Abstract
Host defense (antimicrobial) peptides (HDPs) are produced by virtually all organisms and have an important role in protection against microbial infections. Some naturally occurring peptides such as the human cathelicidin LL-37 and the bovine peptide indolicidin have been shown to inhibit bacterial biofilm development. Rearrangement and substantial modification of the amino acid sequence of these and other HDPs has led to the identification of small synthetic peptides with increased, broad-spectrum anti-biofilm activity that is independent of activity vs. planktonic cells. Some of these peptides have also been shown to act in synergy with antibiotics commonly used in the clinic to prevent biofilm formation and eradicate pre-existing biofilms. Recently, the mechanism of action of one of these peptides (i.e., 1018) was shown to involve binding to and causing degradation of the second messenger stress response nucleotide ppGpp, which plays an important role in biofilm formation and maintenance. Here, we review recent progress in the field of anti-biofilm peptides and propose future directions to further develop these therapeutic agents.
Keywords: bacterial biofilms, anti-biofilm peptides, synergy, anti-infectives, antibiotic resistance
Introduction
Host defense peptides (HDP) are molecules produced by all living organisms. They have been conserved throughout evolution and serve as defense mechanisms against insults such as those imposed by microbial infections and inflammation [1, 2]. These peptides are generally small in size (from 12 to at least 50 amino acids in length) and are cationic due to the presence of excess lysine and arginine amino acid residues [1, 2]. HDPs also contain a high proportion (usually >50%) of hydrophobic residues, which allows them to interact with, and often translocate across bacterial and host membranes, thus enabling their antimicrobial, immunomodulatory and anti-biofilm activities. Here, I will review recent progress on the anti-biofilm properties of synthetic peptides derived from HDPs. Biofilms are multicellular aggregates of surface-associated microorganisms that are estimated to cause at least 65% of all infections in humans, being particularly prevalent in infections affecting medical devices such as catheters, infections on body surfaces (e.g. skin infections, wounds, mucosa, etc.), and in chronic infections [3–7]. Biofilm-related infections are very difficult to treat in the clinic due to their adaptive resistance to most antibiotics and consequent recalcitrance to treatment with conventional antibiotics. Moreover, there are currently no drugs available that selectively target bacterial biofilms and all antibiotics were developed for treating planktonic (free swimming) cell infections [8]. Consequently, there is an urgent need for new strategies to treat biofilm infections.
Based on the observation that the human cathelicidin LL-37 exhibits anti-biofilm activity [9], recent findings have identified small synthetic peptides with increased potency towards biofilms formed by both Gram-negative and Gram-positive bacterial pathogens [10–17]. Results from some of these studies have concluded that the anti-biofilm properties of peptides are independent of their direct antimicrobial activity, since potent anti-biofilm peptides that lacked anti-planktonic cell activity were identified and vice versa, while Burkholderia cenocepacia which is completely resistant to peptides in its planktonic state is susceptible when growing as biofilms [11,17]. In addition, anti-biofilm peptides have been shown to synergize with different classes of conventional antibiotics to eradicate biofilms [18, 19]. Here, I will outline some of the most significant advances made in recent years in the field of anti-biofilm peptides.
Synthetic peptides with anti-biofilm activity
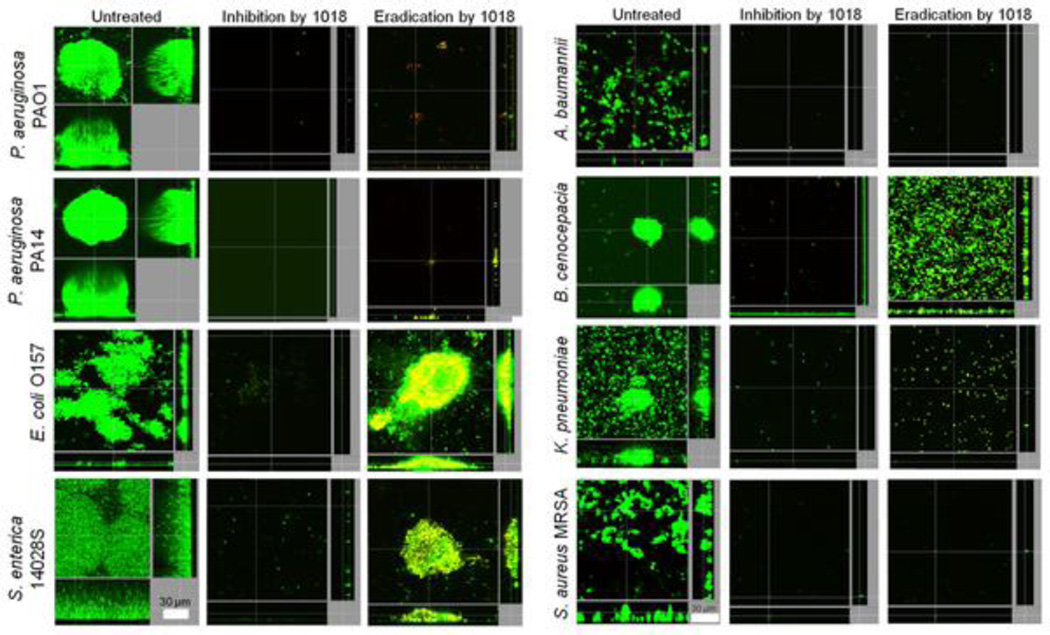
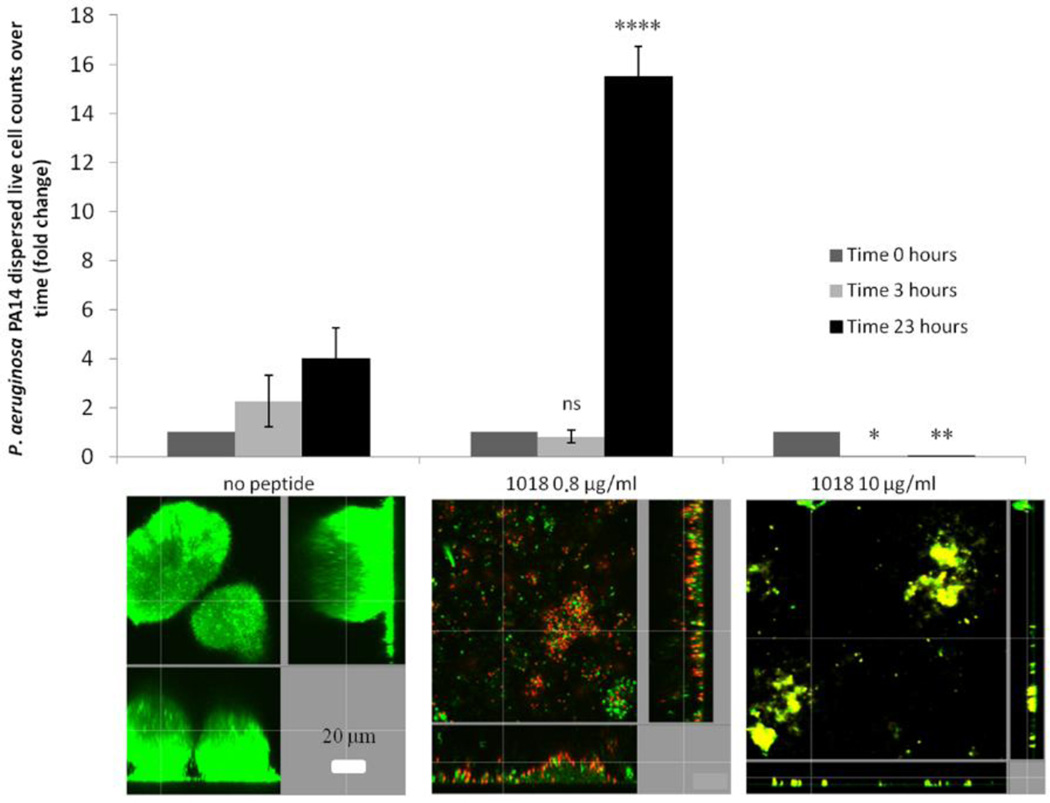
Synthetic peptides exhibiting activity against biofilms are increasingly being reported in the literature [10–17]. The smallest appears to be a cationic peptide of only 9 amino acids in length that can prevent biofilm formation by Gram negatives Pseudomonas aeruginosa and Burkholderia cenocepacia and the Gram-positive microorganism Listeria monocytogenes, despite its very high MICs for bacteria grown under planktonic conditions [11]. More recently, a synthetic dodecapeptide (called 1018; VRLIVAVRIWRR-NH2), based loosely on the amino acid sequence of a peptide (termed Bac2a; RLARIVVIRVAR-NH2) derived from the naturally occurring bovine HDP bactenecin, was identified as an anti-biofilm peptide [17]. Despite presenting very modest direct antimicrobial activity (similar to parent peptide Bac2a) against both Gram-negative and Gram-positive bacteria, peptide 1018 was found to exhibit potent broad-spectrum anti-biofilm activity against P. aeruginosa, Escherichia coli, Acinetobacter baumannii, Klebsiella pneumoniae, methicillin-resistant S. aureus, Salmonella Typhimurium, and Burkholderia cenocepacia at sub-MIC concentrations (Figure 1) [17]. Interestingly, the effect of the peptide on biofilm development varied according to the peptide concentration used. For instance, treatment with very low doses of the peptide (0.8 µg/ml) triggers dispersal of cells from pre-formed biofilms, while it induces cell death within biofilms at higher concentrations (10 µg/ml) [17] (Figure 2). Furthermore, peptide 1018 was shown to target the common stress response nucleotides (p)ppGpp (termed hereafter ppGpp which is the actual effector that binds to RNA polymerase), which play an important role in biofilm development contributing to both biofilm formation and maintenance [17]. Indeed, NMR and thin layer chromatography studies showed that the peptide acted on live bacterial cells to cause degradation of ppGpp and its precursor pppGpp, and in vitro directly interacted with ppGpp [17]. These results indicate that peptide 1018 targets ppGpp and marks it for degradation, thus providing a mechanistic explanation for the broad-spectrum activity of the peptide, since ppGpp is produced by both Gram-negative and Gram-positive bacteria [20]. It is also worth noting that peptide 1018 was originally identified as an immunomodulatory peptide that can selectively enhance chemokine production and polarize cellular differentiation while suppressing the pro-inflammatory response [21, 22]. A range of synthetic peptides based on the amino acid sequence of 1018 were developed and shown to have anti-biofilm and immunomodulatory properties [23].
Figure 1. Broad-spectrum anti-biofilm activity of peptide 1018.
Inhibition of biofilm development was tested by adding peptide 1018 into the flow-through medium at day 0 for a total of 3 days. Eradication conditions involved allowing biofilms to grow for 2 days before treating them with peptide 1018. After 3 days, bacteria were stained green with the all bacteria stain Syto-9 and red with the dead-bacteria stain propidium iodide (merged images show as yellow to red) prior to confocal imaging. Each panel shows reconstructions from the top in the large panel and sides in the right and bottom panels (xy, yz and xz dimensions). Representative images are shown in each case. Taken from reference [17].
Figure 2. Concentration-dependent effect of peptide 1018 on P. aeruginosa biofilm development.
Two-day old P. aeruginosa biofilms were treated with either 0.8 or 10 µg/ml of peptide 1018 and viable dispersed cells were collected from the effluent of the flow cell and viable counts were determined after the indicated times of treatment. Representative confocal microscopy images are shown in each case. Bacteria in flow cell chambers were stained with Syto-9 and propidium iodide as described in the legend of Figure 1. Reprinted from reference [17].
Synergistic interactions with conventional antibiotics
Anti-biofilm peptide 1018 showed strong synergy with different classes of conventional antibiotics to prevent biofilm formation and eradicate pre-existing biofilms [19]. Indeed, when low doses of the peptide were added in the presence of the clinically-important conventional antibiotics ceftazidime, ciprofloxacin, imipenem, or tobramycin, the concentration of antibiotics required to treat biofilms formed by Gram-negative P. aeruginosa, Escherichia coli, Acinetobacter baumannii, Klebsiella pneumoniae and Salmonella enterica, and the Gram-positive bacterial pathogen methicillin-resistant S. aureus, was reduced substantially (up to 64-fold) [19]. Other cationic peptides have also been shown to synergize with different antibiotics to inhibit biofilms formed by methicillin-resistant S. aureus and P. aeruginosa [12, 18]. This represents a new approach to potentiate antibiotic action against biofilms. Peptides that show synergy with clinically relevant antibiotics hold great potential as an adjunctive therapy with antibiotics against drug-resistant infections. Testing these combinations of antibiotics plus peptide in animal models of biofilm-related infections will likely be a major focus of future research.
Therapeutic potential of anti-biofilm peptides
There are several factors limiting the translation of this approach to the clinic. These include potential toxicities, the relatively cost of peptide production, their stability to proteases that abound in the body, and the lack of knowledge about the optimal method of therapeutic administration. In this regard, for example, peptide 1018 exhibits a low toxicity profile both in vitro and in vivo as shown in different studies looking at its effect in animal models [22]. Its small size addresses the cost of goods limitation. In addition, radiolabeling studies have shown that peptide 1018 is rapidly removed from the blood but hits a stable (for 4 hours) level of around 5 µg/ml within 2 minutes of delivery. Moreover it distributes rapidly into the blood, liver, brain and spleen, reaching steady state concentrations that are again stable for up to 4 hours [24]. These positive therapeutic properties, added to the potent anti-biofilm activity of the peptide, make it a good candidate against infected catheters and skin infections/wounds caused by biofilms.
Future directions
Antibiotic resistance is a major health problem worldwide as our entire arsenal of antibiotics is rapidly loosing effectiveness against bacteria that are resistant to multiple antibiotics [25, 26]. This situation is even more worrying in the case of biofilm-related infections, since biofilms are even more resistant to antibiotics compared to free-swimming (planktonic) bacteria and are extremely prevalent in clinical settings, while there are currently no available drugs that effectively target biofilms. Another limitation of the majority of anti-biofilm peptides previously described in the literature is that they are composed entirely of L-amino acids, which can be recognized by bacterial or host proteases that abound during infections and can break down peptides, thus hindering their biological activity [27]. Indeed, bacterial resistance strategies to antimicrobial peptides have been described that include enzymatic degradation of L-amino acid peptides, while host proteases can also degrade such peptides during treatment [28], thus limiting their activity in vivo. Future research is aimed at overcoming this limitation by designing D-enantiomeric peptides, which cannot be recognized by proteases [29] and this strategy has been shown to improve in vivo efficacy in treating model infections. Further rational design of previously identified anti-biofilm peptides will allow deeper characterization of structure-activity relationships, which will likely lead to the identification of improved peptides that selectively target biofilms formed either by individual Gram-negative and/or Gram-positive bacteria, or mixed biofilms such as for example can occur in the oral cavity. Additionally, these studies should take into account important properties, such as the ability to penetrate bacterial cells, synergize with antibiotics and prevent ppGpp accumulation. Finally, efforts should be directed towards establishing different animal models of infection using a multi-host approach. For example, the effect of the peptides may be evaluated in murine biofilm infection models or in invertebrates such as Caenorhabditis elegans, Drosophila melanogaster or Galleria mellonella that enable high throughput screening of potential anti-biofilm drugs. These models will provide a good experimental setting to assess the in vivo anti-biofilm activity of the novel peptides and to test their ability to synergize with different classes of antibiotics.
Acknowledgments
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R21AI098701 and by a grant from the Canadian Institutes for Health Research MOP-74493. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. R.E.W.H. holds a Canada Research Chair in Health and Genomics. C.D.L.F.-N. received a scholarship from the Fundación “la Caixa” and Fundación Canadá (Spain).
Additionally, C.D.L.F.-N and R.E.W.H. are co-inventors of a provisional patent application on the use of cationic anti-biofilm and innate defense regulator (IDR) peptides (U.S. Patent Application No. 61/870,655).
Contributor Information
César de la Fuente-Núñez, Email: cesar@hancocklab.com.
Robert E.W. Hancock, Email: bob@hancocklab.com.
References
- 1.Hancock REW, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. http://dx.doi.org/10.1038/nbt1267 PMid:17160061. [DOI] [PubMed] [Google Scholar]
- 2.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. http://dx.doi.org/10.1038/415389a PMid:11807545. [DOI] [PubMed] [Google Scholar]
- 3.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. http://dx.doi.org/10.1126/science.284.5418.1318 PMid:10334980. [DOI] [PubMed] [Google Scholar]
- 4.O'Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. http://dx.doi.org/10.1146/annurev.micro.54.1.49 PMid:11018124. [DOI] [PubMed] [Google Scholar]
- 5.López D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harb. Perspect. Biol. 2010;2:a000398. doi: 10.1101/cshperspect.a000398. http://dx.doi.org/10.1101/cshperspect.a000398 PMid:20519345 PMCid:PMC2890205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Fuente-Núñez C, Reffuveille F, Fernández L, Hancock REW. Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013;16:580–589. doi: 10.1016/j.mib.2013.06.013. http://dx.doi.org/10.1016/j.mib.2013.06.013 PMid:23880136. [DOI] [PubMed] [Google Scholar]
- 7.Römling U, Kjelleberg S, Normark S, Nyman L, Uhlin BE, Åkerlund B. Microbial biofilm formation: a need to act. J Intern Med. 2014;276:98–110. doi: 10.1111/joim.12242. http://dx.doi.org/10.1111/joim.12242 PMid:24796496. [DOI] [PubMed] [Google Scholar]
- 8.Bjarnsholt T, Ciofu O, Molin S, Givskov M, Høiby N. Applying insights from biofilm biology to drug development - can a new approach be developed? Nat Rev Drug Discov. 2013;12:791–808. doi: 10.1038/nrd4000. http://dx.doi.org/10.1038/nrd4000 PMid:24080700. [DOI] [PubMed] [Google Scholar]
- 9.Overhage J, Campisano A, Bains M, Torfs EC, Rehm BH, Hancock REW. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008;76:4176–4182. doi: 10.1128/IAI.00318-08. http://dx.doi.org/10.1128/IAI.00318-08 PMid:18591225 PMCid:PMC2519444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean SN, Bishop BM, van Hoek ML. Natural and synthetic cathelicidin peptides with anti-microbial and anti-biofilm activity against Staphylococcus aureus. BMC Microbiol. 2011;11:114. doi: 10.1186/1471-2180-11-114. http://dx.doi.org/10.1186/1471-2180-11-114 PMid:21605457 PMCid:PMC3397408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Fuente-Núñez C, Korolik V, Bains M, Nguyen U, Breidenstein EBM, et al. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012;56:2696–2704. doi: 10.1128/AAC.00064-12. http://dx.doi.org/10.1128/AAC.00064-12 PMid:22354291 PMCid:PMC3346644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dosler S, Karaaslan E. Inhibition and destruction of Pseudomonas aeruginosa biofilms by antibiotics and antimicrobial peptides. Peptides. 2014 doi: 10.1016/j.peptides.2014.09.021. pii: S0196-9781(14)00290-3. [DOI] [PubMed] [Google Scholar]
- 13.Nagant C, Pitts B, Nazmi K, Vandenbranden M, Bolscher JG, Stewart PS, Dehaye JP. Identification of peptides derived from the human antimicrobial peptide LL-37 active against biofilms formed by Pseudomonas aeruginosa using a library of truncated fragments. Antimicrob Agents Chemother. 2012;56:5698–5708. doi: 10.1128/AAC.00918-12. http://dx.doi.org/10.1128/AAC.00918-12 PMid:22908164 PMCid:PMC3486595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng X, Sambanthamoorthy K, Palys T, Paranavitana C. The human antimicrobial peptide LL-37 and its fragments possess both antimicrobial and antibiofilm activities against multidrug-resistant Acinetobacter baumannii. Peptides. 2013;49:131–137. doi: 10.1016/j.peptides.2013.09.007. http://dx.doi.org/10.1016/j.peptides.2013.09.007 PMid:24071034. [DOI] [PubMed] [Google Scholar]
- 15.Gopal R, Kim YG, Lee JH, Lee SK, Chae JD, Son BK, Seo CH, Park Y. Synergistic effects and antibiofilm properties of chimeric peptides against multidrug-resistant Acinetobacter baumannii strains. Antimicrob Agents Chemother. 2014;58:1622–1629. doi: 10.1128/AAC.02473-13. http://dx.doi.org/10.1128/AAC.02473-13 PMid:24366740 PMCid:PMC3957903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pompilio A, Scocchi M, Pomponio S, Guida F, Di Primio A, Fiscarelli E, Gennaro R, Di Bonaventura G. Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients. Peptides. 2011;32:1807–1814. doi: 10.1016/j.peptides.2011.08.002. http://dx.doi.org/10.1016/j.peptides.2011.08.002 PMid:21849157. [DOI] [PubMed] [Google Scholar]
- 17.de la Fuente-Núñez C, Reffuveille F, Haney EF, Straus SK, Hancock REW. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 2014;10:e1004152. doi: 10.1371/journal.ppat.1004152. http://dx.doi.org/10.1371/journal.ppat.1004152 PMid:24852171 PMCid:PMC4031209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mataraci E, Dosler S. In vitro activities of antibiotics and antimicrobial cationic peptides alone and in combination against methicillin-resistant Staphylococcus aureus biofilms. Antimicrob Agents Chemother. 2012;56:6366–6371. doi: 10.1128/AAC.01180-12. http://dx.doi.org/10.1128/AAC.01180-12 PMid:23070152 PMCid:PMC3497160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reffuveille F, de la Fuente-Núñez C, Mansour S, Hancock REW. A broad-spectrum anti-biofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob. Agents Chemother. 2014;58:5363–5371. doi: 10.1128/AAC.03163-14. http://dx.doi.org/10.1128/AAC.03163-14 PMid:24982074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potrykus K, Cashel M. (p)ppGpp: still magical? Annu Rev Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. http://dx.doi.org/10.1146/annurev.micro.62.081307.162903 PMid:18454629. [DOI] [PubMed] [Google Scholar]
- 21.Wieczorek M, Jenssen H, Kindrachuk J, Scott WR, Elliott M, Hilpert K, Cheng JT, Hancock REW, Straus SK. Structural studies of a peptide with immune modulating and direct antimicrobial activity. Chem Biol. 2010;17:970–980. doi: 10.1016/j.chembiol.2010.07.007. http://dx.doi.org/10.1016/j.chembiol.2010.07.007 PMid:20851346. [DOI] [PubMed] [Google Scholar]
- 22.Mansour SC, de la Fuente-Núñez C, Hancock REW. Peptide IDR-1018: modulating the immune system and targeting bacterial biofilms to treat antibiotic-resistant bacterial infections. J Pept Sci. 2014 doi: 10.1002/psc.2708. http://dx.doi.org/10.1002/psc.2708. [DOI] [PubMed] [Google Scholar]
- 23.de la Fuente-Núñez C, Mansour SC, Wang Z, Jiang L, Breidenstein EBM, Elliott M, Reffuveille F, Speert DP, Reckseidler-Zenteno SL, Shen Y, Haapasalo M, Hancock REW. Anti-biofilm and immunomodulatory activities of peptides that inhibit biofilms formed by pathogens isolated from cystic fibrosis patients. Antibiotics. 2014;3:509–526. doi: 10.3390/antibiotics3040509. http://dx.doi.org/10.3390/antibiotics3040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolouri H, Sävman K, Wang W, Thomas A, Maurer N, Dullaghan E, Fjell CD, Ek CJ, Hagberg H, Hancock REW, Brown KL, Mallard C. Innate defense regulator peptide 1018 protects against perinatal brain injury. Ann Neurol. 2014;75:395–410. doi: 10.1002/ana.24087. http://dx.doi.org/10.1002/ana.24087 PMid:24339166. [DOI] [PubMed] [Google Scholar]
- 25.Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug. Discov. 2007;6:29–40. doi: 10.1038/nrd2201. http://dx.doi.org/10.1038/nrd2201 PMid:17159923. [DOI] [PubMed] [Google Scholar]
- 26.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009;48:1–12. doi: 10.1086/595011. http://dx.doi.org/10.1086/595011 PMid:19035777. [DOI] [PubMed] [Google Scholar]
- 27.Sieprawska-Lupa M, Mydel P, Krawczyk K, Wójcik K, Puklo M, Lupa B, Suder P, Silberring J, Reed M, Pohl J, et al. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob. Agents Chemother. 2004;48:4673–4679. doi: 10.1128/AAC.48.12.4673-4679.2004. http://dx.doi.org/10.1128/AAC.48.12.4673-4679.2004 PMid:15561843 PMCid:PMC529204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fjell CD, Hiss JA, Hancock REW, Schneider G. Designing antimicrobial peptides: form follows function. Nat. Rev. Drug Discov. 2011;11:37–51. doi: 10.1038/nrd3591. PMid:22173434. [DOI] [PubMed] [Google Scholar]
- 29.de la Fuente-Núñez C, Reffuveille F, Mansour SC, Reckseidler-Zenteno SL, Hernández D, Brackman G, Coenye T, Hancock REW. D-enantiomeric peptides that eradicate wild-type and multi-drug resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chemistry & Biology. 2015;22:196–205. doi: 10.1016/j.chembiol.2015.01.002. http://dx.doi.org/10.1016/j.chembiol.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]