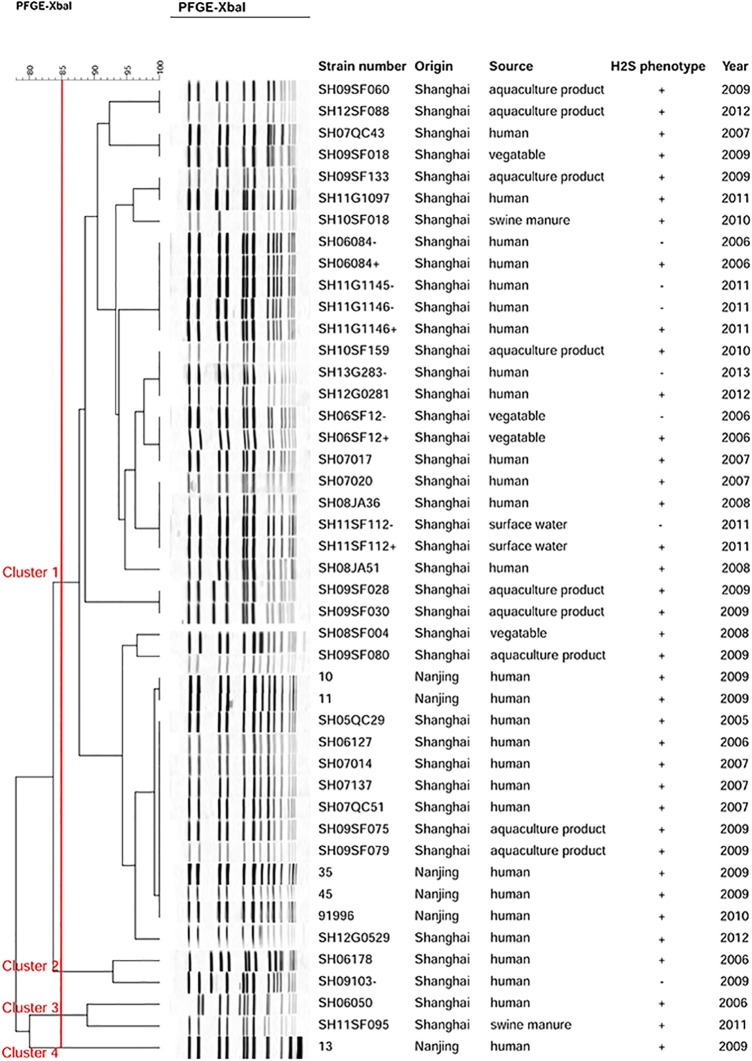
PFGE analysis showed that 45 S. Aberdeen isolates had close genetic relationship, with 88.89% of the isolates belonging to a single clone and few isolates showing lesser relatedness. Cluster analysis primarily divided the isolates into four main clusters with approximately 85% similarity (Fig 2). Cluster 1, the largest cluster, contained 35 Shanghai isolates (including six H2S-negative isolates) and five Nanjing isolates. Most isolates from human cases gave the same PFGE banding patterns as isolates from the environment. SH10SF018, isolated from swine manure, was 96% similar to SH09SF133 and SH11G1097, isolated from aquaculture products and human cases, respectively. Two pairs of S. Aberdeen isolates from the same sample (SH11G1146+/–, SH06084+/–), and one H2S-negative isolate (SH11G1145–) gave the same PFGE banding pattern. Notably, one human isolate (SH07017) shared the same PFGE banding pattern as an H2S-negative isolate (SH06SF12–) from vegetables. Another non-H2S-producing isolate (SH13G283–) from 2013 displayed an identical PFGE profile to that of an H2S-producing isolate (SH12G0281) from 2012. In addition, SH11G112+ and SH11G112–, isolated from the same surface water sample, had the same PFGE profile as H2S-positive S. Aberdeen isolates from humans. Furthermore, the isolate SH09103–, identified in Cluster 2, was 92.86% similar to the human isolate SH06178. One S. Aberdeen isolate from a human in Cluster 3 was 88.89% similar to the other swine manure isolate, and Cluster 4 contained one isolate from Nanjing.
Fig 2. Dendrogram displaying the PFGE profiles of the 43 isolates.
The strain number, origin, source, sequence type (ST), and H2S phenotype are shown for each strain. +, H2S-producing isolate; −, non-H2S-producing isolate.
CRISPR analysis
Four pairs of S. Aberdeen isolates from the same samples had the same spacer content in CRISPR 1 locus (STM1-Der3-Mik1-Abe1-Abe2-Abe3) and CRISPR 2 locus (ParBB1-AbeB1-AbeB2). There are currently no CRISPR 1 spacers designated for S. Aberdeen in the Institute Pasteur CRISPR database dictionary of spacers. In the four pairs of isolates, we identified three new spacers in the CRISPR 1 locus, namely spacers Abe1, Abe2, and Abe3. These four pairs of isolates shared the same CRISPR loci, indicating that they were genetically closely related.
Sequence analysis of the phs operon
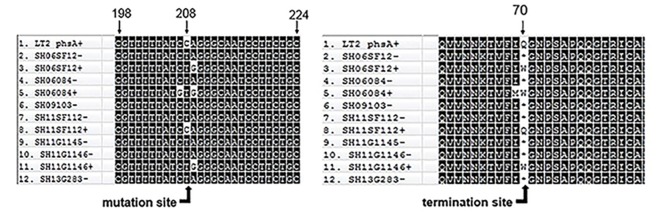
We identified a new mutation site present in seven non-H2S-producing S. Aberdeen isolates. Compared with 38 H2S-producing S. Aberdeen isolates, the seven non-H2S-producing S. Aberdeen isolates displayed a single-nucleotide substitution of C to T at position 208 of the phsA gene, resulting in a codon change from CAG to UAG. The replacement of a sense codon (CAG) with a termination codon (UAG) led to the premature termination of phsA (Fig 3), resulting in the inability of non-H2S-producing S. Aberdeen isolates to produce integral phsA gene products. Although isolates SH06SF12+, SH06084+, and SH11SF1146+ had a mutation at position 208 of phsA, causing replacement of glutamine with tryptophan, this did not lead to inactivation of phsA gene products and thus did not alter the H2S phenotype. Mutations in phsB and phsC were not detected in the seven non-H2S-producing isolates (data not shown).
Fig 3. Sequence alignment of the phs gene and the protein.
A nonsense mutation at position 208 of the phsA gene results in the replacement of a sense codon (CAG) with a termination codon (UAG) leading to the premature termination of phsA. The first sequence, phsA, is based on S. enterica serotype Typhimurium strain LT2 (GenBank AE006468). *, termination codon; +, H2S-producing isolate; −, non-H2S-producing isolate.
Discussion
Based on our laboratory surveillance system, a total of 160 S. Aberdeen isolates were detected from Shanghai and Nanjing. Over half of these strains (58.75%) were isolated from human cases, whereas 24.37% were isolated from spiral shell. Our monitoring data, collected between 2006 and 2011 [7], demonstrated that S. Aberdeen could be enriched by spiral shells. Thus, it is likely that the spiral shell is a potential host of S. Aberdeen. Moreover, spiral shells have become a popular food in Shanghai in recent decades. Typically, spiral shells are stored in wet, aerobic conditions, and spiral shell emunctory organs are often cut before the snails are cooked; both of these actions promote S. Aberdeen growth. A previous study suggested that animal contact is a source of human non-typhoidal salmonellosis and that Salmonella could be parasitic in livestock intestines [24]. Furthermore, two isolates were detected from swine manure, which is often directly excreted into surface water, causing contamination over a period of several years. Thus, we speculate that the spread of S. Aberdeen in China may be related to the inappropriate management of aquaculture and surface water. Additionally, clinical cases are most likely to occur from contaminated food. Therefore, the chain of S. Aberdeen transmission may proceed as follows: swine manure→surface water→spiral shells→humans suggesting that improved monitoring of aquaculture products and surface water is necessary in order to avoid contamination with S. Aberdeen, which may lead to infection in humans and animals.
Seven non-H2S-producing isolates were recovered in this study; five of these isolates were from clinical samples, one was from surface water, and one was from a vegetable. To the best of our knowledge, this is the first report of the identification of H2S-negative S. Aberdeen. Moreover, we identified four pairs of S. Aberdeen isolates with different H2S phenotypes from the same samples. These four non-H2S-producing S. Aberdeen isolates had sequence types, PFGE banding patterns, and CRISPR spacers identical to those of four H2S-producing isolates from the same samples, all indicating a close genetic relationship. Taken together, the molecular typing analysis and antibiotic susceptibility testing suggested that all the S. Aberdeen isolates identified in our laboratory from 2005 to 2013 had the same antibiotic susceptibility, belonged to the same ST, and had closely related PFGE patterns, indicating that these S. Aberdeen isolates were highly clonal.
H2S-negative S. Typhimurium, S. Infantis, and S. Senftenberg isolates have been reported to contain nonsense mutations at positions 1,440, 358, and 1,621 of the phsA gene, respectively [10, 13]. A nonsense mutation in the phsA gene was also identified in low-H2S-producing S. Typhi and S. Paratyphi A in a previous study [25]. Moreover, analysis of H2S-positive versus an H2S-negative variant S. Kentucky, isolated by researchers in Kuwait [11], showed a moaC insertion frameshift mutation, affecting molybdenum cofactor biosynthesis protein C. In our study, a new mutation site within the phsA gene was detected. The phsA gene, one of three open reading frames of the phs operon, is homologous and very similar to several other anaerobic molybdoprotein oxidoreductases. Seven non-H2S-producing S. Aberdeen isolates possess the same mutation at position 208 of phsA, resulting in a sense codon (CAG) being replaced by a termination codon (UAG; Fig 3). Therefore, the non-H2S-producing S. Aberdeen isolates cannot produce the integral phsA gene product, i.e., thiosulfate reductase, and are thus unable to utilize thiosulfate (S2O32−) to produce H2S. Owing to the lack of an H2S phenotype, H2S-negative Salmonella are more likely to be overlooked during laboratory and hospital screening. Accordingly, because the detection methods used for H2S-negative Salmonella isolates are important for preventing the future spread of these pathogens, we have proposed a standard screening procedure (Fig 1) involving enrichment, isolation, species identification, and serotyping to avoid missing or misidentifying H2S-negative Salmonella isolates.
Salmonella triggers inflammation in the gut by using its virulence factors T3SS-1 and T3SS-2; at the same time, the host stimulates oxidation of endogenous sulfur compounds and converts thiosulfate (S2O32−) to tetrathionate (S4O62−) [26]. Previous studies have shown that anaerobic respiration based on tetrathionate, generated by oxidizing thiosulfate, provides a growth advantage for Salmonella to compete with other microbiota in the inflamed intestine [27–29]. Moreover, Salomonella can only use ethanolamine as a carbon source to support growth under anaerobic conditions in the presence of tetrathionate as a respiratory electron acceptor [30]. Thus, H2S-negative Salmonella, which are unable to convert thiosulfate to H2S, may cause increased in tetrathionate-dependent respiration, promoting Salmonella growth in the gut [10, 31]. More comprehensive studies are needed to analyze the virulence and pathogenicity of H2S-negative Salmonella.
Multiple serotypes of H2S-negative Salmonella had been detected in Japan, Kuwait, China, and Hong Kong [10–13]. Seventeen H2S-negative S. Senftenberg isolates [13] and 19 H2S-negative S. Choleraesuis isolates were reported in our previously study [32]. Here, we identified seven H2S-negative S. Aberdeen isolates in China from 2006 to 2011. Therefore, it is possible that H2S-negative S. enterica has been overlooked during hospital and laboratory screening, necessitating improved monitoring of this pathogen to prevent and control its spread.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the Mega-projects of Science and Technology Research (grant nos. 2016ZX10004215 and AWS15J006), the National Nature Science Foundation of China (grant nos. 81371854 and 81373053), and the Beijing Science and Technology Nova program (grant no. Z131107000413061).
References
- 1.Prost E, Riemann H. Food-borne salmonellosis. Annu Rev Microbiol. 1967;21:495–528. Epub 1967/01/01. 10.1146/annurev.mi.21.100167.002431 . [DOI] [PubMed] [Google Scholar]
- 2.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50(6):882–9. Epub 2010/02/18. 10.1086/650733 . [DOI] [PubMed] [Google Scholar]
- 3.Pires SM, Vigre H, Makela P, Hald T. Using Outbreak Data for Source Attribution of Human Salmonellosis and Campylobacteriosis in Europe. Foodborne Pathogens and Disease. 2010;7(11):1351–61. 10.1089/fpd.2010.0564 [DOI] [PubMed] [Google Scholar]
- 4.Barton Behravesh C, Jones TF, Vugia DJ, Long C, Marcus R, Smith K, et al. Deaths Associated With Bacterial Pathogens Transmitted Commonly Through Food: Foodborne Diseases Active Surveillance Network (FoodNet), 1996–2005. Journal of Infectious Diseases. 2011;204(2):263–7. 10.1093/infdis/jir263 [DOI] [PubMed] [Google Scholar]
- 5.Mao X., Hu J., Liu X. Estimation on disease burden of foodborne non-typhoid salmonellosis in China using literature review method. Chin. J. Dis. Control. Prev. 2011; 15, 622–625. [Google Scholar]
- 6.Bicknell SR. Salmonella Aberdeen infection in cattle associated with human sewage. J Hyg (Lond). 1972;70(1):121–6. Epub 1972/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Yang X, Kuang D, Shi X, Xiao W, Gu Z, et al. Prevalence of antimicrobial resistance of non-typhoidal Salmonella serovars in retail aquaculture products. Int J Food Microbiol. 2015;210:47–52. Epub 2015/06/22. 10.1016/j.ijfoodmicro.2015.04.019 . [DOI] [PubMed] [Google Scholar]
- 8.Ikwap K, Erume J, Owiny DO, Nasinyama GW, Melin L, Bengtsson B, et al. Salmonella species in piglets and weaners from Uganda: prevalence, antimicrobial resistance and herd-level risk factors. Prev Vet Med. 2014;115(1–2):39–47. Epub 2014/04/04. 10.1016/j.prevetmed.2014.03.009 . [DOI] [PubMed] [Google Scholar]
- 9.Li W., Zhu Q. Salmonella Aberdeen nosocomial outbreak in NICU: an epidemiological study. Chin. J. Nosocomiol. 2009;19, 1954–1955. [Google Scholar]
- 10.Sakano C, Kuroda M, Sekizuka T, Ishioka T, Morita Y, Ryo A, et al. Genetic analysis of non-hydrogen sulfide-producing Salmonella enterica serovar Typhimurium and S. enterica serovar Infantis isolates in Japan. J Clin Microbiol. 2013;51(1):328–30. Epub 2012/11/09. 10.1128/JCM.02225-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albert MJ, Al Obaid K, Alfouzan W, Sheikh AR, Udo E, Izumiya H, et al. Isolation of Salmonella enterica serovar Kentucky strain ST 198 and its H2S-negative variant from a patient: implications for diagnosis. J Clin Microbiol. 2014;52(11):4090–3. Epub 2014/08/22. 10.1128/JCM.01775-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin D, Yan M, Lin S, Chen S. Increasing prevalence of hydrogen sulfide negative Salmonella in retail meats. Food Microbiol. 2014;43:1–4. Epub 2014/06/16. 10.1016/j.fm.2014.04.010 . [DOI] [PubMed] [Google Scholar]
- 13.Yi S, Xie J, Liu N, Li P, Xu X, Li H, et al. Emergence and prevalence of non-H2S-producing Salmonella enterica serovar Senftenberg isolates belonging to novel sequence type 1751 in China. J Clin Microbiol. 2014;52(7):2557–65. Epub 2014/05/16. 10.1128/jcm.00377-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campioni F, Pitondo-Silva A, Bergamini AM, Falcao JP. Comparison of four molecular methods to type Salmonella Enteritidis strains. APMIS. 2015;123(5):422–6. Epub 2015/02/24. 10.1111/apm.12367 . [DOI] [PubMed] [Google Scholar]
- 15.Fabre L, Zhang J, Guigon G, Le Hello S, Guibert V, Accou-Demartin M, et al. CRISPR typing and subtyping for improved laboratory surveillance of Salmonella infections. PLoS One. 2012;7(5):e36995 Epub 2012/05/25. 10.1371/journal.pone.0036995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shariat N, Dudley EG. CRISPRs: molecular signatures used for pathogen subtyping. Appl Environ Microbiol. 2014;80(2):430–9. Epub 2013/10/29. 10.1128/AEM.02790-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stepan RM, Sherwood JS, Petermann SR, Logue CM. Molecular and comparative analysis of Salmonella enterica Senftenberg from humans and animals using PFGE, MLST and NARMS. BMC Microbiol. 2011;11:153 Epub 2011/06/29. 10.1186/1471-2180-11-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun J, Ke B, Huang Y, He D, Li X, Liang Z, et al. The molecular epidemiological characteristics and genetic diversity of Salmonella Typhimurium in Guangdong, China, 2007–2011. PLoS One. 2014;9(11):e113145 Epub 2014/11/08. 10.1371/journal.pone.0113145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, et al. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis. 2006;3(1):59–67. Epub 2006/04/11. 10.1089/fpd.2006.3.59 . [DOI] [PubMed] [Google Scholar]
- 20.Hunter SB, Vauterin P, Lambert-Fair MA, Van Duyne MS, Kubota K, Graves L, et al. Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. J Clin Microbiol. 2005;43(3):1045–50. Epub 2005/03/08. 10.1128/JCM.43.3.1045-1050.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grissa I, Vergnaud G, Pourcel C. CRISPRcompar: a website to compare clustered regularly interspaced short palindromic repeats. Nucleic Acids Res. 2008;36(Web Server issue):W145–8. Epub 2008/04/30. 10.1093/nar/gkn228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barton LL, Fardeau ML, Fauque GD. Hydrogen sulfide: a toxic gas produced by dissimilatory sulfate and sulfur reduction and consumed by microbial oxidation. Met Ions Life Sci. 2014;14:237–77. Epub 2014/11/25. 10.1007/978-94-017-9269-1_10 . [DOI] [PubMed] [Google Scholar]
- 23.Heinzinger NK, Fujimoto SY, Clark MA, Moreno MS, Barrett EL. Sequence analysis of the phs operon in Salmonella Typhimurium and the contribution of thiosulfate reduction to anaerobic energy metabolism. J Bacteriol. 1995;177(10):2813–20. Epub 1995/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoelzer K, Moreno Switt AI, Wiedmann M. Animal contact as a source of human non-typhoidal salmonellosis. Vet Res. 2011;42:34 Epub 2011/02/18. 10.1186/1297-9716-42-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClelland M, Sanderson KE, Clifton SW, Latreille P, Porwollik S, Sabo A, et al. Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat Genet. 2004;36(12):1268–74. Epub 2004/11/09. 10.1038/ng1470 . [DOI] [PubMed] [Google Scholar]
- 26.Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, et al. Salmonella enterica serovar Typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5(10):2177–89. Epub 2007/09/01. 10.1371/journal.pbio.0050244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levitt MD, Furne J, Springfield J, Suarez F, DeMaster E. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. J Clin Invest. 1999;104(8):1107–14. Epub 1999/10/19. 10.1172/jci7712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohmer L, Hocquet D, Miller SI. Are pathogenic bacteria just looking for food? Metabolism and microbial pathogenesis. Trends Microbiol. 2011;19(7):341–8. Epub 2011/05/24. 10.1016/j.tim.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467(7314):426–9. Epub 2010/09/25. 10.1038/nature09415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, et al. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc Natl Acad Sci U S A. 2011;108(42):17480–5. Epub 2011/10/05. 10.1073/pnas.1107857108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shatalin K, Shatalina E, Mironov A, Nudler E. H2S: a universal defense against antibiotics in bacteria. Science. 2011;334(6058):986–90. Epub 2011/11/19. 10.1126/science.1209855 . [DOI] [PubMed] [Google Scholar]
- 32.Xie J, Yi S, Zhu J, Li P, Liang B, Li H, et al. Antimicrobial Resistance and Molecular Investigation of H2S-Negative Salmonella enterica subsp. enterica serovar Choleraesuis Isolates in China. PLoS One. 2015;10(10):e0139115 Epub 2015/10/03. 10.1371/journal.pone.0139115 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.