Abstract
Background
Iron therapy induces inflammation which could decrease iron absorption. Increased exposure of iron in the gut could also alter microbiome file. Providing antioxidants such as vitamin E with iron therapy has been associated with reduced oxidative potential.
Objective
Test the efficacy of adding vitamin E to therapeutic iron therapy on iron repletion, inflammation markers and gut microbiome in iron deficient infants and toddlers.
Design
This was a randomized, double-blind, control trial in which infants and toddlers (Denver, CO metro area) who were at risk of iron deficiency were screened. Eligible participants were randomized to receive iron therapy (6 mg/kg/d) plus placebo (n = 22) or iron (6 mg/kg/d) plus vitamin E (18 mg/d, n =14) for 8 weeks. Iron and inflammation status, and gut microbiome (16S sequencing) were analyzed in all participants before and after the treatment.
Results
After 8 weeks of treatment, average serum ferritin level returned to normal for both iron + placebo and iron + vitamin E groups at 33.3 ± 20.2 and 33.5 ± 21.5 ug/L, respectively. Serum vitamin E concentration increased in iron + vitamin E group. No change over time was observed regarding serum IL-4, TNF-α or fecal calprotectin. The relative abundance of the genus Roseburia (phylum Firmicutes), a butyrate producer, increased in the Fe + E group (Δ 1.3%, P < 0.01). Also at the genus level, the genus Escherichia decreased by 1.2% on average among all participants (effect of time P = 0.01).
Conclusion
Using a therapeutic iron dose of 6mg/kg/d is effective in treating iron deficiency during an 8-week period, without inducing persistent inflammatory response. Changes of the gut microbiome raised the possibility that antioxidant therapy in conjunction with therapeutic iron supplementation could potentially improve microbial community profiles in the intestinal tract.
Keywords: iron deficiency, vitamin E, microbiome
Introduction
Iron deficiency (ID) is the most common micronutrient deficiency in the world and negatively impacts health in several ways, including impaired growth, increased behavioral problems, and delayed mental and motor development. [1-3]. Despite the fortification of infant and toddler foods in the United States, a significant number of children still develop ID or iron deficiency anemia (IDA) [4]. The current recommended dosage to treat IDA ranges from 3 to 6 mg/kg/day in children (1). This wide range is higher than routine adult supplementation levels (typically ≤ 1 mg/kg/day) and reflects the challenge and imprecision of effective treatment for ID and IDA in children. Studies in adults have shown local (intestinal) and systemic inflammatory changes within days of iron therapy initiation [5]. Systemic inflammation can stimulate the production of hepcidin which blocks iron uptake from the enterocyte [6, 7]. Thus, attenuating iron-induced-inflammation may be beneficial in terms of increasing iron absorption.
Vitamin E is an essential nutrient that functions as an antioxidant and anti-inflammatory agent, and has been studied for many potentially beneficial facets of human health, including potential for protection against heart disease [8], accelerated aging mediated through DNA damage, and impaired immunity [9]. In adults, providing antioxidants along with oral iron therapy has been associated with reduced oxidative potential. Specifically, in one study, a palm oil extract containing approximately 7 mg of Vitamin E administered along with iron therapy was associated with reduced fecal oxidation susceptibility [10]. Alpha-tocopherol is the most studied form of Vitamin E and has been shown to reduce biomarkers of total body oxidative stress and inflammation [9, 11]. Because high dose iron therapy may trigger an inflammatory response that reduces iron absorption, our primary objective was to determine whether adding vitamin E could enhance the efficacy of therapeutic iron for infants and toddlers who have a dietary iron deficiency, potentially through reducing iron-induced inflammation. Our main hypothesis was that in the case of infants and toddlers with ID or IDA, 2 months of supplemental Vitamin E combined with therapeutic iron supplementation at 6 mg/kg/day would be more efficacious than the same dose of iron alone.
Iron is also an important nutrient for many pathogenic intestinal bacteria [12-14], and is often a growth rate-limiting nutrient. However, with high dosage and low absorption rate, iron supplementation could result in increased exposure of iron in the gut, and thereby alter the microbiome profile of the host. Randomized controlled trials have shown that iron fortification produces a more pathogenic gut microbiome profile in African children at risk for ID [15, 16], but no randomized trials of iron therapy and the effect on microbiome have been reported in infants and toddlers from developed countries. From animal models, ID itself has also been suggested to change gut microbiome [17] towards a less-favorable profile. Thus, our secondary objective was to assess the impact of iron supplementation on gut microbiome composition in infants with ID or IDA recruited from the metro Denver, Colorado, area. We hypothesized that a course of high dose therapeutic iron supplementation would result in a microbiome profile with greater abundance of potential pathogens and that the addition of vitamin E would be associated with a more favorable microbiome profile.
Subjects and Methods
Subjects
The study protocol was approved by the Colorado Multiple Institutional Review Board. The legal guardian of the participating infant signed the informed consent before starting the study. Primarily breastfed older infants (≥ 9 months) and young toddlers were recruited from metro Denver area through clinical office referral and flyers mailed by the Colorado Department of Public Health, which has a database of information obtained when mother and newborn infants are being discharged from the delivery hospital, including whether infant was breastfeeding at discharge. Infants of both genders were studied with no limitations on race or ethnicity. Risk factors for iron deficiency include primarily predisposing dietary factors, such as breastfeeding; excessive cow milk intake, low intake of meats, or low intake of fortified foods 1; and overweight status. Inclusion criteria for screening for ID were: full-term, generally healthy infants born after 36 weeks' gestation at an appropriate weight for gestational age. Infants recruited for screening between 9-12 months of age needed to be breastfed through at least the first nine months of life, as standard infant formulas are highly fortified with iron. Older toddlers recruited for screening between 12-24 months of age, needed to have estimated low dietary intake of iron based on diet history reviewed at time of screening (e.g., low meat intake, low iron-fortified food intake, excessive cow's milk intake). Exclusion criteria for screening were: current or recent (within the last 3 months) infant formula use; current or past therapeutic iron supplementation; any disease process that would influence absorption of either iron or Vitamin E, including gastrointestinal malabsorption; any inflammatory process (inflammatory bowel disease, cystic fibrosis, liver or kidney disease, cancer, HIV or any primary immune deficiency disorder); anemia unrelated to iron status (e.g. congenital hematologic disorders); disorders associated with chronic blood loss; inherited disorders of iron homeostasis (e.g. hemochromatosis); or bleeding or coagulation disorders.
Design
This was a double-blind, randomized controlled efficacy trial of iron therapy (6 mg/kg/day) with or without Vitamin E (18 mg/day), in infants and toddlers with ID or IDA between 9 to 24 months of age. The supplementation was administered for 8 weeks. The primary outcome evaluated in this study was change in iron status, as represented by serum ferritin and other iron status biomarkers, including hemoglobin, iron saturation and serum transferrin receptor; biomarkers of intestinal and systemic inflammation status were also assessed. Secondary outcome was gut microbiome profile before and after the treatment. Serum and fecal samples were collected at baseline and at intervention completion. If a child was identified as having risk factors for ID, met exclusion and inclusion criteria, and the legal guardian consented for this study, a blood sample was taken for screening of ID. If the results of the blood tests identified them as having ID (serum ferritin level < 15 ug/L) or IDA (hemoglobin < 11.5 g/dl, adjusted for Denver altitude) while C - reactive protein was < 3 mg/L, they were randomized to one of the two treatment regimens: iron + vitamin E (Fe + E) or iron + placebo (Fe + placebo). Three-day diet records were collected at baseline and post-intervention from each participant and analyzed by the CTRC Nutrition Core using NDSR software for potential confounding factors such as dietary calcium, iron, vitamin E and phytic acid.
A commercial ferrous sulfate solution (Fer-In-Sol, 15 mg elemental Fe/mL; Mead Johnson, Inc, Evansville, IN) was prepared for the study by the research pharmacy at The Children's Hospital Colorado. The volume of the suspension was individualized by the pharmacy to the infant's weight, to maintain consistent iron dosing at 6 mg/kg/day. A commercial Vitamin E preparation (Aquasol E, 50 mg/ml; Hospira, Inc, Lake Forest, IL) was used for the study (FDA IND 111-123). The Vitamin E dose was 18 mg/day for all subjects randomized to the Vitamin E group. This dose represents 3 times the Recommended Dietary Allowance for 12-36 months olds and is less than 10% of the Upper Limit (UL) of 200 mg/day for this age [18]. Thus, this dose given was considered safe for the participants. Additionally, much higher doses, e.g. 50 to > 500 mg/day, have been safely used in infants and children, but these have typically been in subjects with medical problems and/or potential for malabsorption [11]. The control group received an indistinguishable placebo preparation, which was compounded using purified water, polysorbate 80, sorbital, and propylene glycol.
The randomization code (excel generated queue, =rand() function) for subjects' supplementation assignment was maintained by the Children's Hospital of Colorado (CHCO) pharmacy department, staff of which had no direct subject contact. The pharmacy department also made the iron and vitamin E (or placebo) supplements so the research team and the participants could be blinded. Care givers of subjects and the research team members were both blinded to the intervention. After consent, enrollment and initiation of the study procedures, an 8-week supply of supplements was provided from the research pharmacy to the study personnel. Care givers and subjects travelled to the Clinical and Translational Research Center (CTRC) at CHCO for their initial intervention visit. At this visit, parents received the 8 week supply of supplements and were instructed when and how to administer the solutions. A calendar was provided to record daily supplement administration, comments about tolerance, and general health of infant. Supplement bottles were weighed at baseline, each home visit, and the end of the treatment period to monitor compliance.
Administration of the daily iron dose was recommended to be given in two divided doses (i.e. 3 mg/kg/dose × twice a day), to minimize side effects and to optimize absorption. If this regimen compromised compliance, however, care givers were allowed to give total dose at once. The Vitamin E (or placebo) was not expected to directly affect iron absorption in the gastrointestinal tract, and therefore was given at same time as the iron dose or at a different time during the day. Care givers were asked to record the timing of the dose administrations on the log provided.
Compliance was monitored at home visits conducted by the study personnel, who weighed each supplement bottle every 2 weeks to evaluate the amount left in bottle compared to the amount expected. At the home visits, study personnel also guided parents through a health survey, addressed questions or concerns about treatment protocol, and reviewed tolerability of treatment. Phone surveys of health status and compliance were conducted every other week when a home visit was not scheduled.
Sample collections
Blood and stool samples were collected at the beginning and end of the treatment intervention. Blood sample was collected by the CTRC nurses at CHCO. Serum was obtained from the blood sample collected and stored at -80 degree C until analyzed. The following markers were analyzed by the CTRC Core Lab: ferritin, C-reactive protein (Immunoturbidimetric), soluble transferrin receptor (sTfR), transferrin saturation, total iron (colorimetric), iron binding capacity, serum vitamin E-alpha concentration (UPLC), IL-4 (sandwich immunoassay), TNF-alpha (ELISA), and fecal calprotectin level (ELISA).
Each stool sample was collected from disposable diapers fitted with biodegradable liners, which effectively collected stool, but allow passage of urine [19]; two duplicate stool samples were placed in separate sterile fecal collection tubes (Sarstedt, Newton, MA) containing ethanol. The study coordinator assisted mothers with stool collections, using clinical grade gloves and sterile fecal swabs to avoid microbial contamination. Stool samples were stored at -80 degree C until analyzed.
Microbiome analysis
Microbiome sequencing was conducted at the Microbiome Research Consortium at University of Colorado Denver. Total community genomic DNA was prepared from ∼50 mg of stool using the UltraClean Fecal DNA Isolation Kit (MoBio Inc, USA). Bacterial profiles were determined by broad-range amplification and sequence analysis of 16S rRNA genes following our previously described methods [20, 21]. In brief, amplicons were generated using primers that target approximately 300 base pairs of the V1V2 variable region of the 16S rRNA gene. PCR products were normalized using a SequalPrep™ kit (Invitrogen, Carlsbad, CA), pooled, lyophilized, purified and concentrated using a DNA Clean and Concentrator Kit (Zymo, Irvine, CA). Pooled amplicons were quantified using Qubit Fluorometer 2.0 (Invitrogen, Carlsbad, CA). The pool was diluted to 4nM and denatured with 0.2 N NaOH at room temperature. The denatured DNA was diluted to 15pM and spiked with 25% of the Illumina PhiX control DNA prior to loading the sequencer. Illumina paired-end sequencing was performed on the Miseq platform with versions v2.4 of the Miseq Control Software and of MiSeq Reporter, using a 600 cycle version 3 reagent kit.
Illumina Miseq paired-end reads were aligned to human reference genome Hg19 with bowtie2 and matching sequences discarded [22, 23]. As previously described, the remaining non-human paired-end sequences were sorted by sample via barcodes in the paired reads with a python script [21]. The sorted paired reads were assembled using phrap [24, 25]. Pairs that did not assemble were discarded. Assembled sequence ends were trimmed over a moving window of 5 nucleotides until average quality met or exceeded 20. Trimmed sequences with more than 1 ambiguity or shorter than 200 nt were discarded. Potential chimeras identified with Uchime (usearch6.0.203_i86linux32) [26] using the Schloss [27] Silva reference sequences were removed from subsequent analyses. Assembled sequences were aligned and classified with SINA (1.2.11) [28] using the 418,497 bacterial sequences in Silva 115NR99[29] as reference configured to yield the Silva taxonomy. Operational taxonomic units (OTUs) were produced by clustering sequences with identical taxonomic assignments. This process generated 16,325,565 high-quality bacterial 16S rRNA sequences for 73 samples (median sample size: 225,011 sequences/sample; interquartile range: 202,159 – 250,609). All samples had a Good's coverage index >99% at the rarefaction point of 56,615 sequences. The software package Explicet (v2.10.5, www.explicet.org)[30] was used for display, analysis (rarefied values for median Good's coverage, p-values via Two-Part Analysis[31]), and figure generation of results.
Statistical analyses
Statistical analyses were performed using SAS (version 9.3; SAS Institute Inc, Cary, NC). Group data are presented as mean ± SD. Baseline parameters were compared using independent Student's t test between groups and parameters that were different at baseline were included in further analyses. Gender and age were tested as a categorical variable in the subsequent analysis and results remained unaffected. Repeated measures ANOVA (PROC GLM) were used to evaluate the main effects of time, group, and their interactions on the dependent variables. Independent Student's t test was used to compare values between groups as post hoc analysis. Equal variance was checked using Levene's test. Wilcoxon-Mann-Whitney test was conducted when the sample data was not normally distributed. Power calculations were based on a standard deviation of 17 and a 15 ug/L difference of ferritin between the Fe and Fe + E groups, and 28 subjects per group would yield 91% power.
Relative abundances of OTU were calculated for each subject by dividing the sequence counts observed for each OTU by the total number of high-quality bacterial 16S rRNA sequences generated for the subject. OTU relative abundance provided a quantification of the microbiome and basis for further statistical analysis. Non-normally distributed was log transformed. The enteric microbiome was analyzed for differences in the abundances and/or prevalence of particular genera/phyla of interest for effects of time and group via repeated measure ANOVA, with post hoc t tests. P value < 0.02 was considered significant to account for multiple comparisons.
Results
Iron status
Figure 1 is the flow chart of subject recruitment. Forty-four subjects consented and 37 completed the study between April 2011 and December 2013: 22 were in the iron + placebo group (Fe group) and 15 were in the iron + vitamin E group (Fe + E group). Follow-up contact was lost for 5 participants and an additional 2 dropped out (one did not accept taste of the iron supplement; one moved out of state). There were no serious adverse events reported. Baseline characteristics are presented in Table 1. No differences in age, iron status or inflammation status were observed between groups at baseline and all participants were iron deficient with a serum ferritin level of 11 ± 3 ug/L. Dietary intakes of iron, calcium, vitamin E or phytic acid were comparable between groups at baseline and post intervention (Supplementary table 1).
Figure 1. Subject recruitment.
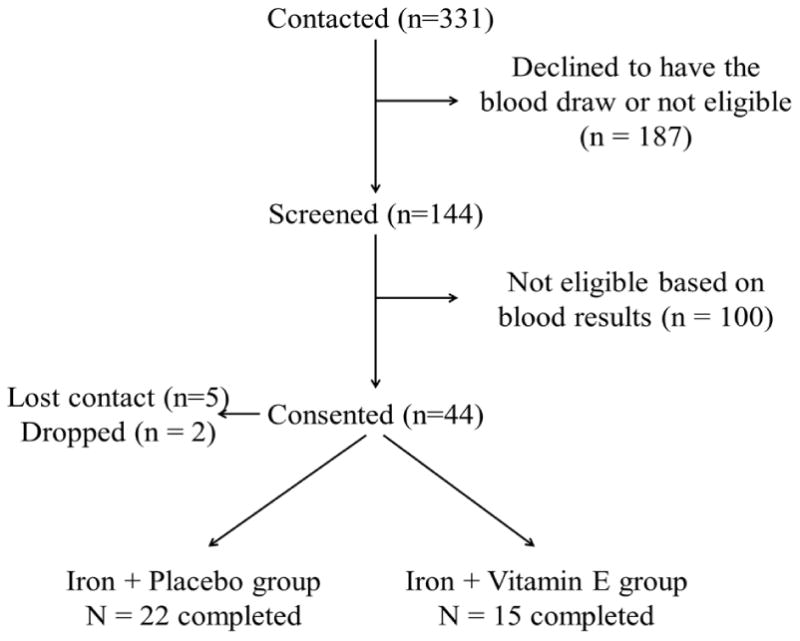
Table 1. Iron status of subjects at baseline and post-intervention1.
| Baseline | Post-intervention | Changes | Normal Range | ||||
|---|---|---|---|---|---|---|---|
| Fe | Fe +E | Fe | Fe +E | Fe | Fe +E | ||
| Age (months)2 | 14 ± 4 | 14 ± 5 | - | - | - | - | - |
| Male (Female)2 | 12 (10) | 8 (7) | - | - | - | - | - |
| Ferritin (ug/L)3 | 10.6 ± 3.3 | 11.5 ± 3.8 | 33.3 ± 20.2 | 33.5 ± 21.5 | 22.9 ± 20.1* | 22.2 ± 20.8* | 17-92 |
| CRP (mg/L)2 | 0.37 ± 0.7 | 0.30 ± 0.4 | 1.36 ± 2.89 | 0.62 ± 1.32 | 0.93 ± 2.9 | 0.28 ± 1.0 | 0-3 |
| Total iron(ug/dL)2 | 81 ± 49 | 69 ± 36 | 125 ± 83 | 100 ± 69 | 41 ± 83 | 22 ± 71 | 50-120 |
| Iron binding capacity (ug/dL)3 | 394 ± 52 | 374 ± 43 | 309 ± 79 | 316 ± 92 | -94 ± 97* | -92 ± 82* | 261-462 |
| %Iron saturation4 | 31 ± 34 | 19 ± 10 | 31 ± 16 | 26 ± 10 | -2.53 ± 32 | 4.92 ± 12.9* | 20-55 |
| Soluble transferrin receptor (ug/L)3 | 2.2 ± 0.9 | 1.9 ± 0.3 | 1.7 ± 0.3 | 1.6 ± 0.3 | -0.60 ± 0.78* | -0.45 ± 0.48* | 0.8-1.8 |
Fe: iron + placebo group (n =22); Fe + E: iron + vitamin E group (n =15).
No difference between groups or over time
Significant change over time (P < 0.01)
Significant group-by-time interaction (P < 0.01)
Significant change over time within group
Overall, over 80% subjects from the iron + placebo (87%) and iron +vitamin E (83%) groups consumed more than 80% of the supplements. After 8 weeks of iron supplementation, average serum ferritin level returned to normal without differences between groups (Table 1). Two subjects from the Fe group remained iron deficient after the treatment (serum ferritin at 9.5 and 9.1 ug/L at the end), despite good reported compliance in consuming the supplement. Iron binding capacity and soluble transferrin receptor concentration significantly decreased over time (Table 1). A significant group-by-time interaction was observed for iron saturation, which increased in Fe + E only (Table 1).
Inflammation markers
Serum vitamin E-alpha concentration at baseline was comparable between groups: 9.8 ± 2.6 and 10.6 ± 2.8 ug/ml for the Fe and Fe + E groups, respectively. As expected, vitamin E-alpha concentration did not change in the Fe group (Δ -0.29 ± 2.77 ug/ml), and increased in the Fe + E group (Δ 1.31 ± 6.08 ug/ml, group-by-time interaction P < 0.01). There were no adverse events reported or safety concerns raised by parents regarding vitamin E intake. Fecal calprotectin concentration was comparable between groups at baseline (Fe group: 49 ± 44 ug/g; Fe + E: 50 ± 58 ug/g). After intervention, calprotectin concentrations were 53 ± 44 and 46 ± 58 ug/g for Fe and Fe + E groups, respectively, which represented a borderline significant group-by-time interaction (P = 0.1). Serum IL-4 concentration did not change over time in the Fe group (0.025 ± 0.015 to 0.025 ± 0.016 pg/ml) or Fe + E group (0.025 ± 0.013 to 0.026 ± 0.013 pg/ml). A similar pattern was observed for serum TNF-α concentration over time in the Fe group (13.2 ± 4.9 to 13.0 ± 4.8 pg/ml) and Fe + E group (14.2 ± 3.3 to 12.0 ± 4.0 pg/ml).
Gut microbiome
Pairs of stool samples collected at baseline and study completion were available from 32 subjects (Fe n=18; Fe + E n=14), all of which were successfully profiled for bacterial diversity using broad-range 16S rRNA sequencing. Good's coverage indices exceeded 99% for all samples, indicating that each sequence dataset adequately represented the biodiversity in the sample from which it was derived. Microbial alpha-diversity increased over time with no difference between groups (data not shown). Changes in the microbiome between treatment groups and time-points were assessed at the phylum, family and genus levels.
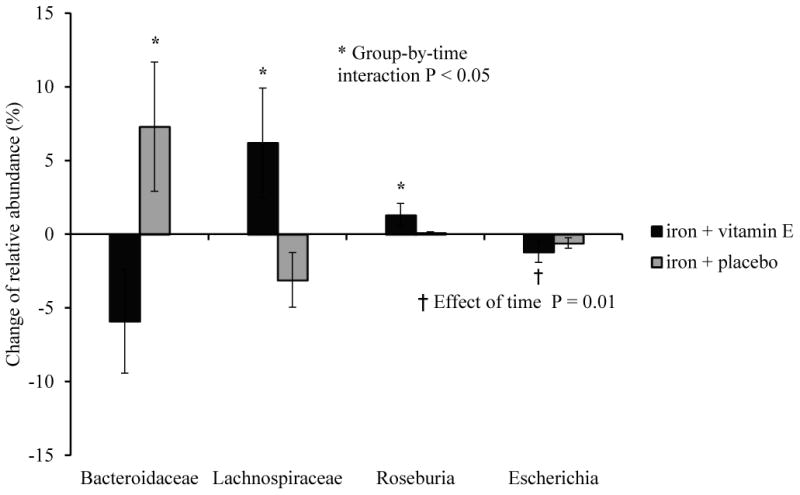
The Fe and Fe + E groups exhibited significantly different changes in microbiome composition over time. These differences were most evident in the highly abundant phyla Bacteroidetes and Firmicutes (Figure 2). With vitamin E added to iron supplementation, the relative abundance of Bacteroidetes decreased by 10% while the Firmicutes increased by 11% on average. These phylum-level effects were driven primarily by changes in the families Bacteroidaceae (phylum Bacteroidetes), which decreased in abundance, and Lachnospiraceae (phylum Firmicutes) which increased in abundance in the Fe +E group, relative to the Fe group. Furthermore, the relative abundance of the genus Roseburia, a butyrate producing member of the phylum Firmicutes [32], increased in the Fe + E group (Δ 1.3%, P < 0.01). Finally the genus Escherichia, which includes both commensal and pathogenic strains of Escherichia coli as well as pathogenic Shigella spp., decreased by 1.2% on average among all participants (Figure 3, effect of time P = 0.01).
Figure 2. Changes of relative abundance between groups at phylum level.
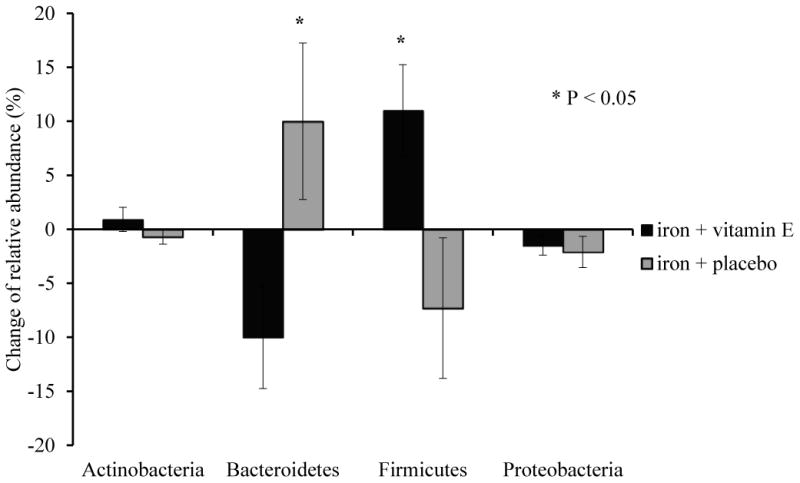
Figure 3. Changes of relative abundance between groups at family and genus levels.
Discussion
Iron deficiency is very common in older infants or toddlers who have been primarily breastfed and/or have limited intake of iron-rich foods or have other risk factors [19]. In the current study, 44 out of 144 (31%) infants and toddlers who completed the screening were iron deficient. Using the upper end of the recommended range of therapeutic iron therapy (6 mg/kg/d) for 2 months was effective in treating ID in these infants and toddlers based on the return of average serum ferritin concentration to normal levels after treatment. Although the intervention is considered relatively aggressive in terms of iron dosage, it did not induce a detectable inflammatory response in the subjects based on the serum concentration of IL-4 and TNF-α, and fecal calprotectin. This is in contrast to one small study in adults, which showed an inflammation response to iron therapy [5], when iron supplements (120 mg/d) were given to 3 adults for only 7 days. Serum IL-4 and hepcidin concentrations increased within 24 hours of iron supplementation and remained elevated for 5 days during the 7 day treatment period, suggesting the iron-induced-inflammation may be an acute response [5]. Our current study only assessed IL-4 concentration before and after the 8-week intervention and IL-4 concentration might have returned to normal before the post-intervention assessment if an acute inflammatory response occurred. There is also a possibility that it could be due to the small sample size.
In the current study, serum ferritin and most other biomarker responses were indistinguishable between groups after treatment, with the exception of percent saturation, which had a significantly greater change in the Fe + E group. Overall, the results suggest that addition of vitamin E to therapeutic iron supplementation did not further improve the efficacy of iron supplement. However, because there was no detectable change in inflammatory status among the subjects in the Fe group, it is unclear whether vitamin E would have any impact on iron-induced inflammation if it existed in this setting. The amount of vitamin E consumed was 18 mg/d, which was 3 times the RDA for vitamin E in 12-36 month olds [33]. The high dose of vitamin E caused only a modest increase in serum vitamin E concentration over time in the Fe +E group, which was still within the normal range for serum vitamin E. This was somewhat surprising, since none of the subjects had evidence of malabsorption.
The findings from animal and human studies support an effect of iron exposure toward an unfavorable enteric microbiome profile, an effect that may differ according to iron status. Recent studies have evaluated the association between iron supplementation and gut microbiome composition, in both animal and human models. In one animal study [17], weaning rats were fed iron-deficient diet for 24 days and iron storage was repleted for 13 days. A control group of rats with normal iron status was studied at the same time. Compared with the iron-sufficient rats, iron depletion significantly altered the abundance of dominant species with a large and significant reduction of the Roseburia spp./E. rectale group. After iron repletion, bacterial communities were partially restored [17]. In another study [34], iron depletion at weaning significantly lowered microbial diversity, which was partially corrected by iron repletion. Both of the animal studies induced relatively severe iron depletion. This was consistent with our observed change in diversity with iron repletion in both groups. However, the effect of time on diversity cannot be excluded because there was no control group without iron therapy.
To our knowledge, no human studies have reported the relation between iron supplements and changes in gut microbiome with vitamin E. The effects of iron fortification have been examined in children. In one study [15], 6-12 y old Ivorian children received iron-fortified biscuits (20 mg/d) or a placebo for 6 months. Enterobacteria increased while lactobacilli decreased in the iron group after 6 months of treatment. The iron group also exhibited an increase in fecal calprotectin concentration. In a recently published trial [35] conducted by the same group among 6-month old Kenyan infants, micronutrient powder containing iron (12.5 mg/d) was given to infants for 4 months. Results showed that iron supplementation increased the abundance of potentially pathogenic bacteria belonging to the genus Escherichia (i.e., E. coli and Shigella spp.), although we note that commensal strains of E. coli commonly colonize the human gut [36-41]. Inflammation status was also increased with iron fortification, reflected by increased fecal calprotectin concentration. The authors concluded from both studies that iron fortification produces a more pathogenic gut microbiome profile with elevated inflammation status in African infants and children. Fortification did not improve the iron status of the older children [15] but was efficacious in improving status in the Kenyan infants [35]. A trial of older Denver breastfed infants found differences in the microbiome among infants randomized to iron fortified cereal compared to those randomized to meats and a significantly lower iron intake. The meat group tended to have greater microbial diversity and more butyrate producers compared to the iron fortified cereal groups [19].
In the current study, iron supplementation did not create a more pathogenic microbial profile among the study participants: there was no increase of Escherichia abundance or decrease of Lactobacillus spp. after the treatment. On the contrary, after 8 weeks of iron supplementation, the abundances of Escherichia spp., whether commensal or pathogens, decreased in both groups. In addition, the Fe + E group benefited from the addition of vitamin E, showing an increase in Roseburia abundance over time. Roseburia spp. are butyrate producers and a previous study in a rat model demonstrated that iron repletion led to increases in both Roseburia abundance and cecal butyrate concentration [17]. Because butyrate stimulates colonic blood flow, is a preferred substrate for colonocytes, and promotes gut mucosal barrier function the increased abundances of Roseburia in the Fe + E group may improve colonic function. Additional research will be required to test this hypothesis [42, 43].
The different findings between our current study and previous ones [15, 35] could be due to a number of factors. For example, the current study was conducted in the U.S., while previous studies were conducted in Africa with high prevalence of malaria and systemic inflammation. The current study was also shorter compared with previous interventions. In addition, the iron supplement dosage was relatively higher in the current study than the previous ones. Findings from the current study suggest that correction of iron deficiency in U.S. infants and toddlers reduced the abundance of putative pathogens and that in addition of vitamin E to therapeutic iron supplement could potentially beneficially alter the gut microbiome in infants and toddlers with iron deficiency, and undergoing iron repletion.
Although the sample size was below the recruitment goal, the non-significance of ferritin concentration between groups (22.9 ± 20.1 vs. 22.2 ± 20.8) is unlikely due to a lack of power. Nonetheless, this is the first study to our knowledge that tested the effects of supplementing iron therapy with the antioxidant, anti-inflammatory vitamin E on iron repletion efficacy, inflammation and gut microbiome in iron deficient U.S. infants and toddlers. Future randomized control trials are needed in a less-developed environment where children are more prone to infection and gut inflammation, to test the potential benefit of adding an antioxidant to iron supplementation strategies. Oxidative stress might be altered by the addition of vitamin E [44], and this should be assessed in future studies. Another limitation of the current study is that the time and frequency of vitamin E administration during the intervention was not recorded, which would be helpful to provide clinical utility, as presumably medication administration at different intervals might have different implications on gut microflora.
In conclusion, findings from the current study suggest that among iron deficient U.S. infants and toddlers, using a therapeutic iron dose of 6 mg/kg/day is effective in treating ID during an 8-week period, without inducing persistent inflammatory response. In addition, difference in the gut microbiome associated with the addition of vitamin E to the iron therapy raises the possibility that concurrent antioxidant intake might offer a potentially beneficial adjunctive component to iron supplementation.
Supplementary Material
What is Known/What is New.
What is Known
Iron fortification may cause inflammation.
Iron-induced inflammation could potentially inhibit iron absorption.
Unabsorbed iron may unfavorably alter gut microbiome.
Vitamin E, as an antioxidant, could potentially reduce iron-induced inflammation.
What is New
Adding vitamin E to iron therapy may potentially create a more favorable gut microbiome profile by promoting the growth of butyrate-producing microbes.
Acknowledgments
Jamie Westcott, MS, and Timothy Schardt, PharmD, BCPS,
Clinical Pharmacist for Investigational Drug Services and Anticoagulation, Pharmacy, Children's Hospital Colorado; No authors declare a conflict of interest.
Grant support: Gerber Foundation; NIH/NCRR Colorado CTSI Grant Number UL1 TR001082; NIH K24 DK083772;
Footnotes
This study is registered at clinicatrials.gov: NCT01700426
Author contributions: Minghua Tang: data collection, sample and data analyses, manuscript writing
Daniel Frank: sample and data analyses, expert consultation on microbiome data interpretation, manuscript, manuscript writing
Laurie Sherlock: grant writing, study design and data collection
Diana Ir: sample and data analyses, microbiome data interpretation
Charles Robertson: sample and data analyses, microbiome data interpretation
Nancy Krebs: grant writing, study design, data collection and manuscript writing
The authors have no conflict of interest
References
- 1.American Academy of Pediatrics, C.o.N. Iron. In: Kleinman R, editor. Pediatric Nutrition Handbook. 2009. pp. 403–422. [Google Scholar]
- 2.Lutter CK. Iron deficiency in young children in low-income countries and new approaches for its prevention. J Nutr. 2008;138(12):2523–8. doi: 10.3945/jn.108.095406. [DOI] [PubMed] [Google Scholar]
- 3.Baker RD, Greer FR. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age) Pediatrics. 2010;126(5):1040–50. doi: 10.1542/peds.2010-2576. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. [August 1, 2010];National Health and Nutrition Examination Survey Data. 2010 Available from: www.cdc.gov/nchs/nhanes/htm.
- 5.Schumann K, et al. Monitoring of hematological, inflammatory and oxidative reactions to acute oral iron exposure in human volunteers: preliminary screening for selection of potentially-responsive biomarkers. Toxicology. 2005;212(1):10–23. doi: 10.1016/j.tox.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Nemeth E, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–3. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 7.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102(3):783–8. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 8.Siekmeier R, Steffen C, Marz W. Role of oxidants and antioxidants in atherosclerosis: results of in vitro and in vivo investigations. J Cardiovasc Pharmacol Ther. 2007;12(4):265–82. doi: 10.1177/1074248407299519. [DOI] [PubMed] [Google Scholar]
- 9.Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr. 2005;25:151–74. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- 10.Orozco MN, et al. Antioxidant-rich oral supplements attenuate the effects of oral iron on in situ oxidation susceptibility of human feces. The Journal of nutrition. 2010;140(6):1105–10. doi: 10.3945/jn.109.111104. [DOI] [PubMed] [Google Scholar]
- 11.Westergren T, Kalikstad B. Dosage and formulation issues: oral vitamin E therapy in children. Eur J Clin Pharmacol. 2010;66(2):109–18. doi: 10.1007/s00228-009-0729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nature reviews. Immunology. 2015;15(8):500–10. doi: 10.1038/nri3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annual review of microbiology. 2000;54:881–941. doi: 10.1146/annurev.micro.54.1.881. [DOI] [PubMed] [Google Scholar]
- 14.Ganz T. Iron in innate immunity: starve the invaders. Current opinion in immunology. 2009;21(1):63–7. doi: 10.1016/j.coi.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmermann MB, et al. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d'Ivoire. The American journal of clinical nutrition. 2010;92(6):1406–15. doi: 10.3945/ajcn.110.004564. [DOI] [PubMed] [Google Scholar]
- 16.Dostal A, et al. Effects of iron supplementation on dominant bacterial groups in the gut, faecal SCFA and gut inflammation: a randomised, placebo-controlled intervention trial in South African children. Br J Nutr. 2014;112(4):547–56. doi: 10.1017/S0007114514001160. [DOI] [PubMed] [Google Scholar]
- 17.Dostal A, et al. Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. The Journal of nutrition. 2012;142(2):271–7. doi: 10.3945/jn.111.148643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otten JJ, Hellwig JP, Meyers LD, editors. Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 19.Krebs NF, et al. Effects of different complementary feeding regimens on iron status and enteric microbiota in breastfed infants. The Journal of pediatrics. 2013;163(2):416–23. doi: 10.1016/j.jpeds.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hara N, et al. Prevention of Virus-Induced Type 1 Diabetes with Antibiotic Therapy. Journal of Immunology. 2012;189(8):3805–3814. doi: 10.4049/jimmunol.1201257. [DOI] [PubMed] [Google Scholar]
- 21.Markle JG, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–8. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 22. [2014 August 14];Homo Sapiens UCSC Hg19 Human Genome Sequence from iGenome 2009. 2014 Available from: http://support.illumina.com/sequencing/sequencing_software/igenome.ilmn.
- 23.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature methods. 2012;9(4):357–9. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ewing B, Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome research. 1998;8(3):186–94. [PubMed] [Google Scholar]
- 25.Ewing B, et al. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998;8(3):175–85. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- 26.Edgar RC, et al. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27(16):2194–200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schloss PD, Westcott SL. Assessing and improving methods used in operational taxonomic unit-based approaches for 16S rRNA gene sequence analysis. Applied and environmental microbiology. 2011;77(10):3219–26. doi: 10.1128/AEM.02810-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pruesse E, Peplies J, Glockner FO. SINA: accurate high throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics. 2012 doi: 10.1093/bioinformatics/bts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quast C, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic acids research. 2013;41(Database issue):D590–6. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson CE, et al. Explicet: graphical user interface software for metadata-driven management, analysis and visualization of microbiome data. Bioinformatics. 2013;29(23):3100–1. doi: 10.1093/bioinformatics/btt526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner BD, Robertson CE, Harris JK. Application of two-part statistics for comparison of sequence variant counts. PloS one. 2011;6(5):e20296. doi: 10.1371/journal.pone.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pryde SE, et al. Molecular analysis of the microbial diversity present in the colonic wall, colonic lumen, and cecal lumen of a pig. Applied and Environmental Microbiology. 1999;65(12):5372–5377. doi: 10.1128/aem.65.12.5372-5377.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otten JJ, Hellwig JP, Meyers LD. DRI, dietary reference intakes : the essential guide to nutrient requirements. Washington, D.C: National Academies Press; 2006. p. 543. xiii. [Google Scholar]
- 34.Pereira DI, et al. Dietary iron depletion at weaning imprints low microbiome diversity and this is not recovered with oral Nano Fe(III) MicrobiologyOpen. 2015;4(1):12–27. doi: 10.1002/mbo3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaeggi T, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut. 2015;64(5):731–42. doi: 10.1136/gutjnl-2014-307720. [DOI] [PubMed] [Google Scholar]
- 36.Bailey JK, et al. Commensal Escherichia coli of healthy humans: a reservoir for antibiotic-resistance determinants. J Med Microbiol. 2010;59(Pt 11):1331–9. doi: 10.1099/jmm.0.022475-0. [DOI] [PubMed] [Google Scholar]
- 37.Patwa LG, et al. Chronic intestinal inflammation induces stress-response genes in commensal Escherichia coli. Gastroenterology. 2011;141(5):1842–51. e1–10. doi: 10.1053/j.gastro.2011.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey JK, et al. Distribution of human commensal Escherichia coli phylogenetic groups. J Clin Microbiol. 2010;48(9):3455–6. doi: 10.1128/JCM.00760-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dyar OJ, et al. High prevalence of antibiotic resistance in commensal Escherichia coli among children in rural Vietnam. BMC Infect Dis. 2012;12:92. doi: 10.1186/1471-2334-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maltby R, et al. Nutritional basis for colonization resistance by human commensal Escherichia coli strains HS and Nissle 1917 against E. coli O157:H7 in the mouse intestine. PLoS One. 2013;8(1):e53957. doi: 10.1371/journal.pone.0053957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leatham MP, et al. Precolonized human commensal Escherichia coli strains serve as a barrier to E. coli O157:H7 growth in the streptomycin-treated mouse intestine. Infect Immun. 2009;77(7):2876–86. doi: 10.1128/IAI.00059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly CJ, et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe. 2015;17(5):662–71. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh N, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40(1):128–39. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lund EK, et al. Oral ferrous sulfate supplements increase the free radical-generating capacity of feces from healthy volunteers. Am J Clin Nutr. 1999;69(2):250–5. doi: 10.1093/ajcn/69.2.250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


